?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
The purpose of this study was to develop a novel and continuous method for preparing a nanosized particle of drug crystals and to characterize its properties.
Materials and methods
A new apparatus was introduced to crystallize nanosized drug crystals of amitriptyline hydrochloride as a model drug. The samples were prepared in the pure state by ultrasonic spray, and elaborated deposition was completed via electrostatic adsorption. Scanning electron microscopy, X-ray powder diffraction, and atomic force microscopy were used to characterize the size of the particles; this was subsequently followed by differential scanning calorimetry.
Results and discussion
Nanoparticles of drug crystals were successfully prepared. The size of the drug crystals ranged from 20 nm to 400 nm; the particle size of amitriptyline hydrochloride was approximately 71 nm. The particles were spherical and rectangular in shape. Moreover, the melting point of the nanoparticles decreased from 198.2°C to 196.3°C when compared to raw particle crystals. Furthermore, the agglomeration effect was also attenuated as a result of electrostatic repulsion among each particle when absorbed, and depositing on the inner wall of the gathering unit occurred under the electrostatic effect.
Conclusion
Ultrasonic spray-assisted electrostatic adsorption is a very effective and continuous method to produce drug nanocrystals. This method can be applied to poorly water-soluble drugs, and it can also be a very effective alternative for industrial production. Once the working parameters are given, drug nanocrystals will be produced continuously.
Introduction
Nanomedicine is the subfield of nanotechnology that uses nanomaterials for cancer diagnosis,Citation1–Citation3 gene transfer,Citation4,Citation5 imaging and drug delivery,Citation6–Citation8 and so on, by engineering their structures, compositions, sizes, and shapes, and by modifying their surfaces with ligandsCitation9 at the atomic and molecular scale. Nano drug delivery is one of the most interesting areas in nanomedicine. Nanoparticles are defined with a nanoscale in the range of 10–1,000 nm; they are smaller than cells and can yield pleasantly surprising effects. Nanocapsules,Citation10,Citation11 nanobubbles,Citation12 nanoliposomes,Citation13,Citation14 micelles,Citation15,Citation16 and nanoparticlesCitation6,Citation17 have been explored with some success for developing high-performance delivery systems in the past few years. However, low encapsulation efficiency and relatively good chemical stability are still not satisfactory. The elaboration of nanosized crystals had been introduced by different methods due to its advantages of precise control of size, dosage form diversification, and high level of drug release (approximately 100%). Three methods (milling, precipitation methods, and homogenization), as well as a possible combination of these methods, can be used to produce nanocrystals of a desired shape and size.
As the classical nanocrystal technology, milling methods – which are known as top-down approaches to nano materials from the first half of the 20th century – by a stainless steel ball or a pearl mill, are completed by filling with a container to achieve particle size diminution. The drug material to be crushed is added in the form of a powder with a grain size diameter of about 50–100 μm, and the milling time can last from about 30 minutes to several hours or several days, depending on the different drugs and many other factors such as the surfactant content, hardness of the drug, viscosity, temperature, energy input, and size of the milling media.Citation18–Citation22 This process is simple and low-energy; however, its weaknesses lie in the fact that the grinding balls contribute to impurities, and it is a slow process. Precipitation methods are the other method used to make nanosized drug materials; hydrosol is essentially a classical precipitation process known as “via humida part-time.” The drug materials are dissolved in a solvent and are subsequently added to a nonsolvent, leading to the precipitation of finely dispersed drug nanocrystals.Citation23,Citation24 Oil/water two-phase system regions are excellent and effective carriers; they are characterized by bioavailability solubility, and improve the bioavailability of only poorly water-soluble drugs. They are not universally applicable for most drug materials. High-pressure homogenization, a simple process that was developed in 1999 for nanoparticle production, is also used for preparing nanosized drugs.Citation25 Only drugs that are poorly water soluble in both aqueous and organic media can be easily formulated into nanoparticulate suspensions. The advantage of this process is that it is a fast method and it is a continuous process. This method needs high-energy techniques and requires a great level of experience. In addition, nanoparticle agglomeration, which reduces the specific surface area, still exists and can reduce the effect of nanoparticles to a certain extent.
The objective of this study was to develop a new and continuous method for the atomization process in the preparation of nano- and submicro-sized drug particles for the first time.
An ultrasonic nebulizer is used to generate the aerosol in which a piezoelectric transducer is located at the central part of the bottom of the container. When the piezoelectric transducer is switched on, ultrafine droplets generate on the upper surface of the liquid due to the qualification caused by ultrasonic waves generated in the liquid. The mechanism of ultrasonic atomization, by which vibration of the liquid surfaces causes atomization, has been studied by Lang.Citation26 The droplet size can be estimated reasonably accurately using Lang’s equation:Citation26
Amitriptyline hydrochloride (AMT · HCl) was chosen as the model drug for this study. This method is an important particle size reduction technology that can be operated both in the laboratory and in industry settings.
Material and methods
Materials
Chemicals
In general, the model drug (raw AMT · HCl; purity =99.6%; pH =5.4) was purchased from Hubei Xindali Biochemical Co., Ltd. (Hubei, People’s Republic of China). Anhydrous ethanol and diethyl ether of analytical grade were obtained from Chengdu United Chemical Industry Supply and Marketing Storage Limited Company (Chengdu, People’s Republic of China). High-purity nitrogen (99.999%) was bought from Changjun GAS Co, Ltd (Mianyang, People’s Republic of China). The ultrasonic nebulizer (402-B) with a series of ultrasonic frequencies was supplied by Jiangsu Yuyue Medical Equipment and Supply Co., Ltd. (Jiangsu, People’s Republic of China); the work voltage was 220 V and the ultrasonic transducer frequency was 1.7 ± 0.17 MHz.
Experimental setup
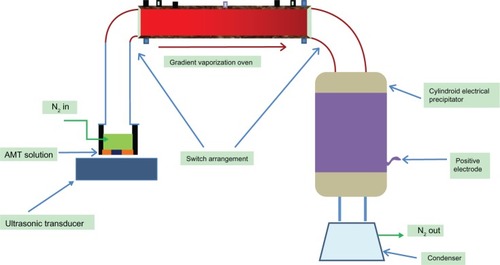
The ultrasonic spray-assisted electrostatic experimental installation, which was designed by our own laboratory, is shown in . It was made of three main parts: 1) an ultrasonic spray, which is used to generate an aerosol on the upper surface of the liquid of the solution; 2) an oven, which can be heated differently with different zones (the half of the oven closest to the entry is hot in temperature, and it attenuates this temperature at the other half of the oven); and 3) an electrostatic adsorption precipitator, which is composed of two electrodes, between which an electrostatic field would exist once the switch is turned on. In consideration of safety, and also to balance the efficiency of the installation, the minimum distance of the two electrodes was 9.0 cm.
Methods
Preparation of AMT·HCl nanoparticles
About 0.71 g of raw AMT · HCl was dissolved in 30.0 mL of anhydrous ethanol (solvent), in a concentration (3 wt%) below the saturation point at 25°C. When the ultrasonic spray, which has been given an ultrasonic frequency, is turned on, it produces an aerosol on the upper part of the solution; the aerosol quantity is affected by the frequency, as well as by the liquid level. Then, the aerosol is transported by an inert gas (nitrogen) flow out of the container and turn into droplets.
When the droplet enters the oven, which was heated differently in different zones, the crystallization process of nano-amitriptyline (AMT) is quickly completed upon evaporation of the solvent. The droplet of different size can get a complete crystal growth, as a result of unequal heating. The AMT nanoparticles flow out of the oven with the help of the gas flow, and continue to be dried at a temperature 80°C ± 1°C (which is higher than the boiling point of anhydrous ethanol). As a result of being charged by an electrode (which applied an electrical tension of 3–11 kV), nano-AMT · HCl was prepared via directional movement from the anode to cathode under the force of electrostatic attraction. The particles produced accumulated close to the bottom of the precipitator. A dry or water-free solvent is very important, because a small amount of water would cause the loss of energy, and cause difficulty in increasing the working temperature. There are few nanoparticles that are stored in the electrostatic adsorption precipitator because most of these particles almost adhere to the inner wall of the oven during the first hour of the experiment. The production rate is constant for a given work parameter, when particles appear in cylindroid electrical precipitator.
Characterization of AMT · HCl nanoparticles
Scanning electron microscopy
The morphology and surface appearance of the nano-AMT · HCl particles were characterized by field scanning electron microscopy (SEM) (Ultra 55; Carl Zeiss Meditec AG, Jena, Germany). The sample was placed on double-sided tape and then observed using field SEM at an acceleration voltage of 15 kV after gold sputtering coating under the vacuum degree of 10−6 Pa for 120 seconds.
Particle size and size distribution
The average size and particle size distribution of AMT · HCl were calculated by means of counting more than 300 particles from the obtained SEM images via the statistics obtained from the SmileView software (JEOL; Tokyo, Japan).
X-ray diffraction
The phase content of AMT · HCl was determined by X-ray diffraction (XRD) (X’Pert PRO; PANalytical, Almelo, the Netherlands) analysis using Cu–Ka radiation at 50 kVand 30 mA, and a monochromatic graphite diffracted beam. The samples were packed into an amorphous silicon holder, and the diffraction angle (2θ) was scanned from 5°C–80°C; the scanning rate was 10°C · minute−1.
Atomic force microscope
The imaging of nano AMT · HCl was carried out in ambient conditions on a SPA300HV atomic force microscope (AFM) (SPA300HV; Japan), which was equipped with a piezoelectric ceramic scanning head. The tapping mode was used with a length of 10 × 10 μm and a spring constant of 40 N · m−1.
Differential scanning calorimetry
Samples of raw AMT · HCl and prepared AMT · HCl were analyzed with differential scanning calorimetry (DSC) (Diamond DSC; PerkinElmer, Waltham, MA, USA). The conditions of the DSC were as follows: sample mass: 2.0 mg; heating rate: 10 K · minute−1; and nitrogen atmosphere (flow rate: 30 mL · minute−1).
Results and discussion
Working parameters
The experimental installation described above runs under the adjustment of several different work parameters, which were all identified with the obtained nanosized medicinal materials and the output of the installation. The droplet size, the main influencing factor (which is decided by the surface tension and the density of the solution that changes with different solvent types), and the excitation frequency offered by ultrasonic transducer have been discussed. The other most important parameters that were identified and studied are discussed in the following sections.
Liquid level in reactor
The liquid level in the reactor is the first variable involved in the reduction of particle size, as shown in Equationequation 1[1] , as the droplet can generate via ultrasonic atomization. Conversely, the amount of the droplet that is transported into the system is decided by the liquid level. The level that yields the greatest amount of aerosol can be obtained by a series of experiments.
Solution concentration
Solution concentration significantly affects the particle size of the product. Generally speaking, there was a positive correlation between the concentration and the average particle size. This means that the high solution concentration yields a large AMT · HCl particle size; namely, the particle size decreased with the reduction of the solution’s concentration.
Inert gas flow
Inert gas is used to increase the distance between each droplet and to transport the aerosol and the particle after the nanocrystals form. There is a balance between the transportation and the deposition. Relatively high inert gas flow will result in a loss of the materials, and to the contrary, low inert gas flow will lead to a low production rate or no production.
Working temperatures
The working temperature of the oven is the key parameter for preparing nanoparticles. In fact, there is a course of crystallization that occurs – the droplet enters into the oven, the liquid evaporates, and the material crystallizes and continues to be dried in the second part of the oven. The temperature of the first part of the oven is about 20°C–40°C higher than the boiling point of the solvent (for fast evaporation); otherwise, possible aggregation would occur. In addition, the temperature of the second part of the oven is normally 10°C–20°C lower than the first part of the oven in order to reduce the rate of crystallization. This is due to the idea that crystallization that occurs too quickly will make an unexpected deposit in the oven instead of in the precipitator.
Electrical precipitator temperature
The electrical precipitator is the place where the sample deposits as time goes on; as such, the precipitator should be provided in order for the particle to exist at a temperature that is normally 1°C–3°C higher than the boiling point of the solvent.
The results presented below were all obtained with the following working parameters:
inert gas: N2;
inert gas flow: 60 mL · minute−1;
solvent: anhydrous ethanol;
drug: AMT · HCl;
ultrasonic transducer frequencies: 1.7 ± 0.17 MHz;
ultrasonic transducer current/voltage: 200 mA/220 V;
liquid level in the reactor: 3.0 ± 0.1 cm; and
working temperatures: 27°C (reactor), 90°C–110°C (oven), and 80°C ± 1°C (electrical precipitator).
Characterization of nano-AMT HCl particles
Nano AMT · HCl particles were successfully prepared via an ultrasonic spray-assisted electrostatic adsorption method. illustrates the product deposition.
Morphology characterization
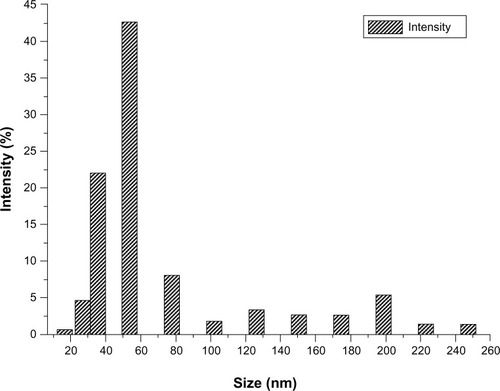
For the SEM analysis, the sample is dispersed in diethyl ether by applying ultrasonic waves of 40 KHz for 30 minutes and vacuum drying for 12 hours. SEM was used to image the morphology of both the raw AMT · HCl and prepared AMT · HCl; the photographs are shown in . The image shows a great difference between the raw and prepared samples, which is probably caused by the electrostatic interaction and agglomeration. Raw AMT has a polyhedral morphology and a particle size of about 1–40 μm (), while the prepared AMT size is nearly reduced to 20–300 nm. In addition, there are two groups of nano-AMT · HCl particles () after ultrasonic processing: a smaller particle size (with an average size of 70 nm), and another group with an average particle size of 270 nm. No obvious particle aggregation was observed. The possible reason behind this is that the aerosol size distribution generated by the piezoelectric transducer during the aerosol production process, as well as the chaotic and complex disintegration, is variable.Citation27 Thus, small aerosol size droplets crystallize small particles, and at the same time, big aerosol size droplets crystallize big particles during crystallization. In addition, the repulsive force existing in the electrostatic field of each particle reduces the agglomeration between each of the particles. Though the effects of ultrasound can help diminish particle size to a certain extent, the main contribution of being nanosized involves the rupture of the capillary surface waves and their subsequent ejection, in the aerosol process. The suitable particle size distribution of the prepared nanoparticles is also given and the average particle size is about 70 nm (as shown in ), and the particle size distribution is relatively narrow with 60–300 nm of smaller sized particles when compared with the distribution of the raw AMT · HCl particles (which is determined by counting more than 300 particles from the obtained SEM images).
Figure 3 Characterization of raw AMT · HCl and nano-AMT · HCl.
Notes: Scanning electron microscopic images of (A) raw AMT · HCl; (B) raw AMT · HCl after ultrasonic wave dispersion for 30 minutes; (C) nano-AMT · HCl after ultrasonic wave dispersion for 30 minutes; and (D) magnification of the red flag area of (C).
Abbreviation: AMT · HCl, amitriptyline hydrochloride; EHT, extra-high tension; Mag, magnification.
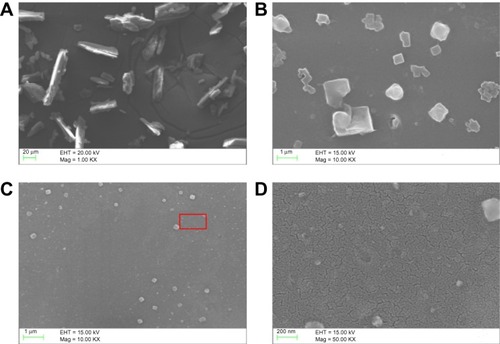
Figure 4 Size distribution of nano-AMT · HCl particles.
Abbreviation: AMT · HCl, amitriptyline hydrochloride.
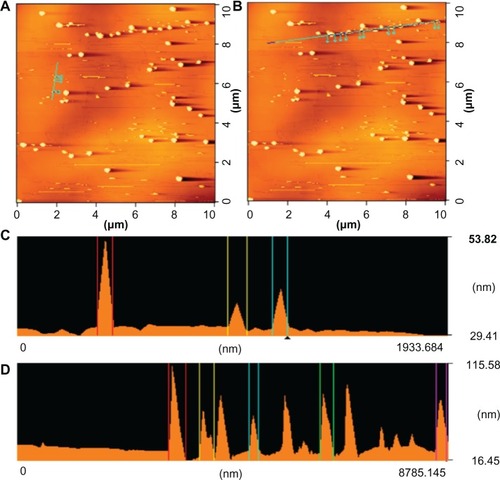
The sample was prepared by dispersing the nanoparticles in a nonsolvent like diethyl ether, and by applying ultrasonic waves (40 KHz). After complete dispersion, a 3.0 mL droplet was placed on an atomic flat mica support. The liquid was then evaporated at 60°C. After the evaporation process, the sample was fixed on the scanning head of the AFM. and show the AFM of a nano-AMT · HCl sample obtained by using the crystallization process with an ultrasonic transducer frequency of 1.7 MHz. The micrograph of provides a global AFM registration of a relatively large scanning area; shows that the threshold of the particles is about 53 nm. The image indicates that the particles have their respective sizes determined by the other techniques. There are two kinds of particles sizes: elementary particles of about 40–90 nm in size, and agglomerates of about 100–400 nm in size (). AFM microscopy, which does not need a vacuum or heating, is considered the most suitable method for the characterization of nanoparticle sizes. The sample is not destroyed during the observation, and can therefore be observed.
Figure 5 Characterization of nano-AMT · HCl.
Notes: (A) Global AFM-registration of nano-AMT · HCl, and (B) three-dimensional image of nano-AMT · HCl by dispersing the nanoparticles in a nonsolvent diethyl ether.
Abbreviations: AMT · HCl, amitriptyline hydrochloride; AFM, atomic force microscope.
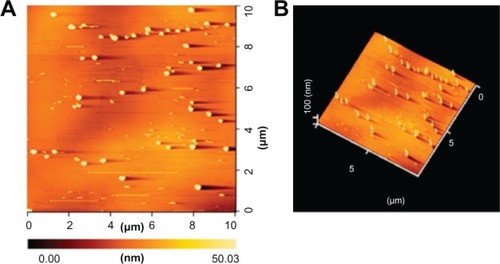
Figure 6 Height measurements on different nano-AMT · HCl particles.
Notes: Atomic force microscope of (A and C) elementary particles of about 40–90 nm (66 nm, 67 nm, and 86 nm surfaces analyzed from left to right), and (B and D) agglomerates of about 100–400 nm (360 nm, 291 nm, 188 nm, 274 nm, and 205 nm surfaces analyzed from left to right).
Abbreviation: AMT · HCl, amitriptyline hydrochloride.
Physicochemical property characterization
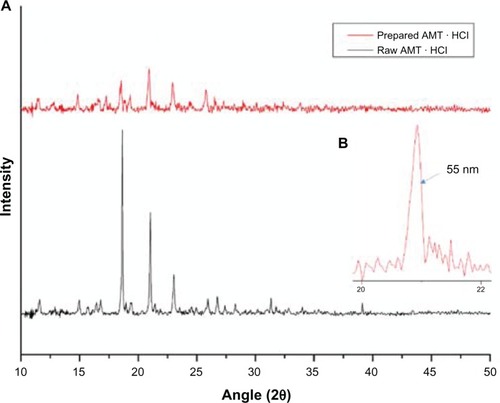
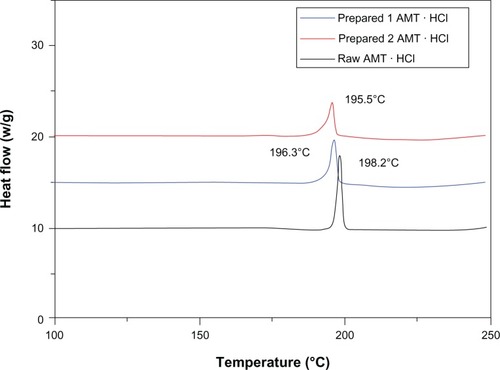
Another technique that is employed to characterize the obtained product is XRD. XRD was used to determine the phase content, the mean size of the elementary crystallites, and the amorphous degree of the product. The peaks () of the raw AMT · HCl at 2θ were 18.50°, 20.96°, and 22.96°, and the crystalline structure of raw AMT · HCl was also observed. The peaks of the prepared AMT · HCl shift at 2θ = 20.94°, 22.96°, and 25.80°. Most of the peaks of the nano-AMT · HCl are presented indicating that the prepared AMT · HCl might be present in a well-crystalline state. As shows, the mean particle size can be calculated by Scherrer’s equation.Citation31 The obtained result is in the order of 55.0 nm. The results were consistent with the results of the AFM, but were 17 nm less than with SEM. The possible reason for this is that the applied vacuum and the heating of the sample by the electronic beam induced the instability of the sample. In addition, as the image was taken, time had to be spent to register the whole image, so that the particles formed agglomerates. shows the results of DSC. Raw AMT · HCl exhibited an obvious melting process, with a peak of 198.2°C, which implied the crystalline form of AMT · HCl. Furthermore, a tendency of the melting point to decrease was confirmed from 198.2°C to 196.3°C. In order to further explain the experimental results, another sample of a different size was prepared with a different work parameter, and this sample was also tested; the result showed that a similar change was observed by DSC characterization, which agrees well with previously published measurements.Citation32–Citation34 The possible reason for the decrease in melting temperature is that more and more atoms are on the surface of the particle, which increases the free surface energy, and consequently decreases the crystal energy by decreasing the particle size. Therefore, precise measuring of the prepared AMT · HCl is reasonable and desirable.
Conclusion
In this work, a new method used to prepare Nano-AMT · HCl was successfully conducted using the ultrasonic spray-assisted electrostatic adsorption method. The mean particle size of nano-AMT is 53 nm, and it presents with a narrow particle size distribution. This method can be used for other drug materials, such as doxorubicin and so on, but differences exist in terms of the solvent, temperature, and other working parameters. Different characterization methods showed that the nanosized drug crystals can be produced continuously via this method. Ultrasonic spray-assisted electrostatic adsorption is a very effective and continuous method to produce drug nanocrystals. This method is also applied to poorly water-soluble drugs, and it is a very effective alternative for industrial production.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project number 11002128, 11272292, and 11172276), and by the Analytical and Testing Center, Southwest University of Science and Technology.
Disclosure
The authors report no conflicts of interest in this work.
References
- PsimadasDGeorgouliasPValotassiouVLoudosGMolecular nanomedicine towards cancer: 111In-labeled nanoparticlesJ Pharm Sci201210172271228022488174
- SumerBGaoJTheranostic nanomedicine for cancerNanomedicine (Lond)20083213714018373419
- ThierryBTextorMNanomedicine in focus: opportunities and challenges aheadBiointerphases201271–41922589062
- GilertAMachlufMNano to micro delivery systems: targeting angiogenesis in brain tumorsJ Angiogenes Res201022020932320
- LawWCMahajanSDKopwitthayaAGene silencing of human neuronal cells for drug addiction therapy using anisotropic nanocrystalsTheranostics20122769570422896771
- DamgéCMaincentPUbrichNOral delivery of insulin associated to polymeric nanoparticles in diabetic ratsJ Control Release2007117216317017141909
- SivasankarMKumarBPRole of nanoparticles in drug delivery systemInternational Journal of Research in Pharmaceutical and Biomedical Sciences2010124166
- RekhiGSAdvances in solid dose oral drug deliveryON Drug Delivery: Oral Drug Delivery and Advanced Excipients20101418
- XiaYNanomaterials at work in biomedical researchNat Mater200871075876018813296
- SchradeACaoZLandfesterKZienerUPreparation of raspberry-like nanocapsules by the combination of Pickering emulsification and solvent displacement techniqueLangmuir201127116689670021563812
- ChenYChenHZengDCore/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug deliveryACS Nano20104106001601320815402
- WangYLiXZhouYHuangPXuYPreparation of nanobubbles for ultrasound imaging and intracelluar drug deliveryInt J Pharm20103841–214815319781609
- YangSQianXXuYTaoXZhaoCIn vitro evaluation of pH-sensitive doxorubicin nanoliposomes modified with carboxymethyl chitosanProceedings of the Shanghai International Nanotechnology Cooperation Symposium30 Oct–Nov 1 2011Shanghai, People’s Republic of China20114650
- SamadikhahHRMajidiANikkhahMHosseinkhaniSPreparation, characterization, and efficient transfection of cationic liposomes and nanomagnetic cationic liposomesInt J Nanomedicine2011662275228322072865
- ChengRFengFMengFDengCFeijenJZhongZGlutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene deliveryJ Control Release2011152121221295087
- DaumNTschekaCNeumeyerASchneiderMNovel approaches for drug delivery systems in nanomedicine: effects of particle design and shapeWiley Interdiscip Rev Nanomed Nanobiotechnol201241526522140017
- SherlockSPTabakmanSMXieLDaiHPhotothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystalsACS Nano2011521505151221284398
- Merisko-LiversidgeELiversidgeGGCooperERNanosizing: a formulation approach for poorly-water-soluble compoundsEur J Pharm Sci200318211312012594003
- RotelloVMNanoparticles: building blocks for nanotechnology1st edNew York, NYSpringer-Verlag2004
- JunghannsJUAHMüllerRHNanocrystal technology, drug delivery and clinical applicationsInt J Nanomedicine20083329531018990939
- SalahNHabibSSKhanZHHigh-energy ball milling technique for ZnO nanoparticles as antibacterial materialInt J Nanomedicine2011686386921720499
- BiazarEBeitollahiARezayatSMEffect of the mechanical activation on size reduction of crystalline acetaminophen drug particlesInt J Nanomedicine2009428328720054432
- ShahNIyerRMMairHJImproved human bioavailability of vemurafenib, a practically insoluble drug, using an amorphous polymer-stabilized solid dispersion prepared by a solvent-controlled coprecipitation processJ Pharm Sci2013102396798123280631
- KolePLVenkateshGKotechaJSheshalaRRecent advances in sample preparation techniques for effective bioanalytical methodsBiomed Chromatogr2011251–219921721154887
- MullerRHBeckerRKrussBPetersKinventorsPharmaceutical nanosuspensions for medicament administration as systems with increased saturation solubility and rate of solution1121999 United States patent US 5858410
- LangRJUltrasonic atomization of liquidsJ Acoust Soc Am196234168
- RajanRPanditACorrelations to predict droplet size in ultrasonic atomisationUltrasonics200139423525511432434
- McCubbinTKJrThe particle size distribution in fog produced by ultrasonic radiationJ Acoust Soc Am195325510131014
- RajagopalESParticle size distributions in ultrasonic emulsificationProceedings of the Indican Academy of Sciences – Section A1959496333339
- AvvaruBPatilMNGogatePRPanditABUltrasonic atomization: effect of liquid phase propertiesUltrasonics200644214615816321416
- ZhuXBirringerRHerrUX-ray diffraction studies of the structure of nanometer-sized crystalline materialsPhys Rev B1987351790859090
- ChenJSunQZouYXueGDSC studies on the melting crystallization of polyethylenes prepared from alkanes of varying molecular sizePolymer2002432568876891
- LiuHArmandJYBouzonJVergnaudJMEffect of sample size and heating rate on the DSC process for reactions of high enthalpyThermochim Acta1988126158192
- ArmandJYGonnetRJonesRBouzonJTouchardMVergnaudJMEffect of heating rate and sample size on heat transfer through the sample in DSCThermochim Acta19861032341351