 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Magnetic particle imaging (MPI) is a novel imaging method that was first proposed by Gleich and Weizenecker in 2005. Applying static and dynamic magnetic fields, MPI exploits the unique characteristics of superparamagnetic iron oxide nanoparticles (SPIONs). The SPIONs’ response allows a three-dimensional visualization of their distribution in space with a superb contrast, a very high temporal and good spatial resolution. Essentially, it is the SPIONs’ superparamagnetic characteristics, the fact that they are magnetically saturable, and the harmonic composition of the SPIONs’ response that make MPI possible at all. As SPIONs are the essential element of MPI, the development of customized nanoparticles is pursued with the greatest effort by many groups. Their objective is the creation of a SPION or a conglomerate of particles that will feature a much higher MPI performance than nanoparticles currently available commercially. A particle’s MPI performance and suitability is characterized by parameters such as the strength of its MPI signal, its biocompatibility, or its pharmacokinetics. Some of the most important adjuster bolts to tune them are the particles’ iron core and hydrodynamic diameter, their anisotropy, the composition of the particles’ suspension, and their coating. As a three-dimensional, real-time imaging modality that is free of ionizing radiation, MPI appears ideally suited for applications such as vascular imaging and interventions as well as cellular and targeted imaging. A number of different theories and technical approaches on the way to the actual implementation of the basic concept of MPI have been seen in the last few years. Research groups around the world are working on different scanner geometries, from closed bore systems to single-sided scanners, and use reconstruction methods that are either based on actual calibration measurements or on theoretical models. This review aims at giving an overview of current developments and future directions in MPI about a decade after its first appearance.
Introduction
There is one prefix that has opened up innumerable new research fields and promises amazing new possibilities. This prefix is “nano,” meaning a billionth of the unit it is put before, and, in the case of a nanometer, it means just a few atoms wide. Today, particles of these dimensions, so called nanoparticles, play a key role in many fields of our daily lives. Their particular properties are utilized from plant construction to medicine. Magnetic particle imaging (MPI) is one result of this development.
It was more than a decade ago, in 2001, that the concept of MPI – built around superparamagnetic iron oxide nanoparticles (SPIONs) – was conceived by Gleich at the Philips Research Laboratory in Hamburg, Germany. MPI takes advantage of the response of SPIONs to an oscillating magnetic field to determine their spatial distribution and local concentration. In 2005, Gleich and Weizenecker wrote a pivotal paper in which they reported the first MPI images and proved the feasibility of this method.Citation1,Citation2 This was the starting signal for a development that was and still is led by numerous research groups all over the globe.
MPI is the first medical application in which nanoparticles are not just supportive contrast agents, as in magnetic resonance imaging (MRI), but the only source for signal and thus the only visualized element. That is why the used SPIONs are referred to as tracers rather than contrast agents. One crucial characteristic of SPIONs in comparison with protons in a field of 1.5 T – the main source of the MRI-signal – is their 108 times higher magnetization and their 104 times faster relaxation.Citation3 These two characteristic variables can be translated into the above mentioned outstanding temporal resolution and into a higher signal-to-noise-ratio (SNR). Due to the fact that tissue is diamagnetic, it does not generate any interfering signal, leading to an image of the tracer distribution that features a superb contrast.Citation4–Citation6 Consequently, MPI does not visualize anatomical structures if they are not labeled by the tracer.
What sets MPI apart from medical imaging modalities currently in use is its inherent combination of capabilities. MPI promises a very high temporal resolution with high acquisition rates of up to 40 volumes per second as well as a high spatial resolution of up to about 1 mm.Citation1,Citation7 Since the strength of the MPI signal is proportional to the concentration of nanoparticle tracers in the field of view (FOV), quantitative data could be acquired. Furthermore, MPI is sensitive, works without ionizing radiation, and offers a three-dimensional image of the SPIONs’ distribution with a great contrast.Citation2,Citation8
This unique combination predestines MPI for a variety of medical applications, eg, cardiovascular diagnostic and interventional procedures as well as cell labelling and tracking.
In this review, an introduction into the basic principles of MPI will be provided – from the signal generation and acquisition over the encoding of the signal to the final reconstruction of an image. The SPIONs, the centerpiece of MPI, are presented together with an update on MPI tracers in development. Furthermore, the actual implementation of the method with an overview of currently available scanners and preclinical demonstrators is offered. In the context of upscaling the preclinical systems to commercially available clinical systems, some safety issues will be highlighted. A presentation of prospective medical applications that exploit the unique potential of MPI will conclude this review.
For a more extensive insight into the physics and chemistry of MPI and SPIONs, we would like to refer to more comprehensive writings, eg, Knopp and BuzugCitation2 as well as Gleich.Citation9
Magnetic particle image: the basic concept
Signal generation and acquisition
MPI exploits the special characteristics and the response of SPIONs when exposed to certain magnetic fields generated by a complex coil topology in the MPI scanner. The tracer’s response is picked up by receiving coils and used as the fundamental signal for the three-dimensional visualization of the tracer’s distribution in space.
By applying a direct and an alternating current to these coils, static and varying magnetic fields are generated, respectively ().
Figure 1 MPI, basic concept.
Notes: Left: response of SPIONs within the FFP/FFL. The response consists of the excitation frequency f and higher harmonics of it. Middle: a graphical depiction of an FFP and an FFL. Only SPIONs within and in close vicinity to the nonsaturated areas respond to the excitation field. The signals’ origin can be allocated to the FFP/FFL. Right: SPIONs outside the FFP/FFL are magnetically saturated and do not respond to the excitation field in a significant way.
Abbreviations: M, magnetization of SPIONs; H, magnetic field strength; HD, magnetic field strength of the drive field; t, time; u, voltage; û, Fourier transform of voltage signal; f/f0, higher harmonics of excitation frequency; MPI, magnetic particle imaging; SPION, superparamagnetic iron oxide nanoparticle; FFP, field-free point; FFL, field-free line.
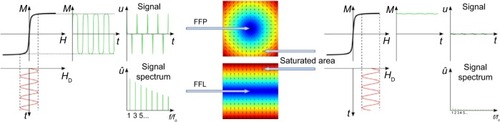
The varying magnetic fields featuring specific frequencies f and high enough amplitudes A are used to excite the SPIONs, ie, change their magnetization direction. The applied fields are therefore known as excitation fields. As the SPIONs’ magnetization curve is nonlinear, the Fourier transform of their magnetization over time, M(t), includes the excitation frequency as well as higher harmonics of this frequency.Citation1 The presence of these higher harmonics allows a separation of the signal originating from the tracer and the one coming from the scanner’s excitation field. The response of the SPIONs is picked up by dedicated receiving coils. The changing magnetization of the superparamagnetic particles causes an electrical induction in the receiving coils, which represents the acquired information about the tracer material.
Spatial encoding
In a setup as described so far, the excitation of the particles would not be limited to a defined region in the imaging area, and all particles exposed to the excitation field would be excited. A possible way to narrow down the area where the particles are excited is to superimpose the excitation fields with a static magnetic gradient field (; middle). The size of this dynamic imaging region and, consequently, the achievable resolution is strongly dependent on the applied gradient strength G for the x, y, and z direction. At this point, a second important feature of the SPIONs has to be mentioned – they are magnetically saturable if exposed to magnetic fields with a high enough amplitude. Such a field, referred to as a selection field, is generated by a Maxwell-coil pair and features a magnetic field-free point (FFP).Citation1,Citation10–Citation12 Due to the selection field, the SPIONs outside the FFP are magnetically saturated (; right), and only tracer material directly in or in close vicinity to this well-defined FFP is influenced by the excitation field (; left). An alternative encoding concept to the FFP-based approach is the use of a magnetic field-free line (FFL), which promises an increase in the sensitivity of the system.Citation13–Citation15
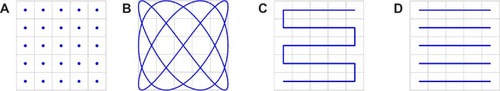
In order to acquire a signal over the whole FOV, the FFP or FFL has to be moved relatively to this area. To realize an adequate covering of the FOV in a reasonable time, specific trajectories are used as a data acquisition path.Citation16 depicts four different trajectories. The movement can be realized either by a mechanical shift of the object or by additional magnetic fields. These fields, referred to as drive fields, are varying magnetic fields and enable the movement of the FFP or FFL, respectively. It should be noted that in actual implemented scanner systems, the excitation fields and the drive fields are implemented using either separate coils or the same coils.Citation17
Figure 2 Four different methods of FFP movement to achieve a spatial coverage of the FOV.
Notes: From left to right: (A) The single-voxel methodCitation1,Citation101 where for each voxel an FFP has to be generated. (B) The Lissajous trajectory, providing a good coverage of the FOV and therefore used for fast electromagnetic movement of the FFP via drive and focus fields in many current MPI systems. (C) An 1D movement of the FFP, with the excitation field as performed by scanners of the Berkeley group.Citation26,Citation102–Citation104 (D) The whole FOV is covered by a mechanical movement of the object of interest. The traveling wave method,Citation12 where the FFP is moved electromagnetically in one direction. With a shift of the FFP within the analyzed plane, several line scans can be obtained.
Abbreviations: FFP, field-free point; FOV, field of view; MPI, magnetic particle imaging; 1D, one dimensional.
Reconstruction principles
To reconstruct the spatial distribution of the particle concentration from the received voltage signal, an adequate image reconstruction has to be performed. In order to meet the requirements of reconstruction, which are a reasonable reconstruction effort and reconstruction time as well as a sufficient image quality, different approaches have been proposed to date.Citation18–Citation20 In general, it is possible to differentiate between two main methods that are used for reconstruction with current existing imaging systems. The first is a system matrix-based reconstruction that depends on a supplemental calibration scan that is performed by moving a delta-sample by robotic means,Citation1,Citation21,Citation22 and the second is a direct reconstruction known as x-space MPI, which is based on some idealized assumptions.
A system matrix includes the information about the particle behavior at every position within the scanner and, therefore, represents a calibration of the system. Using the system matrix, it is possible to disassemble the signal of all particles encoded in the received signal into the individual signals of each spatial position. With the system matrix S, the receive signal, u, and the particle concentration c, it follows that Sc = u. In order to solve this system of equations, typically, an iterative solver combined with regularization typically is used to solve the minimization problem:
A major drawback of the system matrix based approach is the time necessary to perform the calibration measurement. One possibility to omit this tedious procedure is to use x-space reconstruction.Citation20 The basic concept is based on assumptions regarding the particles’ behavior and the pureness of the applied magnetic fields. If these assumptions are fulfilled, a direct reconstruction of the particle concentration c is possible. A simplified formula as published in Goodwill and ConnollyCitation20 can be given by
To date, a system matrix based reconstruction has to be chosen in order to make use of the full potential of MPI in terms of real-time imaging. That is because the dedicated system matrix includes the deviations of the magnetic fields and the complex particle characteristics in the calibration and thereby allows the encoding of the information in the region of interest with a fast Lissajous trajectory. Several image reconstruction results have been published so far. The first in vivo results presented by Weizenecker et alCitation7 as well as several other studies with dynamic imagesCitation23–Citation25 have been reconstructed with dedicated system matrices (see ).
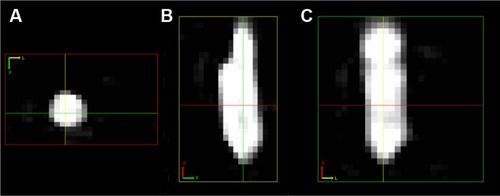
Figure 3 MPI image of a balloon catheter filled with SPIONs.
Notes: From left to right: An image of a commercially available and routinely used interventional device in axial (A), sagittal (B), and coronal (C) plane reconstruction. The contour of the catheter is clearly distinguishable.Citation24
Abbreviations: MPI, magnetic particle imaging; SPION, superparamagnetic iron oxide nanoparticle.
While image quality with x-space is equally convincing, the effort and the time for image reconstruction are much lower than for the system matrix based approach. Current image acquisition procedures take several minutes to enable a good x-space reconstruction.Citation6,Citation26
Magnetic particle spectrometer
A magnetic particle spectrometer (MPS), as shown in , is in concept very similar to an MPI device. In contrast to the imaging scanner, the spatial distribution of nanoparticles is known, and only their physical characteristics are to be studied. Recent research has shown the possibility of a spectrometer to measure the particle core diameter,Citation27 the particle hydrodynamic diameter,Citation28 its temperature,Citation29 and its binding status.Citation30 As these measurements can be used to predict the imaging performance in MPI scanners, the MPS is a helpful tool in particle synthesis as well as in the development of dynamic particle models. Current developments in the signal chain have led to higher sensitivity and higher signal purity.Citation31,Citation32
Figure 4 Transmit and receive setup of a magnetic particle spectrometer.
Notes: The nanoparticle samples are placed in the center of the send and the receive coil. The coils are manufactured of a high frequency litz wire and are glued and pressed to avoid vibrations.

As the spatial distribution is known and the sample size seldom exceeds 20 μL, the selection field can be excluded from the signal chain. The consequences are reduced costs, smaller size, and lower complexity of the device. The field generator is optimized in terms of maximum homogeneity to suppress modeling errors due to different particle excitations. With sufficient homogeneity, the second optimization parameter is the sensitivity of the coil array. This provides a high SNR and makes it possible to measure even high-diluted samples, which can differ in their physical properties from undiluted samples. As in future in vivo applications, where the tracer will be highly diluted in the blood flow of the patient, the sensitivity of the spectrometric device is a crucial parameter.
A measurement is performed by applying a given sequence of time-varying magnetic fields to the particles and recording the particle response. Then the physical parameters are determined by fitting the physical models to this measured response. The amplitude of the particle response is proportional to the amount of particles enclosed in the measured sample. This can be used to measure the uptake of nanoparticles in a specific tissue or organs, ie, lymph nodes.Citation33 This uptake directly corresponds to the efficacy of functionalized particles.
Superparamagnetic iron oxide nanoparticles
SPIONs are the centerpiece of MPI as its principle is based on three of the SPIONs’ characteristics. First, SPIONs are superparamagnetic. A superparamagnetic material does not show any remanent magnetization when a magnetic field used for excitation is turned off. This is due to the Brownian and Néel relaxation, which changes the magnetization direction under thermal excitation even at room temperature. Thus, the SPIONs’ magnetization follows the excitation field (see ), ie, the drive field. Second and third, as mentioned above, SPIONs exhibit a nonlinear magnetization curve and can be magnetically saturated. This allows the differentiation of the SPIONs’ signal from the drive field’s signal and thus the detection and allocation of the SPIONs’ signal to a precise location in the field of view, ie, spatial encoding.
Figure 5 Magnetic nanoparticles synthesized at the Institute of Medical Engineering of the Universität zu Lübeck.
Notes: The fluidal sample shown here is magnetized by a permanent magnet due to a parallel orientation of the SPIONs’ magnetization. Without this external magnetic field the SPIONs would return to a random orientation of each particle’s magnetization.
Abbreviation: SPION, superparamagnetic iron oxide nanoparticle.

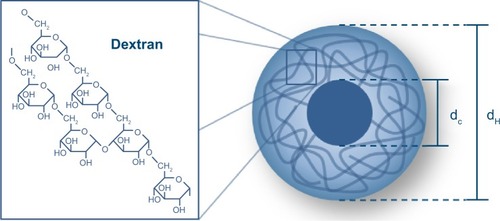
Besides these fundamental characteristics, spatial resolution and sensitivity are heavily influenced by the properties of the SPIONs as well. Here, the iron core diameter is the key parameter (see ). Sensitivity depends, among other factors, on the magnetic moment of the SPIONs, which can be increased by enlarging the iron core diameter, as the strength of the signal increases by the third power of the iron core diameterCitation34 as long as superparamagnetic characteristics are retained. The spatial resolution relies, besides the strength of the gradient selection field, mainly on the particle’s magnetization curve. The steeper the slope of the SPIONs’ magnetization curve, the smaller the space to which the SPIONs’ signal can be confined and the better the spatial resolution.Citation21,Citation35 Hence, the particles’ relaxation characteristics are the key parameter for spatial resolution. Either Néel or Brownian relaxation or a combination of both describes the particles’ response to the time-varying magnetic field. In short, in Néel relaxation, the particles magnetization switches internally whereas in Brownian relaxation the particle physically rotates.Citation34 Which mechanism dominates depends on the iron core diameter and the frequency and the strength of the drive field.Citation34,Citation36
Figure 6 Schematic drawing of a spherical and dextran-coated magnetic nanoparticle.
Notes: The magnetic core (with core diameter dC) is surrounded by a magnetically neutral coating (with hydrodynamic diameter dH), which is necessary to prevent agglomeration of the particles.
For SPIONs in MPI, Néel relaxation seems to be the dominating mechanism. Of course, the relaxation is also dependent on the SPIONs’ environment, ie, if they are suspended in fluid, as is most often the case in medical applications, or fixed in solid structures, where the Brownian relaxation is consequentially blocked. In principle, a large iron core diameter is desirable for a high magnetic moment and a steep magnetization curve. On the contrary, if the iron core diameter exceeds a critical size, the particles lose their superparamagnetic characteristics. Thus, the most suitable iron core diameter has to be a compromise.
At the beginning of MPI, drive field amplitude and frequency were around 10–20 mT and 25 kHz respectively. Here, an ideal iron core diameter of 30 nm was proposed.Citation1 However, as the relaxation characteristics are also dependent on the drive field amplitude and frequency, the SPIONs’ ideal iron core diameters may vary for different drive field settings. It is believed by some, that for a maximum performance, an MPI tracer should contain homogeneously distributed SPIONs with the respective ideal iron core diameter. Another important factor for the performance of SPIONs in MPI seems to be the particles’ anisotropy. Here, first simulations indicate that a high anisotropy may diminish the performance, whereas smaller anisotropy may enhance it.Citation37 The change of the particles’ magnetization characteristics at different states, ie, after internalization in cells and degradation or integration in solid structures, also has to be kept in mind.Citation38,Citation39
As in other imaging modalities, a SPIONs’ hydrodynamic diameter (see ) also influences the pharmacokinetics and thus the application.Citation40,Citation41 In the bloodstream, nanoparticles are rapidly marked by endothelial cells in the reticuloendothelial system (RES).Citation42 This effect is mostly dependent on the hydrodynamic diameter and the surface of the SPIONs. SPIONs with a hydrodynamic diameter smaller than 50 nm, known as ultrasmall SPIONs (uSPIONs), circulate longer and can extravasate. Larger particles are usually collected by the RES of liver and spleen. The iron oxide is eliminated slowly just like endogenous iron. Only 16%–21% of the injected dose of iron is excreted in the feces after 84 days.Citation43 Most of the iron is stored in the iron storage protein ferritin. Ferritin can be found in especially high concentrations in tissues that contain cells of the RES, eg, liver, spleen, bone marrow, and lymph nodes.
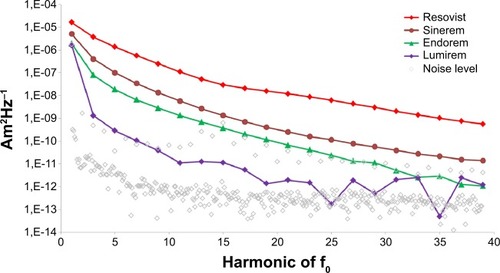
In the beginning of MPI, existing SPION contrast agents for MRI were evaluated regarding their MPI performance using MPS. Only Resovist (Bayer Pharma AG) showed an acceptable MPI performance (see ). All other tracers showed a very weak MPI signal, mostly due to too small iron core diameters.Citation44 The good performance of Resovist was surprising, as Gleich and Weizenecker could show that, according to Langevin theory, only particles with a diameter of about 30 nm contribute significantly to the MPI signal, and in Resovist, these particles amount only to 3% of the iron mass of the Resovist solution.Citation1 Subsequent studies showed that in Resovist, the smaller SPIONs form aggregates behaving like monodomain particles, ie, superparamagnetic, with an iron core diameter of 24 nm.Citation45 These aggregates account for 30% of the nanoparticles in Resovist and might explain its good MPI performance.Citation45 Since Resovist is the only commercially available SPION formulation with an acceptable MPI performance, it became the standard of reference in the MPI community. The iron concentration of undiluted Resovist is 0.5 mmol/mL. MPI scans undertaken with clinically approved concentrations of Resovist for MRI examinations in humans, have provided great results in in vivo MPI, eg, the visualization of blood flow in a beating mouse heart.Citation7 Unfortunately Bayer Pharma AG abandoned Resovist in 2009, and it is currently only available in Japan, distributed by I’rom Pharmaceutical (Tokyo, Japan). However, as Resovist is not an ideal MPI tracer with respect to its iron core size and especially its particle size distribution, many research groups started to develop dedicated MPI tracers beforehand.Citation46
Figure 7 MPI performance of SPION contrast agents for MRI.
Notes: Resovist shows the highest MPI signal of all commercially available SPION tracers. On the axis of abscissae, the higher harmonics of the excitation frequency of f0 =25 kHz are stated. The signal strength (spectral magnetic moment/Am2Hz−1) is shown on the axis of ordinates. Measurements were performed as described by Lüdtke-Buzug et al.Citation44
Abbreviations: MPI, magnetic particle imaging; MRI, magnetic resonance imaging; SPION, superparamagnetic iron oxide nanoparticle.
A variety of strategies has been proposed for the creation of appropriate nanoparticular systems, the most important for MPI are precipitation and thermal decomposition.Citation46,Citation47 To prevent the particles of agglomeration during storage or application, the iron oxide particle cores have to be coated with a biocompatible hull. Dextran, carboxydextran, or other polymeric carbohydrates are frequently used as coating materials. For medical applications, magnetic iron oxide particles in the liquid form must be stabilized. The stabilizer ensures the stability of a colloidal suspension of particles during application. The stabilizer counteracts against the van der Waals interactions as well as magnetic attraction between the particles.
One common approach for MPI is the synthesis of homogeneously distributed single-core SPIONs with a dedicated iron core diameter for ideal MPI characteristics. Khandhar et al have already presented first results of a SPION tracer with an MPS performance twice as good as Resovist. Furthermore, this group predicts an improvement of the spatial resolution of 20% based on the MPS measurements. Starmans et alCitation49 published results of iron oxide nanoparticles micelles (ION-Micelles) outperforming Resovist by a factor of at least 4–6 in MPS. Some groups aim at creating multicore nanoparticles with a big fraction of large aggregates and thus a good MPI performance. Other groups separate multicore aggregates from the smaller particles to achieve a larger fraction of efficient particles.Citation50–Citation54 A list of recently published results in tracer design for MPI is provided in . Besides these conventional approaches, the use of bacterial magnetosomes as biogenic MPI tracers has been recently proposed. First experimental results show a superior performance compared with Resovist in MPS.Citation55
Table 1 Currently published results in tracer design for MPI
The use of Resovist as a standard of reference in tracer and hardware development for MPI is very important as it allows for a comparison and interpretation of the results of different research centers, as many working groups use different MPS systems and MPI scanners for evaluation. However, it has to be kept in mind that different batches of Resovist may show a deviating MPS performance up to a factor of three,Citation56 which is a potential source of error.
Scanner geometries and performances
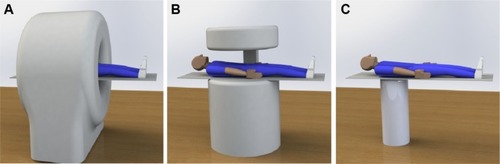
To date, there are mainly three geometries for MPI systems (see A–C): closed-bore scanners, where the subject is inserted in the center of a tube-like device; open-bore systems, where the subject lies between two magnets and is accessible from the side; and single-sided coil arrangements intended for integration in beds, tables, or developed as handheld devices.Citation57 With the exception of one commercially available imager,Citation58 most of the MPI systems currently running are working prototypes, acquiring 1D, 2D, or 3D images. An overview of MPI systems and their actual performance is given in .
Table 2 Overview of MPI systems and selected imaging results achieved so far
Figure 8 Concept of the three main scanner geometries.
Notes: (A) Closed-bore scanner. (B) Open-bore scanner. (C) Single-sided scanner.
The presented performance data include the available free bore, the FOV, the acquisition time per image, the maximal gradient amplitude, as well as the used tracer and the tracer concentration. Spatial resolution and sensitivity have deliberately been left out due to the heterogeneity of criteria used for their determination. Moreover, many research groups have just roughly estimated the spatial resolution or have not explicitly published these data at all. So far, the experimentally proven spatial resolution of MPI lies in the range of one to several millimeters.
The sensitivity of an MPI system depends on many variables, eg, voxel size, scanning time, and coil design and is thus hardly comparable between different scanner geometries in MPI and especially between different modalities like MRI and MPI. However, Gleich theoretically determined the detection limit of MPI for an MPI system with a coil of square shape and a side length of 10 cm with a sample placed 10 cm from the center of the coil.Citation9 Referring to this model, he calculated that it is possible to detect 25 pg of iron oxide [324 nmol(Fe)/L] in 1 second scanning time in a human with MPI. Refering to this, Knopp and Buzug described that for Resovist, if applied according to prescription in a human with a blood volume of 6 liters, the concentration of Resovist would be 116 mol(Fe)/L and thus about 360 times higher than the detection limit of MPI with a voxel size of 1 mm3 and a scanning time of 1 second.Citation2 Ultimately, Gleich depicted the detection limit of MPI as 13 nmol(Fe)/L for a voxel size of 1 mm3 and 13 pmol(Fe)/L for a voxel size of 1 cm3.Citation9 In comparison, Gleich described the sensitivity of MRI to be roughly 50 μmol(Fe)/L, independent of the voxel size.Citation9
Finally, the exact relation between the scanner parameters, the particle characteristics, and the sensitivity as well as the spatial and temporal resolution of the acquired images is still under investigation.Citation59 What can be already stated with a fair amount of certainty is that the spatial resolution strongly depends on the gradient of the magnetic field strength and the SPIONs’ properties.Citation21 With higher gradient field strength, the FFP and FFL narrow allowing to assign the SPION’s signal to a smaller region in space and ultimately to enhance the spatial resolution.
Scaling up MPI
To date, most bore diameters are designed to accommodate mouse- and rat-sized animals. As MPI is intended to be used in a clinical environment, its development is closely connected to human physiology and medical ambitions. To mention just one of the requirements for its clinical use, the scanned volume has to have a clinically relevant size, eg, the volume of the human heart. A whole-body MPI system for high speed imaging is currently being developed by Philips,Citation60 aiming at a gradient amplitude of 2 T/m and a field of view with a diameter of 200 mm.Citation61
Safety considerations
Enlarging the scanning volume is mainly limited by the physiological effects of time-varying magnetic fields. The achievable size of the covered imaging area is kept relatively small due to two phenomena well known to scientists who work in the field of MRI. These phenomena are the peripheral nerve stimulation (PNS) and tissue heating with the specific absorption rate as a measure for the rate at which energy is absorbed by the human body when exposed to an electromagnetic field. The occurrence of both would impair patients’ welfare.Citation62–Citation64 But the experience gained over the last decades with MRI security and also instrument heating could and should be of good use for the safety of MPI during its transition from preclinical into clinical use.Citation65
The frequencies applied in most present MPI systems are in the range of 10 kHz to 100 kHz, and their peak amplitudes are around 10 mT to 100 mT. As the human body is conductive, the applied time-varying magnetic fields induce eddy currents, which may lead to the aforementioned PNS.Citation64,Citation66 A conclusion that can be drawn from current studies on PNS is that the amplitude of the drive field in a clinical MPI system will have to be clearly below 10 mT.Citation63 This is much lower than on preclinical systems that use amplitudes up to twice as high.Citation67,Citation68 Tissue heating seems to become an issue above the frequency range of about 25 kHz.Citation66,Citation69
The reduction of the drive field amplitude leads to a reduction of the volume that can be quickly encoded.Citation60 Nonetheless, an additional extension of the imaging area within the aforementioned limits seems achievable through the introduction of additional fields. These specific fields, introduced as focus fields, enlarge the relatively small “core scanning volume” to achieve a bigger in size “clinical volume”, ie, total FOV.Citation70 As one result of these considerations, the drive frequencies for a clinical system have been shifted from the traditional 25 kHz range to 150 kHz, increasing the available drive field amplitude.Citation71 While first studies have been conducted,Citation65,Citation66,Citation69 further ones are needed to fully understand the complex interaction of multiple magnetic fields and their effect on the human body.
Safety considerations concerning the biocompatibility of SPIONs are of equal importance. Nanoparticle-derived adverse health effects have always been an issue in the field of nanoparticle science. In order to use MPI in a clinical environment on humans, the safety of the utilized tracers has to be ensured. Therfore, extensive studies on the pharmacokinetics and the influence of potential MPI tracers on human cells and organs have to be conducted.
Proposed mechanisms for the induction of the cytotoxicity of uncoated iron oxide nanoparticles are the release of (toxic) irons, surface catalyzed reactions that lead to cytotoxic products or stress and stimuli caused by the particles’ presence.Citation72 Recent in vitro studies investigating the uptake and cytotoxicity of dextran-coated MPI tracers on human adult stem cells indicate a high stability and biocompatibility of dextran-coated SPIONs.Citation73
Medical applications
As already mentioned in the “Introduction”, MPI has the advantage of three-dimensional imaging with a very high temporal resolution, a high sensitivity and spatial resolution, and the absence of hazardous ionizing radiation. Furthermore, MPI operates without contrast agents containing nephrotoxic (iodine based) or serious systemic side effects, ie, nephrogenic systemic fibrosis (gadolinium based) in patients with renal insufficiency.Citation74 The used SPIONs are eliminated via the iron metabolism. Thus, only a significant overdose of SPIONs will lead to toxic effects in terms of iron overload. In fact, SPIONs are even utilized for treatment of iron deficiency anemia in adult patients with chronic kidney disease.Citation75 It could already be demonstrated that MPI is possible with clinically approved doses of SPIONs.Citation7 Also, SPIONs can be tailored to achieve a longer blood circulation, even loading of erythrocytes for very long circulating SPIONs (so called blood pool tracers) is possible.Citation25,Citation76 This may allow repeated examinations without the need of tracer reapplication.
MPI is a truly quantitative method, as the strength of the MPI signal is proportional to the SPIONs’ concentration, allowing quantification of tissue perfusion and stenosis.Citation77 Especially for vascular and perfusion imaging, the missing background signal of the body is an advantage. The FOV of MPI can be tailored to the specific need, ie, from a larger FOV for a general survey to a smaller FOV with a substantially higher spatial resolution for evaluation of pathologies in detail.
Considering these facts, MPI seems suitable for a wide range of applications, ie, vascular, gastrointestinal, pulmonary imaging, and the wide range of cellular and targeted imaging or imaging of the RES.Citation9 Currently, it seems that cardiovascular imaging, cellular and targeted imaging, and imaging of the RES are the focus of research regarding potential applications.Citation4,Citation5
Vascular imaging
Current methods in vascular imaging are conventional x-ray angiography and digital subtraction angiography (DSA) and computed tomography and magnet resonance angiography (CTA and MRA). For diagnostic purposes, MRA and especially CTA are the gold standard; for interventional purposes, DSA is considered the method of choice. Unfavorably, conventional DSA and CTA burden patients and physicians with a considerable amount of ionizing radiation. Furthermore, DSA provides only two-dimensional images and does not allow exact quantification of pathologies like stenosis. CTA and MRA overestimate vascular stenosisCitation78,Citation79 and are limited in time resolved imaging, MRA due to limitations in temporal resolution and CTA due to restrictions owing to radiation protection. MPI can overcome most of these limitations. Visualization of the vasculature and quantitative evaluation of pathologies like stenosis are surely the first step.Citation77 But MPI could, furthermore, provide information about tissue perfusion and functional parameters to assess myocardial viability and function, for example. The advantage of MPI compared with MRI is that the whole organ could be assessed three-dimensionally with such a high temporal resolution that breath hold sequences would not be necessary anymore or at least be substantially shorter. Furthermore, all the information could be collected in one go as a “one stop shop.” First in vivo experiments could demonstrate the high temporal resolution by visualizing the beating of a mouse heart in real-time using Resovist.Citation7 Of course, in MPI morphological information can only be obtained of contrasted structures.Citation68 Here, MRI provides more information; that is why MPI/MRI hybrid systems are already being investigated to combine the advantages of both systems.Citation68,Citation80
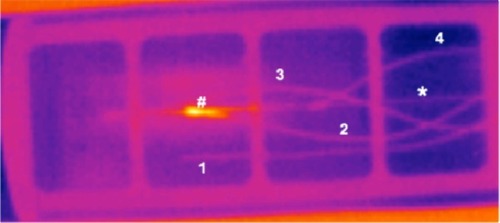
With an open or single-sided scanner geometry (see section “Scanner geometries and performances”), MPI allows supreme patient access. In combination with the very good SNR, temporal and spatial resolution, a tracer with a good safety profile, and the absence of ionizing radiation, MPI is an interesting option for vascular interventions as well. But as MPI is only visualizing the SPIONs, devices for vascular interventions need to be labeled for use in MPI. This can be achieved by loading the lumen with SPIONs, applying a SPION-based coating to the devices or even integrating SPIONs into the structure of the catheters.Citation24 Furthermore, the labeling of the devices has to be discernible from the intravascular contrast, which can be achieved by using different concentrations or even different SPIONs. Finally, the safety of the interventional devices has to be considered.Citation81,Citation82 Besides the necessity of biocompatibility, potential heating of instruments in the oscillating magnetic field has to be kept in mind as in MRI. In MPI, steel-based instruments seem to be prone to substantial heating effects (see ), at least in vitro.Citation82
Figure 9 Thermal image of an interventional device seconds after removing it from an MPI scanner.
Notes: Phantom (pink frame) allows exact positioning of instruments (*) and temperature sensors (1 to 4) inside the bore of the MPI scanner. Reference sensor has no contact to instruments. The other sensors measured heating at the FFP (2) and also distal (3) and proximal (4) of the FFP. Hotspot of punctual heating is shown at the FFP (#) in an instrument with ferromagnetic characteristics.Citation82
Abbreviations: MPI, magnetic particle imaging; FFP, field-free point.
These application scenarios are most often described for use in diagnostic, interventional cardiovascular, and peripheral vascular radiology. However, cerebrovascular applications seem to be interesting as well. Perfusion imaging in diagnosis of ischemia, in particular, is still suboptimal and requires CT-perfusion scans with high doses of ionizing radiation. Furthermore, patients with intracranial hemorrhage, especially subarachnoid hemorrhage after rupture of an intracranial aneurysm, often develop spasms of the intracranial arteries, which can lead to serious brain infarction. The diagnosis of intracranial arterial spasms using Doppler ultrasound is difficult. DSA is still gold standard but always requires an interventional procedure and, as well as the alternative perfusion CT, is socialized with high and repeated doses of ionizing radiation. Here, perfusion MPI could be a valuable addition. In a scenario with a single-sided scanner geometry that is integrated in the headboard of the bed and blood pool tracers, it might even be possible to monitor the brain perfusion permanently. Of course, this could work also for monitoring the reperfusion of brain tissue during thrombolysis therapy of acute brain infarction.
Cellular and targeted imaging
Although the use of SPIONs in clinical MRI has declined in the last few years, there are many promising SPION-based approaches to clinical imaging, especially regarding the wide range of targeted imaging. SPIONs are generally collected by the body’s RES. Larger SPIONs like Resovist are cleared very fast from the bloodstream by the RES in liver and spleen, which can be used for the detection of hepatocellular carcinoma, for example.Citation83 Smaller SPIONs circulate longer, extravasate, and are then collected by the cells of the RES to accumulate in lymph nodes. This principle was used in lymph node staging of pelvic cancer.Citation84,Citation85 Furthermore, the affinity of SPIONs toward cells of the RES has been used for inflammation imaging, eg, in arthritis or even vulnerable atherosclerotic plaques.Citation86–Citation88 SPIONs were used also for tumor imaging, utilizing the enhanced permeability and retention effect of the tumor vessels or the defective blood–brain barrier in terms of passive targeting.Citation89 All these applications have in common that they rely on general characteristics of the SPIONs and “the underlying specific pathology”. In the setting of MPI, the use of SPIONs in combination with a handheld MPI probe, similar to ultrasound, for the detection of the sentinel lymph node in breast cancer diagnostics has been already proposed as a clinical application.Citation90
For more specific applications, SPIONs can be modified by different coatings and especially by adding ligands, such as antibodies, peptides, polysaccharides, and other molecules for active targeting, that is, the SPIONs only bind to specific cells. Another possible approach is to label specific cells with SPIONs ex vivo and monitor their behavior in vivo, eg, their migration, by visualizing the intracellular SPIONs (cellular imaging). Both approaches are already being extensively researched for many disease entities. The possibilities seem endless, the detection of tumors as the most prevalent application, but others such as detection and monitoring of inflammation, cardiovascular disease, apoptosis, transplant rejection reactions, or neurodegenerative disorders are also being investigated. Recently Ittrich et al summarized these applications.Citation91 Most of these approaches are, in principle, designed for SPION-based in vivo MR imaging. However, no targeted or cellular imaging approach has reached clinical routine yet. The main reason is most certainly the limited sensitivity of MRI.Citation92
Sensitivity for SPION detection in MPI exceeds that in MRI, as MPI visualizes SPIONs directly by detecting the particle signal, which is 22×106 times stronger than the proton’s magnetization in MRI.Citation93 Saritas et al describe a detection limit for their current MPI scanner system of about 500 stem cells when labelled with Resovist; due to further development in scanner and SPION technology they see “potential for orders-of-magnitude improvement.”Citation93 Bulte et al specify the detection limit of Resovist labeled mesenchymal stem cells below 100 cells.Citation94 Again, due to further development especially in the field of MPI-dedicated SPIONs, this number will improve. These data show the potential of MPI for cellular and targeted imaging in vivo due to its sensitivity. When very high temporal resolution is not necessary, as most often is the case in targeted/cellular imaging, it can be traded in for further enhancing MPI sensitivity. Nevertheless, MPI is not as sensitive as nuclear imaging. However, the main advantages of MPI are that there is no ionizing radiation involved and that the shelf life of SPIONs is by orders of magnitudes longer than that of radionuclides, which will improve handling, work flow, and lower costs. Moreover, production costs of SPIONs are less than those of radionuclides in the first place.
In terms of cellular imaging, the internalization of Resovist in red blood cells for a substantially prolonged blood circulation time and MPI contrast could already be demonstrated as an example for in vivo cellular imaging in MPI.Citation25 The use of SPIONs to label head and neck squamous cell carcinoma cells for visualization of their migration has recently been proposed.Citation95 First experiments toward using MPI as a tool for theranostics have been published;Citation96,Citation97 functionalized MPI tracers have already been described as well.Citation49,Citation98
Until now, most of these scenarios still need to be assessed in vivo. A big step toward standardized in vivo MPI research has been made by the development of the first commercially available MPI scanner for small animals. Currently, two of those systems are being installed at German Universities in Hamburg and Berlin.Citation99 Many working groups are engaged in development of dedicated SPIONs for MPI with promising results, another very important step to improve MPI performance on the one hand and enable standardized and reproducible research on the other hand.
Conclusion and outlook
MPI is a potent new imaging modality with a unique combination of capabilities that has the potential to enrich today’s arsenal of imaging options in modern medicine. The development is pointed toward the ultimate goal of a clinical human-sized scanner system.
The next step on this path is the implementation of a first clinical demonstrator. Once the technical challenges of upscaling will have been overcome, the first clinical demonstrator will help to actually evaluate the performance of MPI at a whole-body scale. Then, different acquisition schemes will have to be studied on a clinically relevant scenario, taking into account the different trade-offs to be made, particularly regarding PNS and energy absorption (specific absorption rate [SAR]).Citation63,Citation71,Citation100
As realized right from the beginning of the story of MPI, it is the SPION that will be the crucial part for the ultimate success of MPI as a method. With new biocompatible particles that are optimized for use in MPI, the system’s performance will increase dramatically, especially in terms of spatial resolution and sensitivity. With that in mind and besides clinical applications like vascular imaging and interventions, MPI has the potential to pick up SPION-based concepts for cellular, targeted imaging, and theranostics and to enable their translation into clinical imaging.
With dedicated MPI tracers on the way, without any unsolvable technical challenges ahead, and with a growing knowledge about safety issues, there is no reason why the transition from current experimental systems to clinically suitable scanners and ultimately to human MPI should not succeed.
Acknowledgments
The authors would like to thank Dr rer. nat. Jürgen Rahmer and Philips for the data acquisition and reconstruction of , , , and 10.
The research leading to these results has received funding from the Federal Ministry of Education and Research, Germany (BMBF), 13N11090, the European Union and the State Schleswig-Holstein (Programme for the Future – Economy: 122-10-004), and the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 604448.
Disclosure
The authors report no conflicts of interest in this work.
References
- GleichBWeizeneckerJTomographic imaging using the nonlinear response of magnetic particlesNature200543570461214121715988521
- KnoppTBuzugTMMagnetic Particle Imaging: An Introduction to Imaging Principles and Scanner InstrumentationHeidelberg, GermanySpringer2012
- GoodwillPWTamrazianACroftLRFerrohydrodynamic relaxometry for magnetic particle imagingAppl Phys Lett201198262502
- BorgertJSchmidtJDSchmaleIFundamentals and applications of magnetic particle imagingJ Cardiovasc Comput Tomogr20126314915322682260
- GoodwillPWSaritasEUCroftLRX-Space MPI: Magnetic Nanoparticles for Safe Medical ImagingAdv Mater201224283870387722988557
- GoodwillPWLuKZhengBConollySMAn x-space magnetic particle imaging scannerRev Sci Instrum201283303370822462930
- WeizeneckerJGleichBRahmerJDahnkeHBorgertJThree-dimensional real-time in vivo magnetic particle imagingPhys Med Biol2009545L1L1019204385
- KnoppTSattelTFBiedererSModel-based reconstruction for magnetic particle imagingIEEE Trans Med Imaging2010291121819435678
- GleichBPrinciples and Applications of Magnetic Particle ImagingWiesbaden, GermanySpringer Vieweg2013
- SattelTFKnoppTBiedererSSingle-sided device for magnetic particle imagingJ Phys Appl Phys2009422022001
- KaethnerCAhlborgMKnoppTSattelTFBuzugTMEfficient gradient field generation providing a multi-dimensional arbitrary shifted field-free point for magnetic particle imagingJ Appl Phys2014115404491010449105
- VogelPRückertMAKlauerPKullmannWHJakobPMBehrVCTraveling wave magnetic particle imagingIEEE Trans Med Imaging201433240040724132006
- WeizeneckerJGleichBBorgertJMagnetic particle imaging using a field free lineJ Phys Appl Phys20084110105009
- KnoppTSattelTFBiedererSBuzugTMField-free line formation in a magnetic fieldJ Phys Math Theor2010431012002
- ErbeMWeberMSattelTFBuzugTMExperimental validation of an assembly of optimized curved rectangular coils for the use in dynamic field free line magnetic particle imagingCurr Med Imaging Rev2013928995
- KnoppTBiedererSSattelTTrajectory analysis for magnetic particle imagingPhys Med Biol200954238539719098358
- BuzugTMBringoutGErbeMMagnetic particle imaging: introduction to imaging and hardware realizationZ Med Phys201222432333422909418
- GrüttnerMKnoppTFrankeJOn the formulation of the image reconstruction problem in magnetic particle imagingBiomed Tech (Berl)201358658359124088606
- KnoppTRahmerJSattelTFWeighted iterative reconstruction for magnetic particle imagingPhys Med Biol20105561577158920164532
- GoodwillPWConollySMMultidimensional x-space magnetic particle imagingIEEE Trans Med Imaging20113091581159021402508
- RahmerJWeizeneckerJGleichBBorgertJSignal encoding in magnetic particle imaging: properties of the system functionBMC Med Imaging20099419335923
- RahmerJWeizeneckerJGleichBBorgertJAnalysis of a 3-D system function measured for magnetic particle imagingIEEE Trans Med Imaging20123161289129922361663
- RahmerJGleichBWeizeneckerJBorgertJ3D real-time magnetic particle imaging of cerebral blood flow in living miceProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)May 1–7 2010Stockholm, Sweden714
- HaegeleJRahmerJGleichBMagnetic particle imaging: visualization of instruments for cardiovascular interventionRadiology2012265393393822996744
- RahmerJAntonelliASfaraCNanoparticle encapsulation in red blood cells enables blood-pool magnetic particle imaging hours after injectionPhys Med Biol201358123965397723685712
- GoodwillPScottGStangPLeeGCMorrisDConollySDirect imaging of spios in mice using magnetic particle imaging: instrument construction and 3D imagingProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)April 18–24, 2009Honolulu, Hawaii, USA596
- BiedererSKnoppTSattelTFMagnetization response spectroscopy of superparamagnetic nanoparticles for magnetic particle imagingJ Phys D: Appl Phys200942205007
- WawrzikTSchillingMLudwigFDebye-based frequency-domain magnetization model for magnetic nanoparticles and its application to viscosity dependent MPS measurementsPresented at: The 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany
- RauwerdinkAMHansenEWWeaverJBNanoparticle temperature estimation in combined ac and dc magnetic fieldsPhys Med Biol20095419L51L5519741275
- WawrzikTLudwigFSchillingMMagnetic particle imaging: exploring particle mobilitySpringer Proc Phys20121402125
- GraeserMKnoppTGrüttnerMSattelTFBuzugTMAnalog receive signal processing for magnetic particle imagingMed Phys201340404230323556916
- ReevesDBWeaverJBMagnetic nanoparticle sensing: decoupling the magnetization from the excitation fieldJ Phys Appl Phys201447045002
- RuhlandBBaumannKKnoppTMagnetic Particle Imaging durch Superparamagnetische Nanopartikel zur Sentinellymphknotendetektion beim MammakarzinomGeburtshilfe Frauenheilkd20096909A096 German
- DhavalikarRRinaldiCOn the effect of finite magnetic relaxation on the magnetic particle imaging performance of magnetic nanoparticlesJ Appl Phys20141157074308
- GleichBWeizeneckerJBorgertJExperimental results on fast 2D-encoded magnetic particle imagingPhys Med Biol2008536N81N8418367783
- DeisslerRJWuYMartensMADependence of Brownian and Néel relaxation times on magnetic field strengthMed Phys201441101230124387522
- WeizeneckerJGleichBRahmerJBorgertJMicro-magnetic simulation study on the magnetic particle imaging performance of anisotropic mono-domain particlesPhys Med Biol201257227317732723079678
- RahmerJRahnHHenrichFOdenbachSGleichBBorgertJImaging of iron oxide nanoparticles embedded in polyurethanePresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- AramiHKrishnanKMIntracellular performance of tailored nanoparticle tracers in magnetic particle imagingJ Appl Phys20141151717B306
- PankhurstQAConnollyJJonesSKDobsonJApplications of magnetic nanoparticles in biomedicineJ Phys Appl Phys20033613R167
- PankhurstQAThanhNTKJonesSKDobsonJProgress in applications of magnetic nanoparticles in biomedicineJ Phys Appl Phys20094222224001
- AlmeidaJPChenALFosterADrezekRIn vivo biodistribution of nanoparticlesNanomedicine (Lond)20116581583521793674
- GourtsoyiannisNCClinical MRI of the AbdomenBerlinSpringer-Verlag2011
- Lüdtke-BuzugKHaegeleJBiedererSComparison of commercial iron oxide-based MRI contrast agents with synthesized high-performance MPI tracersBiomed Tech (Berl)201358652753323787462
- EberbeckDWiekhorstFWagnerSTrahmsLHow the size distribution of magnetic nanoparticles determines their magnetic particle imaging performanceAppl Phys Lett20119818182502
- KratzHEberbeckDWagnerSTaupitzMSchnorrJSynthetic routes to magnetic nanoparticles for MPIBiomed Tech (Berl)201358650951523950566
- Lüdtke-BuzugKFrom synthesis to clinical Application. Magnetic nanoparticlesChem Unserer Zeit20124613239
- KhandharAPFergusonRMAramiHKrishnanKMMonodisperse magnetite nanoparticle tracers for in vivo magnetic particle imagingBiomaterials201334153837384523434348
- StarmansLWBurdinskiDHaexNPIron oxide nanoparticle-micelles (ION-micelles) for sensitive (molecular) magnetic particle imaging and magnetic resonance imagingPLoS One201382e5733523437371
- LudwigFEberbeckDLöwaNCharacterization of magnetic nanoparticle systems with respect to their magnetic particle imaging performanceBiomed Tech (Berl)201358653554523751379
- EberbeckDDennisCLHulsNFKryckaKLGrüttnerCWestphalFMulticore magnetic nanoparticles for magnetic particle imagingIEEE Trans Magn2013491269274
- YoshidaTOthmanNBEnpukuKCharacterization of magnetically fractionated magnetic nanoparticles for magnetic particle imagingJ Appl Phys201311417173908
- DutzSBuskeNLöwaNEberbeckDTrahmsLTracers for magnetic particle imaging consisting of agglomerated single coresPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany138
- GehrkeNBrielALudwigFRemmerHWawrzikTWellertSNew perspectives for MPI: a toolbox for tracer researchBuzugTMBorgertJMagnetic Particle Imaging140BerlinSpringer-Verlag201299103
- KraupnerAHeinkeDUebeRBacterial magnetosomes as a new type of biogenic MPI tracersPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany141142
- IdeARoohiFPietschHSchuetzGSynthetic approach for iron oxide nanoparticles suitable as tracer for magnetic particle imagingPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany40
- KaethnerCAhlborgMGräfeKBringoutGSattelTBuzugTMAsymmetric scanner design for interventional scenarios in magnetic particle imagingIEEE Trans Magn2014
- BrukerBruker Announces the World’s First Preclinical Magnetic Particle Imaging (MPI) System2013919Billerica, MABruker2013 Available from: http://www.bruker.com/news-records/single-view/article/bruker-announces-the-worlds-first-preclinical-magnetic-particle-imaging-mpi-system.htmlAccessed July 6, 2014
- KnoppTBiedererSSattelTFErbeMBuzugTMPrediction of the spatial resolution of magnetic particle imaging using the modulation transfer function of the imaging processIEEE Trans Med Imaging20113061284129221317081
- BorgertJSchmidtJDSchmaleIPerspectives on clinical magnetic particle imagingBiomed Tech (Berl)201358655155624025718
- BontusCGleichBDavidBMendeOBorgertJConcept of a generator for the selection- and focus field of a clinical MPI scannerPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany67
- ReillyJPMagnetic field excitation of peripheral nerves and the heart: a comparison of thresholdsMed Biol Eng Comput19912965715791813751
- SaritasEUGoodwillPWZhangGZConollySMMagnetostimulation limits in magnetic particle imagingIEEE Trans Med Imaging20133291600161023649181
- DoesselOBohnertJSafety considerations for magnetic fields of 10 mT to 100 mT amplitude in the frequency range of 10 kHz to 100 kHz for magnetic particle imagingBiomed Tech (Berl)201358661162124176960
- SchmaleIGleichBRahmerJBontusCSchmidtJBorgertJMPI Safety in the View of MRI Safety NormsPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany15
- DoesselOBohnertJConsiderations on safety limits for magnetic fields used in magnetic particle imagingPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany14
- GleichBWeizeneckerJTimmingerHFast MPI demonstrator with enlarged field of viewProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)May 1–7, 2010Stockholm, Sweden218
- FrankeJHeinenUMatthiesLFirst hybrid MPI-MRI imaging system as integrated design for mice and rats: description of the instrumentation setupPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- SaritasEUGoodwillPWZhangGZYuWConollySMSafety limits for human-size magnetic particle imaging systemsSpringer Proc Phys2012140325330
- SchmaleIRahmerJGleichBFirst phantom and in vivo MPI images with an extended field of viewProceedings of the SPIE Symposium on Medical Imaging: Biomedical Applications in Molecular, Structural, and Functional ImagingWeaverJBMolthenRC20117965796510
- RahmerJBorgertJGleichBStrategies for fast MPI within the limits determined by nerve stimulationPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany16
- BrunnerTJWickPManserPIn vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubilityEnviron Sci Technol200640144374438116903273
- SchneiderDLüdtke-BuzugKBiomaterials for regenerative medicine: Cytotoxicity of superparamagnetic iron oxide nanoparticles in stem cellsSpringer Proc Phys2012140117122
- WangYAlkasabTKNarinOIncidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelinesRadiology2011260110511121586680
- Ferumoxytol (Feraheme) – a new parenteral iron formulationMed Lett Drugs Ther201052133423
- HaegeleJDuschkaRLGraeserMMagnetic particle imaging: kinetics of the intravascular signal in vivoInt J Nanomedicine201494203420925214784
- HaegeleJRahmerJDuschkaRMagnetic Particle Imaging (MPI): Visualization and Quantification of Vascular Stenosis PhantomsProceedings of the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany5758
- NonentMBen SalemDSerfatyJMOverestimation of moderate carotid stenosis assessed by both Doppler US and contrast enhanced 3D-MR angiography in the CARMEDAS studyJ Neuroradiol201138314815520728218
- BuerkeBPueskenMWittkampGBone subtraction CTA for transcranial arteries: intra-individual comparison with standard CTA without bone subtraction and TOF-MRAClin Radiol201065644044620451010
- VogelPLotherSRückertMMRI meets MPI: A bimodal MPI-MRI tomographIEEE Trans Med Imaging201433101954195925291350
- HaegeleJBiedererSWojtczykHToward cardiovascular interventions guided by magnetic particle imaging: first instrument characterizationMagn Reson Med20136961761176722829518
- DuschkaRLWojtczykHPanagiotopoulosNSafety measurements for heating of instruments for cardiovascular interventions in magnetic particle imaging (MPI) – first experiencesJ Healthc Eng201451799324691388
- ReimerPBalzerTFerucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applicationsEur Radiol20031361266127612764641
- HeesakkersRAHovelsAMJagerGJMRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort studyLancet Oncol20089985085618708295
- HeesakkersRAJagerGJHovelsAMProstate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imagingRadiology2009251240841419401573
- SigovanMBousselLSulaimanARapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal modelRadiology2009252240140919703881
- RuehmSGCorotCVogtPKolbSDebatinJFMagnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbitsCirculation2001103341542211157694
- LefevreSRuimyDJehlFSeptic arthritis: monitoring with USPIO-enhanced macrophage MR imagingRadiology2011258372272821339348
- IyerAKKhaledGFangJMaedaHExploiting the enhanced permeability and retention effect for tumor targetingDrug Discov Today20061117–1881281816935749
- FinasDBaumannKSydowLLymphatic tissue and superparamagnetic nanoparticles – magnetic particle imaging for detection and distribution in a breast cancer modelBiomed Tech (Berl) Epub201397
- IttrichHPeldschusKRaabeNKaulMAdamGSuperparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapyRofo2013185121149116624008761
- DasslerKRoohiFLohrkeJCurrent limitations of molecular magnetic resonance imaging for tumors as evaluated with high-relaxivity CD105-specific iron oxide nanoparticlesInvest Radiol201247738339122659596
- SaritasEUGoodwillPWCroftLRMagnetic particle imaging (MPI) for NMR and MRI researchersJ Magn Reson201322911612623305842
- BulteJWWalczakPBernhardSDeveloping cellular MPI: initial experiencePresented at: the 1st International Workshop on Magnetic Particle Imaging (IWMPI)March 18–19, 2010Lübeck, Germany201205
- PriesRLindemannALuedtke-BuzugKWollenbergBNovel developed superparamagnetic dextran coated iron oxide nanoparticles (SPION) as a potential tool for HNSCC tumor cell detection and its influence on the biological propertiesPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany159
- BleulRLöwaNThiermannRContinously manufactured magnetic polymerosomes as potential theranostic tools in nanomedicinePresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany149150
- BuskeNScheleroNDähneLKrumbeinIReichenbachJRDutzSFerrofluids of modified Ultra Small Magnetic Particles for application in TheranosticsPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany140
- AramiHKrishnanKMHighly stable amine functionalized iron oxide nanoparticles designed for magnetic particle imaging (MPI)IEEE Trans Med Imaging201349735003503
- Deutsche ForschungsgemeinschaftInformation für die Wissenschaft Nr. 65: Großgeräteinitiative 2012: Magnetic Particle Imaging (MPI)BonnDeutsche Forschungsgemeinschaft2012 Available from: http://dfg.de/foerderung/info_wissenschaft/archiv/2011/info_wissenschaft_11_65/index.htmlAccessed Jun 21, 2014
- SchmaleIGleichBSchmidtJHuman PNS and SAR study in the frequency range from 24 to 162 kHzPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- IshiharaYHonmaTNoharaSItoYEvaluation of magnetic nanoparticle samples made from biocompatible ferucarbotran by time-correlation magnetic particle imaging reconstruction methodBMC Med Imaging2013131523734917
- GoodwillPWCroftLRKonkleJJA 7 T/M 3D X-space MPI mouse and rat scannerPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- GoodwillPWScottGCStangPPConollySMNarrowband magnetic particle imagingIEEE Trans Med Imaging20092881231123719211340
- KonkleJJGoodwillPWSaritasEUZhengBLuKConollySMTwenty-fold acceleration of 3D projection reconstruction MPIBiomed Tech (Berl)201358656557623940058
- LudwigFWawrzikTYoshidaTOptimization of magnetic nanoparticles for magnetic particle imagingIEEE Trans Magn2012481137803783
- Resovist [package insert]Leverkusen, GermanyBaryer AG2007
- EberbeckDWiekhorstFWagnerSTrahmsLHow the size distribution of magnetic nanoparticles determines their magnetic particle imaging performanceAppl Phys Lett20119818182502
- LudwigFEberbeckDLowaNCharacterization of magnetic nanoparticle systems with respect to their magnetic particle imaging performanceBiomed Tech (Berl)201358653554523751379
- HaegeleJSattelTErbeMMagnetic particle imaging (MPI)Rofo2012184542042622198836
- WeaverJBRauwerdinkAMTremblyBSSullivanCRImaging magnetic nanoparticles using the signal’s frequency spectrumProceedings of the SPIE Medical Imaging 2008February 16–21, 2008San Diego, California, USA69160Y
- HongHLimJChoiCJShinSWKrauseHJMagnetic particle imaging with a planar frequency mixing magnetic detection scannerRev Sci Instrum201485101370524517773
- BrukerBruker announces first Customer Installation of its Preclinical Magnetic Particle Imaging (MPI) Scanner at the University Medical Center Hamburg, Germany Available from: http://www.bruker.com/de/news-records/single-view/article/bruker-announces-first-customer-installation-of-its-preclinical-magnetic-particle-imaging-mpi-scan.htmlAccessed January 19, 2015
- RahmerJGleichBWeizeneckerJBorgertJReal-time volumetric in vivo magnetic particle imaging of cerebral perfusionWorld Molecular Imaging Congress (WMIC)September 23–26, 2009Montreal, Canada Abstract 0529
- RahmerJGleichBBontusCRapid 3D in vivo magnetic particle imaging with a large field of viewProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)May 7–13, 2001Montreal, Canada3285
- RahmerJGleichBBontusCResults on rapid 3D magnetic particle imaging with a large field of viewProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)May 7–13, 2001Montreal, Canada629
- RahmerJGleichBSchmidtJContinuous focus field variation for extending the imaging range in 3D MPISpringer Proc Phys2012140255259
- RahmerJGleichBWeizeneckerJFast continuous motion of the field of view in magnetic particle imagingPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- NothnagelNDSanchez-GonzalezJHalkolaARahmerJMeasurement of system functions with extended field-of-viewPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany74
- WawrzikTLudwigFSchillingMTwo-dimensional magnetic particle imagingBuzugTMBorgertJKnoppTMagnetic Nanoparticles: Particle Science, Imaging Technology, and Clinical Applications1SingaporeWorld Scientific Publishing Company2010100105
- WawrzikTLudwigFSchillingMAssembly for One-Dimensional Magnetic Particle ImagingProceedings of the International Federation for Medical and Biological EngineeringSeptember 7–12, 2009Munich, Germany898900
- WawrzikTKuhlmannCLudwigFSchillingMScanner setup and reconstruction for three-dimensional magnetic particle imagingProceedings of the SPIE Symposium on Medical Imaging: Biomedical Applications in Molecular, Structural, and Functional ImagingFebruary 9–14, 2013Lake Buena Vista, Florida, USA86721B-86721B-86728
- WawrzikTLudwigFSchillingMThree-dimensional scanner for magnetic particle imagingBiomed Tech (Berl)201156557563
- SchillingMLudwigFKuhlmannCWawrzikTMagnetic particle imaging scanner with 10-kHz drive-field frequencyBiomed Tech (Berl)201358655756323828410
- GoodwillPConollySDirect imaging of SPIOs in mice using magnetic particle imaging: instrument construction and 3D imagingProceedings of the World Molecular Imaging Congress (WMIC)September 23–26, 2009Montreal, Canada0222
- GoodwillPCroftLRKonkleJJThird generation x-space MPI mouse and rat scannerSpringer Proc Phys2012140261265
- LuKGoodmanJEConollySExperimental demonstration of multichannel magnetic particle imaging for improved resolutionPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany8283
- GoodwillPKonkleJZhengBConollySProjection X-space MPI mouse scannerSpringer Proc Phys2012140267271
- GoodwillPWKonkleJJZhengBSaritasEUConollySMProjection x-space magnetic particle imagingIEEE Trans Med Imaging20123151076108522552332
- ZhengBVazinTYangWQuantitative stem cell imaging with magnetic particle imagingPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- ZhengBVazinTGoodwillPSchafferDVConollySIn vivo MPI neural cell monitoring in the rat brainPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany61
- KonkleJGoodwillPCarrasco-ZevallosOMConollySExperimental 3D x-space magnetic particle imaging using projection reconstructionSpringer Proc Phys2012140243247
- KonkleJJGoodwillPWCarrasco-ZevallosOMConollySMProjection reconstruction magnetic particle imagingIEEE Trans Med Imaging201332233834723193308
- BenteKWeberMGraeserMTwo dimensional magnetic particle imaging with a dynamic field free line scannerPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany66
- WeberMErbeMBenteKSattelTFBuzugTMPower loss optimized field free line generation for magnetic particle imagingPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA
- WeberMErbeMBenteKSattelTFBuzugTMScanner Construction for a Dynamic Field Free Line in Magnetic Particle ImagingBiomed Tech (Berl) Epub201397
- WeberMBenteKGräserMTechnical aspects of a two dimensional rotatable field free line imager for magnetic particle imagingPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany99
- BenteKWeberMGraeserMTwo dimensional magnetic particle imaging with a dynamic field free line scannerIEEE Trans Magn2014
- SattelTBiedererSKnoppTSingle-sided coil configuration for magnetic particle imagingProceedings of the World Congress on Medical Physics and Biomedical EngineeringSeptember 7–13, 2009Munich, Germany281284
- SattelTKnoppTBiedererSErbeMLüdtke-BuzugKBuzugTMResolution distribution in single-sided magnetic particle imagingBuzugTMBorgertJKnoppTMagnetic Nanoparticles: Particle Science, Imaging Technology, and Clinical Applications1SingaporeWorld Scientific Publishing Company2010106112
- GräfeKBringoutGGraeserMSattelTBuzugTMSystem matrix recording and phantom measurements with a single-sided MPI scannerPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany9293
- GräfeKBringoutGGraeserMSattelTBuzugTMSystem Matrix Recording and Phantom Measurements with a Single-Sided Magnetic Particle Imaging DeviceIEEE Trans Magn2014
- GräfeKGrüttnerMSattelTFGraeserMBuzugTMSingle-sided magnetic particle imaging: magnetic field and gradientProceedings of the SPIE Symposium on Medical Imaging: Biomedical Applications in Molecular, Structural, and Functional ImagingFebruary 9–14, 2013Lake Buena Vista, Florida, USA867219-867219-867216
- SattelTFErbeMBiedererSSingle-sided magnetic particle imaging device for the sentinel lymph node biopsy scenarioProceedings of the SPIE Symposium on Medical Imaging: Biomedical Applications in Molecular, Structural, and Functional ImagingFebruary 4–9, 2012San Diego, California, USA83170S-83170S-83177
- GräfeKSattelTFLüdtke-BuzugKFinasDBorgertJBuzugTMMagnetic particle imaging for sentinel lymph node biopsy in breast cancerSpringer Proc Phys2012140237241
- IshiharaYKusayamaYResolution improvement of the molecular imaging technique based on magnetic nanoparticlesProceedings of the SPIE Medical ImagingFebruary 7–13, 2009Lake Buena Vista, Florida, USA72584I
- MuraseKHiratsukaSSongRTakeuchiYDevelopment of a system for magnetic particle imaging using neodymium magnets and gradiometerJpn J Appl Phys2014536067001
- MuraseKSongRHiratsukaSMagnetic particle imaging of blood coagulationAppl Phys Lett201410425252409
- KlauerPRückertMAVogelPKullmannWHJakobPMBehrVCMagnetic particle imaging: linear gradient array for imaging with a traveling waveProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)May 7–13, 2011Montreal, Canada3763
- VogelPRückertMAKlauerPKullmannWHJakobPMBehrVCSlicing frequency mixed traveling wave for 3D magnetic particle imagingSpringer Proc Phys2012140231235
- VogelPRückertMAKlauerPKullmannWHJakobPMBehrVC3D magnetic particle imaging with a traveling waveProceedings of the International Society for Magnetic Resonance in Medicine (ISMRM)May 5–11Melbourne, Australia2742
- VogelPRückertMAKullmannWHJakobPMBehrVCSlice scanning mode for traveling wave MPIPresented at: the 3rd International Workshop on Magnetic Particle Imaging (IWMPI)March 23–24, 2013Berkeley, California, USA52
- VogelPRückertMAKlauerPKullmannWHJakobPMBehrVCProjected traveling wave MPIPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany7576
- VogelPRückertMKlauerPKullmannWHJakobPMBehrVCSuperspeed traveling wave MPIPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany7778
- VogelPRückertMAKlauerPKullmannWHJakobPMBehrVCRotating Slice Scanning Mode for Traveling Wave MPIIEEE Trans Magn2014
- VogelPRückertMAKlauerPKullmannWHJakobPMBehrVCSuperspeed traveling wave MPIIEEE Trans Magn2014
- VogelPLotherSRückertMMagnetic particle imaging trifft auf magnetic resonance imaging (MPI meets MRI)Proceedings of the 44 Jahrestagung der Deutschen Gesellschaft für Medizinische PhysikSeptember 18–21, 2013Cologne, Germany131133
- VogelPRückertMAJakobPMBehrVCμMPI – initial experiments with an ultra high resolution MPIIEEE Trans Magn2014
- VogelARückertMJakobPMBehrVCUltra high resolution MPIPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany6869
- FrankeJHeinenUWeberAInitial results of the first commercial preclinical MPI scannerPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany86
- SattelTFWoywodeOWeizeneckerJRahmerJGleichBBorgertJSetup and validation of an MPI signal chain for a drive field frequency of 150 kHzPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany79
- BringoutGAhlborgMGräserMShielded drive coils for a rabbit sized FFL scannerPresented at: the 4th International Workshop on Magnetic Particle Imaging (IWMPI)March 27–29, 2014Berlin, Germany98
