Abstract
The latest development of protein engineering allows the production of proteins having desired properties and large potential markets, but the clinical advances of therapeutical proteins are still limited by their fragility. Nanotechnology could provide optimal vectors able to protect from degradation therapeutical biomolecules such as proteins, enzymes or specific polypeptides. On the other hand, some proteins can be also used as active ligands to help nanoparticles loaded with chemotherapeutic or other drugs to reach particular sites in the body. The aim of this review is to provide an overall picture of the general aspects of the most successful approaches used to combine proteins with nanosystems. This combination is mainly achieved by absorption, bioconjugation and encapsulation. Interactions of nanoparticles with biomolecules and caveats related to protein denaturation are also pointed out. A clear understanding of nanoparticle-protein interactions could make possible the design of precise and versatile hybrid nanosystems. This could further allow control of their pharmacokinetics as well as activity, and safety.
Introduction and background
Nanotechnology has the potential to create new materials and devices with wide-ranging applications in medicine,Citation1–Citation3 agriculture,Citation4 and energy or electronic production.Citation5,Citation6
The size-dependent optical, electrical, and magnetic properties of nanoparticles make nanotechnology a promising candidate for bioapplications such as in vivo imaging, sensing, catalysis, therapeutics, and cell targeting.Citation7–Citation9
Based on different approaches, physicians, physicists, chemists, biologists as well as bioengineers share a common interest to treat severe diseases through nanotechnology. Theoretically, nanoparticles can be tailored to reach the right target at the right time. Pathogenic agents such as viruses or bacteria, and cancer cells could be precisely targeted and affected without disturbing healthy tissues. This crucial task has been one of the highest priorities for the past 10 years.
Among several medical applications, nanoparticles could be largely employed as carriers of therapeutical biomolecules.Citation10 The combination of nanoparticles with biomolecules such as proteins or specific polypeptides offers opportunities for the design of very precise and versatile hybrid systems mostly useful in helping to fight cancer and immunological diseases.Citation11–Citation13
There are more than 50,000 different proteins in the human body.Citation14 Proteins are present in complex biological processes such as muscle contraction, immune protection and transmission of nerve impulses. All enzymes and most hormones are proteins; hence, proteins are vital sources for the body’s metabolism and their lack can result in several diseases (eg, lack of insulin in type 1 diabetes).
The latest development of protein engineering allows the production of proteins having desired properties and great potential market;Citation15 however, protein fragility is one of the major drawbacks for their utilization. Consequently, the discovery and development of new therapeutic proteins have also created new opportunities for drug-delivery systems involving the design of appropriate nanocarriers such as liposomes, micro-, and nanoparticles.Citation16–Citation19
The oral route is a comfortable way for drug administration especially when repeated or routine dosing is necessary.Citation20 Nevertheless, the development of oral carriers for many proteins remains a challenge due to the fact that bioavailability of these molecules is limited.Citation21 Indeed, most polypeptides and proteins are quickly degraded in the gastrointestinal (GI) tract by proteolytic enzymes.Citation22,Citation23 Moreover, the intestinal epithelium is a major barrier to the absorption of hydrophilic drugs that cannot easily diffuse across the cells through the lipid-bilayer cell membranes.
Numerous investigations have shown that nanocarriers can improve the stability of therapeutic agents against enzymatic degradation and achieve desired therapeutic levels in target tissues for the required duration. Nanoparticle drug-delivery systems (nano-DDS) could permit an optimal pharmacokinetic profile and meet specific needs. For example, nanoparticles as oral protein carriers could protect the active ingredient in the GI tract and/or prolong the residence time of its contents on the mucous membrane. After administration, nano-DDS can be taken up and transported across the intestinal mucosa by enterocytes or M cells in the Peyer’s patches because of their small size.Citation24
Several articles and reviews on the use of nanoparticles or microparticles for oral drug delivery are dedicated to insulin.Citation25–Citation28 In 1980, Couvreur and colleagues performed the first study on hypoglycemic effects after oral and parenteral administration of insulin-loaded nanoparticles to diabetic rats.Citation26
Proteins are also difficult to be delivered via topical or transdermal routes and therefore their parenteral administration is still largely applied.
Besides the general complications of the parenteral route (such as local infections, thrombophlebitis, rarely tissue necrosis), small proteins (<30 kD) are quickly filtered out by the kidneys. Without an appropriate drug carrier, proteins can also cause unwanted allergic reactions, can be targeted by the immune system and be rapidly degraded.
For example, rapid clearance from the circulation can be an explanation of the modest in vivo antitumor effects of the antiangiogenic RGD (Arg–Gly–Asp) peptides.Citation29
Bone morphogenetic proteins (BMPs) induce bone formation after implantation; their orthopedic application in repair of bone fractures and defects is focused in local device and spinal fusion procedures. The problem of BMP is its rapid diffusion from the administration site when applied without a carrier. Currently, one of the most effective and biocompatible carriers for BMP delivery is the type I bovine absorbable collagen sponge (ACS). However the BMP release rate is difficult to control and to maintain constant for long term because of a high initial burst release of this device. The use of new nanotechnologies could maintain BMPs at the treatment site preventing extraneous bond formation and optimizing the drug release.Citation30
Synthetic antigenic peptides are specific sections or a variant sequence of viral/bacterial proteins able to induce an immune response in the host. These small peptides are very useful in the vaccine development compared to the use of the whole protein/antigen. To date, several antigenic peptides have been identified but delivery problems still limit their application. Even in this case, the design of an effective delivery system is an important challenge in nanotechnology field.
An efficient protein carrier should solve different problems allowing the access to the target sites, at the right time and for the proper duration. In order to choose the best nanosystem, five factors must be considered: nature of the protein, route of administration, pattern of drug release, method of delivery and formulation.Citation31,Citation32
Proteins such as albumin, antibody, growth factors, transferrin, cytokines and low-density lipoprotein can be also used as active ligands to help nanoparticles loaded with chemotherapeutic or other drugs to reach particular sites in the body.Citation33–Citation35 Abraxane® (Abraxis Bioscience, Los Angeles, CA; AstraZeneca, Wilmington, DE), albumin–bound nanoparticle of paclitaxel, is an example of US Food and Drug Administration (FDA)-approved protein-based active ligand for the treatment of metastatic breast cancer.Citation35
Monoclonal antibodies (mAb) has been widely used as bioprobes in diagnostics as well as delivery drug to specific tumors.Citation36,Citation37 OX26 mAb can help nanoparticles to cross the blood–brain barrier and diffuse in the brain tissue in order to transport drugs (eg, the anticaptase peptide, Z-DEVD-FMK) for the treatment of neurological and psychiatric disorders.Citation38 Nanoparticles can be also coated with mAb for cell surface antigen and used as a bait for detection or isolation of various kind of cells including lymphocyte and tumor cells.Citation39,Citation40
Despite many potential applications, the interaction of nanoparticles with biomolecules and living systems is still not fully understood.Citation41–Citation50 Continuous study on this subject contributes to the current knowledge and stimulates the development of novel therapies such as nonviral vectors for gene therapies or as precise anticancer molecules.Citation37,Citation51–Citation53 Furthermore, by clarifying these aspects, specific protein-based nanovectors with optimized functions could be developed. This review aims to provide an overall picture on current progress and general aspect of the most successful approaches used to combine proteins with nanosystems. This combination is mainly achieved by absorption, bioconjugation or strong binding via avidin–biotin technology and encapsulation. These methods and the correlated problems of protein denaturation are discussed in turn in this review.
Nanoparticle–protein absorption, bioconjugation, and encapsulation
Absorption of proteins on nanoparticles surface
The interaction between biological and synthetic materials impacts on a vast range of medical issues from implants to pharmacokinetic aspects. The study of the materials bio-compatibility starts, therefore, with the analysis of protein absorption on surfaces.Citation54 Synthetic materials for biomedical applications are immediately covered by proteins when put in contact with a biological environment.Citation55,Citation56 After protein binding, nanoparticles are quickly cleared by the mononuclear phagocytic system (MPS), also known as the reticuloendothelial system (RES).Citation57,Citation58 These macrophages, which are typically Kupffer cells of the liver, cannot directly identify the nanoparticles themselves, but rather recognize specific opsonin proteins bound to the surface of the particles.Citation59
The interaction between proteins and nanoparticles surface leads to the formation of proteins “corona” around nanoparticles that largely defines their biological identity as well their potential toxicity.Citation49,Citation50,Citation58,Citation60–Citation64 Recently, Lynch and Dawson postulated the importance of the “protein corona” as the vehicle and the biological identity of a nanoparticle for its transport through cell membranes.Citation60
The nanoparticle surface is immediately occupied by proteins with high concentrations and high association rate constants and successively by proteins having lower concentrations but a higher affinity.Citation47 Competitive absorption of proteins is influenced by several factors such as electrostatic interactions, protein stability, and kinetic parameters.Citation65
As the protein corona could affect the nanoparticle behavior, including its biological effect, the nanoparticle could also have an effect on the protein behavior. Some nanoparticles seem able to promote the protein assembly into amyloid fibrils in vitro by assisting the nucleation process.Citation66 Bellezza and colleagues found that nanoparticles affect the morphology of the myoglobin absorbed onto phosphate-grafted zirconia nanoparticles, inducing prefibrillar-like aggregates.Citation67 This phenomenon could have important implications for medical application of nanoparticles because the self-assembly of a variety of proteins and peptides is known to be the cause of human amyloid diseases where fibrous protein aggregates are formed, resulting in amyloid plaque deposition in the extra-cellular tissues.Citation68–Citation74 Moreover, fibrillar structure seems to be related to heavy human disorders such as Alzheimer’s disease, Parkinson’s disease, and spongiform encephalopathies.
However this action seems strictly related to the type of nanosystems chosen. For instance, there are nanosystems such as C60 hydrated fullerenes that can relax fibrillar structures.Citation60,Citation68 Certainly, the control of the protein absorption on nanoparticle surfaces is an important issue to control their fate in biological systems.Citation75
In order to prevent or control the opsonization, several methods of disguising nanoparticles have been developed. In these methods, generally, nanoparticles are coated with biocompatible polymers that have the double function of preventing their aggregation and retarding the protein absorption.Citation57,Citation76,Citation77
A common strategy to improve blood compatibility and to increase the blood circulation half-life of the nanoparticles is the construction of a protein-coated surface resistant to the absorption of the other opsonines.Citation78 A thin layer of protein appears to minimize adhesion and aggregation of nanoparticles, avoiding subsequent macrophage recognition or, in the worst case, a thrombus formation. Moreover, it is possible to properly tune the cells uptake of the nanoparticles using specific proteins.Citation35
Proteins are mainly amphiphatic molecules that typically adhere to the surface of a biomaterial in a nonspecific way. In various cases, this nonspecific adhesion is sufficient to artificially immobilize proteins on the nanoparticles surface, and no surface modification is necessary.
Despite of the large number of studies, the absorption of a protein on whatever the solid surface is still a complex and not well understood process.Citation60,Citation79–Citation85 In the case of nanoparticles, size and radius of curvature become significant when compared to the protein size resulting in new interactions not shown with the bulk materials.Citation47
The high hydrophobicity of many proteins seems to play an important role in their absorption on the nanoparticles surface.Citation86 Several models of protein absorption on surfaces identify two main steps in the process. The first step could involve the arrival of the protein at the interface, through a diffusion process following the Brownian law of motion, and its further collision with the solid surface. Depending on the balance of the energetic interaction, proteins can remain on the solid surface or return to solution. If the protein has been absorbed, the second step could lead to conformational changes (because of van der Waals interactions), surface charge, protein dipole moment, and protein size or solution ionic strength.Citation84,Citation85,Citation87–Citation90 This second step often involves irreversible changes in the protein structure up to denaturation.Citation91–Citation93
Proteins can be divided in two groups: hard and soft proteins. The first group includes proteins with high internal stability, while proteins in the second group have a low internal stability. Soft proteins seem to be able to change their conformation better than the hard ones. This characteristic results in a gain in conformational entropy when absorbed on solid surfaces, improving the efficacy of the absorption process when compared to the hard proteins. On the other hand, it seems that some degree of denaturation upon absorption is more probable for soft proteins than for the hard ones, especially on hydrophobic surfaces.Citation84,Citation94–Citation97
During the artificial absorption of protein to nanoparticles surface, the use of a large excess of the target material could allow the retention of sufficient biological activity and native epitopes, even if some proteins are denatured. However, problems associated with denaturation of the protein over time, or its exchange with other proteins in solution, could make this strategy satisfactory only for short-term uses.
The success of an absorption strategy to deliver drug or therapeutical proteins using protein-based nanoparticles as a carrier can be influenced by several factors such as the type of nanoparticles, delivery route and the nature of proteins to be absorbed. For this reason, nanoparticle–protein affinity needs to be intensely examined case-by-case.
The knowledge of how the protein-based nanoparticles interact with other proteins present in the blood is fundamental for the understanding of their biological and toxicological properties.Citation77 Many methods based on established techniques could be applied such as size-exclusion chromatography, isothermal titration calorimetry, surface plasmon resonance, atomic force microscopy, differential scanning calorimeter, and circular dichroisim (CD) spectroscopy.Citation47,Citation67
Even if several existing characterization methods for measuring the nature and the amount of absorbed protein on solid surfaces could be applied to nanoparticle systems,Citation96 the development of new physical and biophysical methods may be necessary to fully understand the relationship between proteins and nanomaterials.
Bioconjugation of proteins on nanoparticle surfaces
Conjugation of biomolecules on nanoparticle surfaces has attracted widespread interest in biotechnology and medicine.Citation7,Citation98–Citation100 The conjugation of specific proteins with nanoparticles has introduced a new advancement in molecular and cellular biology which has further led to a vast improvement of in vivo gene delivery, clinical diagnosis, medical/cancer imaging, receptor-targeted delivery.Citation40,Citation101–Citation105
A preferred method used in many areas of biochemistry to couple specific protein to solid surface is the bioconjugation by covalent binding. While protein absorption on solid surfaces such as nanoparticles can be reversible depending on pH, salt concentration, temperature or other environment physicochemical characteristics, protein covalent bounds are highly stable. To fulfill the purpose of stable covalent binding, a large number of reactions have been proposed and many protein modifications using new techniques have been developed.Citation7,Citation106–Citation111
The choice of the bioconjugation procedure depends strictly on physicochemical and biochemical properties of nanomaterials and proteins. Protein made by various side chains and residues can interact by multiple coating ligands with the same nanoparticles or even with more nanoparticles. Moreover, nanoparticles can be more or less polydispersed and have different physicochemical surface properties such as area, porosity, and charge. These aspects are very important since the hydrophobicity, charge and site affinity could affect the interaction and thus jeopardize the stability of final covalent-coupled products.
The most popular approach for coupling covalently nanoparticle to protein is based on the existence on proteins of specific and reactive functional groups such as amino–NH2 (lysine), carboxylic acid–COOH (aspartic, glutamic), hydroxyl–OH (serine, tyrosine) and –SH (cysteine).Citation112
Proteins can be chemically coupled to different kinds of nanoparticles using established reagents such bifunctional cross-linker molecules. In this case, nanoparticles need to be functionalized with functional groups such as carboxylic acid, hydroxyl, sulfhydryl and amino groups.
Proteins, including antibodies, generally have several primary amines in the side chain of lysine residues and the N-terminus of each polypeptide that are available as targets for N-hydroxysuccinimide-ester and carbodiimide reagents. Cysteine residues on proteins can react with maleimides and iodoacetamides reagents to give thioether-coupled products.Citation113 These reagents react rapidly at physiological pH and can be usually coupled with thiol groups selectively in the presence of amine groups. Maleimides and iodoacetamides have the same application but the first reagent seems to have better selectivity than the second one, not apparently reacting with histidine or methionine.
Cross-linking reagents contain reactive ends to specific functional groups (such as primary amines, sulfhydryls) on proteins or other molecules. They can be divided into homobifunctional (same reactive groups) and heterobifunctional (different reactive groups) which chemical cross-links may or may not be reversed.Citation114 Homobifunctional cross-linkers have a disadvantage of potentially connecting two neighboring groups, either on the nanoparticle surface or on the protein inducing undesired cross-linking. Heterobifunctional crosslinkers allow sequential conjugations, minimizing polymerization. For example, sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC) can be used to couple thiol-containing biomolecules with amine–coated nanoparticles, or vice versa. Whereas the hetero-bifunctional cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) is commonly used to link –NH2 and –COOH groups ().Citation114–Citation118
Table 1 The most popular cross-linker reagents for coupling protein to nanoparticle based on their respective functions
Many cross-linkers are available in the market and they can be chosen for specific needs (such as chemical specificity, spacer arm length, cleavability). Among several cross-linkers, the zero-length ones such as carbodiimides are widely used allowing covalent bonds between nanoparticles and proteins without insertion of an exogenous spacer. Nevertheless, the direct attachment of a protein to a surface without a spacer can cause steric constraint modifying the protein reactivity compared to the protein in solution. In addition, without a spacer, multiple contacts between protein and nanoparticle surface are more probable favoring total or partial protein denaturation and thus decreasing protein activity.Citation119
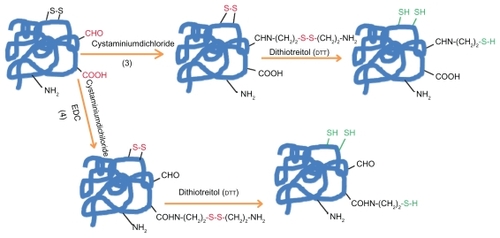
When protein does not have the suitable residue necessary for the specific conjugation, the most common way to get it is the chemical introduction of sulfhydryl groups. This process () can be mainly made by the following four methods: 1) reduction of protein disulfide bonds using reductive agents such as dithiotreitol (DTT = Clelands reagent). 2) Coupling of protein primary amino groups with 2-iminothiolane (Trauts reagent). 3) Quenching of reactive protein aldehyde residues with cystaminiumdichloride reagents or 4) coupling of cystaminiumdichloride to carboxyl groups via 1-ethyl-3-(3-dimethyl-aminopropyl)carbodiimide (EDC); both cases followed by the disulfide bonds reduction with DTT as outlined above.Citation112,Citation120–Citation123
Figure 1a The introduction of sulfhydryl groups by: 1) the reduction of protein disulfide bonds using reductive agents such as dithiotreitol (DTT = Cleland’s reagent). 2) Coupling protein primary amino groups with 2-iminothiolane (Traut; s reagent).
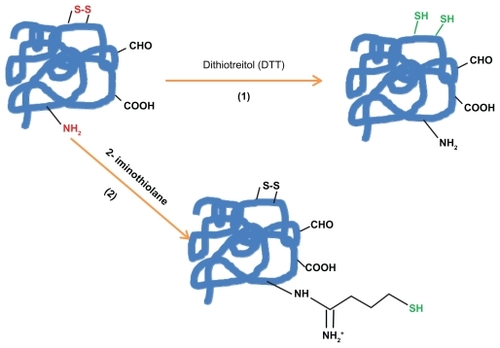
Figure 1b The introduction of sulfhydryl groups by: 3) Quenching of reactive protein aldehyde residues with cystaminiumdichloride reagents or 4) coupling of cystaminiumdichloride to carboxyl groups via 1-ethyl-3-(3-dimethyl-aminopropyl)carbodiimide (EDC); both cases followed by the disulfide bonds reduction with DTT.
The avidin/streptavidin–biotin bound is the strongest noncovalent biological interaction known; for this reason this technology is commonly used in biological labs.Citation124,Citation125
Biotinylated proteins/antibodies/enzymes can be efficiently coupled on amino nanoparticle surfaces by streptavidin-biotin technology accomplished by streptavidin activation through carbodiimide (EDC) chemistry. Biotin binds strongly to this biochemically modified surface in the most specific and sensitive way. Furthermore, streptavidin through carbodiimide (EDC) chemistry can be covalently coupled with different ligands such as mAb and enzymes which make the biotin–streptavidin system widely used in a variety of biotinylated nanoparticles.Citation38,Citation126–Citation128
Proteins having cysteine residues can be directly attached to some metal nanoparticle surfaces such as gold and silver by stable metal–sulfur bonds.Citation129,Citation130 In the other cases, the covalent coupling of proteins on nanoparticle surfaces is always a long experimental procedure.
Covalent bioconjugation procedure can be summarized in: 1) Coating of nanoparticles with the selected active functional groups. 2) Chemical activation of thiol groups on the protein side with specific reductive agents, if necessary. 3) Total removal of the reduction agent in excess; this step can create unplanned reactions and spoil the whole coupling process. 4) Post conjugation procedures such as removal of unbound protein/remnant excess.
In addition to the disadvantage of the long experimental procedure, covalent bioconjugation can affect the protein structure and function resulting in its partial denaturation (). Moreover, modification of enzymes under strong denaturing conditions can result in their complete loss of activity.
Figure 2 Protein–nanoparticle interactions: main factors that can affect proteins resulting in their denaturation. In this example, proteins are conjugated on amino functionalized nanoparticles using the cross-linker SPMB.
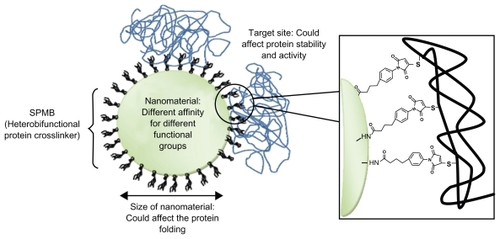
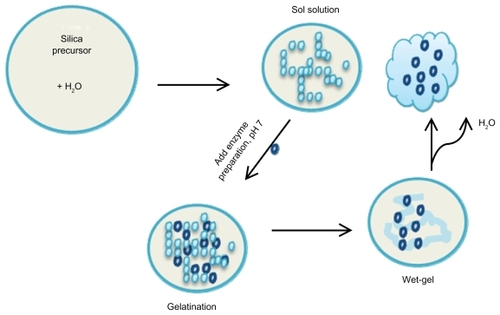
Figure 3 Entrapment of enzymes using sol-gel chemistry: A schematic overview of the sol-gel process. Several silicate precursors can be used to modify the surface chemistry of the sol-gels such as TMSO, APTES, MTMOS, and ETMOS.
Abbreviations: TMSO, tetramethyl orthosilicate; APTES, 3-aminopropyltriethoxysilane; MTMOS, methyltrimethoxysilane; ETMOS, ethyltrimethoxysilane.
Proteins can be denaturated during manipulations or formulations mainly by two mechanisms: conformational denaturation (eg, reversible unfolding and irreversible aggregation via noncovalent interactions) and chemical denaturation (covalent bonds such as deamidation, hydrolysis, oxidation, β-elimination, incorrect disulfide formation, Maillard reaction, and transamidation).
Even if the first denaturation mechanism can happen during the nanoparticles bioconjugation process, the second one is often necessary to obtain high efficacy of the coupling. For example, the –SH or –S-groups in cysteine –SH or disulfide –S–S– bridges are important in maintaining the conformation of the proteins. As a result, the engineering introduction of sulfhydryl groups in the protein changes its natural disulfide bonds resulting in partial conformational and chemical denaturation.
The DTT reagent, widely used to reduce disulfide bonds in biochemical systems, can alter protein function not only by thiol-disulfide exchanging but also by interacting with protein domains in the absence of cysteine residues.Citation131
While carboxyl groups seem to play an important role in enzymes catalytic activity,Citation132 their modification likely results in a change of protein secondary and tertiary structure.
The ɛ-amino groups of lysine are often specifically targeted because of their high reactivity and their modification seems to have fewer effects on protein properties. Unfortunately, the high abundance of these groups in many proteins can lead to increased heterogeneity and restricted conformational flexibility owing to multipoint attachment on a nanoparticles surface.
It is also possible that other reagents used during the coupling chemical process can contribute to the protein denaturation and to its activity loss. Therefore biological function checking as well as close monitoring of the quality and quantity of conjugated protein are extremely important to be assessed before being used.Citation111
Gold or silver nanoparticles too, due to the similar strength bond between Au/Ag–S and S–S, can potentially break up protein disulfide –S–S– bridges leading to denaturation.
Specific ELISA kits can be used to explore the activity of the proteins coupled to nanosystems. However there are nanoparticles such as quantum dots (QD) that can have an overlap in the absorption spectra and the ELISA essay end product. In this case the proper specific activity of the protein needs to be assessed directly by in vitro testing.Citation133
Protein encapsulation
Therapeutic biomolecules based on peptides, proteins or enzymes can be extremely fragile and easily aggressed by external agent such as proteases. Encapsulation of these fragile drugs in nanocarriers is a possible strategy for preventing their aggression and denaturation. This process can also improve the drug pharmacokinetic pathway and reduce immunological reactions.Citation19
An optimal drug delivery system should be biocompatible, biodegradable and should not cause any immunological adverse reaction in the human body. Among several candidates, liposomes are considered as the most promising vectors for proteins delivery due to their biocompatibility and their capacity to improve the drug pharmacokinetic. Liposomes, also known as lipid-based vesicles, are generally composed of concentric amphiphilic lipids, such as phospholipids, containing a water compartment. These carriers are versatile and their physico–chemical characteristics can be properly tuned.Citation19 Liposomes synthesized from dehydrated–rehydrated vesicles are widely used due to the ease of this preparation process and the low amount of stress applied to the proteins.Citation134
Liposome formulations are most frequently considered for parental administration of the drug, but may also be a potential formulation principle for alternative routes such as topical and nasal administration.
Several liposomes have immunoadjuvant properties and their application in vaccines based on recombinant protein subunits and synthetic–peptide antigens is attractive. The first liposome based vaccine (against hepatitis A) that has been licensed for human use is commercially known as Epaxal Berna® vaccine.Citation135
The main drawback of liposomes is their instability in biological media as well as their sensitivity to many external parameters such as temperature or osmotic pressure. Theoretically, it could be possible to increase their stability following several strategies such as the polymerization of a two–dimensional network in the hydrophobic core of the membrane, coating the liposome with a polyelectrolyte shell or adding surface active polymers to form mixed vesicular structures.Citation136–Citation138 However, poor loading and partial protein/enzyme denaturation during the entrapment process can occur.
Another well established technique to encapsulate biological species such as enzymes, antibodies and other proteins in a functional state is based on the sol–gel chemistry method.Citation139 Silica is indeed considered a very appealing material for drug delivery systems because it is relatively inexpensive, chemically inert, thermally stable, and biocompatible. Amorphous silica, used for decades as a food additive and for specific applications, is generally regarded as safe. Up until now, the FDA has not established if existing silica safety data can be applied to nanoscale forms of the material. In this approach, polypeptides, especially enzymes, could be entrapped inside silica matrix allowing the retention of enzymatic activity.Citation139–Citation141
On the other hand, process difficulties such as uncontrolled release, denaturation and the hardness control of the protein orientation can be found.Citation142,Citation143 The control of the drug release of such silica nanoparticles is the most important and difficult parameter that needs to be properly tuned. The encapsulation efficacy of insoluble protein is greatly different compared to the soluble one and the existence of soluble and insoluble part of polypeptides in the same therapeutic protein subunit complicates the synthesis process. Additionally, in the crowded environment of a silica matrix, the physical and chemical properties of the silica can directly influence protein structure and activity. Furthermore, functional activity of proteins entrapped into the sol-gel matrix needs to be accurately analyzed case-by-case using several techniques such as CD spectropolarimetry.Citation144,Citation145
The drug release and the capability of the carrier to be metabolized can be important factors to be considered when chronic or repeated treatments are necessary. The disadvantage associated with inorganic and synthetic carriers are the poor or slow biodegradability and possible inflammatory responses.Citation146
Biodegradable polymers nanosystems are an attractive alternative to liposomes since they have the advantages of longer circulation in the blood stream and generally higher drug carrying capacity.Citation147 Polymers such as poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) have been extensively investigated for their biocompatibility and potential capability of releasing therapeutically proteins in a controlled way even over a prolonged period of time.Citation148–Citation154 These polymers are degradable by bulk erosion through hydrolysis of the ester bonds. The hydrolysis rate depends on several nanoparticles physicochemical parameters and can be tailored according to the desired release pattern of the protein to be incorporated.
PLA and PLGA are FDA-approved as excipients to achieve sustained release of the active ingredient. However, their application in protein delivery systems is often characterized by low entrapment efficiency, burst release, instability of encapsulated hydrophilic protein and partial protein release.Citation155–Citation158 To improve the performance of these polymer nanoparticles, polysaccharides such as alginate (ALG) and chitosan (CS) could be applied.Citation151,Citation159 CS and its derivatives have been intensively studied as carriers for proteins and drugs. More specifically these nanoparticles can be totally made by CS or used in several copolymer combinations.Citation25,Citation160
Copolymers made by the combination of CS/ALG are able to generate a more “friendly” environment which protects peptides and proteins from stressing conditions and allows their stabilization during encapsulation, storage and release.Citation161–Citation166
Glycol chitosan nanoparticles modified with hydrophobic bile acid analogs self-assemble into polymeric nanoparticles with hydrophilic shells of glycol chitosan and hydrophobic cores of bile acid derivatives have been reported as possible vehicle for RGD (Arg–Gly–Asp) peptide.Citation29,Citation30
Regardless of the nanomaterial chosen for protein encapsulation, an important issue that needs to be considered is the understanding of protein–protein interactions. There are large numbers of transient protein–protein interactions that occur in the cell, which in turn control a large number of cellular processes. These transient interactions of protein complexes can cause several effects such as activation/inactivation of certain proteins, resulting in the formation of a new binding site.Citation167,Citation168 Kinetics properties of enzymes can be also altered by denaturation during the entrapment process allowing potential change of the protein specificity to its substrate.Citation169–Citation171
Gaining a clear picture of these basics knowledge will definitely lead to a change of object design to increase the protein load, to control the protein release and to retain the protein integrity and efficacy.
Conclusion
Although new proteins are available for medical purposes, their administration as therapeutics still remains difficult. Nanosystems seem to be the optimal solution to improve protein bioavailability, biodistribution and safety. Moreover, the combination of nanoparticles with proteins could also be a valid system to achieve the design of efficient nanovectors for drug delivery. Indeed, nanoparticles can be properly tuned for specific applications and could be precisely designed to meet biological needs. However, to completely fulfill this purpose, it is necessary to better clarify the nature of interaction between nanoparticles and biomolecules. The control of the protein denaturation is another important parameter that needs a deeper understanding. Further investigations should help to manage these hybrid nanosystems, opening new therapeutic and diagnostic perspectives as well as new challenges in the near future.
Disclosure
The authors report no conflicts of interest in this work.
References
- NavalakheRMNandedkarTDApplication of nanotechnology in biomedicineIndian J Exp Biol20074516016517375555
- EngelEMichiardiANavarroMNanotechnology in regenerative medicine: the materials sideTrends Biotechnol200826394718036685
- HeathJRDavisMENanotechnology and CancerAnnu Rev Med20085925126517937588
- MelendiPGFernaRPachecoRFNanoparticles as smart treatment–delivery systems in plants: Assessment of different techniques of microscopy for their visualization in plant tissuesAnn Bot200810118719517998213
- GidaspowDJiradilokVNanoparticle gasifier fuel cell for sustainable energy futureJ Power Source2007166400410
- ChenDQiaoXQiuXSynthesis and electrical properties of uniform silver nanoparticles for electronic applicationsJ Mat Sci20094410761081
- MedintzILUyedaHTGoldmanERQuantum dot bioconjugates for imaging, labeling and sensingNat Mater2005443544615928695
- CorotCRobertPIdéeJMRecent advances in iron oxide nanocrystal technology for medical imagingAdv Drug Deliv Rev2006581471150417116343
- De la FuenteJMBerryCCRiehleMONanoparticle targeting at cellsLangmuir2006223286329316548590
- AlonsoMJCohenSBernsteinHNanoparticulate drug carrier technologyMicroparticulate Systems for the Delivery of Proteins and VaccinesNew York, NYMarcel Dekker1996203242
- DrexlerKEMolecular engineering: an approach to the development of general capabilities for molecular manipulationProc Natl Acad Sci U S A1981785275527816593078
- RajagopalKSchneiderJPSelf-assembling peptides and proteins for nanotechnological applicationsCurr Opin Struct Biol20041448048615313243
- SinhaRKimGJNieSShinDMNanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug deliveryMol Cancer Ther200651909191716928810
- NelsonDLCoxMMLehninger Principles of Biochemistry4th EditionNew York, NYW H Freeman2004
- LiangFHHongMHHoRMNovel method using a temperature– sensitive polymer (methylcellulose) to thermally gel aqueous alginate as a pH-sensitive hydrogelBiomacromolecules200451917192515360306
- TanMLChoongPFMDassCRRecent developments in liposomes, microparticles and nanoparticles for protein and peptide drug deliveryPeptides109 [Epub ahead of print]
- FerrariMDowningGMedical nanotechnology: shortening clinical trials and regulatory pathwaysBiodrugs20051920321016128604
- AlmeidaAJSoutoESolid lipid nanoparticles as a drug delivery system for peptides and proteinsAdv Drug Delivery Rev200759478490
- Martins SarmentoBFerreiraDCSoutoEBLipid-based colloidal carriers for peptide and protein delivery: liposomes versus lipid nanoparticlesInt J Nanomed20072595607
- ChenHLangerROral particulate delivery: status and future trendsAdv Drug Del199834339350
- DelieFBlanco–PríetoMJPolymeric particulates to improve oral bioavailability of peptide drugsMolecules200510658018007277
- BorchardGLuegenHLde BoerAGThe potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan–glutamate and carbomer on epithelial tight junctions in vitroJ Control Release199639131138
- MalikDKBabootaSAhujaARecent advances in protein and peptide drug delivery systemsCurr Drug Deliv2007414115117456033
- des RieuxAFievezVGarinotMNanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approachJ Control Release200611612717050027
- RekhaMRSharmaCPSynthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorptionJ Control Release200913514415119331862
- CouvreurPLenaertsVKanteBOral and parenteral administration of insulin associated to hydrolysable nanoparticlesActa Pharm Technol198026220222
- AboubakarMCouvreurPPinto-AlphandaryHInsulin-loaded nanocapsules for oral administration: in vitro and in vivo investigationDrug Develop Res200049109117
- CarinoGPJacobJSMathiowitzENanospheres based oral insulin deliveryJ Control Release20006526126910699286
- KimJHKimYSParkKSelf–assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapyBiomaterials2008291920193018289669
- ZhangSDoschakMRUludaHPharmacokinetics and bone formation by BMP-2 entrapped in polyethylenimine–coated albumin nanoparticlesBiomaterials2009305143515519540582
- SinhaVRTrehanABiodegradable microspheres for protein deliveryJ Control Release20039026128012880694
- FrokjaerSHovgaardLPharmaceutical Formulation Development of Peptides and ProteinsAndover, UKTaylor and Francis2000
- MoYLimLYPaclitaxel-loaded PLGA nanoparticles: potentiation of anticancer activity by surface conjugation with wheat germ agglutininJ Control Release200510824426216213056
- AcharyaSDilnawazFSahooSKTargeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapyBiomaterials2009305737575019631377
- MieleESpinelliGPMieleEAlbumin-bound formulation of paclitaxel (Abraxane® ABI–007) in the treatment of breast cancerInt J Nanomed2009499105
- HuhYMIn vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystalsJ Am Chem Soc2005127123871239116131220
- DeNardoSJDe NardoGLMiersLADevelopment of tumor targeting bioprobes (In–chimeric L6 mAb nanoparticles) for alternating magnetic field cancer therapyClin Cancer Res20051170877092
- AktaşYYemisciMAndrieuxKDevelopment and brain delivery of chitosan–PEG nanoparticles functionalized with the monoclonal antibody OX26Bioconjug Chem2005161503151116287248
- ZhaoXHilliardLRMercherySJA rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticleProc Natl Acad Sci U S A200410150275032
- NatarajanAXiongCYGruattnerCDevelopment of multivalent radioimmunonanoparticles for cancer imaging and therapyCancer Biol Ther2008238290
- BhatiaSNBalisUJYarmushMLEffect of cell–cell interactions in preservation of cellullar phenotype: cocultivation of hepatocytes and nonparenchymal cellsFASEB J1999131883190010544172
- AndersonJMBiological responses to materialsAnnu Rev Mater Res20013181110
- HenchLLPolakJMThird-generation biomedical materialsSci200229510141017
- AllenLTFoxEJPBluteIInteraction of soft condensed Interaction of soft condensed materials with living cells: phenotype/transcriptome correlations for the hydrophobic effectProc Natl Acad Sci U S A20031006331633612746496
- HeungsooSSeongbongJMikosAGBiomimetic materials for tissue engineeringBiomaterials2003244353436412922148
- TsudaYKikuchiAYamatoMControl of cell adhesion and detachment using temperature and thermoresponsive copolymer grafted culture surfacesJ Biomed Mater Res200469A7078
- CedervallTLynchILindmanSUnderstanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticlesProc Natl Acad Sci U S A20071042050205517267609
- NelAXiaTMadlerLLiNToxic potential of materials at the nanolevelScience200631162262716456071
- LynchIDawsonKALinseSDetecting cryptic epitopes created by nanoparticlesSci STKE2006327pe1416552091
- LynchICedervallTLundqvistMThe nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st centuryAdv Colloid Interface Sci2007134135167174
- AhmadAEvansHMEwertKNew multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid-DNA complexes for gene deliveryJ Gene Med2005773974815685706
- HapcaACNobsLBBucheggerFDifferential tumor cell targeting of anti–HER2 (Herceptin®) and anti-CD20 (Mabthera®) coupled nanoparticlesInter J Pharm2007331190196
- KocbekPObermajerNCegnarMTargeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibodyJ Control Release2007120182617509712
- TirrelMKokkoliEBiesalskiMThe role of surface science in bioengineered materialsSurf Sci20025006183
- VromanLAdamsALIdentification of rapid changes at plasma–solid interfacesJ Biomed Mater Res1969343675784967
- YoungBRLambrechtLKMosherDFCooperSLPeppasNAPlasma proteins: Their role in initiating platelet and fibrin deposition on biomaterialsBiomaterials: Interfacial phenomena and applicationsWashington, DCAmerican Chemical Society1982199317349
- GrefRMinamitakeYPeracchiaMTBiodegradable long– circulating polymeric nanospheresScience1994263160016038128245
- OwensDEPeppasNAOpsonization, biodistribution, and pharmacokinetics of polymeric nanoparticlesInt J Pharm20063079310216303268
- FrankMFriesLThe role of complement in inflammation and phagocytosisImmunol Today1991123223261755943
- LynchIDawsonKAProtein–nanoparticle interactionsNano Today200834047
- DuttaDSundaramSKTeeguardenJGAdsorbed proteins influence the biological activity and molecular targeting of nanomaterialsToxicol Sci200710030331517709331
- IllumLHunneybalIMDavisSSThe effect of hydrophilic coatings on the uptake of colloidal particles by the liver and by peritonealmac-rophagesInt J Pharm1986295365
- KaulGAmijiMLong-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular deliveryPharm Res2002191061106712180540
- AggarwalPHallJBMcLelandCBNanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacyAdv Drug Del Rev200961428437
- AraiTNordeWThe behavior of some model proteins at solid–liquid interfaces. II. Sequential and competitive absorptionColloid Surf1990511728
- LinseSCabaleiro–LagoCXueWFNucleation of protein fibrillation by nanoparticlesProc Natl Acad Sci U S A20071048691869617485668
- BellezzaFCipicianiAQuotadamoMAStructure, stability, and activity of myoglobin adsorbed onto phosphate–grafted zirconia nanoparticlesLangmuir200723130071301218020378
- PodolskiIYPodlubnayaZAKosenkoEAEffects of hydrated forms of C60 fullerene on amyloid b–peptide fibrillization in vitro and performance of the cognitive taskJ Nanosci Nanotechnol200771479148517450915
- ChienPWeissmanJSDePaceAHEmerging principles of conformation–based prion inheritanceAnnu Rev Biochem20047361765615189155
- ChitiFDobsonCMProtein misfolding, functional amyloid, and human diseaseAnn Rev Biochem20067533336616756495
- DobsonCMThe structural basis of protein folding and its link with human diseasePhilos Trans R Soc Lond B Biol Sci200135613314511260793
- KooEHLansburyPTJrKellyJWAmyloid diseases: abnormal protein aggregation in neurodegenerationProc Natl Acad Sci U S A1999969989999010468546
- FloegeJEhlerdingGBeta–2–microglobulin associated amyloidosisNephron1996729268903856
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks)Risk Assessment of Products of NanotechnologiesBrussels, BelgiumEuropean Commission2009171
- ChunALNanoparticles: Coat of proteinsNat Nanotechnology2008
- KleinJProbing the interactions of proteins and nanoparticlesProc Natl Acad Sci U S A20071042029203017284585
- LundqvistMStiglerJEliaGNanoparticle size and surface properties determine the protein corona with possible implications for biological impactsProc Natl Acad Sci U S A2008105142651427018809927
- MartinsMCLRatnerBDBarbosaMAProtein absorption on mixtures of hydroxyl-and methyl-terminated alkanethiols self–assembleted monolayersJ Biomed Mater Res Pt A200367A158171
- PhillipsMCEvansMTAGrahamDEStructure and properties of protein films adsorbed at the air–water interfaceColloid Polym Sci1975253424427
- BrashJLHorbettTAProteins at Interfaces: Physicochemical and biochemical studies Advanced Symposium Series, 343Washington, DCAmerican Chemical Society1987 http://catalogue.nla.gov.au/Record/36034
- HorbettTABrashJLProteins at Interfaces II: Fundamentals and application Advanced Symposium Series, 602Washington, DCAmerican Chemical Society1995 http://searchworks.stanford.edu/view/3082531
- HladyVBuijsJProtein absorption on solid surfacesCurr Opin Biotechnol1996772778791316
- SigalGBMrksichMWhitesidesGMEffect of surface wetability on the absorption of proteins and detergentsJ Am Chem Soc199812034643473
- WanerMJGilchristMSchindlerMImaging the molecular dimensions and oligomerization of proteins at liquid/solid interfacesJ Phys Chem B199810216491657
- GrayJJThe interaction of protein with solid surfacesCurr Opin Struct Biol20041411011515102457
- WangDDoumaMSwiftBThe absorption of globular protein onto a fluorinated PDMS surfaceJ Colloid Interface Sci2009331909719027912
- AndradeJDHladyVWeiAPAbsorption of complex proteins at interfacesPure Appl Chem19926417771781
- AndradeJDHladyVProtein absorption and materials biocompatibility: A tutorial review and suggested hypothesisAdv Polym Sci198679163
- CollierTOJenneyCRDefifeKMProtein absorption on chemically modified surfacesBiomed Sci Instrum1997331781839731356
- MoulinAMO’SheaSJBadleyRAMeasuring surface–induced conformational changes in proteinsLangmuir19991587768779
- HoltSAMcGillivrayDJPoonSProtein deformation and surfactancy at an interfaceJ Phys Chem B200010474317438
- HendersonMJPerrimanAWRobson–MarsdenHProtein–poly(silicic)acid interactions at the air/solution interfaceJ Phys Chem B2005109208782088616853707
- PerrimanAWHendersonMJHoltSAEffect of the air–water interface on the stability of β–LactoglobulinJ Phys Chem B2007111135271353717994721
- NordeWMacRitchieFNowickaGProtein absorption at solid liquid interfaces: Reversibility and conformation aspectsJ Colloid Interface Sci1986112447456
- HayensCANordeWStructures and stabilities of adsorbed proteinsJ Colloid Interface Sci1995169313328
- NakanishiKSakiyamaTImauraKOn the absorption of proteins on solid surfaces, a common but very complicated phenomenonJ Biosc Bioeng200191233244
- RoachPFarrarDPerryCCInterpretation of protein absorption: surface–induced conformational changesJ Am Chem Soc20051278168817315926845
- NiemeyerCMSemi-synthetic DNA–protein conjugates: Novel tools in analytics and nanobiotechnology. Nucleic acids chemistry and biologyBiochem Soc Trans200432515314748711
- ShinkaiMFunctional magnetic particles for medical applicationJ Biosci Bioeng20029460661316233357
- WangLZhaoWTanWBioconjugated silica nanoparticles: Development and applicationsNano Res2008199115
- BharaliDJIlonaKEwaKSOrganically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brainProc Natl Acad Sci U S A2005102115391154416051701
- ArrueboMFernandez-PachecoRVelascoBAntibody-functionalized hybrid superparamagnetic nanoparticlesAdv Funct Mater20071714731479
- AyturTFoleyJAnwarMA novel magnetic bead bioassay platform using a microchip–based sensor for infectious disease diagnosisJ Immunol Methods2006314212916842813
- ZhaoXTapec-DytiocoRWangKCollection of trace amounts of DNA/mRNA molecules using genomagnetic nanocapturersAnal Chem2003753476348314570200
- ThirumamagalBTSZhaoXBBandyopadhyayaAKReceptor-targeted liposomal delivery of boron-containing cholesterol mimics for boron neutron capture therapy (BNCT)Bioconjug Chem2006171141115016984121
- HermansonGTBioconjugate TechniquesSan Diego, CAAcademic Press1996
- GrahamDEHarichKCWhiteRHReductive dehalogenation of monobromobimane by tris(2–carboxyethyl)phosphineAnal Biochem200331832532812814640
- SimonianALGoodTAWangSSNanoparticle-based optical biosensors for the direct detection of organophosphate chemical warfare agents and pesticidesAnal Chim Acta20055346977
- SunEYJosephsonLKellyKADevelopment of nanoparticle libraries for biosensingBioconjug Chem20061710911316417258
- GrüttnerCMüllerKTellerJSynthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapyJ Magn Magn Mater2007311181186
- Aubin-TamMESchifferliKAStructure and function of nanoparticle-protein conjugatesBiomed Mater20083303400118689927
- WeberCReissSLangerKPreparation of surface modified protein nanoparticles by introduction of sulfhydryl groupsInt J Pharm2000211677811137340
- DerekBSmythDGReactions of N-ethylmaleimide with peptides and amino acidsBiochem J1964915895955840721
- JamesonDMWongSChemistry of Protein Conjugation and Cross-linking2nd EdBaton Rouge, FLCRC Press2009
- MadisonLDRosenzweigSAJamiesonJDUse of the heterobifunctional cross–linker rn–maleimidobenzoyl N–hydroxysuccinimide ester to affinity label cholecystokinin binding proteins on rat pancreatic plasma membranesJ Biol Chem198425914811482
- MattsonGConklinEDesaiSA practical approach to crosslinkingMol Biol Reports199217167183
- GolemisEProtein–Protein Interactions. A molecular cloning manualCold Spring Harbor, NYCold Spring Harbor Laboratory Press2002
- XingYRaoYQuantum dot bioconjugates for in vitro diagnostics and in vivo imagingCancer Biomark2008430731919126959
- JonkheijmPWeinrichDSchröderHChemical strategies for generating protein biochipsAngew Chem Int Ed20084796189647
- ClelandWWDithiothreitol, a new protective reagent for SH groupsBiochemistry1964348048214192894
- TrautRRBollenASunTTMethyl 4-mercaptobutyrimidate as a cleavable cross–linking reagent and its application to the Escherichia coli 30S ribosomeBiochemistry197312326632734581787
- LangerKCoesterCWeberCPreparation of avidin–labeled protein nanoparticles as carriers for biotinylated peptide nucleic acidEur J Pharm Biopharm20004930330710799823
- WangSMamedovaNKotovNAAntigen/antibody immunocomplex from CdTe nanoparticle bioconjugatesNano Lett20022817822
- GonzalezMBagatolliLAEchabeIInteraction of biotin with streptavidin. Thermostability and conformational changes upon bindingJ Biol Chem199727211288112949111033
- GreenNMAvidin and streptavidinMethod Enzymol19901845167
- ParkaJALeeJJKimISMagnetic and MR relaxation properties of avidin–biotin conjugated superparamagnetic nanoparticlesColloid Surf A2008313314288291
- LangerKCoesterCWeberCPreparation of avidin–labeled protein nanoparticles as carriers for biotinylated peptide nucleic acidEu J Pharm Biopharm200049303307
- CoesterCKreuterJvon BriesenHPreparation of avidin– labelled gelatin nanoparticles as carriers for biotinylated peptide nucleic acid (PNA)Int J Pharm200019614714910699706
- GoleADashCSomanCOn the preparation, characterization, and enzymatic activity of fungal protease–gold colloid bioconjugatesBioconjug Chem20011268469011562186
- KudelskiAInfluence of electrostatically bound proteins on the structure of linkage monolayers: absorption of bovine serum albumin on silver and gold substrates coated with monolayers of 2-mercaptoethanesulphonateVib Spectrosc200333197204
- AlliegroMCEffects of dithiothreitol on protein activity unrelated to thiol-disulfide exchange: for consideration in the analysis of protein function with Cleland’s reagentAnal Biochem200028210210610860505
- ShenoyBCAppu RaoAGRaghavendra RaoMREffect of chemical modification on structure and activity of glucoamylase from Aspergillus candidus and Rhizopus speciesJ Biosci198711339350
- DiagaradjanePOrenstein-CardonaJMColon-CasasnovasNEImaging epidermal growth factor receptor expression in vivo: pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobeClin Cancer Res20081473174118245533
- KiselMAKulikLNTsybovskyISLiposomes with phosphatidylethanol as a carrier for oral delivery of insulin: studies in the ratInt J Pharm200121610511511274812
- GluckRLiposomal hepatitis A vaccine and liposomal multiantigen combination vaccinesJ Lipo Res19955467469
- GregoriadisGEngineering liposomes for drug delivery: progress and problemsTrends Biotechnol1995135275378595139
- ColletierJPChaizeBWinterhalterMProtein encapsulation in liposomes: efficiency depends on interactions between protein and phospholipid bilayerBMC Biotechnol200221811818033
- RuysschaertTGermainMda Silva GomesJFPLiposome-based nanocapsulesIEEE Trans Nanobioscience20043495515382644
- BesangerTRBrennanJDEntrapment of membrane proteins in Sol Gel derived silicaJ Sol Gel Sci Techn200640209225
- TanWWangKHeXBionanotechnology based on silica nanoparticlesMed Res Rev20042462163815224383
- NandiyantoABDKimSGIskandarFSynthesis of spherical mesoporous silica nanoparticles with nanometer–size controllable pores and outer diametersMicropor Mesopor Mat2009120447453
- LuBSmythMRO’KeneddyJRImmunological activities of IgG antibody on pre-coated Fc receptor surfacesAnal Chim Acta199633197102
- VanderbergETBrownRSKrullUJVelikyIEMcLeanRJCImmobilized enzymes and cells in biochemical reactionsImmobilized Biosystems: Theory and Practical ApplicationsAmsterdam, HollandElsevier1983
- NarayananSSSarkarRPalSKStructural and functional characterization of enzyme quantum dots conjugates: covalent attachment of CdS nanocryrtal to α-chemo trypsinJ Phys Chem C20071111153911543
- MenaaBHerreroMRivesVLavrenkoMEggersDKFavourable influence of hydrophobic surfaces on protein structure in porous organically-modified silica glassesBiomaterials2008292710271818359512
- SeehermanHWozneyJLiRBone morphogenetic protein delivery systemsSpine2002271623
- JanesKACalvoPAlonsoMJPolysaccharide colloidal particles as delivery systems for macromoleculesAdv Drug Deliv Rev200147839711251247
- GombotzVRPettitDKBiodegradable polymers for protein and peptide drug deliveryBioconjug Chem199563323517578352
- AndersonJMShiveMSBiodegradation and biocompatibility of PLA and PLGA microspheresAdv Drug Deliv Rev19972852410837562
- CrottsGParkTGProtein delivery from poly(lactic-coglycolic acid) biodegradable microspheres: release kinetics and stability issuesJ Microencapsul1998156997139818948
- ZhengCHGoaJQZhangYPLiangWQA protein delivery system: biodegradable alginate-chitosan-poly(lactic-co-glycolic acid) composite microspheresBiochem Biophys Res Commun20043231321132715451441
- Sun-WoongKOjuJByung-SooKTissue Eng20051143844715869422
- LeeESParkKHParkISGlycol chitosan as a stabilizer for protein encapsulated into poly(lactide–co–glycolide) microparticleInt J Pharm200733831031617363202
- XuPGullottiETongLIntracellular drug delivery by poly(lactic–co–glycolic acid) nanoparticles, revisitedMol Pharm2009619020119035785
- FuKGriebenowKHsiehLFTIR characterization of the secondary structure of proteins encapsulated with PLGA microspheresJ Control Release19995835736610099160
- DiwanMParkTGPegylation enhances protein stability during encapsulation in PLGA microspheresJ Control Release20017323324411516501
- KangJSchwendemanSPComparison of the effects of Mg(OH)2 and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(d,l-lactide-co-glycolide) implantsBiomaterials20022323924511762843
- Van de WeertMHenninkWEJiskootWProtein instability in poly(lactic–co–glycolic acid) microparticlesPharm Res2004171159116711145219
- VilaASanchezATobioMDesign of biodegradable particles for protein deliveryJ Control Release200278152411772445
- CalvoPRemunan–LópezCVila–JatoJLChitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccinesPharm Res199714143114369358557
- CalvoPRemunan-LopezCVila-JatoJLNovel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriersJ Appl Polymer Sci199763125132
- CalvoPRemunan-LopezCVila-JatoJLChitosan and chitosan/ethylene oxide–propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccinesPharm Res199714143114369358557
- AmidiMRomeijnSGBorchardGPreparation and characterization of protein–loaded N-trimethyl chitosan nanoparticles as nasal delivery systemJ Control Release200611110711616380189
- SarmentoBFerreiraDCJorgensenLProbing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticlesEur J Pharm Biopharm200765101717101268
- LiTShiXWDuYMQuaternized chitosan/alginate nanoparticles for protein deliveryJ Biomed Mater Res A20078338339017450586
- SchoubbenABlasiPGiovagnoliSNovel composite microparticles for protein stabilization and deliveryEur J Pharm Sci20093622623418950709
- NakaiHRichardsonCCThe gene 1.2 protein of bacteriophage T7 interacts with the E. coli dGTP triphosphohydrolase to form a GTP-binding proteinJ Biol Chem1990265441144192155228
- EvansPRFarrantsGWHudsonPJPhosphofructokinase: Structure and controlPhilos Trans R Soc Lond B Biol Sci198129353626115424
- HillRLBrewKLactose synthetaseAdv Enzymol197543411490812340
- SrerePAComplexes of sequential metabolic enzymeAnn Rev Biochem198756891242441660
- PrelichGTanCKKosturaMFunctional identity of proliferating cell nuclear antigen and a DNA Polymerase-δ auxiliary proteinNature19893265175202882424