Abstract
Background
In this study, 293T cells were genetically engineered to secrete tissue inhibitor of metalloproteinase-2 (TIMP2) and encapsulated into alginate microcapsules to continuously release TIMP2 protein.
Methods
The anti-invasive potential of the microcapsules was studied in vitro using brain tumor cells. The TIMP2 gene was transfected to 293T cells, and genetically engineered 293TIMP2 cells were encapsulated into alginate microcapsules. Release of TIMP2 protein was detected with Western blot analysis and the anti-invasive potential against U87MG cells was tested using gelatin zymography and a Matrigel assay.
Results
Cell viability within the alginate microcapsules was maintained at a cell density of 5 × 106. Because polycationic polymers are helpful for maintaining the mechanical strength of microcapsules with good cell viability, the alginate microcapsules were reinforced with chitosan (0.1% w/v). Expression of TIMP2 protein in cell lysates and secretion of TIMP2 into the conditioned medium was confirmed by Western blot analysis. Alginate microcapsules encapsulating 293TIMP2 cells released TIMP2 protein into the medium efficiently, where the TIMP2 protein participated in degradation of the matrix metalloproteinase-2 enzyme and inhibited invasion of U87MG cells.
Conclusion
Alginate microcapsules encapsulating 293TIMP2 cells are promising candidates for anti-invasive treatment of glioma.
Introduction
Brain tumors develop in tens of thousands of adults each year, and the incidence has increased rapidly in recent decades.Citation1–Citation3 Brain tumors are specifically distinguished from other tumors based on their invasive properties, ie, local invasion of brain tumors is more common than metastasis. It is rare that glioma cells enter the subarachnoid space and/or intravasate into the cerebral microvasculature.Citation4 Further, the grade of brain tumor is not significantly correlated with the degree of local invasion. Therefore, the invasive properties of high-grade gliomas in the surrounding normal brain tissue are lethal factors. Infiltrated brain tumor cells, which often escape surgical resection, frequently lead to tumor recurrence.Citation5,Citation6 The infiltrative nature of high-grade gliomas is responsible for much of the morbidity and mortality associated with these tumors. Surgical debulking of the tumor often constitutes only a temporizing measure, because microscopic-infiltrated foci of tumors will eventually lead to recurrence, often in areas that are surgically inaccessible. As a result, patients afflicted with high-grade gliomas face a poor prognosis, with less than 10% surviving beyond 2 years.Citation1–Citation6
Among various mechanisms, degradation of the extracellular matrix by proteolytic enzymes is a classic feature of the invasive process. Such features are commonly expressed by the infiltrating cells of brain tumors. Matrix metalloproteinases (MMPs), which can degrade almost all components of the extracellular matrix, are known to have an important role in invasion of brain tumors.Citation7–Citation11 Because the mechanism of local invasion by malignant glioma cells is distinguished from the mechanisms underlying proliferation, therapeutic strategies against invasive behavior are needed. Among the various kinds of MMPs, activated gelatinase A (MMP-2) has a major role in glioma invasion.Citation12–Citation16 Van Meter et al reported that tissue inhibitors of MMPs (TIMPs) block the action of MMPs and significantly decrease invasiveness.Citation16 Further, when glioma cells are transfected with gene constructs encoding TIMP-1 or TIMP2, invasion is decreased.Citation14 Merzak et al also reported that TIMP2 expression in malignant glioma cell lines decreases the ability to invade.Citation15
Alginate microcapsules encapsulating cells that are genetically engineered to continuously produce a therapeutic protein (endostatin) have been reported to inhibit angiogenesis of gliomas.Citation17–Citation19 Read et al reported that genetically engineered human embryonic kidney cells producing endostatin, an angiogenesis inhibitor, could be encapsulated in alginate beads that released endostatin for several months.Citation18,Citation19 Further, these alginate beads effectively inhibited development of vascular structures in an animal brain tumor model.
In the current study, 293T was genetically modified to secrete TIMP2 and these genetically engineered 293T cells were encapsulated in alginate microcapsules. We expected that alginate beads encapsulating 293TIMP2 cells would produce TIMP2 continuously and that this protein could inhibit invasion of brain tumor cells in vitro.
Materials and methods
Materials
Alginic acid sodium salt, ethidium homodimer-1, calcein AM, and calcium chloride were purchased from Sigma Chemical Co (St Louis, MO, USA). Chitosan 5 was purchased from Wako Pure Chemical Co (Osaka, Japan). Chitosan was pretreated with acid solution to make water-soluble chitosan as follows: chitosan was dissolved in 0.1N HCl solution for 3 hours, then dialyzed (using 12,000 g/mol dialysis tubes) against excess deionized water to remove HCl salt with exchange of water at 3-hourly intervals for 2 days. The chitosan solution was then lyophilized or used for bead preparation. [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] was purchased from Amresco (Solon, OH, USA).
Preparation of alginate beads
293E or 293TIMP2 cells were maintained under exponential growth conditions in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum. Cells were trypsinized and harvested by centrifugation. The cells were resuspended in sodium alginate-saline (1.2% wt/vol) to a final ratio of 5 × 106 cells/mL of alginate. The suspension was dropped through a 23G needle into a solution of HEPES-buffered calcium chloride (13 mM HEPES, 1.5% [wt/vol] CaCl2 [pH 7.4]; Sigma Chemical Co) with chitosan 1 mg/mL and allowed to gel for 20 minutes. Chitosan was used to reinforce the alginate microcapsule.Citation20 The alginate beads were washed three times with HEPES solution (13 mM), then cultured in DMEM supplemented with 10% fetal bovine serum in a 5% CO2 incubator.
Cells and cell culture
U87MG glioma cells and 293T cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in DMEM supplemented with 10% fetal bovine serum.
Viability of encapsulated cells
Viability of the encapsulated cells was measured using Alamar Blue™ (AbD Serotec, Kidlington, Oxford UK) as reported by Baruch et al.Citation21 A volume of microcapsules equivalent to 100,000 encapsulated cells at the day of encapsulation was placed in a 24-plate. The microcapsules were incubated in 1 mL of 10% (v/v) Alamar Blue for 4 hours, after which two 100 μL samples of conditioned medium from each well were distributed to a 96-well plate and read using a microplate fluorometer.
TIMP2 transfection procedure
According to the sequence of GenBank, the primers were designed and synthesized by Bioneer Corporation (Deajeon, Korea) as follows: sense primer, 5′-ATGGGCGCCGCGGCCCGCACC-3′; and antisense primer, 5′-TTATGGGTCCTCGATGTCGAGAAACTCC-3′.
The 293 T cells were maintained under exponential growth conditions in DMEM supplemented with 10% fetal bovine serum in the absence of antibiotics. The optimum cell density for transfection is normally between 50% and 80% confluence for adherent cells. The pcDNA™6/myc-HisA empty vector and pcDNA™6/myc-HisA-TIMP2 (both Bioneer Corporation, Daejeon, Korea) were transfected into 293T cells using Lipofectamine™ 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA). The transfectants are referred to herein as 293E and 293TIMP2, respectively. Cells in serum-free DMEM were mixed with 6 mg of plasmid DNA and 6 mL of Lipofectamine 2000 serum-free medium according to the manufacturer’s protocol. After a 5-hour incubation at 37°C in 5% CO2, the transfection mixture was replaced with DMEM supplemented with 10% fetal bovine serum. After 48 hours of incubation, the medium was replaced with DMEM containing 10% fetal bovine serum and 12 mg/mL blasticidin, and cultured in a CO2 incubator. The blasticidin clones were isolated, and the level of expression of TIMP2 protein was determined by Western blot analysis. The stable transfectants were maintained in DMEM supplemented with 10% fetal bovine serum and 6 mg/mL blasticidin.
Live/dead cell staining
Live/dead cell staining was performed according to a previously reported method.Citation22 The live/dead cell staining working solution was prepared as follows: 2 mM ethidium homodimer-1 solution was diluted to 4 μM in 0.1 M phosphate-buffered saline (pH 7.4); calcein AM stock solution was then added to this solution (final concentration, 2 μM calcein AM). Alginate beads was washed with phosphate-buffered saline three times, and incubated with live/dead staining working solution at 37°C for 30 minutes in dark conditions. Samples were observed under a confocal laser scanning microscope (TCS-SP2; Leica, Wetzlar, Germany). Calcein AM produced green fluorescence-labeled live cells and ethidium homodimer-1 produced red fluorescence-labeled dead cells.
Western blotting
For Western blotting, cells cultured in 10 cm dishes were washed with 0.1 M phosphate-buffered saline (pH 7.4) and 2 mL of serum-free DMEM was added. After one or 3 days, the medium was harvested. For 3-day culture, 1 mL of DMEM was supplemented at day 2 to avoid drying of the culture medium. The cells were harvested by centrifugation and washed three times with phosphate-buffered saline.
Cells were lysed in a lysis buffer (50 mM Tris [pH 8.0], 5 mM ethylenediamine tetra-acetic acid, 150 mM NaCl, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate, 1% NP-40, 1 mM phenylmethylsulphonyl fluoride, and 1 mg/mL protease inhibitor cocktail). The protein concentrations were determined using a protein assay kit (Bio-Rad, Hercules, CA, USA). Next, 50 μg of protein from the whole cell lysates or conditioned medium was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Pall Corporation, Port Washington, NY, USA). Subsequently, the membrane was incubated for 2 hours at room temperature in a solution of TBST (10 mM TrisCl [pH 8.0], 150 mM NaCl, and 0.05% Tween 20) supplemented with 5% nonfat dry milk, and probed overnight at 4°C with mouse anti-TIMP2 antibody 1 (1:1,000 dilution). The bound antibodies were visualized with an anti-mouse second antibody (1:20,000 dilution) conjugated with horseradish peroxidase using enhanced chemiluminescence reagents (Amersham Biosciences, Sunnyvale, CA, USA). ß-actin was used as an internal control. We used densitometer (1-Dmain) software to estimate the level of expression of TIMP2.
Matrigel invasion assay
Prior to performing the Matrigel® invasion assay, U87MG cells were exposed to 293 E and 293TIMP2-encapsulated alginate beads (number of beads approximately 100) for 2 days. The Matrigel invasion assay was performed using a Transwell chamber, as reported previously,Citation23 and 8 μm pore size polycarbonate membranes (Costar, Cambridge, MA, USA) coated with serum-free DMEM-diluted extracellular matrix (Matrigel, DMEM to extracellular matrix [3:1]; Becton Dickson, Bedford MA, USA). Cells were seeded at a density of 5 × 104 cells in 350 μL of serum-free DMEM in the upper compartment of the Transwell, and allowed to invade polycarbonate membranes for 24 hours. The lower chamber was filled with DMEM that contained 10% fetal bovine serum. After incubation, the noninvaded cells on the upper surface of the membrane were removed and the invaded cells on the lower surface of the membrane were stained with Hemacolor® (Merck, Darmstadt, Germany). The number of invaded cells in four randomly selected microscopic fields per membrane was counted.
Gelatin zymography
Gelatin zymography was performed as reported previouslyCitation24 The U87MG cells were seeded onto 10 cm tissue culture plates and cultured in 5% CO2 at 37°C in the presence of serum. The cells were grown and enriched on a culture plate (approximately 70% of the plate). Subsequently, the medium was replaced by 3 mL of serum-free DMEM per plate containing >100 alginate beads encapsulating 293E cells or 293TIMP2 cells. After 48 hours of incubation, the conditioned medium was collected and ultracentrifuged to remove the cell debris. After this procedure, the cells were harvested to study intracellular MMP expression, as described below. The centrifuged medium was used for the gelatin zymography assay, as follows: 50 μg of the total protein from the solution was mixed with the sample buffer (50 mM Tris-Cl, 2% sodium dodecyl sulfate, 0.1% bromophenol blue, and 10% glycerol); the protein was electrophoresed on 8% denaturing sodium dodecyl sulfate polyacrylamide gels containing 2 mg/mL of gelatin (type A, Sigma); the gel was washed three times for 30 minutes in 2.5% Triton X-100, and incubated for 20 hours at 37°C in 50 mM of a buffer solution containing Tris-Cl (pH 7.5), 10 mM CaCl2, and 200 mM NaCl; and the gel was stained with Coomassie Brilliant Blue R-250 (0.2% Coomassie Brilliant Blue R-250, 20% methanol, and 10% acetic acid in H2O), then destained (20% methanol and 10% acetic acid in H2O).
Statistical analysis
The Student’s t-test (Statgraphics, SigmaPlot) was used to assess the statistical differences between experimental groups. P<0.05 was taken as being statistically significant.
Results
Transfection of TIMP2-overexpressed 293T cells
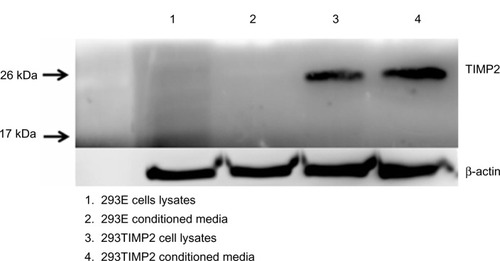
293T cells were cloned with the TIMP2 gene. An empty vector without the TIMP2 gene was also transfected to 293T cells (293E). As shown in , 293TIMP2 cells expressed TIMP2 protein based on Western blotting, and the conditioned medium also showed TIMP2 protein, while 293E cells did not express TIMP2.
Preparation of alginate microcapsule encapsulating 293TIMP2 cells
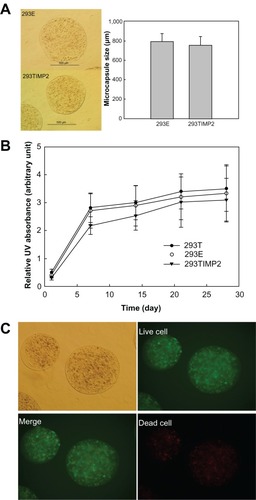
Alginate microcapsules were prepared with the transfected cells; 5 × 106 cells were encapsulated into alginate microcapsules and their size is shown in . As shown in , the average size of the microcapsule was <800 μm in the 293E and 293TIMP2 microcapsules. The viability of cells in the microcapsules is shown in . Even though the viability of 293TIMP2 cells was less than that of the 293T and 293E cells in the microcapsules, cell viability was not significantly changed. Live and dead cells were stained after 4 weeks of culture, as shown in . After 4 weeks of culture, the cells encapsulated in the alginate microcapsules showed good viability.
Figure 2 (A) Mean diameter of alginate microcapsules encapsulating 293TIMP2 cells. (B) Viability of 293TIMP2 cells in alginate microcapsules. (C) Live/dead cell staining of 293TIMP2 microcapsules. The microcapsules were cultured in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum for 4 weeks. Results are expressed as the mean (standard deviation) of three separate experiments.
Abbreviation: 293TIMP2, 293T cells genetically modified to secrete tissue inhibitor of metalloproteinase-2.
To demonstrate continuous secretion of TIMP2 from the alginate microcapsules, the microcapsules encapsulating 293TIMP2 cells were cultured in serum-free medium and secretion of TIMP2 was checked by Western blotting. As shown in , the intensity of TIMP2 was increased on day 2 of culture compared with day 1, even though TIMP2 expression was not significantly changed on day 3. These results indicate continuous release of TIMP2 from the microcapsule and TIMP2 accumulation in the medium. The activity of the TIMP2 protein released from the alginate microcapsules encapsulating 293E and 293TIMP2 cells against MMP-2 is shown in . Alginate microcapsules encapsulating 293TIMP2 cells might affect the MMP-2 secretion from U87MG cells, ie, MMP-2 activity at day 2 and 3 was significantly lower than that of day 1, as shown in . These results might be due to the fact that TIMP2 secreted from microcapsules may affect activation of proMMP-2 and then active MMP-2 can be degraded, which reduces the total amount of MMP-2.
Figure 3 Secretion of TIMP2 from 293TIMP2 alginate microcapsules. The extent of TIMP2 secretion was analyzed by Western blotting.
Abbreviations: TIMP2, tissue inhibitor of metalloproteinase-2; 293TIMP2, 293T cells genetically modified to secrete tissue inhibitor of metalloproteinase-2; conc, concentration.
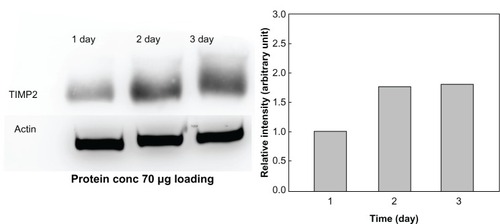
Figure 4 Gelatin zymography. U87MG cells were cultured in 10 cm dishes (cell density 70%–80% of dish area) with Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum. U87MG cells cultured with serum-free DMEM were treated with 293E or 293TIMP2 microcapsules (>100 microcapsules).
Abbreviations: TIMP2, tissue inhibitor of metalloproteinase-2; 293TIMP2, 293T cells genetically modified to secrete tissue inhibitor of metalloproteinase-2.
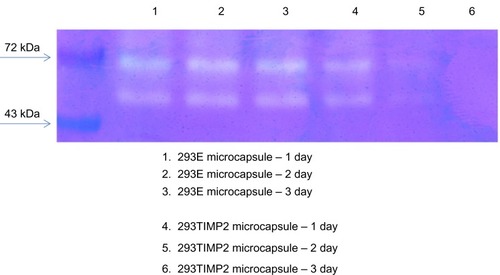
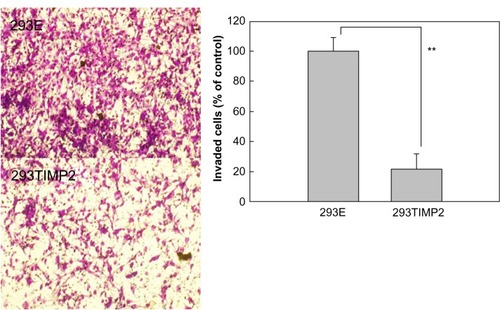
MMP-2 activity was not significantly changed by treatment with 293E microcapsules. shows the effect of alginate microcapsules encapsulating 293TIMP2 cells on invasion of U87MG cells. More than 100 microcapsules were treated with U87MG cells for 2 days. Invasion of U87MG cells was tested with the Matrigel assay. As shown in , the number of cells invading the lower surface of the membrane was decreased significantly when 293TIMP2 microcapsules were treated. These results indicate that alginate microcapsules encapsulating 293TIMP2 cells are prime candidates for inhibiting invasion by brain tumors.
Figure 5 Effect of 293E and 293TIMP2 microcapsules on invasiveness of U87MG cells. The invasion test was performed using the Matrigel® assay. U87MG cells cultured in 10 cm dishes were exposed to 293E or 293TIMP2 microcapsules (>100 microcapsules) for 2 days. Next, 5 × 106 cells were seeded into the upper chamber and the cells invading the lower surface were stained with Hemacolor®. The number of invading cells was counted in four randomly selected microscopic fields per membrane. These experiments were repeated five times and are expressed as the mean (standard deviation).
Note: **P<0.001.
Abbreviations: TIMP2, tissue inhibitor of metalloproteinase-2; 293TIMP2, 293T cells genetically modified to secrete tissue inhibitor of metalloproteinase-2.
Discussion
Local invasiveness of tumor cells is one of the main issues in the treatment of brain tumors; no correlation between degree of local invasion and grade of brain tumor has been demonstrated.Citation4 Infiltrated cells which escape surgical resection frequently lead to recurrence of tumors after surgical debulking.Citation5,Citation6
In tumor cell invasion, a three-step process has been proposed,Citation7–Citation10 as follows: receptor-mediated matrix adhesion; degradation of matrix by tumor-secreted hydrolytic enzymes (such as MMPs); and movement of tumor cells into the newly created space. Degradation of the extracellular matrix by activated gelatinase A (MMP-2) or gelatinase B (MMP-9) in brain tumors is an important factor in invasion of brain tumors.Citation7–Citation16 MMP-2 is known to have a strong influence on glioma invasion.Citation12,Citation13 Otherwise, TIMP-1 and TIMP2 are known to block the action of MMP-2 and MMP-9, which significantly decreases the invasiveness of tumor cells. Overexpression of TIMP2 in glioma cells results in decreased expression of MMP, which decreases the invasiveness of glioma cells.Citation14,Citation15 For example, Huang et al reported that mRNA levels of MMP-2 and membrane type I MMP were significantly reduced in glioma cells.Citation25 Further, Lu et al reported that adenovirus-mediated transfection of TIMP2 to U87MG cells reduces relative MMP-2/9 activity and suppresses the invasiveness of U87MG cells.Citation26 Noninvasive cells such as MCF-7 show high levels of TIMP2 and very low levels of MMP-2, while highly invasive cells such as U87MG cells express MMP-2 together with TIMP2.Citation27 Wisniewski et al also reported on the correlation between invasiveness of glioma cells, MMP-2 activity, and TIMP2 expression.Citation28 Bernardo and Fridman reported that TIMP2 is known to regulate MMP-2 activity in the extracellular environment of tumor tissues after pro-MMP-2 activation by membrane type 1 MMP.Citation29 They showed that net MMP-2 activity correlates with the level of TIMP2 expression. From this point of view, inhibition of MMP expression by TIMP2 is an ideal target for decreasing tumor cell invasion.
In recent decades, microcapsules or microbeads using sodium alginate has received considerable attention for the treatment of tumors.Citation19,Citation30 Read et al reported that cells genetically engineered to produce endostatin and encapsulated in alginate beads released endostatin continuously for several months.Citation19 They also showed that endostatin released from beads inhibited vascularization in a rat BT4C glioma model and prevented growth of solid tumors.Citation19 Further, release of endostatin from alginate beads was maintained over 12 months.Citation30 Another group also reported that continuous release of endostatin from microcapsules encapsulating genetically engineered cells is effective in inhibiting vascularization and tumor growth using an U87MG xenograft mouse model.Citation17
Antiangiogenesis therapy using continuous release of endostatin has been successful.Citation17,Citation19 However, there are several reports on the side effects, ie, antiangiogenesis therapy using endostatin can enhance glioma invasion. Lamszus et al reported that antiangiogenesis therapy using an orthotopic model significantly enhanced the number and total area of small satellite tumors clustered around the primary tumor mass.Citation31 Thus, antiangiogenesis therapy can enhance glioma invasion. From these points of view, we have focused on inhibition of glioma invasion. TIMP2 expression is inversely correlated with MMP-2 expression. We determined that 293T cells (human embryonic kidney cells) are genetically modified with the TIMP2 gene to secrete TIMP2 protein. The 293T cell, ie, the normal human embryonic kidney cell, was selected for genetic modification because it has several advantages for this purpose.Citation32,Citation33 For example, the rapid growth rate of 293 cells is suitable for producing therapeutic protein. Ease of transfection and stable maintenance of transformed cells are other reasons for this choice. For all these reasons, researchers have used HEK293 cells for genetic modification and production of therapeutic proteins.Citation17–Citation19 As shown in , the TIMP2 clone of 293T cells (293TIMP2) clearly expressed TIMP2 protein in cell lysates and the conditioned medium.
Alginate microcapsules were used to encapsulate 293TIMP2 cells. Among various polymeric materials, alginate is acceptable for cell encapsulation since it is biocompatible, and has good morphologic/mechanical properties and biochemical characteristics.Citation34,Citation35 Further, because alginate forms hydrogel microcapsules with the aid of cationic chemicals such as Ca2+ or Mg2+ in aqueous solution, the alginate microcapsule is suitable for maintenance of cell viability and secretion of therapeutic protein. A number of researchers have used alginate for encapsulation of genetically engineered cells or sustained release of proteins.Citation17–Citation19,Citation36 Read et al reported that empty alginate capsules or alginate capsules encapsulating 293 cells cause negligible irritation in the mouse brain and have good biological compatibility.Citation19 Further, they argued that viability of 293 cells in alginate capsules was adequately maintained in vivo. Our results show that capsule size and cell viability in 293TIMP2 microcapsules was not significantly different from that of 293E microcapsules, as shown in . shows live and dead cell staining for 293TIMP2 microcapsules after 4 weeks. As shown in , 293TIMP2 cells were viable in alginate microcapsules. Specifically, alginate microcapsules encapsulating 293TIMP2 cells showed increased TIMP2 density on Western blotting analysis and TIMP2 protein density in the medium was increased according to the time course. These results indicate that alginate microcapsules encapsulating 293TIMP2 cells are able to release TIMP2 protein continuously. This phenomenon was demonstrated using U87MG cells, as seen in and . As shown in , when alginate microcapsules encapsulating 293TIMP2 cells were added to U87MG cell cultures, the intensity of MMP-2 decreased, while alginate microcapsules encapsulating 293E cells did not significantly affect MMP-2 activity, indicating that MMP-2 activity in U87MG cells is decreased by TIMP2 protein secreted from alginate microcapsules. The decreased MMP-2 activity in U87MG cells may affect invasiveness. As shown in , invasion was significantly decreased in U87MG cells treated with 293TIMP2 microcapsules. Hur et al reported finding high activity of MMP-2 and weak expression of TIMP2 mRNA in malignant glioma, indicating an imbalance of TIMP2/MMP-2 in glioma cells.Citation37 Our results indicate that alginate microcapsules encapsulating 293TIMP2 cells are superior candidates for anti-invasive treatment of glioma.
Conclusion
293T cells were genetically engineered to secrete TIMP2 (293TIMP2). 293TIMP2 cells were encapsulated into alginate microcapsules to continuously release TIMP2 protein. The anti-invasive potential of the microcapsules was studied in vitro using brain tumor cells. The TIMP2 gene was transfected to 293T cells, and genetically engineered 293TIMP2 cells were encapsulated into alginate microcapsules. The TIMP2 protein released was detected using Western blot analysis and its anti-invasive potential in U87MG cells was tested with gelatin zymography and a Matrigel assay. Cell viability within the alginate microcapsules was adequately maintained at a cell density of 5 × 106. Because polycationic polymers are helpful in maintaining the mechanical strength of microcapsules with good cell viability, the alginate microcapsules were reinforced with chitosan (0.1% w/v). Expression of TIMP2 protein in the cell lysates and secretion of TIMP2 into the conditioned medium was confirmed with Western blot analysis. Alginate microcapsules encapsulating 293TIMP2 cells released TIMP2 protein into the medium effectively and the TIMP2 protein released was effective in inhibiting MMP-2 expression and invasion of U87MG cells. In conclusion, alginate microcapsules encapsulating 293TIMP2 cells are promising candidates for anti-invasive treatment of glioma.
Acknowledgments
This study was supported by a grant (CRI 10065-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
- GreigNHRiesLGYancikRRapoportSIIncreasing annual incidence of primary malignant brain tumors in the elderlyJ Natl Cancer Inst199082162116242213902
- MahaleyMSJrMettlinCNatarajanNLawsERJrPeaceBBNational survey of patterns of care for brain-tumor patientsJ Neurosurg1989718268362585073
- WalkerAERobinsMWeinfeldFDEpidemiology of brain tumors: the national survey of intracranial neoplasmsNeurology1985352192263969210
- SchoenbergBSEpidemiology of primary intracranial neoplasms: disease distribution and risk factorsSalcmanMNeurobiology of Brain Tumors (Concepts in Neurosurgery)Baltimore, MDWilliams & Wilkins19914
- BernsteinJJWoodardCAGlioblastoma cells do not intravasate into blood vesselsNeurosurgery1995361241327708148
- RuoslahtiEBrain extracellular matrixGlycobiology199664894928877368
- CockettMIBirchMLMurphyGHartIRDochertyAJMetalloproteinase domain structure, cellular invasion and metastasisBiochem Soc Trans19942255578206283
- SatoHTakinoTOkadaYA matrix metalloproteinase expressed on the surface of invasive tumour cellsNature199437061658015608
- VincentTLGatenbyRAAn evolutionary model for initiation, promotion, and progression in carcinogenesisInt J Oncol20083272973718360700
- WoodhouseECChuaquiRFLiottaLAGeneral mechanisms of metastasisCancer199780152915379362419
- Wojtowicz-PragaSMDicksonRBHawkinsMJMatrix metalloproteinase inhibitorsInvest New Drugs19971561759195290
- AbeTMoriTKohnoKExpression of 72 kDa type IV collagenase and invasion activity of human glioma cellsClin Exp Metastasis1994122963048039304
- DeryuginaEIBourdonMALuoGXReisfeldRAStronginAMatrix metalloproteinase-2 activation modulates glioma cell migrationJ Cell Sci1997110247324829410885
- MatsuzawaKFukuyamaKHubbardSLDirksPBRutkaJTTransfection of an invasive human astrocytoma cell line with a TIMP-1 cDNA: modulation of astrocytoma invasive potentialJ Neuropathol Exp Neurol19965588968558175
- MerzakAParkerCKoochekpourSSherbetGVPilkingtonGJOverexpression of the 18A2/mts1 gene and down-regulation of the TIMP2 gene in invasive human glioma cell lines in vitroNeuropathol Appl Neurobiol1994206146197898625
- Van MeterTRoopraiHKRucklidgeGJPilkingtonGJFunctional blocking with TIMP-1 and anti-alpha-V integrin: evidence for cooperation of MMPs and integrins in glioma invasion in vitroAnticancer Res19971710519137448
- JokiTMachlufMAtalaAContinuous release of endostatin from microencapsulated engineered cells for tumor therapyNat Biotechnol200119353911135549
- ReadTAFarhadiMBjerkvigRIntravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cellsCancer Res2001616830683711559558
- ReadTASorensenDRMahesparanRLocal endostatin treatment of gliomas administered by microencapsulated producer cellsNat Biotechnol200119293411135548
- SankaliaMGMashruRCSankaliaJMSutariyaVBReversed chitosan-alginate polyelectrolyte complex for stability improvement of alpha-amylase: optimization and physicochemical characterizationEur J Pharm Biopharm20076521523216982178
- BaruchLBennyOGilertAUkobnikMBen ItzhakOMachlufMAlginate-PLL cell encapsulation system co-entrapping PLGA-microspheres for the continuous release of anti-inflammatory drugsBiomed Microdevices2009111103111319517239
- HaqueTChenHOuyangWSuperior cell delivery features of poly(ethylene glycol) incorporated alginate, chitosan, and poly-L-lysine microcapsulesMol Pharm20052293615804175
- JinSGJeongYIJungSRyuHHJinYHKimIYThe effect of hyaluronic acid on the invasiveness of malignant glioma cells: comparison of invasion potential at hyaluronic acid hydrogel and matrigelJ Korean Neurosurg Soc20094647247820041058
- LimSHJeongYIMoonKSAnticancer activity of PEGylated matrix metalloproteinase cleavable peptide-conjugated adriamycin against malignant glioma cellsInt J Pharm201038720921419945519
- HuangHPShihYWWuCHLaiPJHungCNWangCJInhibitory effect of penta-acetyl geniposide on C6 glioma cells metastasis by inhibiting matrix metalloproteinase-2 expression involved in both the PI3K and ERK signaling pathwaysChem Biol Interact200918181419464279
- LuWZhouXHongBLiuJYueZSuppression of invasion in human U87 glioma cells by adenovirus-mediated co-transfer of TIMP2 and PTEN geneCancer Lett200421420521315363547
- ChernovAVSounniNERemacleAGStronginAYEpigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP2 axis in cancer cellsJ Biol Chem2009284127271273419286653
- WisniewskiPEllert-MiklaszewskaAKwiatkowskaAKaminskaBNon-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFkappaB-TIMP2 pathwayCell Signal20102221222019788921
- BernardoMMFridmanRTIMP2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMPBiochem J200337473974512755684
- BjerkvigRReadTAVajkoczyPCell therapy using encapsulated cells producing endostatinActa Neurochir Suppl20038813714114531571
- LamszusKKunkelPWestphalMInvasion as limitation to anti-angiogenic glioma therapyActa Neurochir Suppl20038816917714531575
- BackliwalGHildingerMHasijaVWurmFMHigh-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEIBiotechnol Bioeng20089972172717680657
- ThomasPSmartTGHEK293 cell line: a vehicle for the expression of recombinant proteinsJ Pharmacol Toxicol Methods20055118720015862464
- RajNKSharmaCPOral insulin – a perspectiveJ Biomater Appl20031718319612614083
- WebberREShullKRStrain dependence of the viscoelastic properties of alginate hydrogelsMacromolecules20043761536160
- HanMRKwonMCLeeHYpH-dependent release property of alginate beads containing calcium carbonate particlesJ Microencapsul20072478779617926170
- HurJHParkMJParkICMatrix metalloproteinases in human gliomas: activation of matrix metalloproteinase-2 (MMP-2) may be correlated with membrane-type-1 matrix metalloproteinase (MT1-MMP) expressionJ Korean Med Sci20031530931410895974