Abstract
Recent data in the literature support the role of nicotinamide (NA) as a pharmacologic agent that stimulates pancreatic beta-cells to produce insulin in vitro. There are data showing that carbon nanotubes may be useful in initiating and maintaining cellular metabolic responses. This study shows that administration of multiwalled carbon nanotubes (MWCNTs) functionalized with nicotinamide (NA-MWCNTs) leads to significant insulin production compared with individual administration of NA, MWCNTs, and a control solution. Treatment of 1.4E7 cells for 30 minutes with NA-MWCNTs at concentrations ranging from 1 mg/L to 20 mg/L resulted in significantly increased insulin release (0.18 ± 0.026 ng/mL for 1 mg/L, 0.21 ± 0.024 ng/mL for 5 mg/L, and 0.27 ± 0.028 ng/mL for 20 mg/L). Thus, compared with cells treated with NA only (0.1 ± 0.01 ng/mL for 1 mg/L, 0.12 ± 0.017 ng/mL for 5 mg/L, and 0.17 ± 0.01 ng/mL for 20 mg/L) we observed a significant positive effect on insulin release in cells treated with NA-MWCNTs. The results were confirmed using flow cytometry, epifluorescence microscopy combined with immunochemistry staining, and enzyme-linked immunosorbent assay techniques. In addition, using immunofluorescence microscopy techniques, we were able to demonstrate that MWCNTs enhance insulin production via the macrophage migration inhibitory factor pathway. The application and potential of NA combined with MWCNTs as an antidiabetic agent may represent the beginning of a new chapter in the nanomediated treatment of diabetes mellitus.
Introduction
Over the past three decades, the number of patients with diabetes mellitus has more than doubled globally, making diabetes one of the most important public health challenges for all nations. A 2011 report from the US Centers for Disease Control and Prevention estimated that approximately one million Americans have type 1 diabetes mellitus. Type 1 diabetes is the most common childhood metabolic disease.Citation1
Currently, insulin replacement is the first-line treatment option for patients with type 1 diabetes and severe forms of type 2 diabetes. Treatment by insulin injection is generally effective but nonphysiologic, and has the major disadvantage of producing chronic complications. On the other hand, islet transplantation can maintain normoglycemia without hypoglycemic side effects, with the major clinical impact of releasing diabetics from insulin dependence. However, the transplanted islets tend to lose their functional ability over time.
Therefore, while allotransplantation or xenotransplantation still imposes important problems, the use of tumoral B-cell lines could be a potential solution, especially given that various neoplastic cell lines have demonstrated changes characteristic of differentiation after treatment with specific chemical agents. Hence, sodium butyrate increased the transcription of the glucagon and insulin genes in rat insulinoma cells, suggesting that these cells acquired more differentiated characteristics.Citation2 Moreover, clones displaying the most appropriate patterns of insulin secretion can be selected and amplified at low cost, thus providing a method of producing pure insulin-secreting cells with applications in the study of pancreatic cell biology. However, in addition to retaining their neoplastic nature and presenting primitive differentiating features of the parent cells, the bulk of these cell lines progressively lose their insulin biosynthesis and regulated secretion with successive numbers of passages.Citation3
Despite good progress, several obstacles remain to be overcome before insulin-producing cell transplantation can be translated into a therapy for human patients.Citation4 The evidence suggests that nicotinamide (NA), a derivative of nicotinic acid, has many effects on beta-cell survival and functions, by acting as a free radical scavenger or as a precursor for NA adenine dinucleotide (NAD).Citation5 NA is also an inhibitor of poly(adenosine diphosphate ribose) synthetase, a chromatin-associated enzyme involved in cellular DNA repair and differentiation which, if activated during DNA repair synthesis, could lead to a critical decrease in NAD levels in beta-cells. The activity of poly(adenosine diphosphate ribose) synthetase is linked with the level of cellular differentiation in several cell types. Further, it has been postulated that poly(adenosine diphosphate ribose) synthetase inhibitors, such as NA, may counteract suppression of beta-cell DNA replication, thus inducing beta-cell regeneration.Citation6
NA has also been reported to affect insulin production and cell proliferation in adult mouse islets in culture and following transplantation. Moreover, increases in insulin production and content of cultured fetal pig islet-like cell clusters have been seen as a consequence of beta-cell neoformation through differentiation.Citation7 Hence, in stem cell research, several protocols have used NA as a differentiation factor.Citation8
Preliminary data from the literature support the involvement of macrophage migration inhibitory factor (MIF) in stimulating insulin secretion. This implication is supported by the fact that, as shown by immunocytochemistry, MIF and insulin colocalize within the secretory granules of the pancreatic islet beta-cells, and once released, MIF appears to regulate insulin release in an autocrine fashion. MIF is also highly expressed in several insulin-secreting cell lines, and its production is regulated by glucose in a time-dependent and concentration-dependent manner.Citation9
Carbon nanotubes (CNTs) have many unique physical, mechanical, electrical, and chemical properties, and have been intensively explored for biological and biomedical applications in the past few years.Citation10 Extensive reports have explored CNTs as potential drug carriers and delivery vehicles.Citation11–Citation15 CNTs can be functionalized with pharmacologically active biomolecules by forming stable covalent bonds or supramolecular assemblies based on noncovalent interactions. Once the cargos are delivered into various cells, they are able to overexpress their physiologic functions.Citation16–Citation18
Considering all these data, we hypothesized that combined administration of both NA and CNTs would function as powerful nanobiosystems to improve the capacity of insulin-producing cells to generate and release insulin. This study shows that administration of multiwalled carbon nanotubes (MWCNTs) functionalized with nicotinamide (NA-MWCNTs) leads to significant insulin production and glucose-induced insulin secretion in 1.4E7 beta-cells via the MIF pathway.
Materials and methods
Cell culture
A hybrid beta-cell line (1.4E7) formed by electrofusion of human pancreatic islets cells with PANC-1 cells, purchased from the European Collection of Cell Cultures (Salisbury, UK) was used in the experiments. The 1.4E7 cells were grown in 25 cm3 Corning plastic plates in Roswell Park Memorial Institute (RPMI) (Sigma-Aldrich Co, St. Louis, MO, USA) 1640 culture medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and 1% penicillin-streptomycin until they reached 80% confluence. The cells were maintained in a humidified 5% CO2 incubator at 37°C, and were routinely passaged by washing with phosphate-buffered saline (PBS, Invitrogen, Carlsbad CA, USA) prior to detachment with 0.25% (w/v) trypsin-EDTA (Invitrogen) and splitting at a 1:3 ratio. All ingredients for the media were purchased from Sigma Chemical Co (Deisenhofen, Germany). Exposures to the different nanomaterials were done on subconfluent cells.
Functionalization of carbon nanotubes
Multiwalled carbon nanotubes (>98% carbon basis, optical density [OD] × internal diameter [ID] × L 10–15 nm × 2–6 nm × 0.1–10.0 μm; product number 677248) and NA (N0636) were purchased from Sigma Chemical Co for the experiments involving covalent functionalization of CNTs. A nonconjugated highly purified MWCNT control solution was prepared as previously described.Citation19 The product obtained was diluted in physiologic saline solution (NaCl 0.9%) at a ratio of 1:10 (v/v). A 1 M solution of NA was prepared by dissolving 0.122 g of NA per mL of sterile water, providing a 100 × stock solution. In our experiments, the NA was used at a final concentration of 10 mM.
Functionalization of MWCNTs with NA was performed as previously described.Citation20 Briefly, in the first step, MWCNTs were carboxylated by oxidation in a mixture of sulfuric acid and nitric acid (3:1), and the NA molecules were further attached by formation of amide bonds with carboxylic groups using 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimidehydrochloride and N-hydroxysuccinimide, which generated MWCNT complexes functionalized with nicotinamide (NA-MWCNTs).
Characterization of MWCNTs
The ultrastructural morphology of the MWCNTs was evaluated using a transmission electron microscope (JEM 1010, JEOL, Tokyo, Japan) as previously described.Citation21 The images were captured using a Mega View III camera (Soft Imaging System, Olympus, Münster, Germany). Spectroscopy was performed using an ultraviolet-visible spectrometer (Mini 1240, Shimadzu, Kyoto, Japan).
Exposure conditions
For the experiments, the cells were seeded at a density of 2–4 × 104 cells/cm2 on Nunc® Lab-Tek 4 (Sigma-Aldrich) chamber glass slides (No 177399) or in a six-well plate in complete medium and allowed to attach overnight. After 24 hours of cell attachment, the cells were washed with 0.2 mL per cm2 of PBS. The cells were then exposed to suspensions (10 μL) of the test materials (MWCNTs and MWCNTs-NA, NA), or PBS between 1 μg/mL and 20 μg/mL for 30 minutes, one hour, or 3 hours. All experiments were performed in triplicate for each concentration.
Microscopy analysis of cells
For the microscopy analysis, we used Lab-Tek chamber slides for in situ observation of the cells adherent to the glass (the walls were removed and the culture medium was discharged). Thus, no cell transfer was needed prior to visualization/staining. After being exposed to different formulations of MWCNTs/NA, the cells were thoroughly washed three times with 1 × PBS, and then fixed with 10% formaldehyde solution for 10 minutes, washed three times with PBS, and immunochemically stained. Cells in culture were explored using an inverted microscope with phase contrast (FSX10, Olympus, Munich, Germany).
Fluorescence immunocytochemistry
A standard immunocytochemistry protocol was used. The 1.4E7 cells were treated with 1, 5, or 20 μg/mL MWCNTs, NA-MWCNTs, NA, or PBS alone for 30 minutes, one hour, and 3 hours, respectively, and were then fixed with 3%–4% paraformaldehyde in PBS for 15 minutes at room temperature and ice-cold acetone for 7 minutes, respectively, and subsequently permeabilized with digitonin 0.2% in PBS for 10 minutes. Nonspecific binding of the antibodies was blocked in PBS containing 10% goat serum and 0.3 M glycine. After 30 minutes of incubation, the cells were further incubated with a guinea pig anti-insulin antibody (ab7842, Abcam, Cambridge, MA, USA) diluted to 1:50 in PBS or with a rabbit polyclonal anti-MIF antibody (No 36–7401, Invitrogen) at 4°C for one hour. Next, the cells were washed and then reacted with a goat polyclonal secondary antibody to guinea pig antibody –H&L (TR) (ab6906, Abcam, Cambridge, MA, USA) diluted to 1:100 in PBS or with Alexa Fluor® 488 goat anti-rabbit IgG antibody diluted to 1:1000 at room temperature in the dark for one hour for detection. After being washed with PBS three times for 5 minutes in the dark, the cells were further incubated with DAPI (4′,6-diamidino-2-phenylindole) for one minute.
Image analysis
All image analyses were performed using ImageJ version 1.43u (National Institutes of Health, Bethesda, MD, USA). Quantification of insulin and MIF levels was achieved by applying a linear stretch of the pixel intensity histogram corresponding to each slice in the z-stack in order to increase the image contrast by adjusting the number of low and high intensity pixels. The image was then converted into a z-projection. To remove the spaces between each slice of the z-stack and to create a single image from all slices, the image was further converted into a z-projection. A 7 × 7 top-hat filter was further used followed by a median filter and a threshold step. The thresholded image was then converted to a binary image, which resulted in white structures (insulin/MIF) on a black background that can be analyzed in ImageJ.Citation22
ELISA quantification of insulin production
Quantification of insulin levels in the cell media following treatment was performed using an enzyme-linked immunosorbent assay (ELISA) kit for insulin (E90448Hu, USCN Life Science Inc, Houston, TX, USA) in accordance with the manufacturer’s instructions. Insulin concentrations in culture supernatant following treatment were measured using a solid phase sandwich ELISA based on two monoclonal antibodies targeting against insulin epitopes. Briefly, after appropriate insulin secretion stimulation, insulin standards and appropriately diluted (1:50) samples were added to an insulin antibody-precoated multiwell microplate. Next, the secondary antibody was supplemented to the well and incubated for 30 minutes at room temperature. After a repetitive washing step, addition of tetramethylbenzidine chromogen with short incubation (15 minutes in the dark at room temperature) was performed. The reaction was stopped by addition of H2SO3 reagent. Absorbance at 450 nm was read using a MR-9602G microplate reader and concentrations were calculated using the microplate reader (MG-9620G ELISA) software provided by the manufacturer.
Glucose stimulation test
Following treatment as described the 1.4E7 cells were washed twice with PBS and immersed in 15 mM glucose-containing Dulbecco’s Modified Eagle’s Medium for 2 hours. The medium was then collected and stored at −20°C for ELISA. The secreted insulin in the 15 mM glucose medium was measured according to the ELISA protocol.
Flow cytometric analysis
A FACSCalibur™ flow cytometer (Becton Dickinson, San Jose, CA, USA) was used to quantify the number of insulin-producing cells following treatment. Suspensions of treated 1.4E7 cells (NA-MWCNTs/NA/MWCNTs/PBS, respectively) were analyzed by flow cytometry using a red fluorescence (FL3 channel > 670 nm) which was employed to show the population of insulin-producing cells marked with Texas Red® anti-insulin antibody (Abcam, Cambridge, MA, USA). The primary and secondary antibodies used were guinea pig anti-insulin antibody (ab7842; Abcam) and goat polyclonal secondary antibody to guinea pig antibody–H&L (TR) (ab6906, Abcam, Cambridge, MA, USA). The resulting comparative cytogram was further used to determine the number of insulin-producing cells per mL. All of the parameters were gathered at low speed in logarithmic mode (approximately 15 μL per minute) in order to maintain the counting level under 1,000 events per second. The threshold value for the FSC (forward scatter channel) channel was 52. The population concentration was estimated using CellQuest software programs (quadrant statistics module).
Statistical analysis
All data are expressed as the mean ± standard error of the mean. Continuous data were tested for normality (Kolmogorov–Smirnov test) before hypothesis testing. Fisher’s exact test was used for qualitative data correlations. For all tests, a 0.05 threshold was selected for statistical significance. Statistical data analysis was performed using Statistical Package for the Social Sciences version 17.0 software (SPSS Inc, Chicago, IL, USA).
Results
The main goal of this investigation was to develop and test a new method of insulin production enhancement in 1.4E7 beta-cells (). Preliminary data from the literature support the involvement of MIF in enhancement of insulin secretion. This implication is supported by the fact that MIF is a secretory product of the insulin-producing cell that functions as an autocrine regulator of insulin release.
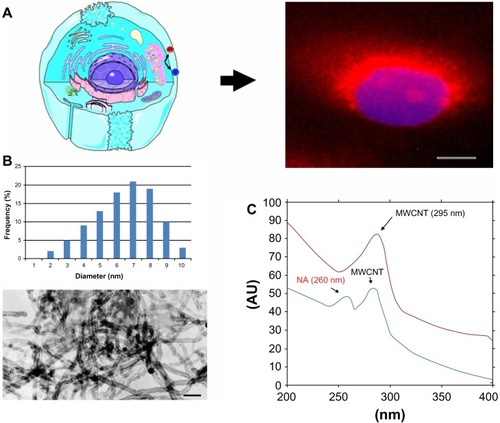
Figure 1 (A) Schematic illustration of insulin production following treatment with NA-MWCNTs.
Notes: Right panel shows a beta-cell with red fluorescence exhibited by insulin following the proposed treatment (cells were marked with Texas Red®-labeled anti-insulin antibody). (B) MWCNTs used for the experiment. The scale bar represents 50 nm. (C) Ultraviolet visible spectroscopy of raw MWCNTs and NA-MWCNTs. Nanotube solutions give a 295.7 nm absorption band, (red spectrum) nicotinamide solution is characteristic of the 260 nm adsorption band.
Abbreviations: NA, nicotinamide; MWCNTs, multiwalled carbon nanotubes; AU, absorbance units
Characterization of MWCNTs
The diameter and length of the MWCNTs used in the experiments were confirmed by transmission electron microscopy (). Oxidation of nanotubes was performed using 3:1 (v/v) sulfuric acid and concentrated nitric acid, which gave them hydrophilicity and stability in aqueous systems due to the formation of COOH and OH groups at the end of or on the lateral sides of the tubes. Next, we covalently conjugated NA onto the surfaces of oxidized MWCNTs. Ultraviolet-visible spectroscopy is a simple but effective method confirming the formation of oxidized NA-MWCNT complexes. Nanotube solutions give an 295.7 nm absorption band, corresponding to transition plasmon MWCNTs (red spectrum). NA solution is characteristic of the 260 nm absorption band, suggesting infrared aromatic structures. Comparing the above-mentioned range, the formation of NA-MWCNT complexes becomes obvious due to the band specific for MWCNTs and the 260 nm band which has a low intensity (blue spectrum)Citation23 ().
Immunofluorescence microscopy
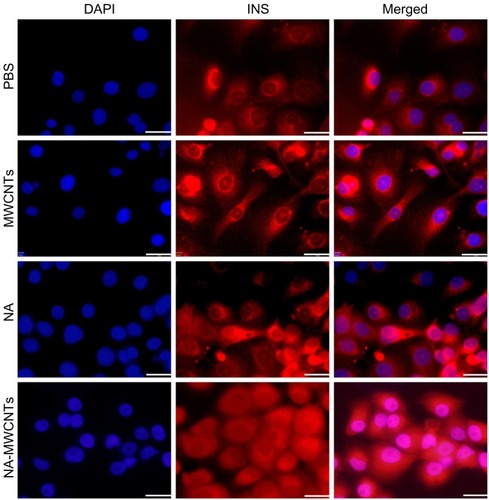
As seen in (row 4) red fluorescence (Texas Red anti-insulin antibody) in cells treated with NA-MWCNTs (5 mg/L for 30 minutes) showed bright, elongated, and punctate fluorescence, evenly spread in the cell cytoplasm, strongly suggesting increased insulin production compared with exposure to 5 mg/L MWCNTs/NA/PBS for 30 minutes. These cells showed a decreased fluorescent signal with less insulin vesicles being visualized. In almost all NA-MWCNT-treated cells, we noticed specific movement of insulin vesicles to the cellular membrane, which resulted in strong diffuse cytoplasmic fluorescence. ImageJ quantification of all fluorescent images following treatment of 1.4E7 cells with NA-MWCNTs revealed significantly increased red fluorescence, suggesting insulin production (Chi square test, P < 0.05). Thus, the average area of red fluorescence/total number of cells between cells treated was 3.4 versus 7.2 for 1 mg/L, 5.9 versus 13.1 for 5 mg/L, and 6.9 versus 17.4 for 20 mg/L.
Figure 2 Immunofluorescence detection of insulin production in 1.4E7 insulin-producing cells. The 1.4E7 cells were harvested at a density of 2–4 × 104 cells/cm2 on Lab-Tek 4 chamber glass slides and treated with 5 mg/L NA-MWCNTs, NA, MWCNTs and PBS, respectively.
Abbreviations: NA, nicotinamide; MWCNTs, multiwalled carbon nanotubes; PBS, phosphate-buffered saline; DAPI, 4′,6-diamidino-2-phenylindole; INS, insulin.
Flow cytometric quantification of insulin-producing cells
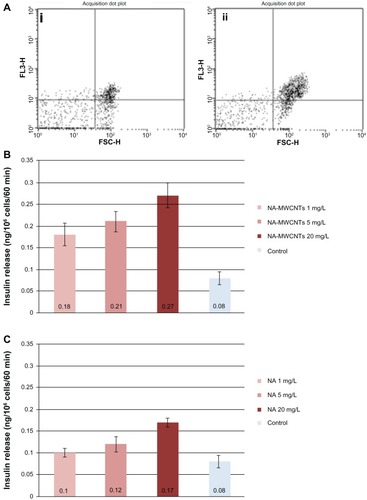
Flow cytometry showed that cultures treated with NA-MWCNTs (5 mg/L) had an increase of 87.3% in insulinpositive cells following incubation (), whereas treatment with 5 mg/L NA in similar conditions () resulted in an increase of only 56.3% in insulin-positive cells. Thus, quantification of flow cytometric data from all cells (red FL3 channel) revealed a significantly decreased fluorescence signal (Chi square test, P < 0.05), suggesting a lower proliferation rate in 1.4E7 cells treated with NA only compared with those treated with NA-MWCNTs.
Figure 3 (A) Flow cytometric quantification of insulin-producing cells following treatment with 5 mg/L NA (i) or NA-MWCNTs (ii) for 30 minutes. (B and C) Quantification of insulin released in culture media following treatment and stimulation with glucose. Statistically significantly increased amount of insulin was found in the NA-MWCNT medium compared with that found in the medium of the cells treated with NA.
Abbreviations: NA, nicotinamide; MWCNTs, multiwalled carbon nanotubes; FSC-H, forward scatter.
ELISA quantification of insulin secretion from beta-cell population
Using ELISA to quantify the amount of insulin released in cell culture medium under glucose stimulation following treatment with NA-MWCNTs/NA/MWCNTs/PBS administered at various concentrations and various incubation times, it was found that 1.4E7 cultures treated with NA-MWCNTs secreted more insulin into the supernatant as compared with cultures exposed to NA. As seen in , 1.4E7 cells treated for 30 minutes with NA-MWCNTs at concentrations ranging from 1 mg/L to 20 mg/L showed significantly increased insulin release (0.18 ± 0.026 ng/mL for 1 mg/L, 0.21 ± 0.024 ng/mL for 5 mg/L, and 0.27 ± 0.028 ng/mL for 20 mg/L). Thus, compared with cells treated with NA alone (0.1 ± 0.01 ng/mL for 1 mg/L, 0.12 ± 0.017 ng/mL for 5 mg/L, and 0.17 ± 0.01 ng/mL for 20 mg/L, ) we observed a significant positive effect on insulin release in cells treated with NA-MWCNTs (Mann–Whitney U test, P = 0.046 for 1 mg/L, P = 0.037 for 5 mg/L, and P = 0.028 for 20 mg/L).
Immunocytochemical localization of MIF in 1.4E7 beta-cells
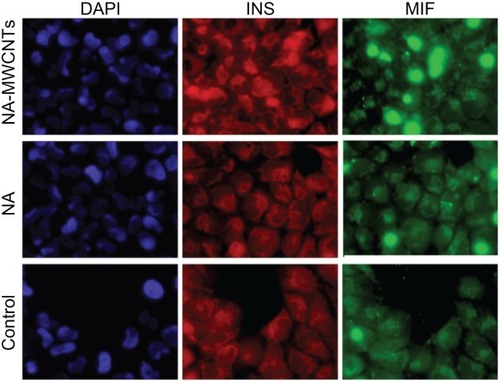
To shed light on the molecular mechanisms involved in the enhancement of insulin production mediated by MWCNTs, 1.4E7 cells were immunostained with anti-MIF and anti-insulin antibodies (). To accomplish this, we allowed cells treated with 5 mg/L NA-MWCNTs for one hour to incorporate Alexa Fluor 488 anti-MIF antibody for 60 minutes at 37°C. This technique allowed us to identify MIF clearly within the cytoplasm of 1.4E7 cells (green fluorescence, , third column) that were also positive for insulin (, second column). Alternatively, as seen in , we showed that anti-insulin antibodies labeled with Texas Red were predominantly distributed in the cytoplasm of these cells overlapping MIF. Of utmost importance, we could clearly observe that MIF was overexpressed in NA-MWCNT-treated cells, strongly suggesting a correlation between NA-MWCNT administration and MIF expression. ImageJ analysis of all fluorescent images revealed significantly increased expression of MIF in 1.4E7 cells (Chi square test, P < 0.05) following treatment with NA-MWCNTs.
Figure 4 Immunocytochemical localization of MIF and insulin in 1.4E7 insulin-producing cells.
Notes: 1.4E7 cells were stained with DAPI, and anti-insulin and anti-MIF antibodies and further analyzed by immunofluorescence (Texas Red® anti-insulin antibody and Alexa Fluor® 488 anti-MIF antibody). Insulin staining is red, MIF staining is green. Fluorescence images were obtained using an Olympus FSX 100 fluorescence inverted microscope (×60).
Abbreviations: NA, nicotinamide; MWCNTs, multiwalled carbon nanotubes; DAPI, 4′,6-diamidino-2-phenylindole; INS, insulin.
Discussion
Carbon nanotubes show many unique intrinsic physical, mechanical, electrical, and chemical properties, and have been intensively explored for biological and biomedical applications in the past few years.Citation10
Because the physical properties of CNTs are essentially preserved by the noncovalent approach, aqueous solutions of CNTsCitation24 engineered by noncovalent functionalization are promising for multiple biomedical applications.
High doses of NA have protective effects on beta-cell survival and function in animal and in vitro studies,Citation25 and NA has been shown to increase the insulin content in insulin-producing cells.Citation7 Moreover, several protocols have used NA as a differentiation factor.Citation26 NA is also suggested to have a stimulatory effect on islet regeneration. Several studies have confirmed that NA promotes replication of mouse islet cells both in culture and after transplantation.Citation6 Further, Otonkoski et al showed that the addition of NA to the culture medium of human fetal pancreatic cells increased both the total cell number and the frequency of beta cells.Citation27 Other authors used a protocol in which insulin-secreting cells (clone IB/3x-99) were cultured in 10 mmol/L NA for 2 weeks followed by another week in 10 mmol/L NA plus 5 mmol/L glucose. They noted that the insulin content of the cells increased 20-fold and that the cells showed a good insulin response to different secretagogues.Citation5
Vaca et alCitation7 demonstrated that the combination of nutrient restriction and NA supplementation decreases the rate of proliferation and substantially increases the insulin content (1.780 ng insulin/mg protein) in D3 mouse embryonic stem cell culture, a value 3-fold greater than the values obtained by other authors, suggesting that NA may increase insulin synthesis. Therefore, NA plus nutrient restriction increases insulin content and improves the last steps in the maturation process under experimental conditions in which undesired proliferation is controlled.
It has been shown previously that the MIF content of pancreatic islets was increased by high glucose concentrations, while immunoneutralization of MIF in a perifusion system of Langerhans islets reduced the first and second phases of glucose-induced insulin secretion by 39% and 31%, respectively. Conversely, exogenously added recombinant MIF was found to potentiate glucose-induced insulin release.Citation9 Therefore, MIF appears to be a glucose-dependent islet cell product that regulates insulin secretion in a positive manner.Citation28 Evidence supporting the role of MIF in upregulating insulin secretion comes also from another experiment demonstrating that administration of recombinant MIF in vitro upregulates PDX-1 and insulin gene transcription in mouse islets. Another glucose-induced insulin secretion test performed in vitro showed that pancreatic islets isolated from MIF knockout mice produced less insulin than wild-type C57BL/6 islets but had a higher insulin content within beta-cells.Citation29 Further, it appears that MIF regulates insulin secretion in physiologic conditions, whereas in pathologic states it alters beta-cell function and induces their apoptosis or necrosis and affects beta-cell neoplasia. Likewise, recent epidemiologic data support the role of MIF in the development of insulin resistance and type 2 diabetes in humans.Citation30 While much progress has been made in the treatment of diabetes mellitus as well as in the study of beta-cell lines, a proper procedure to generate a sufficient mass of insulin-producing cells that display regulated release has not been found as yet. Considering the many potent actions of NA on pancreatic islet cells in animal models and its use in clinical trials to preserve beta-cell function, as well as the exciting properties of CNTs as drug carriers and delivery vehicles, we have characterized the effects of NA-MWCNTs on the function and insulin production of 1.4E7 insulin-secreting cells. Insulinoma cell lines, which display several undifferentiated characteristics even though retaining some of the features of normal islets (for example, peptide hormone biosynthesis and secretion),Citation2 have applications in the study of pancreatic biology, providing a method of producing pure insulin-secreting cell lines. This is an alternative to the use of primary tissue in cell transplantation therapies for type 1 diabetes. Hence, the 1.4E7 line offers clues on how to generate corresponding human beta-cell lines that may be useful for research and for cell replacement therapy in diabetes. As far as we know, we have noted for the first time that 1.4E7 cells treated with NA-MWCNTs show increased insulin production and regulated insulin secretion as compared with administration of NA or MWCNTs alone.
Our immunocytochemistry staining data suggest a strong interaction between NA-MWCNTs and the MIF signaling pathway that regulates insulin secretion, thereby suggesting strategies for developing continuously growing human beta-cell lines. Nevertheless, further investigations are required for a careful assessment of unexpected toxicities and biological interactions of NA-MWCNTs inside the living organism.
Acknowledgments
The authors would like to acknowledge grant support from the Romanian Ministry of Research (PN-II-RU-PD-2011-3-0287, PT-PCCA-2011-3.1-1586).
Disclosure
The authors report no conflicts of interest in this work.
References
- GaleEAThe rise of childhood type 1 diabetes in the 20th centuryDiabetes200251123353336112453886
- PhilippeJDruckerDChickWHabenerJTranscriptional regulation of genes encoding insulin, glucagon, and angiotensinogen by sodium butyrate in a rat islet cell lineMol Cell Biol1987715605633550424
- SoriaBRocheEBernáGLeón-QuintoTReigJAMartínFInsulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic miceDiabetes200049215716210868930
- LeungPSCarlssonPPancreatic islet renin angiotensin system: its novel roles in islet function and in diabetes mellitusPancreas200530429329815841036
- SoriaBSkoudyAMartinFFrom stem cells to beta cells: new strategies in cell therapy of diabetes mellitusDiabetologia200144440741511357469
- SandlerSAnderssonALong-term effects of exposure of pancreatic islets to nicotinamide in vitro on DNA synthesis, metabolism and B-cell functionDiabetologia19862931992022939000
- VacaPBernaGMartinFSoriaBNicotinamide induces both proliferation and differentiation of embryonic stem cells into insulin-producing cells200335520212023
- SegevHFishmanBZiskindAShulmanMItskovitz-EldorJDifferentiation of human embryonic stem cells into insulin-producing clustersStem Cells200422326527415153604
- WaeberGCalandraTRoduitRInsulin secretion is regulated by the glucose-dependent production of islet β cell macrophage migration inhibitory factorProc. Natl. Acad. Sci. USA199794478247879114069
- IancuCMocanLAdvances in cancer therapy through the use of carbon nanotube-mediated targeted hyperthermiaInt J Nanomedicine20116167521904457
- ZhangYBaiYYanBFunctionalized carbon nanotubes for potential medicinal applicationsDrug Discov Today20101511–1242843520451656
- KamNWDaiHCarbon nanotubes as intracellular protein transporters: generality and biological functionalityJ Am Chem Soc2005127166021602615839702
- IancuCMocanLBeleCEnhanced laser thermal ablation for the in vitro treatment of liver cancer by specific delivery of multiwalled carbon nanotubes functionalized with human serum albuminInt J Nanomedicine2011612921289990
- MarchesRMikoryakCWangRHPantanoPDraperRKVitettaESThe importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cellsNanotechnology201122909510121258147
- MocanLTabaranFMocanTSelective ex-vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubesInt J Nanomedicine20116191592821720504
- PatlollaAMcGinnisBTchounwouPBiochemical and histopathological evaluation of functionalized single-walled carbon nanotubes in Swiss-Webster miceJ Appl Toxicol2011311758320737426
- WangCHChiouSHChouCPChenYCHuangYJPengCAPhotothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibodyNanomedicine201171697920620237
- KhazaeiARadMNSBorazjaniMKOrganic functionalization of single-walled carbon nanotubes (SWCNTs) with some chemotherapeutic agents as a potential method for drug deliveryInt J Nanomedicine2010563920856839
- RaffaVCiofaniGNitodasSCan the properties of carbon nanotubes influence their internalization by living cells?Carbon2008461216001610
- ZhouHZhangZYuPSuLOhsakaTMaoLNoncovalent attachment of NAD cofactor onto carbon nanotubes for preparation of integrated dehydrogenase-based electrochemical biosensorsLangmuir20102686028603220121055
- KosynkinDVHigginbothamALSinitskiiALongitudinal unzipping of carbon nanotubes to form graphene nanoribbonsNature2009458724087287619370030
- HicksKAHoweDKLeungADenverDREstesSIn vivo quantification reveals extensive natural variation in mitochondrial form and function in Caenorhabditis briggsaePloS One201278e4383722952781
- LiNChenGFlow injection analysis of trace amounts of NADH with inhibited chemiluminescent detectionTalanta200257596196718968701
- ZhengXZhouFNoncovalent functionalization of single-walled carbon nanotubes by indocyanine green: potential nanocomplexes for photothermal therapyJ Xray Sci Technol201119227528421606588
- HoorensAPipeleersDNicotinamide protects human beta cells against chemically-induced necrosis, but not against cytokine-induced apoptosisDiabetologia1999421555910027579
- LumelskyNBlondelOLaengPVelascoIRavinRMcKayRDifferentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic isletsScience200129255201389139411326082
- OtonkoskiTBeattieGMallyMRicordiCHayekANicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cellsJ Clin Invest199392314598104197
- TosoCEmamaulleeJMeraniSShapiroAThe role of macrophage migration inhibitory factor on glucose metabolism and diabetesDiabetologia200851111937194618612626
- StojanovicISaksidaTStosic-GrujicicSBeta cell function: the role of macrophage migration inhibitory factorImmunol Res2012521–2818822388641
- KleemannRBucalaRMacrophage migration inhibitory factor: critical role in obesity, insulin resistance, and associated comorbiditiesMediators Inflamm2010201061047920169173