Abstract
Use of biodegradable polymers for biomedical applications has increased in recent decades due to their biocompatibility, biodegradability, flexibility, and minimal side effects. Applications of these materials include creation of skin, blood vessels, cartilage scaffolds, and nanosystems for drug delivery. These biodegradable polymeric nanoparticles enhance properties such as bioavailability and stability, and provide controlled release of bioactive compounds. This review evaluates the classification, synthesis, degradation mechanisms, and biological applications of the biodegradable polymers currently being studied as drug delivery carriers. In addition, the use of nanosystems to solve current drug delivery problems are reviewed.
Introduction
The use of biodegradable polymers for biomedical applications is continually increasing and evolving.Citation1–Citation5 The present work reviews the most recent literature on the characteristics, properties, and applications of biodegradable polymers already in use or under investigation as drug nanocarriers.
The main advantage of biodegradable polymers is that the products of degradation are not toxic or are completely eliminated from the body by natural metabolic pathwaysCitation6,Citation7 with minimal side effects.Citation8–Citation12 These degradation products define the biocompatibility of a polymer.Citation13–Citation15 For example, poly(lactic-co-glycolic acid) (PLGA), poly(glycolic acid) (PGA) and poly(lactic acid) (PLA) have been approved by the US Food and Drug Administration (FDA) for certain medical applications, because their products of degradation are eliminated from the body in the form of carbon dioxide and water.Citation13 Nevertheless, these polymers may reduce local pH, affecting the integrity of the cells in their microenvironment, thus limiting their application in tissue scaffolds. Biodegradable polymers can be of natural origin or “biopolymers” produced by living organisms during the cell growth cycle.Citation4,Citation16 They can also be manufactured, which is another advantage of these materials because they show great synthesizing flexibility.Citation17
Biodegradable polymers have the potential to become part of new medical devices with specific and unique physical, chemical, and mechanical properties, such as electrical conductivity, optical properties, chemical reactivity, and mechanical strength.Citation18,Citation19 The most important biomedical goal of biodegradable polymeric materials is the development of matrices to control the release of drugs into specific sites in the body.Citation20 Therefore, there are nanodrugs specifically designed to carry therapeutic molecules that are directly coupled, functionalized, coated, or entrapped in devices produced by controlled manipulations of size and shape at the nanometer scale.Citation21 Additionally, these polymeric materials have been used as orthopedic devices to replace bones or blood vessels and surgical sutures.Citation22,Citation23 Biodegradable polymeric materials provide a platform on which nanoscaled structures can be developed, and this property can be used in numerous medical applications, from surgical implants to binding matrices of drugs.Citation24–Citation26
Nanoparticles made with biodegradable polymers have been an important instrument in the treatment of neurodegenerative diseases, because of their ability to cross the blood-brain barrier and their high drug-loading capacity,Citation17 or in the diagnosis and treatment of cardiovascular disease, because of their size, shape, and an available surface area for biomolecule conjugation.Citation27 Because polymeric nanoparticles have the capability of long-term protection, they can preserve the integrity of drug molecules for more efficient deliveryCitation28 in the case of an unstable active compound. For example, PLGA nanoparticles can contain nitric oxide molecules using (trans-[RuCl([15]ane)(NO)]2+) as a nitric oxide donor,Citation29 PLGA-poly(ethylene glycol) (PEG) protect curcumin from macrophages,Citation30 and PEG reduces the toxicity and increases the stability of gold nanoparticles.Citation31
Biodegradable polymeric nanoparticles influence the pharmacokinetic behavior of drugs by fine-tuning release, like the sustained release of nerve growth factor encapsulated in polyphosphoesters.Citation32 Some biopolymers degrade in specific pH environments, like paclitaxel poly(β-amino ester) nanoparticles which dissolve in the intracellular pH range of 5.1–6.5.Citation33
Thus, convenient characteristics of nanoparticles can be achieved by combining different polymers. For instance, PLGA provides a hydrophobic core that is able to retain oily material,Citation34 while PEG reduces the interaction with untargeted tissues, increasing specificity.Citation35
This review begins by describing the mechanisms of synthesis and degradation of a variety of biodegradable polymers. It also discusses encapsulation techniques used to prepare nanoparticles from these polymers, their biomedical applications, cellular uptake, and factors that affect bioavailability and internalization of nanoparticles.
Classification
Biodegradable polymers can be broadly classified, according to their origin, as natural and synthetic polymers. Natural polymers are the first option in biomedicine, due to their abundance in nature and biocompatibility. However, their full exploitation has been limited because of batch-to-batch variations in properties or risk of viral infections.Citation36,Citation37 This is the case with parvovirus B19 infection transmitted by blood products such as fibrin, which is widely used as a surgical adhesive, hemostatic agent, and sealant.Citation38 Synthetic polymers, on the other hand, have manufacturing flexibility and reproducibility.Citation39
Classification of biodegradable polymers is mainly restricted to their origin.Citation6,Citation37 In , we include subclassifications of common polymers used or currently under study for biomedical applications. Aliphatic polyesters, such as PLA, PLGA, and PGA, are the most used synthetic polymers.Citation40 In this review, we give emphasis to synthetic biodegradable polymers that, in our opinion, have more potential in commercial applications where process manufacture reliability and batch reproducibility are often required.
Table 1 Data on biodegradable polymers based on natural and synthetic origin
Synthesis
shows several of the reactions involved in synthesis of these polymers, including ring opening, polycondensation, bulk synthesis, dehydrative coupling, transesterification, and polymerization.
Table 2 Different pathways involved in polymer synthesis
Ring opening
Synthesis of biodegradable polymers can be done by ring-opening polymerization, which is the most common synthetic pathway of most biodegradable polymers (α-hydroxy acids, α-amino acids, polydepsipeptides, polyesters).Citation6 The six-member cyclic diesters, such as glycolide, L-lactide, and D,L-lactide, are used to synthesize PGA, poly(L-lactic acid), and poly(D,L-lactic acid), respectively by this method. For example, the production of poly(ester amide) starts with synthesis of 6-methylmorpholine-2,5-dione by heating of N-(2-bromopropionyl) glycine sodium salt. Ring-opening polymerization is then carried out in the bulk at 130°C for 28–48 hours using stannous octoate [Sn(Oct)2] as a catalyst ().Citation41
Polycondensation
Biodegradable polymers can also be synthesized by polycondensation (), which is a condensation reaction between polymeric molecules.Citation42 Ring-opening of cyclic diesters, self-polycondensation of hydroxy acids, or polycondensation of diacids and diols, produce polyesters.Citation43 The polycondensation technique is cheaper than open ring polymerization and has several variants, including melt polycondensation, interfacial polycondensation, solution polycondensation, and solid/liquefied state polycondensation.Citation43 However, this method is hampered by difficulty in obtaining high molecular weight polymers, achievement of specific end groups, and preparation of well defined copolyesters.Citation44
Bulk synthesis
As mentioned before, open ring polymerization is the most important method for synthesis of biodegradable polymers, but other methods can be used. Polyurethanes can be produced by bulk synthesis using diisocyanate (). The reaction occurs by dehydrating the macrodiol followed by addition of 4,4′-diphenylmethane diisocyanate under a nitrogen atmosphere. The resulting polymer is precipitated in water and then vacuum-dried.Citation45,Citation46
Dehydrative coupling
Polyanhydrides are synthesized by dehydrative coupling of carboxyl groups (). This reaction occurs by the conversion of the carboxyl group to a mixed anhydride with acetic acid. The prepolymer is then subjected to melt polycondensation. Thereby, generation of high molecular weight species is limited by reversible thermal depolymerization. In addition, acetic anhydride reflux may be unsuitable for heat-sensitive monomers.Citation47
Synthesis of poly(ortho esters)
Poly(ortho esters) (POE), consisting of a family of four members, can be produced as follows: POE I can be synthesized by transesterification between a diol and diethoxytetrahydrofuran; POE II can be synthesized by the reaction of diketene acetal 3,9-bis(ethylidene-2,4,8,10-tetraoxaspiro[5] undecane) (); and POE III can be synthesized by polymerization of a triol with an ortho ester, whereas POE IV is a modification of POE II by hydrolysis of the ortho ester linkage.Citation48
Mechanisms of degradation
As mentioned before, the degradation of biodegradable polymers may lead to complete elimination of degradation products from the organism. Since the products of polymer degradation can produce alterations in a cell, such as inflammatory responses,Citation14 the biocompatibility of the biodegradable polymer is defined by these degradation products.Citation49
The mechanisms of degradation for different polymers depend on the chemistry, molecular weight, and morphology of each type of polymer, and environmental factors such as pH or temperature also play an important role. Degradation occurs mainly by hydrolysis, oxidation, or enzymatic reactions.Citation37,Citation50
The stability of the polymeric material affects its efficiency.Citation49,Citation51 Therefore, knowledge about its mechanisms of degradation is crucial to select a polymer for specific applications. The most important mechanisms of polymeric degradation are discussed below.
Hydrolytic degradation
The hydrolytic mechanism involves reaction of vulnerable bonds in the polymer with water molecules, resulting in reduction of the main or side chains in the polymer. This reaction is a second order nucleophilic substitution, in which the rate of the reaction is proportional to the concentration of water and hydrolytic bonds of the polymer. Chemical groups that react with water and contain O, N, S, or P give a positive charge to the nearby carbon atom.Citation15
The hydrolytic activity depends mainly on the charge value of the reacting carbon atoms, but factors such as conjugate structures, side groups, and dielectric constant or water solubility also affect the hydrolytic activity of the polymer. In polyanhydrides, for example, degradation depends on the backbone of the polymer, and the relationship between the hydrophilic and hydrophobic components can be adjusted to regulate degradation. This condition is possible due to the two hydrolysable sites (, structure 7) of the repeating unit.Citation50
Table 3 Summary of chemical and biotechnologic properties of polymers used in drug delivery
Polymers with hydrolysable backbones, ie, polyesters, polyamides, polyurethanes, and polyanhydrides, are susceptible to hydrolytic biodegradation under particular conditions. In aliphatic polyesters, for instance, the rate of degradation is more related to water accessibility into the matrix rather than the intrinsic rate of ester cleavage. Graft copolymerization affects reduction of the polymeric main and side chains, for instance, polymers such as PLA, which display improved biodegradability and thermal stability when grafted onto chitosan. Copolymerization can reduce the degradation rate, but also increases it. Hydrolytic cleavage degradation of poly(ester amides) can be enhanced by incorporating amino acid units into the polymers.Citation50
Polymer degradation causes erosion of the matrix. If penetration of water into the polymer matrix produces a homogeneous erosion of the material, the behavior is called bulk erosion. On the other hand, if erosion of the material represents a mass loss in the surface while the bulk remains intact, degradation is termed surface erosion.Citation50 A four-step mechanism for degradation of PGA in a buffer solution has been proposed elsewhere.Citation52 Water is initially absorbed into the sample, followed by polymer molecular weight reduction throughout the sample. As soon as a critical molecular weight is reached, the polymer starts to diffuse from the surface and finally a combination of water diffusing into the polymer structure and polymer erosion on the surface causes complete degradation.Citation52
Oxidation
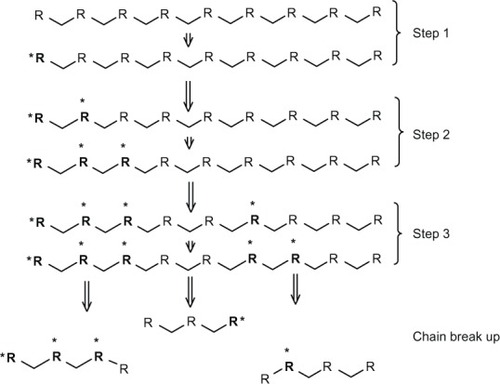
The inflammatory response of cells like leukocytes and macrophages produces reactive oxygen species such as nitric oxide, hydrogen peroxide, or superoxide. These species cause degradation of polymers.Citation49 Some biopolymers, such as polyethylene, polyether, and polyurethanes, are more suitable to degradation by an oxidation reaction, because their structure can easily generate free radicals. On the other hand, polyesters and silicon are less susceptible to oxidation.Citation15,Citation53 This kind of degradation follows the general mechanism shown in : initiation of free radicals generated by inflammatory cells or by thermal, photochemical, and radiation processes; proliferation of free radicals by a series of reactions with oxygen; the free radicals produced react with the polymer and are transferred to different regions of the polymer chain; and finally, cleavage of the polymeric chain at the free radical site forms shorter segments.Citation15 A block copolymer of PEG/poly(propylene sulfide)/PEG that self-assembles to form nanosized vesicles has been developed.Citation54 The mechanism consists of oxidation of central block sulfide moieties to sulfoxides and ultimately sulfones, increasing the hydrophilicity of the initially hydrophobic central block.Citation54
Figure 1 Scheme of general mechanism of oxidation reaction. Step 1 is an initiation that involves generation of free radicals. Step 2 (proliferation) is an increase in the number of free radicals by a series of reactions with oxygen in the surrounding polymer. Step 3 is a transfer of free radicals to different sites in the polymer chain. Finally, the break-up occurs, leading to formation of new chain ends.
Notes: R: Polymer chain. *Free radical.
Enzymatic degradation
On the other hand, enzymatic degradation involves hydrolysis catalyzed by enzymes known as hydrolases, such as proteases, glycosidases, and phosphatases. This mechanism follows surface erosion, in that the polymers lose material from the surface because the enzyme cannot penetrate into the polymer. Enzymatic degradation is affected by factors such as interactions with the polymeric chain (diffusion or adsorption of the enzyme), physicochemical properties of substrates (molecular weight, surface area), properties of the enzyme, environmental conditions (pH, temperature), and presence of activators or inhibitors in the medium.Citation49 For example, rapid swelling and enzymatic degradation of starch reduces its applications in controlled delivery. However, acetylation of starch reduces its enzymatic degradation, improving its potential as a carrier system.Citation55
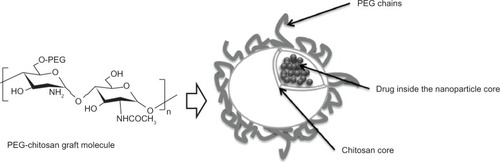
The enzymatic hydrolysis of chitosan-grafted PEG and chitosan nanoparticles has been studied using lysozyme, a 14 kD cationic protein that catalyzes hydrolysis of the 1,4-β-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine. It was demonstrated that nanoparticles made of chitosan showed more enzymatic degradation as compared with nanoparticles made of chitosan-grafted PEG; this reduction in degradation rate can be attributed to the presence of PEG.Citation30 shows a nanosphere of chitosan-grafted-PEG with the active component inside the nanoparticle core.
Figure 2 Scheme of a chitosan nanosphere with grafted PEG. Due to its biodegradability, biocompatibility, and minimal toxicity, chitosan and PEG have been used to develop nanoparticle carrier systems for poorly soluble drugs. The chitosan core can encapsulate drugs, allowing for their subsequent sustained release.
Abbreviation: PEG, poly(ethylene glycol).
Strategies to control drug delivery
Controlled degradation of polymers helps to maintain drug levels within a suitable therapeutic window,Citation6 and since different drugs require different release systems, polymeric matrices must be carefully engineered for a specific drug and a specific target.Citation6 For instance, unmodified chitosan nanoparticles of ammonium glycyrrhizinate showed a burst effect followed by a continuous release profile,Citation56 while modified chitosan/alginate enabled pH-dependent release of insulin after oral administration.Citation57
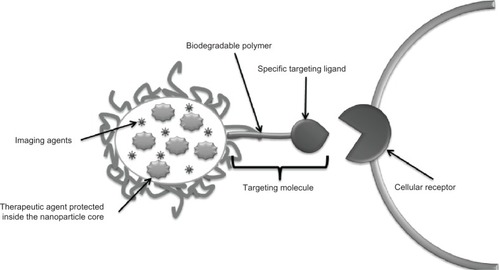
As mentioned earlier, understanding of the degradation mechanisms of a material plays a crucial role in the efficacy of a drug delivery device. The polymeric matrix has to degrade under physiologic conditions in a controlled manner to allow sustained release of the drug.Citation51 The two main strategies for controlled drug delivery are classified as temporal () or targeted () drug delivery systems.Citation6,Citation49,Citation51
Figure 3 Mechanisms for temporal controlled-release drug systems. (A) Dissolution of a polymer with slow break-down that delays exposure of drug to water from the environment of the delivery system. (B) Drug diffusion-controlled release through gaps in insoluble polymeric devices. (C) Controlled flow using osmotic forces on a semipermeable polymer matrix.
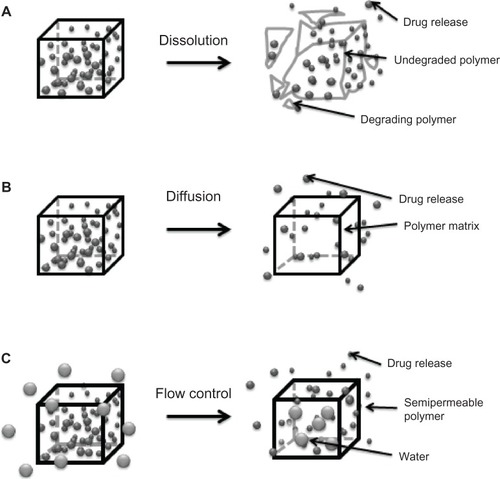
Figure 4 Strategy to create targeted drug delivery systems. Therapeutic tools like genes, proteins, and small drug molecules, as well as imaging tools such as fluorescent probes or magnetic contrast agents are encapsulated inside the nanoparticle core. In parallel, targeting molecules like specific antibodies or recognition peptides are located on the nanoparticle surface.
A temporal delivery system looks for the extended release of drugs or their release in a specific period of time. This mechanism allows the concentration of the drugs in the body to be maintained at a constant level, thereby reducing the frequency of administration. It also protects the molecule from the environment and ensures maximum benefits from the drug.Citation51 Temporal release delays diffusion of the molecule out of the polymeric matrix, inhibiting diffusion or controlling drug flow through the matrix.Citation51 These strategies involve manipulation of some physicochemical properties of the polymers, eg, copolymerizing or blending of polymers in order to change the degradation behavior.
Chitosan/glyceryl monooleate has been used to form paclitaxel-loaded nanoparticles with high entrapment efficiency (98%) and increased mucoadhesive properties. These nanoparticles have a hydrophilic surface and a hydrophobic core, and also have a significant positive charge and sustained-release characteristics. These characteristics intensify the cellular association of paclitaxel and expand the duration of the therapeutic effect of the drug. Delivery starts with a burst release probably due to the tendency of chitosan to swell, followed by slow release. Dose-response studies show that nanoparticles increased MDA-MB-231 cell death when compared with paclitaxel solution alone.Citation58
A targeted drug delivery system is designed to seek a specific site of action in the body.Citation6 This mechanism avoids two main situations, ie, side effects caused by drugs in contact with untargeted tissue and drug loss due to natural distribution in the body.Citation51 A taxane-loaded PGA-PEG nanoparticle conjugated to the A10 RNA aptamer, which binds to prostate-specific membrane antigen, has been developed. This nanoparticle shows enhanced delivery to tumor cells compared with the equivalent nanoparticles lacking the aptamer. The ability of conjugated nanoparticles to maintain a significantly higher concentration in the cell is due to uptake by the targeted ligand.Citation59 Surface modification of the polymeric matrix by addition of enzymatic recognition sites modulates the degradation rate or cellular uptake of the nanoparticle (eg, by means of receptor-mediated gene delivery) enhancing the transfection efficiency.Citation60 Thus, the transfection efficiency of DNA was improved using a folic acid-conjugated stearic acid-grafted chitosan/pDNA complex in SKOV3 cells.Citation60
Encapsulation techniques
Several techniques have been developed to encapsulate drugs using biodegradable polymers in the last 60 years. The major aim of these techniques is to protect active compounds from environmental deterioration and to obtain controlled drug release.Citation20,Citation61 These techniques are classified according to the initial state of the polymer into two main categories, ie, nanoparticles obtained from polymerization of a monomer or nanoparticles obtained from a macromolecule.
The polymerization of a monomer to produce nanoparticles is a fast and easily scalable method, with high entrapment efficiency. However, residual molecules in the medium (surfactant, monomer) can be toxic and require extensive purification of the nanoparticles.Citation62
From polymerization of a monomer
Emulsion polymerization
Emulsion polymerization is a technique that can be classified according to continuous phase as organic or aqueous emulsion polymerization.Citation62 For instance, polymerization of poly(alkyl cyanoacrylate) monomers is a process in which drops of hydrophobic monomers are emulsified in the aqueous phase, and usually requires a pH below 3.5 and constant stirring. Polymerization is confined to the surface of the micelle due to the high ionic strength of the medium.Citation63,Citation64 Initialization can occur by clash with an initiator (ion or free radical) or radiation of the monomer molecule. Continuous organic phase methodology may involve toxic organic solvents, surfactants, monomers, and initiators, which represents a disadvantage for biomedical applications.Citation62
Interfacial polymerization
Interfacial polymerization involves introduction of an alcohol solution of the monomer into an aqueous solution containing surfactants and ions that induce polymerization.Citation62,Citation65 This method has some advantages, such as high efficiency of hydrophilic drug encapsulation (up to 90%) and spontaneous polymer-nanoparticle formation.Citation62 For instance, nanoparticles of poly(n-butylcyanoacrylate) with diameters of 132 nm prepared by interfacial polymerization have shown excellent transdermal penetration. The major disadvantage of this technique is the use of organic solvents in the continuous phase.Citation65
Polycondensation
Polycondensation starts with formation of an oligopolymer membrane at the interface of the oil or water droplet in the emulsion. The inner phase formed through the condensation reaction in the core is forced away from the reaction site, followed by growth of the polymer membrane.Citation66,Citation67 Polymeric nanoparticles of polysaccharides with a size range of 200–300 nm have been produced using this method.Citation68
From macromolecules
Emulsion solvent evaporation
This method involves two steps; the first one is formation of an emulsion in a dispersed organic phase containing dissolved polymers in an aqueous phase while constantly stirring. The final step is gradual evaporation of the solvent under low pressure to dry the particles.Citation62 The modified solvent evaporation method allows production of porous polylactide microspheres that can be aerosolized more easily than nonporous ones, increasing the breathable fractions of inhaled particles. In addition, these particles can maintain overall particle volume while reducing acidic degradation products. These properties allow for diverse medical applications, such as engineering scaffold tissue, drug delivery carriers, and adsorption matrices.Citation69 Emulsion solvent evaporation appears to be appropriate for laboratory conditions, but is not suitable for large-scale production.Citation70
Salting out
This method consists of separation of water-miscible solvents from aqueous solution by the salting out effect. It provides less stress to protein drugs,Citation71 and allows use of a wide selection of solvents to produce drug nanoparticles. This solvent variation affects particle size, as well as the porosity and roughness of the nanoparticle.Citation72 A solution of polymer, drug, and a solvent, normally acetone, is emulsified into an aqueous solution that contains a salting-out agent and a colloidal stabilizer. The salting-out agent can be an electrolyte such as calcium chloride, magnesium acetate, or a nanoelectrolyte such as sucrose. This agent may compete for water of hydration causing phase separation.Citation73 The result of acetone dilution into water is formation of nanoparticles, followed by solvent and salting-out agent elimination via cross-flow filtration. This is an easy procedure for encapsulation of drugs. However, it is only suitable for loading lipophilic drugs and requires several washing steps.Citation62
Emulsion/solvent diffusion
This method is very similar to the salting-out technique. In this case, the polymer is dissolved into an organic solvent (partially miscible with water), and the solution is saturated with water in order to reach an initial thermodynamic equilibrium between water and the organic phase. The nanoparticles are formed by solvent diffusion into the aqueous phase.Citation62 A modified emulsion/solvent diffusion method was used to produce PLGA nanoparticles (280–292 nm). The modified method can produce PLGA nanoparticles with a simple preparation scheme, in which the mixture of water-miscible organic solvent prevents nanoparticle aggregation.Citation74
Supercritical fluids
This is a widely applied technique,Citation75–Citation78 using the advantageous and exceptional thermodynamic, heat-transfer, and mass-transfer transport properties of supercritical fluids.Citation79 They have gas-like viscosity and higher diffusivity than traditional solvents, and the density can be tuned by a change in pressure. However, use of this technique is limited due to the poor solubility of high molecular mass (10,000) polymersCitation20 and difficulty in dissolving strong polar substances in supercritical CO2.Citation62 A supercritical fluid technique was used to produce 5-aminosalicylic acid nanoparticles via a method in which the particle size and morphology could be tuned by adjusting parameters such as concentration, solution flow rate, or temperature. The solution flow rate significantly affected drug loading and entrapment efficiency.Citation19
Solvent displacement
Using this procedure, drugs and polymers are dissolved in a water-miscible solvent. Next, the organic solution is injected into an aqueous solution under stirring. Nanoparticles are formed immediately by solvent diffusion. The organic solvent is then eliminated from the suspension under reduced pressure. The major disadvantage of this technique is finding a drug/polymer/solvent/nonsolvent system in which nanoparticles can be formed with efficient entrapment of the drug. Furthermore, this method is not advantageous for encapsulation of water-soluble drugs, due to the fast diffusing rate from the organic phase into the water phase.Citation62 However, a modified method has been developed to make 300 nm Gantrez™ nanoparticles (poly[methyl vinyl ether-co-maleic anhydride]) with encapsulated ovalbumin which were able to increase antibody titers (immunoglobulins G1 and G2a) in mice.Citation80
Nanoparticles must be free of contaminants for biomedical application. The main processes for nanoparticle purification include ultracentrifugation, dialysis, gel filtration, and cross-flow filtration.Citation62,Citation81
Moreover, chemical factors such as pH can destabilize the system,Citation82 while physical variables such as storage temperature, Brownian motion, and gravitational forces can produce aggregation, diffusion, or sedimentation of the colloidal particles.Citation82 A suitable stabilizer or surfactant can be used in order to prevent aggregation or sedimentation phenomena.Citation83
The physical and chemical stability of nanoparticles can be improved by elimination of water.Citation62 The most common method used is freeze-drying or lyophilization.Citation82 Thereby, the nanoparticle suspension can be primarily frozen followed by elimination of water via sublimation.Citation62,Citation82 Use of a cryoprotectant such as glucose, sucrose, sorbitol, maltose, gelatin, or mannitol is important to avoid changes in the nanoparticles during the freezing step.Citation82 Finally, nanoparticles can be stored at room temperature, but some studies have shown that the best long term-storage is achieved by refrigeration at 4°C.Citation82,Citation84
Biomedical applications
The main goal of nanotechnology as applied in medicine corresponds to use of engineered materials that facilitate interaction of drugs with their biological targets while minimizing side effects.Citation17 In order to use a biodegradable polymer in biomedicine, its degradation products must be nontoxic and should be easily eliminated from the body.Citation15 Meanwhile, biodegradable polymeric nanoparticles have been used in scaffolds to promote tissue regeneration.Citation85,Citation86 Most frequently, they serve as delivery systems for drugs used in the treatment of cancer and neurodegenerative disorders, as antimicrobials, antivirals, and immunosuppressants, and more recently have been used in cardiovascular disease and osteoporosis.Citation39,Citation87–Citation89
Cancer
The toxicity of cytostatic drugs is not confined to malignant cells, so it is desirable to encapsulate them in target-specific controlled-release polymers.Citation89 Nanoparticles provide better penetration of therapeutic substances within the body at a reduced risk and could diminish the multidrug resistance that characterizes many anticancer drugs.Citation90
Among the various biodegradable synthetic and natural polymers used to prepare nanoparticles for delivery of anticancer agents, PLA, PLGA, and poly(caprolactone) are the most often used.Citation90 For example, paclitaxel-loaded PLGA nanoparticles with antibodies capable of epithelial cell adhesion have been developed.Citation91 It was demonstrated that paclitaxel-loaded nanoparticles equipped with such antibodies enhanced cellular uptake compared with nanoparticles without the ligand. Additionally, nanoparticles with epithelial cell adhesion capability showed increased antiproliferative activity of paclitaxel.
Moreover, multidrug resistance, which characterizes many anticancer drugs, like docetaxel, was overcome by docetaxel-loaded polycaprolactone/Pluronic F68 nanoparticles. Such nanoparticles increased the level of uptake of this drug in a docetaxel-resistant human breast cancer cell line.Citation90
Cisplatin-based therapeutic regimens have been limited by severe side effects and toxicity to organs, including the liver, kidney, heart, and nervous system. Nanosized drug carriers have been developed to minimize the side effects of cisplatin and enhance its antitumor efficacy through the form of polymeric micelles, liposomes, and solid lipid nanoparticles during cancer therapy. However, the encapsulation efficiency of cisplatin is still poor.Citation34
Carboxymethyl cellulose core nanoparticles made from PLGA-monomethoxy-PEG copolymers and d-alpha tocopheryl polyethylene glycol 1000 succinate as an emulsifier are homogeneous nanoparticles devoid of debris and aggregation, that enhance the loading efficiency of cisplatin. Hepatic necrosis and atrophy in the kidney in mice treated with free cisplatin has been observed, but not in mice treated with cisplatin-loaded nanoparticles. Further, organs such as the heart, spleen, and lungs did not show any abnormalities.Citation34
Neurodegenerative disorders
Neurodegenerative disorders are mostly recognized to be Alzheimer’s disease and Parkinson’s disease. Their treatment remains a major challenge, since drug delivery to the brain depends on the ability of the formulation to pass the numerous protective barriers surrounding the central nervous system.Citation17 Nanotechnology provides engineered materials with functional organization on the nanometer scale, which are capable of penetrating the blood-brain barrier.Citation92
Curcumin is a water-insoluble natural product with antiamyloid and antioxidant activity, both of which are properties recognized as being highly beneficial in the treatment of Alzheimer’s disease.Citation93,Citation94 New formulations of curcumin attempt to target the drug towards its site of action in the brain and to overcome the problem of insolubility. Thus, a new water-soluble PLGA-coated curcumin nanoparticle has been successfully synthesized and coupled with Tet-1 peptide, giving neuron affinity to the nanoparticles.Citation94
Other formulations designed to treat neurodegenerative disorders include chitosan nanoparticles loaded with subfragments of amyloid-beta, which permeated the blood-brain barrier and were nonimmunogenic;Citation95 a transdermal nanoemulsion gel containing ropinirole to treat Parkinson’s disease, which improved the relative bioavailability of the drug with no toxicity;Citation96 and dopamine incorporated into a smart nanocrystal conjugated with PEG and covered by a carbohydrate shell allowing recognition of glucose transporter 1.Citation97
Detailed reviews on current nanotechnology-based delivery systems applied to the treatment of Alzheimer’s disease and Parkinson’s disease have been recently published.Citation17,Citation98
Cardiovascular disease
Current research on biodegradable polymers focuses on their applications in vascular tissue engineering and diagnosis (imaging), while fewer works involve nanocarriers for drug delivery.Citation39,Citation99
Nanocarriers for drug delivery have been investigated for treatment of atherosclerosis and restenosis.Citation27 A novel sustained-release drug delivery system using tacrolimus-eluting biodegradable nanofibers composed of poly(L-lactide-co-glycolide) and tacrolimus was developed. This formulation reduced intimal hyperplasia and preserved endothelialization even in a venous stricture and might be useful for preventing recurrent pulmonary venous obstruction after correction of total anomalous pulmonary venous connection.Citation100
Subsequently, localized drug delivery from drug-eluting stents has been accepted as one of the most promising treatment methods for preventing restenosis after stenting. A controlled-release formulation of epigallocatechin-3-O-gallate in PLCL-coated stents was made to suppress migration and invasion of vascular smooth muscle cells as well as platelet-mediated thrombosis.Citation101 With this purpose, industry has also manufactured drug-eluting stents containing antiproliferative drugs like paclitaxel and sirolimus that promote integration of the stent within the vessel wall.Citation27
Microbial and parasitic infections
Because of their nature and size, polymeric nanoparticles are easily endocytosed by phagocytic cells, which might contain the pathogen. A recent review includes an indepth description of biodegradable polymers in nanoparticles for the treatment of intracellular microbial infections.Citation89
Viral infections
Numerous groups have proposed biodegradable nanoparticles and microparticles as vaccine delivery systems, aiming at induction of both humoral and cellular immune responses.Citation39,Citation102,Citation103 For example, PLGA has been applied to encapsulate the hepatitis B surface antigen,Citation102 and PEG and Pluronic-poly(ethylenimine) (PEI) has been used to formulate nanogel carriers of nucleoside reverse transcriptase inhibitors decorated with brain-targeting peptide molecules, which demonstrated high efficacy for inhibition of human immunodeficiency virus-1 in the brain.Citation104 Another example of biodegradable nanoparticle-based vaccines is the intranasal formulation of entrapped PLGA nanoparticles with ultraviolet-killed porcine reproductive and respiratory syndrome virus antigens.Citation105
Nanohydrogels of pure chitosan obtained by ammonia-induced physical gelation of a reverse emulsion in a triglyceride were loaded with human immunodeficiency virus-1 p24 and immunoglobulin G and have been proposed as versatile carriers for a variety of biomolecules.Citation106
Osteoporosis
The problems associated with current orthopedic drug delivery systems include limited ability to reach the target site by conventional systemic administration and weak bonding of the drug with its carrier, which causes nonspecific bone formation in unaffected areas. For instance, an implantable system capable of long-term drug release of a bone morphogenetic protein derived and peptide loaded onto nanocrystalline hydroxyapatite and dispersed into PLGA was designed.Citation107 More applications of nanohydroxyapatite have been described elsewhere.Citation108
Mitochondria-targeted drugs
Efficient delivery of various drugs targeting mitochondria can be achieved by designing targeted nanoparticles based on blends of biodegradable polymers, such as a targeted PLGA-block-PEG-triphenylphosphonium polymer with either non-targeted PLGA-block-PEG-OH or PLGA-COOH.Citation109
Bioavailability
Biodegradable polymers in controlled drug release systems play an important role in increasing the bioavailability of poorly soluble and unstable drugs. For instance, encapsulation in polymeric nanoparticles has improved the solubility and bioavailability of poorly water-soluble curcumin through formation of exosome-curcumin complexes.Citation110 These results have a significant impact on target-based drug development for successful in vivo drug delivery to treat inflammation-related diseases. Curcumin-loaded PLGA nanoparticles using PEG 5000 as a stabilizer were prepared. These curcumin nanoparticles were more bioavailable, had a substantial longer half-life than free curcumin, and were nontoxic.Citation111
Chitosan can be used as a coating material to enhance drug bioavailability due to its absorption-enhancing effect.Citation70 For example, the bioavailability of cyclosporin A was increased using chitosan nanoparticles. The increased gastrointestinal permeability of charged chitosan nanoparticles improves their absorption rate.Citation112
Conjugation of PEG to a drug improves water solubility,Citation113 stability in a specific medium such as blood plasma,Citation114 and bioavailability.Citation115 It also improves pharmacokinetic parameters, including volume of distribution, circulation half-life, and renal clearance,Citation116 and also provides protection from recognition by the immune system, prolongs the circulation time, and increases the efficacy of PEGylated nanoparticles in vivo.Citation115
Cellular uptake
The mechanism used by cells to internalize particles varies according to the characteristics of the particles. A general classification of internalization mechanisms includes passive and active transport. Passive transport involves diffusion across the membrane, in which the concentration gradient is the driving force, and osmosis, dialysis and facilitated diffusion are examples of this type of transport.Citation117 Active transport is an energy-dependent movement across the membrane. This mechanism refers to the reversible binding of a membrane component to the material to be introduced into the cell.Citation117 Nanoparticles may cross the cell membrane by either passive transport or an active mechanism of internalization, such as endocytosis.Citation117
Endocytosis is a complex process that includes many alternatives, the best known of which is the clathrin-mediated mechanism.Citation117 General classifications include two main categories, ie, phagocytosis (actin-dependent process, large particle) and pinocytosis (solid and small particles).Citation117
Phagocytosis occurs mainly in specialized mammalian cells such as macrophages, monocytes, and dendritic cells, the function of which is to maintain a clean and sterile immune system.Citation118 The process starts with polymerization of actin, followed by particle internalization mediated by specific receptors, ie, Rho family GTPases, which trigger signaling for ingestion.Citation118 The conventional particle size for phagocytosis starts at around 0.5 μm.Citation119 However, some studies have shown that the optimal size range of drug carriers is between 200 nm and 3 μm.Citation120,Citation121
On the other hand, pinocytosis occurs in almost all cells and involves a variety of mechanisms. The most common uptake pathways are mediated by either clathrin-coated pits or caveolae.Citation117 Descriptions of the main classes of endocytosis are discussed below.
Clathrin-mediated endocytosis
Clathrin-mediated endocytosis occurs in all mammalian cells and is mediated by specific receptors, and is a process whereby “coated pits” invaginate into the cell and form vesicles, usually with particles ranging from 60 nm to 200 nm.Citation117 This process occurs typically in a membrane area rich in clathrin. It has been demonstrated that encapsulation of apotransferrin can enhance uptake of curcumin-loaded nanoparticles, because endocytosis of these nanoparticles is mediated by the transferrin receptor. This transferrin receptor is implicated in clathrin-mediated endocytosis.Citation122 There are several works describing in detail the structure of clathrin and the proteins involved in this pathway, making it possible to model the process of internalization.Citation123 Serum-containing medium decreased the cellular uptake of 20 nm carboxylate polystyrene nanoparticles by 20-fold compared with serum-deprived medium; however, in both cases, internalization occurred via clathrin-mediated endocytosis.Citation124
Caveolae-mediated endocytosis
Caveolae-mediated endocytosis involves bag-shaped compartments in the cell membrane formed by groups of lipids called caveolae, which are capable of producing invagination after interacting with a large number of signaling-associated proteins, such as receptor tyrosine and serine/threonine kinases, G-protein coupled receptors, and steroid hormone receptors, among others. Caveolae are very common in endothelial cells and are considered the main pathway for particles above 200 nm.Citation125 This is the case of nanoparticles modified with albumin, which are internalized and further degraded by caveolae-mediated mechanisms on endothelial cells.Citation126
Macropinocytosis
Macropinocytosis involves the invagination of a highly ruffled area in the plasma membrane, with subsequent internalization of vesicles containing an important amount of fluid from the extracellular region. These vesicles are called macropinosomes (generally bigger than 1 μm) and have poor size selective uptake.Citation127 This mechanism is based on Rac1 (small G proteins) and actin-dependent, and has been reported in the literature.Citation117,Citation128 However, the molecules implicated in the pathway are not known. Studies of the internalization pathway of 90 nm surface-charged PEG-PLA nanoparticles in canine kidney (MDCK type II) cells showed that a fraction of both cationic and anionic nanoparticles internalized through a macropinocytic-like pathway.Citation129
Clathrin-caveolae-independent endocytosis
Recently, other endocytic mechanisms have been reported and classified as clathrin-caveolae-independent,Citation117,Citation130 showing a similar mechanism of caveolae-mediated endocytosis,Citation131 but the complete mechanism is not as yet understood. Different cholesterol-rich microdomains are related to this pathway. The cell invaginates via a cholesterol-dependent pathway and the kinetics of internalization are different from conventional transmembrane protein uptake. The cholesterol microdomains form lipid rafts (40–50 nm) that diffuse through the cell surface and are internalized by endocytic vesicles rich in glycosylphosphatidylinositol anchor proteins. However, the factors regulating this mechanism are still unclear.Citation117,Citation132
Physicochemical properties affecting cellular uptake
Cellular uptake of nanoparticles depends on their physicochemical properties, such as size, shape, charge, and surface hydrophilicity, and the presence of a ligand at the surface.Citation133–Citation135
Size
Particle size can directly affect the mechanism and efficiency of cellular uptake, endocytosis, and further processing of particles in the endocytic pathway,Citation117 with the exception of macropinocytosis, which is hardly a size-selective endocytic pathway.Citation129
Epithelial cells can internalize nanoparticles of a diameter smaller than 100 nm.Citation136 Nonphagocytic cells can internalize nanoparticles with a lower size limit of 200 nm and an upper limit of 1 μm via a clathrin-independent mechanism. However, these particles prefer a caveolae-mediated endocytosis pathway.Citation121 Internalization of particles with a diameter less than 100 nm implies a receptor-mediated, vesicle-coated mechanism, in a relative fast process (30 minutes)Citation121,Citation131 that also depends on the number of receptors in the target membrane cells.Citation60
Some studies have established behavioral patterns with regard to nanoparticle size, in that if the particle size decreases, both cellular uptake and cytotoxicity increase.Citation137 However, nanodrugs with noninternalizing markers have shown toxicity nearby tumor cells, so it is not clear to what extent endocytosis can be correlated to cytotoxicity of drugs.Citation35 Further studies suggest that the increase in cytotoxicity of the compound delivered by liposomes is probably due to a different distribution of liposomes inside the cell compartments, which improves the efficiency of activity.Citation35,Citation138
Toxicity of nanoparticles is also related to their size. Some studies show that the PEG-coated gold nanoparticles with a diameters of 5–10 nm accumulate in the liver; 30 nm particles accumulate preferentially in the spleen, and particles with diameters of 10–60 nm have low toxicity in the liver and kidneys.Citation31
Shape
Internalization pathways for nanoparticles seem to depend on their shape, so endocytosis of rod-shaped polymeric nanoparticles occurs more rapidly and efficiently than that of sphere-shaped particles in nonphagocytic cells.Citation120 This characteristic confers an advantage to nanofibers, which are recently developed drug carriers with applications in gene therapyCitation139 and industrial synthetic chemistry.Citation140 However, due to the multiple variables involved in internalization mechanisms (eg, shape, size, charge), no general tendency can be established as yet.
Charge and hydrophilicity
Nanoparticle charge and hydrophilicity are affected by polymer composition.Citation133,Citation141 Positively charged nanoparticles interact with cells by binding to the cell membrane via electrostatic interactions.Citation142 These interactions are common in polymers such as chitosan.Citation143 Studies using negatively charged carboxy-terminal poly(amidoamine) dendrimers have shown significant cellular uptake in a human KB carcinoma cell line. In addition, it has been determined that the surface curvature of the nanoparticles is not a factor that influences the ligand-receptor interaction, but only the charge of the nanoparticles.Citation142 Cationic PEI nanoparticles have been used to demonstrate that charge on the surface of a nanoparticle modifies its entry through human blood brain barrier endothelial (hCMEC/D3) cells. The charge of the nanoparticle stimulates targeting into a macropinocytotic entry pathway. Additionally, it was found that nanoparticles coupled with a ligand (prion) are internalized via the caveolin 1 and clathrin pathways.Citation144 An optimized formulation of PLGA nanoparticles coated with cationic materials increased DNA encapsulation efficiency and enhanced gene delivery to A549 lung epithelial cells.Citation145
The surface properties of nanoparticles can be modified by adsorption of surfactants, hydrophilic stabilizing, or bioadhesive polymers on the nanoparticle surface. These surface modifications affect the properties of nanoparticles, such as zeta potential, hydrophobicity, colloidal stability, and mucoadhesion.Citation145 It has been suggested that transport of nanoparticles through mucosal surfaces increases with the presence of hydrophilic polymers on the surface of these systems.Citation133 Mucoadhesion extends the residence time of carrier systems in the site of action, increasing the bioavailability of poorly absorbed drugs.Citation133 Polymers such as PEG, methylcellulose, hydroxyethylcellulose, and chitosan are examples of materials that show mucoadhesion.Citation146,Citation147 For instance, studies using chitosan-loaded and modified chitosan-loaded nanoparticles (chitosan-N-acetylcysteine and N-acetyl penicillamine-chitosan) as a drug delivery system against epidermal growth factor receptor in T47D breast cancer cells showed a high degree of stability and mucoadhesive ability. Modified chitosan nanoparticles prevented degradation of the active substance, showed triggered release properties in simulated intracellular reducing environments, and had higher structural stability and mucoadhesiveness when compared with nonmodified chitosan nanoparticles.Citation148
Presence of ligands on the surface
The presence of ligands on the surface of nanoparticles also affects cellular uptake. For example, PEGylated nanoparticles with endocytic and nonendocytic receptors show the important role of receptor-mediated endocytosis in the efficacy of nanoparticles used in the imaging and treatment of cancer.Citation35 Thus, doxorubicin-loaded nanoparticles with endocytic receptors showed better cellular uptake and anticancer efficiency than those with nonendocytic receptors.
Another interesting therapeutic target that can benefit from ligand-conjugated nanoparticles is the endothelial lining. In order to deliver to this site, a new class of nanocarriers called filomicelles has been conjugated with antibodies against specific endothelial surface epitopes. Antibody-conjugated filomicelles show that it is possible to formulate stable and dynamically flexible particles that successfully anchor onto their target despite the effect of blood flow.Citation149
Toxicity
PLGA has shown minimal systemic toxicity and excellent biocompatibility in vitro and in vivo. However, some inflammatory responses have been reported.Citation39,Citation150,Citation151 PGA has low solubility and a high degradation rate, with formation of an acidic product that can also provoke an inflammatory reaction.Citation14 For this reason, use of PGA in biomedical applications is limited, and other options like caprolactone, lactide, and trimethylene carbonate have been developed to eliminate these toxic products.Citation152
Another concern is adducts of biopolymer production which remain after purification methods. For instance, polyurethanes may contain some remnants of toxic methylene diamine that are produced by inefficient mixing, limited polymerization, and insufficient post purification of the polymer.Citation15 Further, the products of hydrolysis of biodegradable polymers (carboxylic acid and/or hydroxyl chain end) may be oxidized, producing species such as short chain carboxylic acid, that may lead to local variations in pH that trigger an inflammatory response.Citation15,Citation153
There are controversies related to the safety or toxicity of polymeric nanomaterials for biomedical application. For example, some authors report that chitosan only reduces the side effects of some drugs such as doxorubicin, but also improves therapeutic efficacy.Citation154 However, other studies showed that membrane damage and leakage of alanine transaminase out of the hepatocyte were due to direct interaction with chitosan nanoparticles.Citation155
On the other hand, polymers such as PEI are cytotoxic.Citation156 It has been demonstrated that PEI causes destabilization of the plasma membrane and activation of effector caspase-3, so PEI appears to be an apoptotic agent.Citation156 However, modifications in its structure may address this concern and make it suitable for medical application.Citation157,Citation158 Further, PEI confers resistance to enzymatic degradation, as shown by micelle-like nanoparticlesCitation159 and Tween 85-modified PEI particlesCitation160 where DNA was completely protected when loaded into these formulations.
shows the results of in vitro toxicity studies for some of the most important biodegradable polymers used in medical applications. The majority of these results report minimal or nontoxic effects of polymer nanoparticles in themselves; however, a complete scan for possible effects of biopolymer-drug combinations at the nanosize level is required for each system.
Table 4 In vitro toxicity studies of commonly used biodegradable polymers in medical applications
In this review, we have focused on the toxicity of biopolymers. Other nanomaterials, such as metallic nanoparticles (gold, iron, titanium, or silica), carbon nanotubes, or fullerenes, are beyond the scope of this discussion.
Polymers with FDA approval
PLA, PLGA, and polycaprolactone are the most widely used biodegradable polymers because of their biocompatibility, biodegradability, and mechanical properties. Nevertheless, a complete list of biodegradable polymers approved by the FDA for biomedical application is shown in .
Table 5 List of biodegradable polymers approved by the US Food and Drug Administration for use in the preparation of nanodrugs updated to September 2012 (http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm)
Conclusion
There have been many advances in the production of more stable, efficient, and safe nanocarriers, and formulations and manufacturing processes will undoubtedly continue to evolve. The treatment of major diseases with worldwide economic importance has been proven to benefit from these nanosystems, although there are still few nanodrugs available on the pharmaceutical market.
Many methods for synthesis and modification of biodegradable polymers have been established, but it is still relevant to develop new technologies to allow use of minimally toxic reagents, protect the active compounds, and scale up to an industrial level.
Goals like increasing the degradation rate of polymeric matrices, reducing undesirable side effects, and improving the efficiency of drugs have been partially achieved by development of polymeric matrices which can be degraded in specific conditions and reach specific receptors that improve their cellular uptake.
Acknowledgments
Thanks are due to the National Secretariat for Science, Technology, and Innovation of Panama for financial support (grant Fie10-08) and to The Institute for Training and Development of Human Resources (IFARHU) from the Panamanian government which, jointly with the National Secretariat for Science, Technology, and Innovation, provided EM with a scholarship. We are grateful to Jagannatha Rao, Marisín Pecchio, Dominique Gutierrez, and Suyin Torres for critically reviewing this work. The authors wish to dedicate this work to the memory of Dr Gustavo Nuñez, whose leadership and creative ideas inspired this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiechtyWBPeppasNAExpert opinion: responsive polymer nanoparticles in cancer therapyEur J Pharm Biopharm20128024124621888972
- RyvolovaMChomouckaJDrbohlavovaJModern micro and nanoparticle-based imaging techniquesSensors (Basel)201212147921482023202187
- UleryBDNairLSLaurencinCTBiomedical applications of biodegradable polymersJ Polym Sci B Polym Phys20114983286421769165
- VromanITighzertLBiodegradable polymersMaterials20092307344
- NittaSKNumataKBiopolymer-based nanoparticles for drug/gene delivery and tissue engineeringInt J Mol Sci2013141629165423344060
- NairLSLaurencinCTPolymers as biomaterials for tissue engineering and controlled drug deliveryAdv Biochem Eng Biotechnol2006102479017089786
- ParkJHYeMParkKBiodegradable polymers for microencapsulation of drugsMolecules20051014616118007283
- MohanrajVChenYNanoparticles: a reviewTrop J Pharm Res20065561573
- ElzoghbyAOSamyWMElgindyNAAlbumin-based nanoparticles as potential controlled release drug delivery systemsJ Control Release201215716818221839127
- MalhotraMLaneCTomaro-DuchesneauCSahaSPrakashSA novel method for synthesizing PEGylated chitosan nanoparticles: strategy, preparation, and in vitro analysisInt J Nanomedicine2011648549421562608
- BhattacharyyaSSPaulSKhuda-BukhshAREncapsulated plant extract (Gelsemium sempervirens) poly (lactide-co-glycolide) nanoparticles enhance cellular uptake and increase bioactivity in vitroExp Biol Med (Maywood)201023567868820511672
- GrenhaAGraingerCIDaileyLAChitosan nanoparticles are compatible with respiratory epithelial cells in vitroEur J Pharm Sci200731738417408932
- LiuHSlamovichEBWebsterTJLess harmful acidic degradation of poly(lacticco-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle additionInt J Nanomedicine2006154154517722285
- CeonzoKGaynorAShafferLKojimaKVacantiCAStahlGLPolyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activationTissue Eng20061230130816548688
- LyuSUnterekerDDegradability of polymers for implantable biomedical devicesInt J Mol Sci2009104033406519865531
- JainAGuptaYJainSKPerspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colonJ Pharm Pharm Sci2007108612817498397
- ModiGPillayVChoonaraYEAdvances in the treatment of neurodegenerative disorders employing nanotechnologyAnn N Y Acad Sci2010118415417220146696
- PatelDNBaileySRNanotechnology in cardiovascular medicineCatheter Cardiovasc Interv20076964365417390307
- HuDLiuLChenWLiSZhaoYA novel preparation method for 5-aminosalicylic acid loaded Eudragit s100 nanoparticlesInt J Mol Sci2012136454646822754377
- SoppimathKSAminabhaviTMKulkarniARRudzinskiWEBiodegradable polymeric nanoparticles as drug delivery devicesJ Control Release20017012011166403
- BawaRNanoBiotech 2008: exploring global advances in nanomedicineNanomedicine200955719230083
- TangpasuthadolVPendharkarSMKohnJHydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part I: study of model compoundsBiomaterials2000212371237811055284
- JeongSIKimBSKangSWIn vivo biocompatibilty and degradation behavior of elastic poly(L-lactide-co-epsilon-caprolactone) scaffoldsBiomaterials2004255939594615183608
- RawatMSinghDSarafSNanocarriers: promising vehicle for bioactive drugsBiol Pharm Bull2006291790179816946487
- KulkarniRKPaniKCNeumanCLeonardFPolylactic acid for surgical implantsArch Surg1966938398435921307
- YanHJiangWZhangYNovel multi-biotin grafted poly(lactic acid) and its self-assembling nanoparticles capable of binding to streptavidinInt J Nanomedicine2012745746522334778
- GodinBSakamotoJHSerdaREGrattoniABouamraniAFerrariMEmerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseasesTrends Pharmacol Sci20103119920520172613
- LeeCHCharacterization of nitric oxide delivery systems produced by various nanotechnologiesMethods Mol Biol201170416918521161637
- SaraivaJMarotta-OliveiraSSCicilliniSAEloy JdeOMarchettiJMNanocarriers for nitric oxide deliveryJ Drug Deliv2011201193643821869934
- El-SherbinyIMSmythHDControlled release pulmonary administration of curcumin using swellable biocompatible microparticlesMol Pharm2012926928022136259
- ZhangXDWuDShenXSize-dependent in vivo toxicity of PEG-coated gold nanoparticlesInt J Nanomedicine201162071208121976982
- XuXYuHGaoSMaHQLeongKWWangSPolyphosphoester microspheres for sustained release of biologically active nerve growth factorBiomaterials2002233765377212109702
- ShenoyDLittleSLangerRAmijiMPoly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluationsMol Pharm2005235736616196488
- ChengLJinCLvWDingQHanXDeveloping a highly stable PLGA-mPEG nanoparticle loaded with cisplatin for chemotherapy of ovarian cancerPLoS One20116e2543321966528
- ChuangKHWangHEChenFMEndocytosis of PEGylated agents enhances cancer imaging and anticancer efficacyMol Cancer Ther201091903191220501805
- MoXIwataHMatsudaSIkadaYSoft tissue adhesive composed of modified gelatin and polysaccharidesJ Biomater Sci Polym Ed20001134135110903034
- IkadaYTsujiHBiodegradable polyesters for medical and ecological applicationsMacromol Rapid Commun200021117132
- HinoMIshikoOHondaKITransmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgeryBr J Haematol200010819419510651745
- LuJMWangXMarin-MullerCCurrent advances in research and clinical applications of PLGA-based nanotechnologyExpert Rev Mol Diagn2009932534119435455
- SachlosECzernuszkaJTMaking tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffoldsEur Cell Mater20035293914562270
- FengYGuoJBiodegradable polydepsipeptidesInt J Mol Sci20091058961519333423
- GaralehMSynthesis of poly(ether-ester) catalyzed by bismuth triflateEur J Sci Res201155383387
- Rodriguez-GalanAFrancoLPuiggaliJDegradable poly(ester amide)s for biomedical applicationsPolymers201136999
- StridsbergKMRynerMAlbertssonACControlled ring-opening polymerization: polymers with designed macromolecular architectureDegradable Aliphatic PolyestersBerlin, GermanySpringer2002
- MerlinDLSivasankarBSynthesis and characterization of semi-interpenetrating polymer networks using biocompatible polyurethane and acrylamide monomerEur Polym J200945165170
- MacocinschiDFilipDVladSOpreaAMGafitanuCACharacterization of a poly(ether urethane)-based controlled release membrane system for delivery of ketoprofenAppl Surf Sci2012259416423
- LeongKWSimonteVLangerRSynthesis of polyanhydrides: melt-polycondensation, dehydrochlorination, and dehydrative couplingMacromolecules198720705712
- SintzelMBHellerJNgSYTabatabayCSchwach-AbdellaouiKGurnyRIn vitro drug release from self-catalyzed poly(ortho ester): case study of 5-fluorouracilJ Control Release1998552132189795063
- AzevedoHSReisRLUnderstanding the enzymatic degradation of biodegradable polymers and strategies to control their degradation rateRuiLReisJSRBiodegradable Systems in Tissue Engineering and Regenerative MedicineBoca Ratio, FLCRC Press2004
- EdlundUAlbertssonACDegradable polymer microspheres for controlled drug deliveryAlbertssonAADusekACAdvances in Polymer ScienceBerlin, GermanySpringer-Verlag2002
- UhrichKECannizzaroSMLangerRSShakesheffKMPolymeric systems for controlled drug releaseChem Rev1999993181319811749514
- HurrellSCameronREPolyglycolide: degradation and drug release. Part I: changes in morphology during degradationJ Mater Sci Mater Med20011281181615348229
- WardRAndersonJMcVenesRStokesKIn vivo biostability of polysiloxane polyether polyurethanes: resistance to biologic oxidation and stress crackingJ Biomed Mater Res A20067758058916506175
- NapoliAValentiniMTirelliNMullerMHubbellJAOxidation-responsive polymeric vesiclesNat Mater2004318318914991021
- ChenLLiXLiLGuoSAcetylated starch-based biodegradable materials with potential biomedical applications as drug delivery systemsCurr Appl Phys20077e90e93
- WuYYangWWangCHuJFuSChitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinateInt J Pharm200529523524515848008
- SarmentoBRibeiroAVeigaFSampaioPNeufeldRFerreiraDAlginate/chitosan nanoparticles are effective for oral insulin deliveryPharm Res2007242198220617577641
- TricklerWJNagvekarAADashAKA novel nanoparticle formulation for sustained paclitaxel deliveryAAPSPharmSciTech20089486493
- ChengJTeplyBASherifiIFormulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug deliveryBiomaterials20072886987617055572
- DuYZCaiLLLiJReceptor-mediated gene delivery by folic acid-modified stearic acid-grafted chitosan micellesInt J Nanomedicine201161559156821845046
- FangaZBhandariaBEncapsulation of polyphenols – a reviewTrends Food Sci Technol201021510523
- Pinto ReisCNeufeldRJRibeiroAJVeigaFNanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticlesNanomedicine2006282117292111
- DuanJZhangYChenWCationic polybutyl cyanoacrylate nanoparticles for DNA deliveryJ Biomed Biotechnol2009200914925419300519
- YangSCGeHXHuYJiangXQYangCZFormation of positively charged poly(butyl cyanoacrylate) nanoparticles stabilized with chitosanColloid Polym Sci2000278285292
- MiyazakiSTakahashiAKuboWBachynskyJLoebenbergRPoly n-butylcyanoacrylate (PNBCA) nanocapsules as a carrier for NSAIDs: in vitro release and in vivo skin penetrationJ Pharm Pharm Sci2003623824512935436
- EssawyHTauerKPolyamide capsules via soft templating with oil drops-1. Morphological studies of the capsule wallColloid Polym Sci201028831733120098514
- CrespyDLandfesterKMiniemulsion polymerization as a versatile tool for the synthesis of functionalized polymersBeilstein J Org Chem201061132114821160567
- HansMLLowmanAMBiodegradable nanoparticles for drug delivery and targetingCurr Opin Solid State Mater Sci20026319327
- HongYGaoCShiYShenJPreparation of porous polylactide microspheres by emulsion-solvent evaporation based on solution induced phase separationPolym Adv Technol200516622627
- NagpalKKumar SinghSKMishraDNChitosan nanoparticles: a promising system in novel drug deliveryChem Pharm Bull (Tokyo)2010581423143021048331
- YunYChoYWParkKNanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein deliveryAdv Drug Deliv Rev20136582283223123292
- Galindo-RodriguezSAllemannEFessiHDoelkerEPhysicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methodsPharm Res2004211428143915359578
- TroyDBRemington: The Science and Practice of Pharmacy21st edPhiladelphia, PALippincott Williams & Wilkin2006
- MurakamiHKobayashiMTakeuchiHKawashimaYPreparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion methodInt J Pharm199918714315210502620
- MakadiaHKSiegelSJPoly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrierPolymers (Basel)201131377139722577513
- CansellFAymonierCDesign of functional nanostructured materials using supercritical fluidsJ Supercrit Fluids200947508516
- GuptaVKKararPKRameshSMisraSPGuptaANanoparticle formulation for hydrophilic and hydrophobic drugsInt J Res Pharm Sci20101163169
- ReverchonEAdamiRNanomaterials and supercritical fluidsJ Supercrit Fluids200637122
- IrfanMSeilerMEncapsulation using hyperbranched polymers: from research and technologies to emerging applicationsInd Eng Chem Res20104911691196
- GomezSGamazoCSan RomanBVauthierCFerrerMIrachelJMDevelopment of a novel vaccine delivery system based on Gantrez nanoparticlesJ Nanosci Nanotechnol200663283328917048548
- KowalczykBLagziIGrzybowskiBANanoseparations: strategies for size and/or shape-selective purification of nanoparticlesCurr Opin Colloid Interface Sci201116135148
- AbdelwahedWDegobertGStainmesseSFessiHFreeze-drying of nanoparticles: formulation, process and storage considerationsAdv Drug Deliv Rev2006581688171317118485
- BiroENemethASSisakCFeczkoTGyenisJPreparation of chitosan particles suitable for enzyme immobilizationJ Biochem Biophys Methods2008701240124618155771
- LemoineDFrancoisCKedzierewiczFPreatVHoffmanMMaincentPStability study of nanoparticles of poly(epsilon-caprolactone), poly(D,L-lactide) and poly(D,L-lactide-co-glycolide)Biomaterials199617219121978922605
- MimaYFukumotoSKoyamaHEnhancement of cell-based therapeutic angiogenesis using a novel type of injectable scaffolds of hydroxyapatite-polymer nanocomposite microspheresPLoS One20127e3519922529991
- VenkatesanJKimSKChitosan composites for bone tissue engineering – an overviewMar Drugs201082252226620948907
- Jagur-GrodzinskiJBiomedical application of functional polymersReact Funct Polym19993999138
- WilczewskaAZNiemirowiczKMarkiewiczKHCarHNanoparticles as drug delivery systemsPharmacol Rep2012641020103723238461
- VilarGTulla-PucheJAlbericioFPolymers and drug delivery systemsCurr Drug Deliv2012936739422640038
- MeiLZhangYZhengYA novel docetaxel-loaded poly (epsilon-caprolactone)/Pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatmentNanoscale Res Lett200941530153920652101
- MitraMMisraRHarilalASahooSKKrishnakumarSEnhanced in vitro antiproliferative effects of EpCAM antibody-functionalized paclitaxel-loaded PLGA nanoparticles in retinoblastoma cellsMol Vis2011172724273722065926
- FogedCNielsenHMCell-penetrating peptides for drug delivery across membrane barriersExpert Opin Drug Deliv2008510511718095931
- RingmanJMFrautschySAColeGMMastermanDLCummingsJLA potential role of the curry spice curcumin in Alzheimer’s diseaseCurr Alzheimer Res2005213113615974909
- MathewAFukudaTNagaokaYCurcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s diseasePLoS One20127e3261622403681
- SongjiangZLixiangWAmyloid-beta associated with chitosan nanocarrier has favorable immunogenicity and permeates the BBBAAPS PharmSciTech20091090090519609682
- AzeemATalegaonkarSNegiLMAhmadFJKharRKIqbalZOil based nanocarrier system for transdermal delivery of ropinirole: a mechanistic, pharmacokinetic and biochemical investigationInt J Pharm201242243644422057087
- MalvindiMADi CoratoRCurcioAMultiple functionalization of fluorescent nanoparticles for specific biolabeling and drug delivery of dopamineNanoscale201135110511922037807
- Di StefanoAIannitelliALaserraSSozioPDrug delivery strategies for Alzheimer’s disease treatmentExpert Opin Drug Deliv2011858160321391862
- WicklineSANeubauerAMWinterPCaruthersSLanzaGApplications of nanotechnology to atherosclerosis, thrombosis, and vascular biologyArterioscler Thromb Vasc Biol20062643544116373609
- MutsugaMNaritaYYamawakiADevelopment of novel drug-eluting biodegradable nano-fiber for prevention of postoperative pulmonary venous obstructionInteract Cardiovasc Thorac Surg2009840240719139028
- ChoHHHanDWMatsumuraKTsutsumiSHyonSHThe behavior of vascular smooth muscle cells and platelets onto epigallocatechin gallate-releasing poly(l-lactide-co-epsilon-caprolactone) as stent-coating materialsBiomaterials20082988489318031806
- BharaliDJMousaSAThanavalaYMicro- and nanoparticle-based vaccines for hepatitis BAdv Exp Med Biol200760141542117713030
- DementoSSteenblockERFahmyTMBiomimetic approaches to modulating the T cell immune response with nano- and micro- particlesConf Proc IEEE Eng Med Biol Soc200920091161116619963488
- VinogradovSVPoluektovaLYMakarovEGersonTSenanayakeMTNano-NRTIs: efficient inhibitors of HIV type-1 in macrophages with a reduced mitochondrial toxicityAntivir Chem Chemother20102111421045256
- DwivediVManickamCBinjawadagiBJoyappaDRenukaradhyaGJBiodegradable nanoparticle-entrapped vaccine induces cross-protective immune response against a virulent heterologous respiratory viral infection in pigsPLoS One20127e5179423240064
- BrunelFVeronLDavidLDomardAVerrierBDelairTSelf-assemblies on chitosan nanohydrogelsMacromol Biosci20101042443220166229
- LiuHWebsterTJCeramic/polymer nanocomposites with tunable drug delivery capability at specific disease sitesJ Biomed Mater Res A2010931180119219777574
- FoxKTranPATranNRecent advances in research applications of nanophase hydroxyapatiteChemphyschem2012132495250622467406
- MarracheSDharSEngineering of blended nanoparticle platform for delivery of mitochondria-acting therapeuticsProc Natl Acad Sci U S A2012109162881629322991470
- SunDZhuangXXiangXA novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomesMol Ther2010181606161420571541
- AnandPNairHBSungBDesign of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivoBiochem Pharmacol20107933033819735646
- El-ShabouriMHPositively charged nanoparticles for improving the oral bioavailability of cyclosporin-AInt J Pharm200224910110812433438
- BanerjeeSSAherNPatilRKhandareJPoly(ethylene glycol)-prodrug conjugates: concept, design, and applicationsJ Drug Deliv2012201210397322645686
- GrefRDombAQuellecPThe controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheresAdv Drug Deliv Rev201216215233
- Zohuriaan-MehrMJAdvances in chitin and chitosan modification through graft copolymerization: a comprehensive reviewIran Polym J200514235265
- van VlerkenLEVyasTKAmijiMMPoly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular deliveryPharm Res2007241405141417393074
- DohertyGJMcMahonHTMechanisms of endocytosisAnnu Rev Biochem20097885790219317650
- AderemAUnderhillDMMechanisms of phagocytosis in macrophagesAnnu Rev Immunol19991759362310358769
- GrovesEDartAECovarelliVCaronEMolecular mechanisms of phagocytic uptake in mammalian cellsCell Mol Life Sci2008651957197618322649
- GrattonSERoppPAPohlhausPDThe effect of particle design on cellular internalization pathwaysProc Natl Acad Sci U S A2008105116131161818697944
- RejmanJOberleVZuhornISHoekstraDSize-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosisBiochem J200437715916914505488
- GandapuUChaitanyaRKKishoreGReddyRCKondapiAKCurcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitroPLoS One20116e2338821887247
- RamananVAgrawalNJLiuJEnglesSToyRRadhakrishnanRSystems biology and physical biology of clathrin-mediated endocytosisIntegr Biol (Camb)2011380381521792431
- SmithPJGiroudMWigginsHLCellular entry of nanoparticles via serum sensitive clathrin-mediated endocytosis, and plasma membrane permeabilizationInt J Nanomedicine201272045205522619541
- KrajewskaWMMaslowskaICaveolins: structure and function in signal transductionCell Mol Biol Lett2004919522015213803
- SchnitzerJEOhPPinneyEAllardJFilipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromoleculesJ Cell Biol1994127121712327525606
- HillaireauHCouvreurPNanocarriers’ entry into the cell: relevance to drug deliveryCell Mol Life Sci2009662873289619499185
- SwansonJAWattsCMacropinocytosisTrends Cell Biol1995542442814732047
- Harush-FrenkelORozenturEBenitaSAltschulerYSurface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cellsBiomacromolecules2008943544318189360
- DammEMPelkmansLKartenbeckJMezzacasaAKurzchaliaTHeleniusAClathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolaeJ Cell Biol200516847748815668298
- MayorSPaganoREPathways of clathrin-independent endocytosisNat Rev Mol Cell Biol2007860361217609668
- dos SantosTVarelaJLynchISalvatiADawsonKAEffects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell linesPLoS One20116e2443821949717
- des RieuxAFievezVGarinotMSchneiderYJPréatVNanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approachJ Control Release200611612717050027
- KamelAOAwadGAGeneidiASMortadaNDPreparation of intravenous stealthy acyclovir nanoparticles with increased mean residence timeAAPS PharmSciTech2009101427143619949904
- AlmeidaAJSoutoESolid lipid nanoparticles as a drug delivery system for peptides and proteinsAdv Drug Deliv Rev20075947849017543416
- InnesNPOgdenGRA technique for the study of endocytosis in human oral epithelial cellsArch Oral Biol19994451952310401530
- NabeshiHYoshikawaTAkaseTEffect of amorphous silica nanoparticles on in vitro RANKL-induced osteoclast differentiation in murine macrophagesNanoscale Res Lett2011646421777482
- EliazRENirSMartyCSzokaFCJrDetermination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomesCancer Res20046471171814744789
- ChenMGaoSDongMChitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA deliveryACS Nano201264835484422621383
- SongJKahveciDChenMEnhanced catalytic activity of lipase encapsulated in PCL nanofibersLangmuir2012286157616222397625
- ZhangJChenXGLiuCSParkHJInvestigation of polymeric amphiphilic nanoparticles as antitumor drug carriersJ Mater Sci Mater Med20092099199919083084
- ShiXThomasTPMycLAKotlyarABakerJRJrSynthesis, characterization, and intracellular uptake of carboxyl-terminated poly(amidoamine) dendrimer-stabilized iron oxide nanoparticlesPhys Chem Chem Phys200795712572017960261
- WangJJZengZWXiaoRZRecent advances of chitosan nanoparticles as drug carriersInt J Nanomedicine2011676577421589644
- GeorgievaJVKalicharanDCouraudPOSurface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitroMol Ther20111931832521045812
- BaoumADhillonNBuchSBerklandCCationic surface modification of PLG nanoparticles offers sustained gene delivery to pulmonary epithelial cellsJ Pharm Sci2010992413242219911425
- de SalamancaAEDieboldYCalongeMChitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo toleranceInvest Ophthalmol Vis Sci2006471416142516565375
- RoySPalKAnisAPramanikKPrabhakarBPolymers in mucoadhesive drug delivery system: a brief noteDes Monomers Polym200912483495
- TalaeiFAziziEDinarvandRAtyabiFThiolated chitosan nanoparticles as a delivery system for antisense therapy: evaluation against EGFR in T47D breast cancer cellsInt J Nanomedicine201161963197521976973
- ShuvaevVVIliesMASimoneEEndothelial targeting of antibody-decorated polymeric filomicellesACS Nano201156991699921838300
- KimJDadsetanMAmeenuddinSWindebankAJYaszemskiMJLuLIn vivo biodegradation and biocompatibility of PEG/sebacic acid-based hydrogels using a cage implant systemJ Biomed Mater Res A20109519119720574982
- NicoleteRdos SantosDFFaccioliLHThe uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory responseInt Immunopharmacol2011111557156321621649
- WangZGHsiaoBSZongXHMorphological development in absorbable poly(glycolide), poly(glycolide-co-lactide) and poly(glycolide-co-caprolactone) copolymers during isothermal crystallizationPolymer20004621628
- NiedermanRZhangJKashketSShort-chain carboxylic-acid-stimulated, PMN-mediated gingival inflammationCrit Rev Oral Biol Med199782692909260044
- MitraSGaurUGhoshPCMaitraANTumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrierJ Control Release20017431732311489513
- LohJWYeohGSaundersMLimLYUptake and cytotoxicity of chitosan nanoparticles in human liver cellsToxicol Appl Pharmacol201024914815720831879
- MoghimiSMSymondsPMurrayJCHunterACDebskaGSzewczykAA two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapyMol Ther20051199099515922971
- GuptaKCKumarPSwamiAPathakAInventorsNovel crosslinked pei nanoparticle based transfection agents for delivery of biomolecules United States Patent US 20100029750 A1242010
- GoyalRTripathiSKTyagiSIn vitro and in vivo evaluation of linear polyethylenimine nanoparticlesJ Biomed Nanotechnol20117525321485799
- Tag KoYKaleAHartnerWPapahadjopoulos-SternbergBTorchilinaVSelf-assembling micelle-like nanoparticles based on phospholipid-polyethyleneimine conjugates for systemic gene deliveryJ Control Release200913313213818929605
- XiaoJDuanXYinQChenLZhangZLiYLow molecular weight polyethylenimine-graft-Tween 85 for effective gene delivery: synthesis and in vitro characteristicsBioconjug Chem20122322223122168476
- Fernandez-CarballidoAPastorizaPBarciaEMontejoCNegroSPLGA/PEG-derivative polymeric matrix for drug delivery system applications: characterization and cell viability studiesInt J Pharm2008352505718036755
- LiuJJiangZZhangSSaltzmanWMPoly(omega-pentadecalactone-co-butylene-co-succinate) nanoparticles as biodegradable carriers for camptothecin deliveryBiomaterials2009305707571919632718
- DeschampsAAGrijpmaDWFeijenJPoly(ethylene oxide)/poly(butylene terephthalate) segmented block copolymers: the effect of copolymer composition on physical properties and degradation behaviorPolymer20014293359345
- GibasIJanikHReview: synthetic polymer hydrogels for biomedical applicationsChem Chem Technol20104297304
- BathejaPSheihetLKohnJSingerAJMichniak-KohnBTopical drug delivery by a polymeric nanosphere gel: formulation optimization and in vitro and in vivo skin distribution studiesJ Control Release201114915916720950659
- ErricoCBartoliCChielliniFChielliniEPoly(hydroxyalkanoates)-based polymeric nanoparticles for drug deliveryJ Biomed Biotechnol2009200957170219789653
- MoebusKSiepmannJBodmeierRAlginate-poloxamer microparticles for controlled drug delivery to mucosal tissueEur J Pharm Biopharm200972425319126428
- LiXYangZYangKSelf-assembled polymeric micellar nanoparticles as nanocarriers for poorly soluble anticancer drug ethaselenNanoscale Res Lett200941502151120652138
- RaiBTeohSHHutmacherDWCaoTHoKHNovel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2Biomaterials2005263739374815621264
- HelleJBarrJNgSYDevelopment and applications of injectable poly(ortho esters) for pain control and periodontal treatmentBiomaterials2002234397440412219830
- GunatillakePAAdhikariRBiodegradable synthetic polymers for tissue engineeringEur Cell Mater2003511614562275
- Bernkop-SchnurchADunnhauptSChitosan-based drug delivery systemsEur J Pharm Biopharm20128146346922561955
- ThanouMVerhoefJCJungingerHEOral drug absorption enhancement by chitosan and its derivativesAdv Drug Deliv Rev20015211712611718935
- BhattacharjeeSErshovDFytianosKCytotoxicity and cellular uptake of tri-block copolymer nanoparticles with different size and surface characteristicsPart Fibre Toxicol201291122546147
- MihaiRFlorescuIPCoroiuVOanceaALunguMIn vitro biocompatibility testing of some synthetic polymers used for the achievement of nervous conduitsJ Med Life2011425025522567047
- BertramJPJaySMHynesSRRobinsonRCriscioneJMLavikEBFunctionalized poly(lactic-co-glycolic acid) enhances drug delivery and provides chemical moieties for surface engineering while preserving biocompatibilityActa Biomater200952860287119433141
- HuYLQiWHanFShaoJZGaoJQToxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo modelInt J Nanomedicine201163351335922267920
- da SilvaLCGarciaTMoriMPreparation and characterization of polysaccharide-based nanoparticles with anticoagulant activityInt J Nanomedicine201272975298622787393