 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigated the potential adverse effects of zinc oxide nanoparticles ([ZnOSM20(+) NPs] zinc oxide nanoparticles, positively charged, 20 nm) on pregnant dams and embryo–fetal development after maternal exposure over the period of gestational days 5–19 with Sprague-Dawley rats. ZnOSM20(+) NPs were administered to pregnant rats by gavage at 0, 100, 200, and 400 mg/kg/day. All dams were subjected to a cesarean section on gestational day 20, and all of the fetuses were examined for external, visceral, and skeletal alterations. Toxicity in the dams manifested as significantly decreased body weight after administration of 400 mg/kg/day NPs; reduced food consumption after administration of 200 and 400 mg/kg/day NPs; and decreased liver weight and increased adrenal glands weight after administration of 400 mg/kg/day NPs. However, no treatment-related difference in: number of corpora lutea; number of implantation sites; implantation rate (%); resorption; dead fetuses; litter size; fetal deaths and placental weights; and sex ratio were observed between the groups. On the other hand, significant decreases between treatment groups and controls were seen for fetal weights after administration of 400 mg/kg/day NPs. Morphological examinations of the fetuses demonstrated significant differences in incidences of abnormalities in the group administered 400mg/kg/day. Meanwhile, no significant difference was found in the Zn content of fetal tissue between the control and high-dose groups. These results showed that oral doses for the study with 15-days repeated of ZnOSM20(+) NPs were maternotoxic in the 200 mg/kg/day group, and embryotoxic in the 400 mg/kg/day group.
Introduction
Recent advances in nanotechnology have spurred increases in the use of nanoparticles (NPs), and concerns over the possible detrimental effects associated with exposure to NPs.Citation1 Zinc oxide nanoparticles (ZnO NPs) are currently engineered and most widely used NPs. Most applications with ZnO powder exploit the reactivity of the oxide, as a precursor to other zinc compounds.Citation2 The applications in material science utilize the high refractive index, high thermal conductivity, and binding properties of ZnO. Possible exposure to ZnO NPs could occur in the industrial settings and through everyday consumer products. ZnO NPs are added into diverse materials and products, such as plastics, ceramics, glass, cement, rubber, lubricants, paints, ointments, adhesive, sealants, pigments, batteries, ferrites, and fire retardants. In addition, ZnO nanomaterials possess ultraviolet (UV)-shielding, antibacterial properties, deodorizing effects, and heat and UV light resistance, which could provide many great potentials for a wide range of applications in many fields: cosmetics and sunscreens,Citation3 food additives, additives in packing,Citation4,Citation5 fungicides in agriculture,Citation6 and biomedical applications such as anticancer drugs.Citation7,Citation8 However, the human risk and toxicity mechanism are not well known.
Since zinc is an essential trace element in the human body and is commonly present in foods or added as a nutritional supplement, ZnO is generally considered to be a material with low toxicity.Citation9 However, ZnO could turn into hazardous material upon inhalation as a gas (for example, metal fume fever), since fumes could be generated from melting and oxidizing at high temperature from zinc or zinc alloys.Citation9 Drinker et alCitation10 and Balance et alCitation11 reported that higher concentrations of freshly generated ZnO, as given in previous human inhalation exposure studies, can produce symptomatic, physiologic, and hematologic effects, as well as elevations in certain peripheral blood and bronchoalveolar lavage cytokines. It should be noted that small-sized particles are more reactive and responsive than bulk-sized particles, because they have a higher proportion of atoms on their surface.Citation12 Also, due to the lower surface energy of ZnO NPs, they can be well dispersed in various solvents as well as in air.Citation13 Therefore, exposures and uptakes of ZnO NPs could occur through various routes.
Few reports were published on the diverse biological systems of ZnO NPs.Citation13,Citation14 Severe damages to liver and lung tissues from acute inhalation of ZnO NPs at dose of 2.5 mg/kg body weight in Wistar rats were reported by Wang et al.Citation14 Also, nano-forms of various particles were more toxic than their micro-counterparts after acute exposure via the oral route in mice.Citation15 In an another study by Wang et al, acute oral toxicity study in mice of ZnO NPs at a high dose range (1–5 g/kg body weight) revealed the increased damages to liver, spleen, and pancreas with increased doses.Citation16 In the dermal toxicity study, 28-day repeated dose of ZnO NPs caused the greater collagen losses in skin of Sprague-Dawley (SD) rats in comparison with tail.Citation17 Liver, lung, and kidney were considered to be the main target organs in a pharmacokinetic and tissue distribution study.Citation18 Also, Lu et alCitation19 suggested that ZnO NPs could disturb the energy metabolisms and cause impairments in mitochondria and cell membrane of rat kidney, in causing ZnO NPs-induced nephrotoxicity. In spite of significant impact of increased usages and productions of ZnO NPs to human health and the environment, the potential adverse effects of ultrafine ZnO on pregnant dams and embryo–fetal development have never been determined.
Currently, there is a serious lack of information on the potential NP hazard to human health, particularly on their possible toxic effects on the endocrine system, and existing data and knowledge of potential endocrine interactions and toxicities are quite limited (for example, reprotoxicity).Citation20,Citation21 Some studies of reproductive function suggest that exposure to some nanomaterials may disrupt endocrine functions such as regulation of serum sex-hormone levels. In contrast, other nanomaterials may prevent endocrine dysfunction via various mechanisms, including antioxidant effects.Citation20 Also, a lot of evidence shows that fetuses are affected more than adults by a variety of environmental toxins because of physiological immaturity. Yamashita et alCitation21 reported that nanosilica induced fetal resorption and restricted fetal growth, and surface modification of nanosilica with carboxyl or amine groups prevented resorption and fetal growth restriction in the study of nanosilica (70 nm).
Therefore, studies of reproductive function are necessary to evaluate the potential endocrine-disrupting risks and the effects on fetuses and pregnancies of nanomaterials. The present study was undertaken to investigate the potential adverse effects of 20-nm positively charged ZnO NPs (ZnOSM20(+) NPs) on pregnant dams and embryo–fetal development in SD rats. The results of this investigation could provide additional relevant information to the safety evaluation of ZnOSM20(+) NP exposures during pregnancy in SD rats.
Materials and methods
This study was performed in compliance with Organization for Economic Cooperation and Development (OECD) test guideline 414 entitled to Prenatal Developmental Toxicity Study,Citation22 and in accordance with the Good Laboratory Practice (GLP) principle.Citation23,Citation24 The GLP process was performed in accordance with the standard operation procedures certified by the Ministry of Food and Drug Safety (MFDS). All of the animals were cared for as specified in the “Guide for the Care and Use of Laboratory Animals” issued by the Animal Care and Use Committee of the NVRQS (National Veterinary Research and Quarantine Service).
Characterization of ZnO NPs
ZnOSM20 NPs (Lot No 141319 for 20 nm), supplied by Sumitomo-Osaka Cement Co. (Tokyo, Japan), had 100.1% assay analysis and also contained <1 ppm Fe not detected and 1.5 ppm arsenic trioxide (As2O3). The ZnO NPs were capped with L-serine molecules, which are widely used capping agents for in organic NPs, providing charge surface property.Citation25–Citation27
Animals and dosage
Crl:CD(SD) rats are commonly used in toxicity studies, as they have a large amount of reference data accumulated over a long time. Male and nulliparous female rats at 10 weeks of age were obtained from a specific pathogen-free colony at Orient Bio Inc. (Gyeonggi-do, South Korea) and used after 12 days of quarantine and acclimatization. The animals were housed in a room that was maintained at a temperature of 20.8°C–23.0°C and a relative humidity of 45.3%–56.9%, with artificial lighting from 8 am to 8 pm and 10–15 air changes per hour. Normally, 1:1 (one male to one female) mating was used in this study. Each morning, the female rats were examined for the presence of sperm or a vaginal plug. Day 0 of pregnancy was defined as the day when a vaginal plug or sperm was found. The mated females were housed individually in clear polycarbonate cages with stainless steel wire lids. They were allowed to drink sterilized tap water with UV irradiation and fed on commercial rodent chow (Cargill Agri Purina, Gyeonggi-do, Korea) ad libitum.
For the oral administration of ZnOSM20(+) NPs, the NPs were suspended in 20 nM 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES) buffer with 2% L-serine (vehicle) and then mixed well. The final pH of the buffer solution was adjusted with hydrochloric acid to 6.5, and 20% of the surface-modified ZnOSM20(+) NPs were used as a stock solution. Before the administration, the suspension was stirred for 10 seconds and then diluted with distilled water. Concentration of dosing solution was measured on gestational day (GD) 5, 11, and 19 using inductive coupled plasma atomic emission spectrometry (ICP-AES) (ULTIMA2; HORIBA JOBIN YVON, Paris, France).
The ZnO NPs were administered daily by gavage to pregnant rats from GD 5 through 19 with a dose volume of 10 mL/kg body weight. The vehicle control group received only HEPES/L-serine buffer solution with gavage. The daily application volume was calculated in advance based on the most-recently recorded body weight of the individual animal.
Dose-range finding study
In a previous dose-range finding study, 14-day repeated oral dose of ZnOSM20(+) NPs showed decreases in body weight, and changes in hematology and biochemistry parameters for 1,000- and 2,000-mg/kg/day groups. Death occurred in two male rats from the 2,000-mg/kg/day group. Decreases in body weight, reductions in food consumption, and changes in hematology and biochemistry parameters were observed from 1,000- and 2,000-mg/kg/day groups. In a subchronic toxicity study of 90-day repeated oral treatment of ZnOSM20(+) NPs, the decreases in food and water consumptions were observed in 125-, 250-, and 500-mg/kg/day groups. Reductions in thymus weight, atrophy of testis and seminal vesicle, and changes of hematology and biochemistry parameters were present in 250- and 500-mg/kg/day groups. Moreover, repeated oral doses of ZnOSM20(+) NPs caused aninar cell apoptosis, submucosal edema, inflammation in the glandular stomach, and decreases in total protein and albumin for 250- and 500-mg/kg/day groups. Either single oral dose or 90-day repeated oral doses (vehicle control and 125, 250, and 500 mg/kg/day) was performed for pharmacokinetic study; the increased zinc concentrations in plasma were seen for 125-, 250-, and 500-mg/kg/day groups.Citation28 Therefore, for the prenatal and developmental toxicity study, the high dose was set to 400 mg/kg/day of body weight, and the middle and low doses was set to 200 and 100 mg/kg/day, respectively.
Experimental groups
A total of 91 healthy female rats were assigned randomly to four experimental groups as follows: three treatment groups of ZnOSM20(+) NPs receiving 100 (n=24), 200 (n=21), and 400 mg/kg/day (n=23), and a vehicle control group (inseminated females per group). The selected doses for this study were based on the results of a dose-range finding study, conducted in our laboratory.
Observation of dams
All pregnant females were observed daily throughout the gestation period for clinical signs (mortality, morbidity, general appearance, and behavior). Maternal body weights were measured daily from GD 0–20, and individual food consumptions were determined on GD 0, 2, 4, 6, 8, 10, 12, 14, 16, and 18. At the scheduled termination day (GD 20), all of the pregnant females were euthanized by isoflurane inhalation and exsanguination from the aorta. A complete gross postmortem examination was then performed. The absolute and relative (organ-to-body weight ratio) weights of the liver, heart, brain, kidneys, ovaries, spleen, lung, uteral cornua, adrenal glands, and pituitary were measured.
Postmortem examination
The ovaries and uteri of each female were removed and examined for the number of corpora lutea and the status of all the implantation sites, ie, live and dead fetuses, early and late resorptions, and total implantations. Uteri with no evidence of implantation were stained with a 2% sodium hydroxide solution to identify the presence of early resorption sites.Citation29 If no stained implantation site was present, the rat was considered “not pregnant”.
On day 20 of gestation, dams were subjected to cesarean section. Following measurement of gravid uterine weight, corpora lutea, implantation, live fetuses, fetal resorptions, and dead fetuses were counted and recorded. Based on the results, the following were calculated:
Resorption was classified as “early” when only a resorption site resembling a dark brown blood clot and with no embryonic tissue was visible, and was considered “late” when both the placental and embryonic tissues were visible at the postmortem examination. All live fetuses were weighed individually, sexed, and examined for any morphological abnormalities, including a cleft palate. Alternate fetuses were selected for either skeletal or visceral examinations. Half of the live fetuses from each litter were fixed in absolute ethanol, eviscerated, and then processed for skeletal staining with alizarin red S and Alcyan blue using for subsequent skeletal examination.Citation30 The other half was preserved in Bouin solution and examined for internal soft-tissue changes using a freehand razor sectioning techniqueCitation31 and Nishimura’s method.Citation32 The observed fetal morphological alterations in this study were classified as developmental malformations or variations. A malformation was defined as a permanent structural change that is likely to adversely affect survival or health.Citation33 The term “variation” was defined as a change that occurred within the normal population under investigation and may be unlikely to adversely affect survival or health. Terminology suggested in an internationally developed glossary of terms was used to classify the structural developmental abnormalities in common laboratory mammals.Citation34
Zn concentration in fetal tissue
To investigate the placenta transfer of ZnOSM20(+) NPs in vivo, four extra female rats were used in the control (control group; n=2) and 400-mg/kg/day groups (ZnOSM20(+) NPs; n=2). Dosing occurred for the period of GD 5–19 in the same manner as that for the main study animals. On GD 20, fetuses were collected via cesarean sections from dams, and Zn contents in the fetal tissues were analyzed. Fetuses were digested in concentrated nitric acid overnight. The next day, nitric acid and perchloric acid were added to each sample and heated at 200°C–250°5 until the solutions were colorless and clear. The concentrated sample solutions were transferred into a 100-mL volumetric flask and filled with purified water to the measuring line. Before analysis, ICP-AES (HORIBA JOBIN YVON) was calibrated every time by running at least six Zn standard concentrations (0.5, 2, 5, 10, 20, and 40 mg/L).
Data analysis
The unit for statistical measurement was the pregnant female or the litter.Citation35 Quantitative continuous data, such as the maternal body weight, food consumption, fetal body weight, and placental weight, were subjected to a one-way analysis of variance, and a Scheffe’s multiple comparison test was carried out for the significant differences.Citation36 The number of corpora lutea, total implantations, live and dead fetuses, and fetal alterations were evaluated statistically by using the Kruskal–Wallis nonparametric analysis of variance,Citation37 followed by the Mann–Whitney U-test where appropriate. The sex ratio and the proportions of litters with malformations and developmental variations were compared using a chi-square test and Fisher’s exact probability test.Citation38 Statistical analyses were performed by comparing the treatment groups with the control group using SPSS 19.0 software (IBM Corporation, Armonk, NY, USA). Differences with a P-value of 0.05 or lower were considered to be statistically significant.
Results
Formulations analysis of test article
summarizes the concentrations of dosing solution for ZnOSM20(+) NPs on GD 5, 11, and 19. Three analyses confirmed that the analyzed concentrations of all dose formulations were within ±15% of the target concentrations. ZnOSM20(+) NPs were stable for 4 hours at room temperature. Concentrations of total zinc were 9.82±0.84 mg/mL (mean ± standard deviation) for the 100-mg/kg/day group, 20.04±1.63 mg/mL for the 200-mg/kg/day group, and 40.80±1.47 mg/mL for the 400-mg/kg/day group.
Table 1 Formulations analysis of dosing solution for ZnOSM20(+) NPs on gestation day 5, 11, and 19
Zn concentration in fetuses
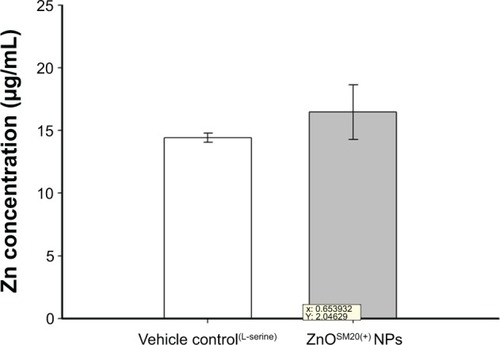
Zn concentrations in fetal tissues are shown in . The measured total Zn levels by ICP-AES were 14.44±0.37 μg/g (mean ± standard deviation) for the control group and 16.47±2.19 μg/g Zn for the 400-mg/kg/day group (). The Zn contents in fetuses after in utero exposure to ZnOSM20(+) NPs was not significantly different from the Zn contents in control fetuses.
Table 2 The Zn content in fetuses after in utero exposure to ZnOSM20(+) NPs
Figure 1 The total Zn levels measured with ICP-AES.
Notes: To investigate the placenta transfer of ZnOSM20(+) NPs in vivo, four extra female rats were used in the control (nontreatment control group; n=2) and 400-mg/kg/day (ZnOSM20(+) NPs; n=2) groups. Dosing occurred on gestational day 5–19 in the same manner as for main study animals.
Abbreviations: ICP-AES, inductive coupled plasma atomic emission spectrometry; ZnOSM20(+) NPs, 20-nm positively charged ZnO nanoparticles.
Effects on dams
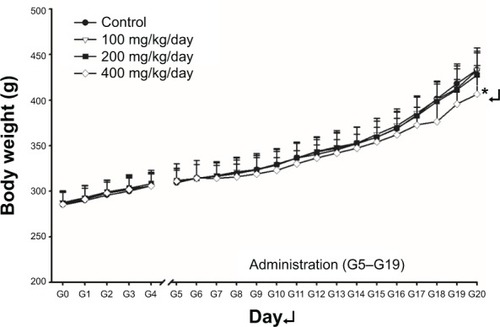
Although seven of 24 dams from the 100-mg/kg/day group, 12 of 21 dams from the 200-mg/kg/day group, and 18 of 23 dams from the 400-mg/kg/day group showed salivation around the mouth in general appearance. Starting from 3–12 days after oral administration, alopecia (localized areas of partial alopecia) was observed in one pregnant rat from the 100-mg/kg/day group, one from the 200-mg/kg/day group, and one from the 400-mg/kg/day group (data not shown). This clinical sign was not recovered for the treatment period. The changes in body weight during the entire experimental period are listed in . As shown by the data in , significant decreases in maternal body weight on GD 20 from the high-dose group was observed in comparison with the vehicle control group. The maternal-body-weight during pregnancy and corrected body weight were also significantly lower in the high-dose group than in the group. Statistically significant decreases in food consumption were noticed on day 18 of gestation in the 200- and 400-mg/kg/day groups in comparison to the vehicle control (). At the scheduled autopsy, one case of caveola of kidney surface in the vehicle control group; one case of splenomegaly in the 200-mg/kg/day group; and hypertrophy of adrenal and lung, edematous bowel, gastro-tympanites, and red reaction of liver in the 400-mg/kg/day group were observed in dams (data not shown). The absolute and relative organ weights of the pregnant rats treated with ZnOSM20(+) are presented in . Significantly decreased liver weight in the 400-mg/kg/day group was observed, and the increased absolute and relative weights of gland were significant in the 400-mg/kg/day group in dose-dependent manner in comparison with the control group.
Table 3 Body weights of the pregnant rats treated with ZnOSM20(+) NPs
Table 4 Food consumptions of the pregnant rats treated with ZnOSM20(+) NPs
Table 5 Absolute and relative organ weights of pregnant rats treated with ZnOSM20(+) NPs
Figure 2 Body weight changes of female rats during the gestation period.
Notes: Pregnant rats were orally treated with ZnOSM20(+) nanoparticles for 15 days (G5–G19) with dose of 100, 200, and 400 mg/kg/day. “G” refers to the number of the days after gestation. “G0” refers to the day on which a rat becomes pregnant. Statistically different from the vehicle control group; *P<0.05.
Effects on embryo–fetal development
summarizes the reproductive findings for the pregnant rats treated with ZnOSM20(+) NPs on GD 5–19. The pregnancy rates were similar for all dosage groups, ranging from 87.5%–100%. Totally resorbed litters were not found in any group. The number of corpora lutea, implantations, and fetal deaths, as well as implantation rates, placental weight, and sex ratios of the live fetuses were similar for the treatment groups and the vehicle control group. Significantly decreased fetal weights of males and females were observed in the 400-mg/kg/day group in comparison to the vehicle control group. No fetus showed an external malformation. Hematoma was shown in all groups, including the vehicle control group. However, the numbers of fetuses with hematomas were not significantly increased in comparison to the control group (). Several types of visceral variations were seen in fetuses of the treatment groups, including misshapen thymus, ureter abnormality (grade I: slight dilation of the renal pelvis, grade II: reduced papilla size and noticeable dilation of the renal pelvis, grade III: very short or no papillae and a marked dilation of the renal space),Citation39 dilated renal pelvis, and urinary bladder hypertrophy (). There were significant increases in the number of fetuses with visceral variations, such as misshapen thymus, ureter abnormality (grade III), and ectopic kidney in the 400-mg/kg/day group. reveals the types and incidences of fetal skeletal malformations and variations. Skeletal malformation, such as cleavage ossification of thoracic centrum, was observed in all groups. Although several types of skeletal variations were observed, including incomplete ossification of skull, dumbbell ossification of thoracic centrum, incomplete ossification of thoracic centrum, asymmetric thoracic centrum, supernumerary rib, short rib, and incomplete ossification of sternebra, no significant difference in the number of fetuses with skeletal variations or in the number of affected fetuses was seen between the groups.
Table 6 Cesarean section data from pregnant rats treated with ZnOSM20(+) NPs
Table 7 External alterations in fetuses from pregnant rats treated with ZnOSM20(+) NPs
Table 8 Visceral alterations in fetuses from rats treated with ZnOSM20(+) NPs
Table 9 Skeletal variations in fetuses from rats treated with ZnOSM20(+) NPs
Discussion
Recently, information on the toxicity of ZnO NPs, including liver damage, membrane injury, cytotoxicity, and inflammatory response,Citation40,Citation41 has been accumulating, but nearly no extensive study on the reproduction and developmental toxicity in mammals was performed. Many studies have been conducted on ZnO NP toxicity, in both environmental species and mammalian cell lines.Citation42–Citation44 On the other hand, ZnO NPs are used extensively in various commercial applications,Citation45,Citation46 since nanomaterials have specific physicochemical and electrical properties.Citation47 As ZnO NPs become a significant source for intended and unintended human exposures, it would be highly desirable and necessary to know more about other possible toxicities of ZnO NPs. This study was conducted to investigate the maternal and developmental toxic potentials of ZnOSM20(+) NPs after oral treatment in SD rats at dose levels of 0, 100, 200, and 400 mg/kg/day from days 5 through 19 of pregnancy. The ZnO NPs were capped with organic ligands, L-serine molecules, which are widely used capping agents for inorganic NPs to enhance the surface charge property.Citation25–Citation27
The results showed that a 15-day repeated oral dosing of ZnOSM20(+) NPs during pregnancy resulted in maternal toxicity at 400 mg/kg/day, but the same dose did not cause serious teratogenic toxicity. Although salivation was observed in all treated groups, it was not considered to be related to the ZnOSM20(+) NP treatment because the salivation was observed sporadically and was not dose dependent. This finding may attribute to irritation or stress of the test subjects. ZnOSM20(+) NPs induced significant maternal toxicity in the 400-mg/kg/day group, which was evidenced by suppressed body-weight gain, decreased liver weight, and increased adrenal gland weights. The suppressed body-weight gain in the high-dose group could have been a direct effect of ZnOSM20(+) NPs on pregnant dams, because the gravid uterine weight of the 400-mg/kg/day group at term was similar to that of the control group.
In general reproductive toxicity studies, it is well known that body and organ weights could be sensitive indicators of potentially toxic chemicals.Citation48–Citation50 The significant decreases in the absolute liver weight in 400-mg/kg/day group were attributed to the administration of ZnOSM20(+) NPs, since these changes were remarkable in comparison with the control group, showing a clear-cut dose–response relationship. Earlier studies revealed that after uptake in the gastrointestinal tract, the biodistribution of engineered NPs was located at the liver and kidney.Citation15,Citation51,Citation52 Significant weight increases of the adrenal glands were observed in the 400-mg/kg/day group, which could be considered as a treatment-related effect, since these changes was remarkably distinguishable in comparison with the control group. Previous studies demonstrated that the function and weight of adrenal glands is adversely effected by various stressful factors.Citation53–Citation56 So the increased weights of adrenal glands are also considered to be related to stress responses, induced by the administration of ZnOSM20(+) NPs, which is consistent with the decreased body-weight gain in the group.
Up to now, several studies have evaluated the toxic effects of ZnO nanomaterials in vitro and in vivo. Yang et alCitation57 demonstrated with primary mouse embryo fibroblast cells that the interrelationship existed between particle size, shape, chemical composition, and toxicology effects of carbon black, single-wall carbon nanotube, silicon dioxide, and ZnO NPs. Xia et alCitation58 reported that ZnO induced toxicity in RAW 264.7 cell lines, leading to the generation of reactive oxygen species, oxidant injury, excitation of inflammation, and cell death. According to the bacterial toxicity study of ZnO NPs,Citation59 ZnO NPs damaged bactericidal wall by increasing membrane permeability. In nematodes,Citation60 the toxicity of manufactured ZnO NPs (1.5 nm) might have caused the intracellular biotransformation. Interestingly, comparable toxicities of nanoparticulate ZnO, bulk ZnO, and ZnCl2 were observed with algae.Citation61
External and skeletal variations were found in few fetuses (–), as to morphological variation or malformation findings from the fetuses of ZnOSM20(+) NP-treated dams. In particular, significant increases in the number of fetuses with visceral variations were observed in the 400 mg/kg of ZnOSM20(+) NPs group, which could be considered as a treatment-related effect, since these changes were remarkable in comparison with the control group. Significant increases in the number of fetuses with visceral variations were observed in the 400 mg/kg/day of ZnOSM20(+) NPs group, which may have not been a direct influence of the test substance. Increases in visceral variations (misshapen thymus and ureter abnormality) were also considered to be indirect effects of maternal toxicity or a spontaneous effect, because these changes were not significant, and the treatment-related fetal malformation was not found at all tested doses. The observed fetal variations in the present study were not considered to be caused by the administration of ZnOSM20(+) NPs since they occurred at a very low rate without exhibiting a dose–response relationship, or they were sporadically observed in fetuses from normal control rats.Citation62–Citation64
The concentration of Zn in the fetuses in the vehicle and ZnOSM20(+) NP-dose groups are shown in . Different distributions of NPs ingested into the body could be found at different regions due to their small size. After the exposure of 400 mg/kg/day of ZnOSM20(+) NPs, a significant increase of Zn contents was not found in the ZnOSM20(+) NP-treated rats in comparison with the control group. Although the increases in Zn concentrations in the exposed fetuses exhibited a trend, their influence on intrauterine fetal growth/development could be considered as weak effects.
In the present study, effects of ZnOSM20(+) NPs on intrauterine growth and on fetal visceral morphology were observed. Lower mean maternal body weights and body-weight gain in comparison to the control group were observed in the group treated with 400 mg/kg/day ZnOSM20(+) NPs in a dose-related manner. Lower mean corrected body weight was noted in this group at necropsy. Furthermore, the reduction in body-weight gain during the late gestation period in the 400-mg/kg/day group was considered to be mainly due to the growth of fetuses, since the fetal weight was markedly decreased in this group. Similarly, decreased body-weight gain during the late gestation period was due to the decreased food consumption, which resulted in reduction of fetal weight in utero. On the contrary, some positive effects of other NPs were noted in similar reproduction/developmental toxicity studies. When silica and titanium dioxide NPs were administered to pregnant mice intravenously, pregnancy complications were observed. Mice also had smaller uteri and smaller fetuses than untreated controls.Citation65 Also, when silicon crystal NPs (50 mg/kg) were injected, these NPs led to reduction in body-weight gain in pregnant rats and newborn rats at different stages of the experiment, but without noticeable effect on other parameters of physical development of rat progeny or teratogenic effects.Citation66 In addition, oral administrations of silver NPs (250 mg/kg) caused a relatively low toxic effect.Citation67
Unpublished 90-day repeated oral-dose and genotoxicity data from our previous studies are briefly summarized, as follows. The ZnO NPs did not cause any significant changes at the endpoints of repeated-dose toxicity, including clinical observation, functional observation, body-weight gain, water and food consumption, urinalysis, hematology, serum biochemistry, organ weight, toxicokinetic study, tissue distribution, and histopathology in the repeated study. In addition, when 14-day recovery groups were set for the high-dose groups in both sexes, no significant result was observed in functional examination, body-weight gain, water and food consumption, urinalysis, necropsy, and organ weight. Hence, it was concluded that no significant induced effect was affected by the treatment of the ZnO NPs. However, dose-dependent depositions of ZnO NPs were also found in the pancreas, glandular stomach, and eyes, indicating potential systemic distributions of ZnO NPs in the mammalian tissues. Furthermore, genotoxicity tests of ZnO NPs did not show any gene mutation potential in vitro, chromosome aberration assay in vitro, or micronucleus test in vivo. Moreover, in vivo micronucleus test and in vivo comet assay in the 90-day study also indicated negative results, supporting that ZnO NPs did not cause any DNA damage.
Conclusion
In summary, prenatal and developmental toxicity of positively charged ZnO NPs were tested in accordance with the OECD test guideline 414 and GLP principle. Pregnant female rats were orally treated with ZnOSM20(+) NPs, from GD 5–19, with doses of 100, 200, and 400 mg/kg/day. The results of this developmental toxicity study suggest that administration of ZnOSM20(+) NPs to pregnant rats had minimal impact on intrauterine fetal growth and development, even in the high dose of 400 mg/kg/day. Based on the results of these studies, a dosage level of 200 mg/kg/day of ZnOSM20(+) NPs was considered the no-observed-adverse-effect level for both maternal toxicity and embryo–fetal development. Although the result of the current study clearly showed the adverse effects of ZnOSM20(+) NPs on pregnant rats and fetuses, information on the effects of ZnO NPs on reproduction/development are not sufficient at this time.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This research was supported by a grant (10182MFDS991) from the Ministry of Food & Drug Safety in 2010–2011.
References
- ClarenceSYGeoffreySSSunnyEINanoparticles toxicity and their of exposuresPak J Pharm Sci201225247749122459480
- HernandezbattezABGonzalezRViescaJLCuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricantsWear20082653–4422428
- SchillingKBradfordBCastelliDHuman safety review of “nano” titanium dioxide and zinc oxidePhotochem Photobiol Sci20109449550920354643
- GerloffKAlbrechtCBootsAWFörsterISchinsRPCytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco-2 cellsNanotoxicology200934355364
- JinTSunDSuJYZhangHSueHJAntimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7J Food Sci2009741M46M5219200107
- HeLLiuYMustaphaALinMAntifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansumMicrobiol Res2011166320721520630731
- RasmussenJWMartinezELoukaPWingettDGZinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applicationsExpert Opin Drug Deliv2010791063107720716019
- JohnSMarpuSLiJHybrid zinc oxide nanoparticles for bio-photonicsJ Nanosci Nanotechnol20101031707171220355561
- US Department of Health and Human ServicesToxicological profile for zincAtlanta, GeorgiaUS Department of Health and Human Services, Agency for Toxic Substances and Disease Registry2005 Available from: http://www.atsdr.cdc.gov/toxprofiles/tp60.pdfAccessed November 27, 2014
- DrinkerPThomasonRMFinnJLMetal fume fever: therehold doses of zinc oxide, preventive measures, and the chronic effects of repeated exposureJ Industrial Hygiene19279331345
- BalancePWongHBernsteinMSBousheyHAAn experimental human model of metal fume feverAnn Intern Med19911149309362024859
- BaekMChungHEYuJPharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticlesInt J Nanomedicine201273081309722811602
- Metal Oxide Nanostrutures and Their ApplicationsChapter 4ZnO NanoparticlesGrowth, Properties, and Applications2010136
- WangLWangLDingWZhangFAcute toxicity of ferric oxide and zinc oxide nanoparticles in ratsJ Nanosci Nanotechnol201010128617862421121374
- WangJZhouGChenCAcute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administrationToxicol Lett2007168217618517197136
- WangBFengWWangMAcute toxicological impact of nano-and submicro-scaled zinc oxide powder on healthy adult miceJ Nanopart Res2008102263276
- SurekhaPKishoreASSrinivasARepeated dose dermal toxicity study of nano zinc oxide with Sprague-Dawley ratsCutan Ocul Toxicol2012311263221830917
- YanGHuangYBuQZinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in ratsJ Environ Sci Health A Tox Hazard Subst Environ Eng201247457758822375541
- LuXLiuYKongXLobiePEChenCZhuTNanotoxicity: a growing need for study in the endocrine systemSmall201399–101654167123401134
- IavicoliIFontanaLLesoVBergamaschiAThe effects of nanomaterials as endocrine disruptorsInt J Mol Sci201314167321680123949635
- YamashitaKYoshiokaYSafety assessment of nanomaterials in reproductive developmental filedYakugaku Zasshi20121323331335 Japanese22382838
- OECD Guidelines for Testing of Chemicals section 4: Test No. 414, Prenatal and Developmental Toxicity Study [website on the Internet]ParisOECD2001 Available from: http://www.oecd-ilibrary.org/environment/test-no-414-prenatal-development-toxicity-study_9789264070820-enAccessed November 27, 2014
- OECDPrinciple of Good Laboratory PracticeParisOECD1997 Available from: http://www.oecd.org/officialdocuments/displaydocumentpdf/?cote=env/mc/chem(98)17&doclanguage=enAccessed November 27, 2014
- MFDSGLP principle by Ministry of Food and Drug Safety (MFDS Gosi 2013-40. MFDS Available from: http://www.mfds.go.kr/index.do?mid=1013&division=&searchkey=title%3Acontents&searchword=%BA%F1%C0%D3%BB%F3&x=0&y=0Accessed December 5, 2014 Korean
- DegenAKosecMEffect of pH and impurities on the surface charge of zinc oxide in aqueous solutionJ Eur Ceram Soc200020667673
- OhJMParkDHChoyJHIntegrated bio-inorganic hybrid systems for nano-forensicsChem Soc Rev201140258359521152667
- KimK-MKimT-HKimH-MColloidal Behaviors of ZnO Nanoparticles in Various Aqueous MediaToxicol Environ Health Sci201242121131
- ChungHYuJBaekMToxicokinetics of zinc oxide nanoparticles in ratsJ Phys Conf Ser2013429012037
- YamadaTHaraMOhbaYInoueTOhnoHStudies on implantation traces in rats. II. Staining of cleared uteri, formation and distribution of implantation tracesJikken Dobutsu1985343249260 Japanese2415372
- MenegolaEBrocciaMLGiaviniEAtlas of rat fetal skeleton double stained for bone and cartilageTeratology200164312513311514942
- WilsonJGMethods for administering agents and detecting malformations in experimental animalsTeratology: Principles and Techniques1965262277
- SuzukiMMurakamiUTabuchiAAbstracts of papers presented at the thirteenth annual meeting of the congenital anomalies research association of Japan. Hiroshima, Japan, July 12–13, 1973Teratology19738183111
- ChahoudIBuschmannJClarkRClassification terms in developmental toxicology: need for harmonization 1Reprod Toxicol1999131778210080303
- MakrisSLSolomonHMClarkRTerminology of developmental abnormalities in common laboratory mammals (version 2)Congenit Anom (Kyoto)200949312324620002907
- WeilCSSelection of the valid number of sampling units and a consideration of their combination in toxicological studies involving reproduction, teratogenesis or carcinogenesisFood Cosmet Toxicol1970821771825421015
- ScheffeHA method for judging all contrasts in the analysis of variance*Biometrika1953401–287110
- KruskalWHWallisWAUse of ranks in one-criterion variance analysisJournal of the American Statistical Association195247583621
- FisherRAStatistical methods for research workersOliver and Boyd1970
- BryantPLSchmidJEFentonSEBuckalewARAbbottBDTeratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the expression of EGF and/or TGF-alphaToxicol Sci200162110311411399798
- SharmaVSinghPPandeyAKDhawanAInduction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticlesMutat Res20127451–2849122198329
- EsmaeillouMMoharamnejadMHsankhaniRTehraniAAMaadiHToxicity of ZnO nanoparticles in healthy adult miceEnviron Toxicol Pharmacol2013351677123262039
- MillerRJLenihanHSMullerEBTsengNHannaSKKellerAAImpacts of metal oxide nanoparticles on marine phytoplanktonEnviron Sci Technol201044197329733420469893
- SinhaRKaranRSinhaAKhareSKInteraction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cellsBioresour Technol201110221516152020797851
- WangHJGrowcockACTangTHO’HaraJHuangYWAronstamRSZinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signaling pathwayToxicol In Vitro20102471953196120708676
- JiSYeCSynthesis, growth mechanism, and applications of zinc oxide nanomaterialsJournal of Materials Science and Technology2008244457472
- WangZLZinc oxide nanostructures: growth, properties and applicationsJ Phys Condens Matter200416R829
- AjayanPMZhouOZApplications of carbon nanotubesTopics in Applied Physics200180391425
- AndersenHLarsenSSpliidHChristensenNDMultivariate statistical analysis of organ weights in toxicity studiesToxicology19991362–3677710514000
- BaileySAZidellRHPerryRWRelationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint?Toxicol Pathol200432444846615204968
- ChungMKKimCYKimJCReproductive toxicity evaluation of a new camptothecin anticancer agent, CKD-602, in pregnant/lactating female rats and their offspringCancer Chemother Pharmacol200759338339516896929
- ChenZMengHXingGAcute toxicological effects of copper nanoparticles in vivoToxicol Lett2006163210912016289865
- CuiYLiuHZhouMSignaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticlesJ Biomed Mater Res A201196122122921105171
- OdioMRMaickelRPComparative biochemical responses of rats to different stressful stimuliPhysiol Behav19853445955994011740
- Kioukia-FougiaNAntoniouKBekrisSLiapiCChristofidisIPapadopoulou-DaifotiZThe effects of stress exposure on the hypothalamic-pituitary-adrenal axis, thymus, thyroid hormones and glucose levelsProg Neuropsychopharmacol Biol Psychiatry200226582383012369253
- KimHYLeeSBChungYHEvaluation of subchronic inhalation toxicity of dimethyl disulfide in ratsInhal Toxicol200618539540316513596
- Ulrich-LaiYMFigueiredoHFOstranderMMChoiDCEngelandWCHermanJPChronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific mannerAm J Physiol Endocrinol Metab20062915E965E97316772325
- YangHLiuCYangDZhangHXiZComparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and compositionJ Appl Toxicol2009291697818756589
- XiaTKovochichMLiongMComparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress propertiesACS Nano20082102121213419206459
- HuangZZhengXYanDToxicological effect of ZnO nanoparticles based on bacteriaLangmuir20082484140414418341364
- MaHBertschPMGlennTCKabengiNJWilliamsPLToxicity of manufactured zinc oxide nanoparticles in the nematode Caenorhabditis elegansEnviron Toxicol Chem20092861324133019192952
- FranklinNMRogersNJApteSCBatleyGEGaddGECaseyPSComparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubilityEnviron Sci Technol200741248484849018200883
- MoritaHAriyukiFInomataNSpontaneous Malformations in Laboratory Animals: Frequency of External, Internal and Skeletal Malformations in Rats, Rabbits and MiceCongenit Anom1987272147206
- HoodRDHandbook of developmental toxicologyInforma Healthcare1997
- KimJLeeSBaeJParkJKimYChungMHistorical control data for developmental toxicity study in Sprague-Dawley ratsJ Toxicol Public Health2001178390
- YamashitaKYoshiokaYHigashisakaKSilica and titanium dioxide nanoparticles cause pregnancy complications in miceNat Nanotechnol20116532132821460826
- DurnevADSolominaASDaugel-DaugeNOEvaluation of genotoxicity and reproductive toxicity of silicon nanocrystalsBull Exp Biol Med20101494445449 English, Russian21234440
- HongJSKimSLeeSHCombined repeated-dose toxicity study of silver nanoparticles with the reproduction/developmental toxicity screening testNanotoxicology20148434936223432083
