?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hydrotalcite-like compounds are two-dimensional inorganic nanolayers also known as clay minerals or anionic clays or layered double hydroxides/layered hydroxy salts, and have emerged as a single type of material with numerous biomedical applications, such as drug delivery, gene delivery, cosmetics, and biosensing. Inorganic nanolayers are promising materials due to their fascinating properties, such as ease of preparation, ability to intercalate different type of anions (inorganic, organic, biomolecules, and even genes), high thermal stability, delivery of intercalated anions in a sustained manner, high biocompatibility, and easy biodegradation. Inorganic nanolayers have been the focus for researchers over the last decade, resulting in widening application horizons, especially in the field of biomedical science. These nanolayers have been widely applied in drug and gene delivery. They have also been applied in biosensing technology, and most recently in bioimaging science. The suitability of inorganic nanolayers for application in drug delivery, gene delivery, biosensing technology, and bioimaging science makes them ideal materials to be applied for theranostic purposes. In this paper, we review the structure, methods of preparation, and latest advances made by inorganic nanolayers in such biomedical applications as drug delivery, gene delivery, biosensing, and bioimaging.
Introduction
In recent years, inorganic metal nanolayers have been receiving increased attention from scientists of various fields, due to their fascinating properties, such as ease of preparation, ability to intercalate variety of anions like inorganic anions, organic anions, biomolecules, and genes, and tendency to protect intercalated anions from physicochemical degradation and sustained release of intercalated anions. These inorganic nanolayers have a hydrotalcite-like structure. The most common class of these inorganic nanolayers is well known as layered double hydroxides (LDHs), and another type is referred to as layered hydroxide salts (LHS). Several books on this research field have provided valuable insight into their functionality and versatile applications. One popular book by RivesCitation1 has presented a great overview of inorganic nanolayers. In addition, Duan and EvansCitation2 clearly explain the inherent molecular dynamics in play, whereas Carillo and Griego et alCitation3 discussed various applications and methods of preparation. Many reviews on LDHs, such as Del Hoyo,Citation4 Khan et alCitation5 (drug-delivery applications of LDHs), Wang and O’HareCitation6 (synthesis and applications of LDHs), and Rives et alCitation7,Citation8 (drug-delivery applications, drug intercalation of LDHs and sustained release), have been published.
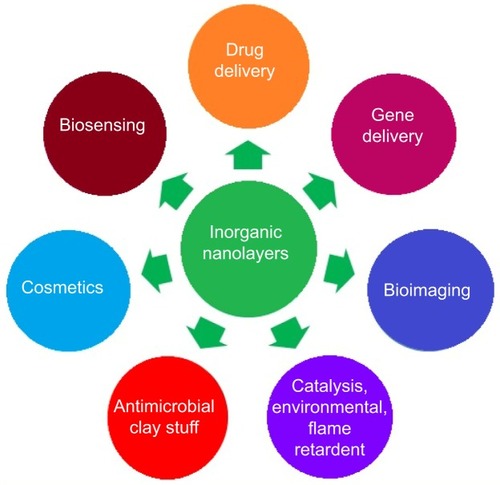
Drug delivery, gene delivery, biosensing science, and bioimaging technology are the key tools of modern-day biomedical sciences. Different materials are used for the application of the aforementioned biomedical tools but there are certain issues related to different materials, such as cytotoxicity, lack of biodegradation, and different types of material being used for individual application.Citation9 Inorganic nanolayers have many advantages for biomedical applications, such as having been proven to possess very high in vitro and in vivo biocompatibility and biodegradability, and a single material can be applied for drug delivery, gene delivery, biosensing, and in bioimaging sciences. shows the different applications of inorganic nanolayers. This review addresses several aspects, such as structure, preparations, various biomedical sustained-release properties, in vitro and in vivo biocompatibilities, and theranostic applications. There have been many reviews regarding inorganic nanolayers, but most of them have focused on one dimension, ie, their drug-delivery applications.
This is a comprehensive review that provides detailed information about the structure, preparation, and most recent advances in biomedical applications, such as drug delivery, gene delivery, biosensing, and bioimaging, of inorganic nanolayers.
Structure
Structure of brucite
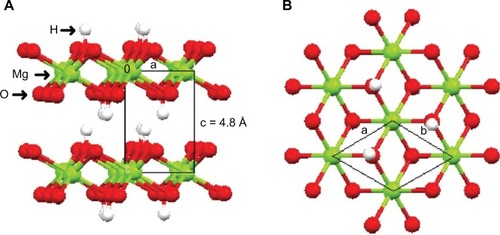
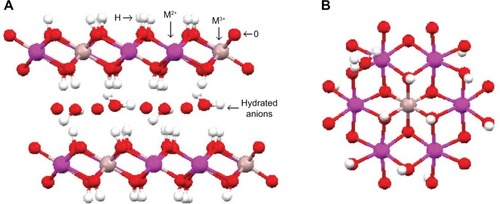
In layered magnesium hydroxide (brucite) [Mg(OH)6], the divalent M2+ ions are octahedrally surrounded by hydroxide ions. These octahedral units of magnesium hydroxides share their edges and form infinite layers in which O–H forms the bond perpendicularly to the layers. In these layers, hydroxyl anions are arranged in a closed-packed manner with triangular symmetry in two-dimensional (2-D) planes. The octahedral holes of the O–H alternate planes are occupied by Mg2+ ions, accounting for the triangular lattice similar to the one occupied by O–H ions, resulting in neutral layer.Citation10–Citation14 The structure of brucite is shown in .
Figure 2 Schematic representation of the brucite structure.
Notes: (A) Side and (B) top view of the layer. Reprinted from Arizaga GG, Satyanarayana KG, Wypych F. Layered hydroxide salts: synthesis, properties and potential applications. Solid State Ionics. 2007;178:1143–1162, Copyright 2007, with permission from Elsevier.Citation11
Structure of layered double hydroxides
Compositional changes can be made in the brucite structure by the isomorphic replacement of divalent cations with trivalent cations (M3+). This results in overall positive charge in the layer and must be counterbalanced by negatively charged anions. In nature, hydrotalcite is a mineral with a similar isomorphic structure. It consists of hydroxycarbonate of magnesium and aluminum, which can be easily crushed to powder. The foliated structure has the formula Mg6Al2 (OH)16CO34H2O.Citation15,Citation16
Substitution of divalent magnesium cations by the trivalent Al3+ results in overall positive charge in the hydro-talcite.Citation15 LDHs are often described as hydrotalcite-like compounds, as both hydrotalcite-like compounds and LDHs have isomorphic substitution of the trivalent cations, with a structure similar to brucite, such as layers with positive charge and charge-neutralizing anions and solvent molecules within the interlayer galleries.Citation2,Citation7,Citation8,Citation17,Citation18 The general formula for LDHs is [M2+1−xM3+x(OH)2]x+ (Am−)x/m·nH2O, where M2+ are divalent cations, such as Mg2+, Zn2+, Ni2+, and Ca2+, M3+ are trivalent cations, ie, Al3+, Fe3+, and Cr3+, and A is a counter anion with negative charge (m).Citation2,Citation8,Citation10,Citation12,Citation18 They have an octa hedral structure, in which metal cations are accommodated in the centers of the edge-sharing octahedral; each cation contains six OH− ions that are pointed toward the corners and form infinite 2-D sheets.Citation1,Citation2,Citation7,Citation8 shows the schematic structure of LDHs with side view and top view.
Figure 3 Schematic representation of the structure of layered double hydroxide.
Notes: (A) Side and (B) top view of the layer. Reprinted from Arizaga GG, Satyanarayana KG, Wypych F. Layered hydroxide salts: synthesis, properties and potential applications. Solid State Ionics. 2007;178:1143–1162, Copyright 2007, with permission from Elsevier.Citation11
Structure of layered hydroxides
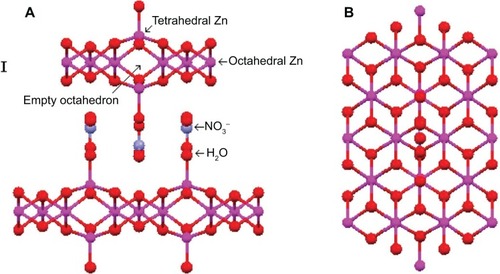
One way of modification of brucite-like structures is by induction of trivalent cations similar to LDHs. Another way of structure modification is by replacing the hydroxyl group (OH−1) with suitable anions or water molecules. These types of compounds are called layered hydroxides or LHS, with the general formula M2+ (OH−)2 × (Am−)x/m·nH2O, where M2+ is the metal cation (eg, Mg2+, Zn2+, Ca2+, Cd2+, Co2+, Ni2+, and Cu2+) and Am− is the counterion. In the layered hydroxide structure, additional anions must be located in the second coordination sphere.Citation2,Citation11,Citation18 shows the structure of LHS with both the side view () and top view ().
Figure 4 Structures of zinc hydroxide nitrate.
Notes: (A) Side view and (B) top view. Reprinted from Arizaga GG, Satyanarayana KG, Wypych F. Layered hydroxide salts: synthesis, properties and potential applications. Solid State Ionics. 2007;178:1143–1162, Copyright 2007, with permission from Elsevier.Citation11
Preparation methods of layered double hydroxides
LDHs can easily be prepared by a number of methods, such as:
coprecipitation (direct method)
ion exchange (indirect method)
reconstruction (memory-effect method)
sol–gel synthesis
preparation of LHS.
Coprecipitation (direct method)
A coprecipitation technique is most commonly applied for direct one-pot synthesis of LDHs with a variety of divalent and trivalent cations and different anions ranging from inorganic anions, such as Cl−, NO3−, and CO3− and a variety of organic molecule dyes and even large biomolecules have been intercalated.Citation1,Citation2,Citation8,Citation10,Citation11 Moreover, coprecipitation methods can be applied for the large-scale production of the material of interest.Citation2
In coprecipitation, both the divalent cations and trivalent cations along with the anions to be intercalated are mixed in aqueous solution, and pH is increased by adding basic solution.Citation2,Citation18 Coprecipitation is often the preferred method of choice over other methods, as most of the anions can easily be intercalated using this method.Citation2 The coprecipitation of cations is ensured by applying a supersaturation condition, which can be easily reached by controlling the pH of the solution.Citation15 Thermal treatment is often performed after coprecipitation to ensure maximum yield and to improve the crystallinity of the sample. Thermal treatment can be done by aging the solution at 25°C–100°C. Alternatively, it can be performed by hydrothermal methods, wherein the sample is subjected to high pressure ranging from 10 to 150 MPa and high temperature in autoclave bombs with duration ranging from a few hours a to few days.Citation2,Citation19,Citation20
Ion exchange (indirect method)
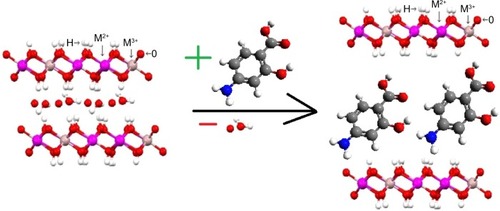
Ion exchange is also commonly used for LDH synthesis, and has been successfully applied for the intercalation of a number of different types of anions.Citation1,Citation2,Citation8,Citation11 It is also known as an indirect method, in which the first LDHs are prepared with host anions, most commonly NO3−, CO32−, and Cl− In the later stages, anions present in the interlayer region are exchanged with the desired anions.Citation1,Citation2 The host–guest exchange mainly depends on the electrostatic forces between positively charged LDH layers and the exchanging anions, and shows the typical ion-exchange process.Citation21
Anions of interest can be intercalated in two possible ways, as described in the equations:
In the first example, the anions to be deintercalated are univalent, such as Cl−, NO3−, or ClO4−. There exists weak electrostatic interaction with layers, and hence these anions can be easily replaced by anions with higher electrostatic interaction with layers.
In the second example, there is strong interaction between the positive layers and anions, as they have higher negative charge, such as CO32− or carboxylate. But these divalent anions are susceptible to acid attack, and can be easily exchanged with univalent anions, such as (Cl−), (NO3−), or (ClO4−).Citation1,Citation2 Different anions have been intercalated by ion-exchange methods, such as the inorganic anions Cl−, NO3−, SO42−, CrO42−, HAsO42−, HPO42−, and CO32−, pharmaceutical agents like para-aminosalicylic acid (PAS), and cetirizine.Citation1,Citation2,Citation22–Citation25
Regeneration of layered double hydroxides by structural memory effect
LDHs can be completely transferred to the respective metal oxide by heating the LDHs at high temperature, a process known as calcination. In this method, LDHs are heated at between 500°C and 600°C. As a consequence, interlayer water molecules, intercalated anions, and hydroxyl groups of LDHs are completely removed, leaving behind mixed metal oxides.Citation23,Citation26–Citation28 It should be noted that preferably LDHs should be heated at about 400°C–550°C; heating at higher temperature will affect the regeneration of LDHs.Citation2,Citation18 When these mixed metal oxides are exposed to water and anions of interest, LDHs are reformed. However, due to the structural memory effect, if only the metal oxides that are not formed from LDHs are used, they will not generate LDHs.Citation2,Citation18,Citation26–Citation28 Calcination and anions of interest can be introduced after calcination and rehydration process.Citation2,Citation18 This is a very useful method, especially for the intercalation of organic molecules, and many different anions have been successfully incorporated in LDHs, such as caprolactam, organic chromospheres, dyes, surfactants, and metal complex anions, and this is even a very useful method for the intercalation of nonionized species, eg, sugar molecules like pentoses.Citation29–Citation33 The whole process of rehydration should be carried out under an inert nitrogen atmosphere.Citation2,Citation18
Hydrothermal method
In the hydrothermal method, metals either in hydroxide forms or in the form of salts or anions of choice are mixed, and the pH of the solution is raised to the desired level. For Mg/Al LDHs, for example, the pH is usually set at 9–10 and for Zn/Al LDHs, the pH is set at 7–7.5.Citation20,Citation34,Citation35 After rising the pH, the sample solution is enclosed in stainless steel autoclave hydrothermal bombs, then the sample is either heated to a certain temperature ranging from 50°C to 180°C or it is subjected to simultaneous rotations.Citation34–Citation36 This method is particularly useful for the intercalation of anions with low affinity for LDHs compared to the competing anions of starting salts, such as Cl− and NO3−2. Intercalation of low-affinity anions into LDHs using the hydrothermal procedure can be more effective by the application of insoluble hydroxides, such as magnesium hydroxides and aluminum hydroxides, instead of their salts, which contain counteranions like Cl− and NO3−36. By this method, very fine LDHs are formed. Zhao et al synthesized very fine nickel/aluminum LDH nanorods with a size range of 15–40 nm.Citation34 By this method, properties of LDHs can easily be tuned by controlling the temperature, pressure, and contact time.Citation35
Preparation of metal layered hydroxides
Metal layered hydroxides (layered hydroxyl salts [LHS]) can be prepared in two ways: by using salt of the chosen metal and by using metal oxide.Citation37–Citation39 In the first method, salt is used as the starting material, which is dissolved in aqueous solution and its pH is increased by the slow addition of basic solutions, such as sodium hydroxides, aqueous solution of ammonia, or aqueous carbonate, until precipitation occurs.Citation40 This method is very much similar to the coprecipitation method used in LDH preparation, where aqueous salt solutions are coprecipitated with counteranions of interest.Citation23,Citation24
Metal layered hydroxides can also be prepared using metal oxide as the starting material. In this method, metal oxide is dissolved in the solution of the desired anions, and the pH is raised until precipitation occurs.Citation37,Citation39 The results are more fruitful if the desired anion solution is acidic in nature.Citation37,Citation39 Varieties of organic and inorganic anions have been intercalated using these two methods.Citation39–Citation42
Application of layered double hydroxides
Catalytic
LDHs have been used as catalysts as well as catalyst support in a number of reactions.Citation43 They have been applied in the oxidation of ethanol, methanol, selective oxidation of glycerol, selective oxidative halogenations, and oxidation of water for oxygen production and several other applications.Citation43–Citation49
Environmental remediation
LDHs are considered to be an ideal material for the removal of toxic material from water and also from the atmosphere.Citation50,Citation51 Motor vehicles, such as cars and motorcycles, are now integral to modern requirements. However, their increased use has also deteriorated our environment, due to the toxic gases being released as a by-product of combustion. Nitrogen oxides and soot exhaust from our vehicles are the most common causes of environmental and health problems.Citation50 LDHs with different metal compositions have been applied in the adsorption of nitrogen oxides and soot.Citation52–Citation57 Removal of toxic material, both organic and inorganic, is very difficult task, which LDHs have successfully accomplished.Citation58
Moreover, LDHs have been successfully applied in the removal of toxic metals in the form of their oxides, such as selenium, arsenic, chromium in wastewater, and radioactive uranium (VI), and have also been utilized for the removal of other heavy metals.Citation58–Citation61 Also, halogen anions and perchlorate anions have been removed by LDH application.Citation62–Citation65 Organic toxins are very difficult to remove from water, but this has been rendered possible by LDH application. Another study on the removal of guar gum and humic acid demonstrated very positive removal from water with the application of LDHs.Citation66 LDHs have also been successfully utilized in the removal of many organic compounds from water, such as N,N-dimethyl aniline, 2-chlorophenol, phenolic compounds, dodecylbenzenesulfonate, and Remazol Blue 19. Even bacteria and viruses have been removed from water by the application of LDHs.Citation32,Citation67–Citation70
In water-treatment plants, disinfection is an important procedure, and currently halogens and ozone are employed as disinfectants. However, there are certain issues with respect to these disinfectants, such as the fact that they can form genotoxic and carcinogenic by-products in the presence of organic compounds.Citation71 Nowadays, scientists are looking for alternative disinfectants. One of the important disinfectants is lysozyme (LYZ), also called muramidase, which can break down the bacterial cell wall, resulting in bacterial eradication.Citation72 Yang et al developed a novel disinfectant by the intercalation of LYZ into LDHs (LYZ LDHs), and its antibacterial capability was evaluated against Staphylococcus aureus. The antibacterial activity of LYZ LDHs was found to be consistently above 94% in the pH range of 3–9, which was much higher compared to free LYZ. This was due to the double action of LYZ LDHs, ie, adsorption of LDHs and antibacterial action of LYZ.Citation73 The LYZ LDH formulation is considered to be a green antibacterial agent, as it does not produce any by-product.
Flame retardancy
One of the important characteristics of LDHs is their high thermal stability, which makes them an ideal halogen-free retardant material.Citation74 They have been applied alone and in combination with polymers for fire control.Citation74,Citation75 Gao et al developed flame-retardant nanocomposite-based LDHs with different anions like nitrate, carbonate, chloride, and sulfonate, and combined them with a high-density polyethylene polymer.Citation75 There are many other LDHs and polymer composites that have been developed and applied as flame retardants.Citation74,Citation76–Citation79
Layered double hydroxides in biosensing
Biosensing is an emerging technology that has a number of applications in biomedical diagnosis, food sciences, and environmental sciences. The sensors are small devices that can detect chemical or biochemical changes in the medium around them and can convert them into the analytically useful signal. The sensor contains two basic components: 1) a receptor that can recognize the occurrences of events in the analytes of interest and 2) a transducer that converts the events into measurable signals.Citation80 In biosensors, transducers are attached with biological materials (enzymes, affinity ligands, antibody, oligonucleotides, receptors, peptides, etc), which can serve as a biological recognizer.Citation81
The high thermal stability and tendency of inorganic nanolayers to protect immobilized bioactive molecules and their biocompatibility make them ideal material to be applied in biosensing technology. In this section, the application of LDHs in biosensing technology is highlighted.
Layered double hydroxide-based chemiluminescence flow cell sensor
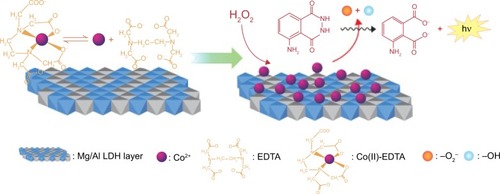
Zhang et al designed a chemiluminescence (CL) flow cell sensor by immobilizing Co(II)-ethylenediaminetetraacetic acid (EDTA)-intercalated Mg/Al LDHs on a clear quartz tube. The developed microcell enhanced the CL signal from the luminol–H2O2 reaction, and has the strong potential to be applied in chemical/biological sensing for H2O2, as well as for other oxidase-based reactions producing H2O2. The limit of detection for H2O2 was found to be 0.14 μM with a signal-to-noise ratio of 3 (S/N =3).Citation82
The likely mechanism of CL in the enhanced luminol–H2O2 system-based Co(II)-EDTA complex-intercalated LDHs is shown in . There is an equilibrium between the Co(II) and EDTA, accompanied by a constant source of Co(II) ions to the luminol–H2O2 system. The Co(II) ions released then react with H O, producing lots of OH− and O2− ions, which can further react with luminol to emit light.Citation82–Citation84
Figure 6 Possible chemiluminescence mechanism for the Co(II)-ethylenediaminetetraacetic acid (EDTA)-intercalated Mg/Al layered double hydroxide (LDH)-enhanced luminol–H2O2 system.
Note: Reprinted from Zhang LJ, Chen YC, Zhang AM, Lu C. Highly selective sensing of hydrogen peroxide based on cobalt-ethylenediaminetetraacetate complex intercalated layered double hydroxide-enhanced luminol chemiluminescence. Sens Actuators B Chem. 2014;193:752–758, Copyright 2014, with permission from Elsevier.Citation82
Hemoglobin and DNA-fabricated layered double hydroxide-based biosensor
Liu et al designed a biosensor by fabricating hemoglobin, deoxyribonucleic acid (DNA), and Ni/Al LDHs. The hemoglobin-DNA LDH-based biosensor showed sensitivity toward H2O and NO2 in the linear range of 4.85–10−7 to 1.94–10−4 M and 2.5–10−7 to 3.0–10−5 M, respectively.Citation85
Acetylcholinesterase: layered double hydroxide-based biosensor
Gong et al designed highly sensitive amperometric biosensors for the biosensing of organophosphate pesticides by the fabrication of LDHs with acetylcholinesterase (AChE). They found that LDHs facilitate a biocompatible microenvironment that assists in maintaining the bioactivity of AChE because of the intrinsic properties of LDHs, specifically regular structure, good chemical, mechanical, and thermal stability, and swelling properties. They found that AChE LDH-based electrodes greatly enhanced catalysis, oxidation producing thiocholine, and facilitated improved sensitivity with a detection limit of 0.6 ng·mL−1 (S/N =3).Citation86
Luminol-layered double hydroxide-based biosensor for glucose detection
Wang et al developed a biosensor by the fabrication of Mg/Al LDHs with luminol for glucose detection in human serum. They combined the Mg/Al LDH–luminol with silica gel-fabricated glucose oxidase. The concentration was determined by CL, which can detect glucose in a linear range of 0.005–1.0 mM. The detection limit for glucose was found to be 0.1 mM, with S/N =3.Citation87 Colombari et al designed a biosensor for the amperometric detection of glucose by combining Mg/Al LDHs with glassy electrode carbon.Citation88
Ionic liquid: layered double hydroxide-based biosensor for protein detection
Lou et al developed a biosensor based on ionic liquid 1-carboxyl-methyl-3-methylimidazolium tetrafluoroborate (CMMIMBF4) and LDHs for redox protein detection. The designed biosensor facilitated faster electron transfer in the redox process and was highly suitable for the detection of redox proteins.Citation89
Gold-layered double hydroxide-based biosensor for toxic nitrite compound
Nitrite is the most commonly found compound in foods and the environment. It has been reported to be toxic and carcinogenic, and can even block oxygen supply via the blood by irreversible oxidation of hemoglobin to methemoglobin.Citation90,Citation91 Yin et al developed a biosensor by the fabrication of the gold electrode with Cu-Mg-Al LDHs, which were able to detect the nitrite amperometrically. The designed LDH-based bio-sensor showed excellent bioelectrocatalytic activity for nitrite oxidation in a linear range of 0.75–123 μM, with a detection limit of 2×10−7 M and S/N =3. The biosensor successfully determined nitrite content in food samples.Citation81
Layered double hydroxide-based biosensor for cysteine sulfoxide detection in food samples
Anifantaki et al designed a biosensor by fabricating alliinase with LDHs for cysteine sulfoxide detection. The developed biosensor has the strong potential to be applied in food science.Citation92 Mansouri et al designed a conductometric biosensor for polypeptide biosensing by the intercalation of trypsin into Zn/Al LDHs and Mg/Al LDHs.Citation93
Layered double hydroxides in bioimaging
Bioimaging is one of the latest scientific techniques applied for disease diagnosis and examination of disease, and is also being applied in medical science to study normal physiology and anatomy. In bioimaging techniques, different processes are used to develop human body image, tissues, and anatomical area down to the molecular level.
LDHs can be ideal material to be applied in bioimaging science, due to their proven high biocompatibility (both in vitro and in vivo studies), positively charged surface, and ease of interaction with negatively charged cell membranes, which improve the efficiency of transfer.Citation94 LDHs are most recently explored in the bioimaging science and only few studies are carried out using LDHs in bioimaging science.
Layered double hydroxide-based upconversion fluorescence for bioimaging and drug delivery in cancer therapy
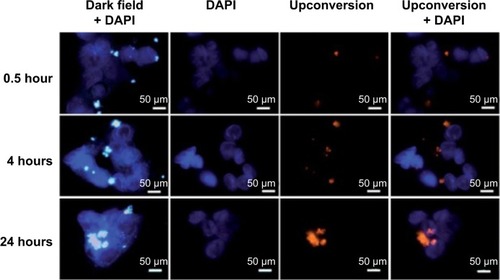
Chen et al designed a multifunctional nanodelivery system for drug delivery to and bioimaging of tumors by the fabrication of Y2O3:Er3+ Yb3+ nanoparticles (NPs; near-infrared fluorescent nanophosphors) and fluorouracil (5-FU; anticancer drug) intercalated into Mg/Al LDHs, collectively abbreviated as (Y2O3:Er3+,Yb3+@SiO2@LDH-5FU).Citation95 The developed nanodelivery systems showed powerful red upconversion fluorescence upon excitation with a 980 nm laser, which permitted the easy tracking of the nanodelivery system after internalization in cancer cells. The resultant nanodelivery system showed better anticancer efficacy and stronger red fluorescence, as shown in . The better therapeutic outcome with the nanodelivery system compared to the free 5-FU can be attributed to the better internalization of positively charged NPs (due to LDHs’ positive surface) in the cell membrane.Citation95
Figure 7 CytoViva microscopy images of MCF-7 cells incubated with Y2O3:Er3+, Yb3+@SiO2@LDH-5-FU at 100 mg·mL−1 for different time durations of 0.5 hour, 4 hours, and 24 hours.
Note: Reproduced from Chen C, Yee LK, Gong H, Zhang Y, Xu R. A facile synthesis of strong near infrared fluorescent layered double hydroxide nanovehicles with an anticancer drug for tumor optical imaging and therapy. Nanoscale. 2013;5:4314–4320, with permission of The Royal Society of Chemistry.Citation95
Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; LDH, layered double hydroxide; 5-FU, fluorouracil.
Layered double hydroxide-based multifunctional core/shell nanospheres for bioimaging and targeted delivery in cancer
Li et al designed novel biodegradable multifunctional core/shell nanospheres with targeted, controlled release and fluorescent properties for cancer therapy. First, they prepared a core based on iron magnetic supraparticles (MSPs), which were then coated with a shell of Ni/Al LDH. Next, the doxorubicin (anticancer drug)-carboxyl group, modified (DOX-COOH), was loaded on the shell of MSP LDH nanospheres. In addition, they immobilized the iminodiacetic acid-modified folate over MSP LDH-(DOX-COOH) in order to target cancer cells. Fluorescence microscopy found that the designed nanospheres targeted the cancerous cells and were highly cytotoxic to the cancerous cells compared to normal HEK 293T cells.Citation96
Layered double hydroxide-based luminescent systems for bioimaging
Yan et al designed a highly luminescent nanocomposite by the fabrication of sodium fluorescein dye with Mg/Al LDHs. The nanocomposite was found to exhibit excellent fluorescence intensity, maintain fluorescence even in the form of dry powder, and was able to form transparent free-standing film (fluorescence active in ultraviolet [UV] light).
Tanaka et al intercalated a fluorescent compound fluores-cein (Fluo) into Mg/Al LDHs and studied their internalization and intracellular behavior in mammalian cells. They found that the Fluo LDHs displayed high green fluorescence in whole cells, including the nucleus. Furthermore, the study revealed that anion fluorescein was released from Fluo LDHs and endosomes by the proton-assisted dissolution of Fluo LDHs.Citation97
Musumeci et al fabricated LDHs with different organic dyes for understanding the biological interactions and their biocompatibility with LDHs. They intercalated LDHs with organic dyes, namely fluorescein isothiocyanate (FITC), fluorescein, 8-aminopyrene-1,3,6-trisulfonic acid, and 8-ami-nonaphthelene-1, 3,6-trisulfonic acid. Confocal imaging after incubation of LDH–dye nanocomposites with cells revealed that for biological tracking and labeling purposes, organic dyes were suitable in the following order: 1) fluorescein, 2) sulfonic acid-derived pyrene, and 3) naphthalene-based dyes.Citation98
Posati et al doped Zn/Al LDHs with europium (III) ions via microemulsion methods, and luminescence measurements revealed that the resulting material, europium (III)–Zn/Al LDHs, was found to be very luminescent.Citation99
Layered double hydroxides in UV protection and sunscreen formulations
Most skin cancers are caused by solar UV radiation, and skin cancer is responsible for human deaths. According to the World Health Organization, about 60,000 people died from cancer in the year 2000.Citation100 We are exposed to sunrays every day; visible light is beneficial for us, but at the same time we are also exposed to UV (carcinogenic) radiation. Sunscreen formulation is the most commonly applied defense against UV radiation, but these sunscreen formulations contain organic UV absorbers that have certain limitations, eg, they cannot cover the whole UV region (200–400 nm). These organic UV absorbers undergo photodegradation under UV radiation, and the degradation products are toxic.Citation42 Therefore, there is a need to design new sunscreen protection formulations free from the aforementioned demerits. The photostability of these organic UV absorbers can be achieved by intercalating them with inorganic nanolayers. Inorganic nanolayers are highly biocompatible, and most importantly they also absorb in the UV region, resulting in a hybrid material to cover the larger UV region (200–400 nm).
Mohsin et al designed an efficient sunscreen formulation by the intercalation of an organic UV absorber – cinnamate anion – into zinc layered hydroxides (ZnLHs). The developed formulation covered the whole UV region, and was found to be biocompatible with HDF cells. In addition, cinnamate anion was thermally stabilized in the interlayers of ZnLHs.Citation42
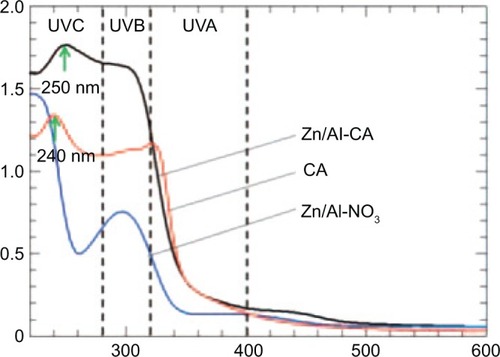
Mohsin et al also recently designed three UV-absorbing nanocomposites by separate intercalation of three organic UV absorbers, namely cinnamic acid (CA), benzophenone-4, and Eusolex® 232, into Zn/Al LDHs. The study revealed that the UV-absorption range of all of the nanocomposites was longer compared to the free individual organic absorbers. shows the UV-absorption spectrum of CA, Zn/Al-CA, and Zn/Al LDHs. It can be easily seen that Zn/Al-CA covered a larger UV region compared to the CA alone. Likewise, the UV absorption of the two other nanocomposites showed similar trends. Most importantly, a cytotoxic study revealed that all three nanocomposites were found to be biocompatible with HDF cells.Citation101
Figure 8 Solid-state absorbance spectra of Zn/Al-NO3, organic sunscreen guest cinnamic acid (CA), and the corresponding nanocomposite.
Note: Reprinted with permission from Mohsin SM, Hussein MZ, Sarijo SH, Fakurazi S, Arulselvan P, Taufiq-Yap YH. Characterisation and cytotoxicity assessment of UV absorbers-intercalated zinc/aluminium-layered double hydroxides on dermal fibroblast cells. Sci Adv Mater. 2014;6:648–658, Copyright © American Scientific Publishers.Citation101
Abbreviation: UV, ultraviolet.
Mohsin et al have recently also developed a UV-absorbing nanohybrid (sunscreen formulation) by intercalating the strong UV absorber benzophenone-9 into Zn/Al LDHs. The sunscreen formulation was found to be stable in deionized water, artificial seawater, and in skin pH conditions. The UV-absorption spectrum was found to be broader compared to the free benzophenone-9, and the nanocomposite was also determined to be biocompatible with HDF cells.Citation102
Wang et al designed novel UV-absorbing thin film by doping Fe3+ with Mg/Al LDHs, and the resulting material covered the whole UV region (200–400 nm). Furthermore, the thin film was easily fabricated with highly transparent polymer polysiloxanes and UV-blocking composite material. The designed transparent UV-blocking composite can potentially be applied in protective coatings for glass curtain walls, automotive glass, outdoor wood objectives, priceless scrolls of calligraphy, cultural relic paintings, and some UV-sensitive materials.Citation100
Li et al designed a UV-absorber organic–inorganic nanocomposite by the intercalation of 2,4-dihydroxybenzophe-none into dodecylbenzenesulfonate-fabricated LDHs. The UV-absorption capability of the designed nanocomposite was extended over the entire UV region, which suggests it can be potentially applied as a UV absorber.Citation103
Drug-delivery applications of layered double hydroxides
LDHs have been the focus of several researchers, due to their potential applications in the biomedical field, and the research trends have proved that LDHs are the ideal material for this field. In this section, recent advances in drug-delivery application for the cure of various diseases are discussed.
Also in this section, the latest developments in the drug-delivery applications of inorganic nanolayers are discussed. Many nonsteroidal anti-inflammatory drugs have been intercalated into inorganic nanolayers; Rives et al comprehensively reviewed the application of LDHs to the delivery of nonsteroidal anti-inflammatory drugs.Citation8 Many other drugs, such as antidiabetes, cardiovascular, antibiotics, antioxidants, antiosteoporosis, vitamins, amino acids, and peptides, have been intercalated into inorganic nanolayers, which were comprehensively reviewed by Rives et al recently.Citation7
Layered double hydroxides in anticancer formulations
Cancer has been threatening humans for centuries, and despite technological advancements, cancer claims millions of human lives every year, with about 8.2 million deaths in 2012.Citation104,Citation105 The statistics of increasing cancer cases suggest that by the year 2025, cancer-related deaths will increase by up to 80% in less developed countries.Citation104 Cancer is a set of diseases caused by the uncontrolled growth of abnormal cells and spread of these abnormal cells. It has been reported that if the spread of these abnormal cells is not controlled, they ultimately lead to death.Citation110 Of drugs, such as decrease in bloodstream cell count, hair loss, cardiac dysfunction and heart failure, hyperglycemia, bone marrow depression, vomiting, nausea and diarrhea, tiredness, mouth soreness, constipation or diarrhea, loss of appetite, skin changes or reactions, pain or nerve changes, and changes in fertility and sexuality.Citation106–Citation109 Resistance of cancer cells toward effective therapeutic drugs is another major issue in cancer therapy.Citation110,Citation111
Drug-delivery systems could be very handy in reducing the adverse side effects of anticancer drugs, and could target cancer cells and release the drugs in a sustained manner. All of these factors would enhance therapeutic efficacy.
Methotrexate layered double hydroxides
Methotrexate (MTX) is an anticancer drug that can deactivate the metabolism of diseased cells effectively via programmed cell death (apoptosis), and is being used against different human cancers, eg, osteosarcoma (bone cancer) and leukemia.Citation112 Jae-Min et al evaluated the anticancer efficacy of free MTX and MTX LDHs against bone cancer cell lines (Saos-2 and MG-63), and found that MTX LDHs were more effective in suppressing the cancer cells compared to free MTX. Oh et al revealed that LDHs were highly cytocompatible with human fibroblast cells even up to 500 μg·mL−1.Citation113
Zhang et al recently chose MTX LDHs as a model nanohybrid to study the effect of different hydrothermal conditions on particle size and the effect of particle size on control release and suppression of cancer cells.Citation114 They found a direct relationship between the diameter of LDHs and prolonged hydrothermal treatment at higher temperatures. In addition, hydrothermal treatment had no effect on percentage drug loading.Citation114 Furthermore, they studied the effect of particle size on in vitro drug release, and found a direct relation between the size of LDHs and sustained release: the bigger the size, the longer the release. They considered the shelf life of MTX LDHs, and found that release was excellent even after 18 months, which makes LDHs suitable for drug delivery.Citation114
Ifosfamide layered double hydroxides
The intercalation of neutral or nonionic molecules into LDHs is very difficult and challenging, because the positive layers of LDHs have very low affinity for neutral molecules. Alternative methods for neutral species intercalation are either the memory-effect method or the direct assembly of neutral molecules with negatively charged surfactants like sodium dodecyl sulfate, sodium dodecylbenzenesulfonate, cholate, and deoxycholate.
Ifosfamide (IFO) is an anticancer agent commonly applied for the treatment of many pediatric and adult tumors, including soft-tissue sarcomas and lymphomas.Citation115,Citation116 IFO is a nonionic drug, and nonionic drugs are difficult to intercalate into LDHs. Nie and Hou intercalated IFO into Mg/Al LDHs by the surfactant-assisted method, in which they applied sodium dodecyl sulfate and sodium dodecyl-benzenesulfonate.Citation117 They intercalated IFO into surfactant-modified Mg/Al LDHs separately. They conducted in vitro release in phosphate-buffered saline (PBS, pH 7.5), and found that IFO release was much more sustained from the LDH-surfactant-IFO nanohybrid compared to the physical mixture of free IFO and LDHs. The kinetic study of IFO release from LDHs was revealed to follow a pseudo-second order process.Citation117
Camptothecin-layered double hydroxides
Camptothecin (CPT) is highly potent against a variety of cancers, such as lung cancer, ovarian cancer, pancreatic cancer, and stomach cancer.Citation118,Citation119 However, the chemical instability of CPT and its poor water solubility limit its clinical applications.Citation118,Citation119
Recently, Wu et al successfully intercalated the neutral anticancer drug CPT into Mg/Al LDHs by coassembling it with sodium cholate, and then intercalated the CPT-sodium cholate by the delaminating method.Citation120 The loading of CPT was found to be 13%, and PBS solution of pH 7.4 was found to be much more sustained compared to the physical mixture of CPT and sodium cholate LDHs.Citation120 In addition, they also determined release kinetic models, which satisfactorily adopted the parabolic kinetic model, which suggests that release possibly follows a diffusion mechanism. However, ironically, they did not study the anticancer effect, which is crucially important for drug-delivery applications.
Protocatechuic acid layered double hydroxides
Protocatechuic acid (PA; 3,4-dihydroxybenzoic acid) is an anticancer agent widely used for the treatment of different types of cancers, namely cervix, breast, human leukemia (pa-2000-leukemia), liver, lung, and prostate cancers.Citation121 Barahuie et al intercalated PA into Mg/Al LDHs (PA–Mg/Al LDHs) by coprecipitation and ion-exchange methods, and studied various drug-delivery aspects.Citation122 Anticancer assay revealed that PA–Mg/AL LDHs had higher anticancer activity compared to the free PA against the HeLa cervical cancer cell line and MCF-7 breast cancer cells. In addition, nanocomposites did not show any cytotoxicity to normal 3T3 fibroblast cells. The in vitro release study revealed that PA release from PA–Mg/Al LDHs was much more sustained compared to their physical mixture.Citation122 The in vitro release of PA from Mg/Al LDHs was found to follow a second-order kinetic model more satisfactorily.Citation122
Etoposide layered double hydroxides
Etoposide (VP16) is a derivative of podophyllotoxin, and has been reported to have significant anticancer activities against various cancer cells, such as small-cell lung carcinoma, gastric cancer cells, hematologic malignancies, childhood malignancies, and germ cell tumors.Citation123–Citation127
Qin et al prepared a nanohybrid formulation of VP16 by intercalating it into Mg/Al LDHs (VP16–Mg/Al LDHs).Citation128 They found that the nanohybrid (VP16–Mg/Al LDHs) possessed better antitumor activity against MKN45 and SGC-7901 cells. The release of VP16 from LDHs was found to be sustained and followed a diffusion mechanism, as it followed the parabolic kinetic model.Citation28
Ciprofloxacin zinc layered hydroxides
Latip et al designed a anticancer nanodelivery formulation by intercalating ciprofloxacin into ZnLHs. The release of ciprofloxacin was found to sustain in human body-simulated PBS of pH 7.4. The ciprofloxacin ZnLH nanocomposite showed stronger anticancer effect against A549 cancer cells compared to free ciprofloxacin.Citation129 The enhanced anticancer effect can be attributed to the nanoscale size of the nano-composite and sustained release of the ciprofloxacin. Many other anticancer drugs have been intercalated into LDHs, such as ferulic acid,Citation130 cetirizine,Citation131 ellagic acid (EA),Citation41 5-FU–cyclodextrin complex,Citation132 and doxifluridine.Citation133
Layered double hydroxides in antimicrobial formulations
Inorganic nanolayers have been applied in designing antimicrobial formulations by intercalating a variety of antimicrobials into them. Here, we discuss some of the latest developments made in the application of inorganic nanolayers in antimicrobial formulations.
Ciprofloxacin layered double hydroxides
Hesse et al intercalated the antimicrobial drug ciprofloxacin into Mg/Al LDHs and evaluated its in vivo antimicrobial effect in rabbit ears against the bacterium Pseudomonas aeruginosa, and the material was found to show excellent antimicrobial effect even after 1 week.Citation134
Hippuric acid zinc layered hydroxides
Hippuric acid has been reported to have antimicrobial and anti-tumor properties.Citation39,Citation135 Hussein Al Ali et al developed nanocomposite formulations by the intercalation of hippuric acid into ZnLHs.Citation136 The antimicrobial study revealed that hippuric acid ZnLHs showed strong activity against P. aeruginosa, and most importantly hippuric acid ZnLHs showed better antimicrobial properties against drug-resistant bacteria, namely methicillin-resistant S. aureus, compared to free hippuric acid.Citation136
Benzylpenicillin layered double hydroxides
Wang et al designed an antimicrobial film by intercalating benzylpenicillin (antimicrobial agent) into Mg/Al LDHs, and then fabricated that nanohybrid with graphene oxide.Citation137 They found that sustained release of benzylpenicillin from the film was directly related to the thickness of the film, and the film showed strong antimicrobial activity against Micrococcus lysodeikticus and sulfate-reducing bacteria.Citation137
Amino acid layered double hydroxides
Wang et al designed antimicrobial formulations by intercalating different complex amino acids based on the Schiff base ligands salicylidene with alanine, phenylalanine, glycine, tyrosine, aspartic acid, and glutamic acid for complexes and gallium ion (G3+).Citation138 The designed nanocomposites retained antimicrobial activity against P. aeruginosa, with a very good minimum inhibitory concentration (65–137 μmol·L−1).Citation1380Many different antimicrobial agents, eg, amoxicillinCitation139 and cefazolin,Citation140 have been successfully intercalated into LDHs.
Layered double hydroxides as antimicrobial biomaterial
The development of biomaterial with antimicrobial properties is another fascinating area of research, as many diseases are caused by microbes. Biomaterial with antimicrobial properties finds a variety of applications, such as in medical implants, medical devices, food packaging, and household products.Citation141 Inorganic nanolayer composition based on metals is renowned for its antimicrobial effect. Some compounds, such as silver, zinc, and copper, can be very useful for the production of different LDH-based ceramic materials in floor tiles, kitchens, and even bathrooms.Citation142 In this section, we discuss applications of LDH-based biomaterial as antimicrobial agents.
Silver-based layered double hydroxides with antimicrobial properties
Mishra et al incorporated silver into Zn/Al LDHs and compared their antibacterial activity with Zn/Al LDHs, silver-based LDHs, and their calcined product. They found that Ag LDHs before and after calcination remained active against the bacterial species Escherichia coli and S. aureus.Citation142 The antimicrobial effect of calcined Ag LDHs (spinel form) would be a useful property that can be applied in household items to prevent microbes, which in itself is an important avenue of research in the field of ceramics. In addition, the antimicrobial drugs intercalated into this type of LDH would result in a synergetic antimicrobial effect.
Biogenic silver nanoparticle-fabricated layered double hydroxides
Silver NPs have been reported to have strong antimicrobial properties, but toxicity associated with them limits their applications.Citation143–Citation145 In order to make silver NPs more biocompatible, Marcato et al fabricated biogenic silver NPs (AgNPbio) with Mg/Al LDHs.Citation146 They evaluated the biocompatibility of free AgNPbio, LDHs, and AgNPbio LDHs against a lung fibroblast cell line (V79), and found that AgNPbio caused 50% cell death at a concentration of 45 μmol·L−1. However, even at a concentration of 45 μmol·L−1, LDHs alone and AgNPbio LDHs did not show any toxicity.Citation146 In addition, AgNPbio LDH hybrid material was found to retain the antimicrobial effect, as the minimum inhibitory concentration of AgNPbio remained unchanged against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria: 6.6 μg·mL−1 and 12 μg·mL−1, respectively.Citation146 This fascinating hybrid material is suitable to be applied in biomedical devices, cosmetics, and household items.
Carja et al previously adopted a similar approach, and developed hybrid material by fabricating AgNPs on the surface of LDHs (AgNP LDHs).Citation147 They evaluated the antimicrobial effect of AgNPs alone and hybrid AgNP LDHs with respect to time, and found that AgNP LDHs retained the inhibition zone against the Gram-positive S. aureus (American Type Culture Collection [ATCC] 25923) and the Gram-negative E. coli (ATCC 35218). However, the AgNP zone of inhibition was found to almost disappear with time.Citation147
Titanium-based layered double hydroxides with antimicrobial properties
Zhao et al designed nanoscale material with photocatalytic activity.Citation141 They developed Zn-Ti LDH nanosheets in the size range of 40–80 nm with a band gap of about 2.3 eV.Citation141 They found that photochemical activity and nanosize confer antimicrobial activity under visible light. The material was found to possess a strong antimicrobial effect against Saccharomyces cerevisiae (85% inhibition), S. aureus (65% inhibition), and E. coli (100% inhibition).Citation141
ZnO nanoparticle-fabricated layered double hydroxides with antimicrobial properties
Zhang et al designed antimicrobial nanocomposite by combining Zn/Al LDHs, waterborne polyurethane, and ZnO NPs. The designed nanocomposite showed strong antimicrobial activity against the Gram-negative E. coli and the Gram-positive S. aureus.Citation148
Layered double hydroxides in antituberculosis nanodelivery formulations
Tuberculosis (TB) is an airborne infectious disease caused by the bacterium Mycobacterium tuberculosis (MTB). It can be classified as pulmonary TB when MTB infects the lungs, and the other form is extrapulmonary TB, in which MTB attacks other organs of the human body, namely the liver, kidneys, intestines, bones, tonsils, spleen, and brain.Citation149 TB has been intimidating humans for centuries, and in the Global Tuberculosis Report 2013 by the World Health Organization, there were approximately 8.6 million people infected with TB, and about 1.3 million died in the year 2012.Citation150
There are several issues in TB treatment, such as longer treatment duration (6–24 months), multidrug prescription, frequent dosage of anti-TB drugs, and adverse effects of anti-TB drugs, and all of these factors result in patients’ noncompliance with the treatment. Patient noncompliance is the most common reason for the failure of TB chemotherapy.Citation151 There has been no new anti-TB drug that has been introduced in the market, and in a situation like this, biocompatible drug-delivery systems with sustained release seem to be the best option.
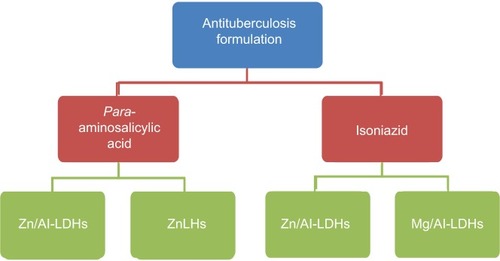
Para-aminosalicylic acid layered double hydroxides
Saifullah et al designed an anti-TB nanocomposite formulation with sustained-release properties by intercalation of the anti-TB drug PAS into ZnLHs using zinc oxide as a precursor.Citation37 They have also designed a similar sustained-release PAS ZnLH nanocomposite with higher drug loading by using zinc nitrate as a precursor.Citation152 Both of these nanocomposite formulations were found to be highly biocompatible with normal human lung fibroblast cells (MRC-5) and 3T3 fibroblast cells. In addition, the nanocomposites showed higher efficacy against bacteria MTB compared to the free drug PAS. The nanocomposites showed antimicrobial activities against S. aureus, P. aeruginosa, E. coli, and Candida albicans.Citation152,Citation153
Saifullah et al also reported the development of anti-TB nanocomposite formulations by intercalating PAS into Zn/Al LDHs by coprecipitation and ion-exchange methods.Citation23 The developed nanodelivery formulation was found to release the PAS in a sustained manner in human body-simulated PBS solutions. They also reported better biocompatibility of the nanocomposites with human normal lung cells (MRC-5) and fibroblast cells (3T3). The nanocomposites were found to be more effective in eradicating MTB compared to free PAS, and were also found to show antimicrobial activities against S. aureus, P. aeruginosa, E. coli, and C. albicans.Citation23,Citation153 sows the anti-tuberculosis formulations based on inorganic nanolayers and anti-TB drugs.
Isoniazid layered double hydroxides
Saifullah et al recently designed an anti-TB nanodelivery formulation based on the anti-TB drug isoniazid (INH) and Mg/Al LDHs with sustained-release properties. The designed nanodelivery formulation of INH–Mg/Al LDHs was found to be very effective in eradicating the MTB, and most importantly the formulation was found to be more biocompatible when compared to free INH.Citation154 Saifullah et al also designed another anti-TB sustained-nanodelivery formulation by intercalating INH into Zn/Al LDHs. The designed INH–Zn/Al LDHs showed better therapeutic results against MTB compared to free INH. The as-synthesized INH–Zn/Al LDHs were also found to be more biocompatible with human normal lung cells (MRC-5) and fibroblast cells (3T3).Citation155 INH-based Zn/Al LDHs and Mg/Al LDHs were also found to show antimicrobial activities against S. aureus, P. aeruginosa, E. coli, and C. albicans.Citation154,Citation155
The better therapeutic efficacy against MTB, high biocompatibility with human normal lung cells, and sustained release of the anti-TB drugs will reduce adverse effects as well as dosing frequency, and will shorten the treatment duration. The sustained-release biocompatible nanodelivery formulation will make TB chemotherapy more patient-friendly.
Layered double hydroxides in gene delivery
The genes are the hereditary units being transferred from parents to children, and are responsible for transporting the characteristics of parents to the children. There are instances where parents with defective genes can pass the disease to their children.Citation156 Gene therapy is a modern scientific technique that uses genes to prevent and treat disease. It is believed that in the future, this technique will revolutionize medical science in the treatment of all diseases by introducing genes into patient cells instead of drugs and surgery.Citation157 Scientists are adopting several different approaches in gene therapy, such as the replacement of a defective gene (responsible for disease) with a healthy one, and the introduction of new genes into the body to fight disease.Citation157 The in vitro and in vivo delivery of bioactive molecules, such as proteins, peptides, and nucleic acids, with the specific focus to transfer these biomolecules into the cytoplasm/nucleus by passing the cell membrane is an important area of biomedical research. There are many issues related to the direct delivery of biomolecules, such as poor solubility and enzymatic degradation. Though there are many hurdles in the development of efficient gene carriers, it is an exciting area of modern biomedical research. Many viral and nonviral carriers have been designed traditionally.Citation158 If the gene of interest is introduced into the body directly, it will not work. Therefore, certain carriers are required, which can transfer the gene safely without harming the gene or the human body. Some viruses are currently being tested for the delivery of genes, and these viruses are modified in such a way that they do not harm the body.Citation157
LDHs offer several advantages over other gene carriers, such as easy preparation, easy intercalation of the biomolecules, targeted delivery, easier removal from the body after delivery without accumulation, high biocompatibility, and the ability to enter the cell via clathrin-mediated mechanism.Citation159–Citation161
Layered double hydroxides in pEGFP-N1-DNA transfection mouse neuron cells
Li et al studied the cellular uptake of LDHs into mouse motor neuron cells (NSC 34) and determined the effect of LDH concentration, size, and incubation time by labeling the LDHs with FITC.Citation162 The study revealed that cellular uptake was directly proportional to LDH concentration and incubation time. They also found that LDHs of 20 nm size were better at internalizing in the cell cytoplasm and cell nucleus; however, LDHs greater than 20 nm were internalized in the cell cytoplasm.Citation162 They fabricated LDHs of 20 nm size with plasmid enhanced green fluorescent protein (pEGFP)-N1 DNA, and then successfully transfected them to NSC 34 cells. The found 20 nm LDHs did not cause any cytotoxicity to NSC 34 cells up to 200 mg·mL−1.
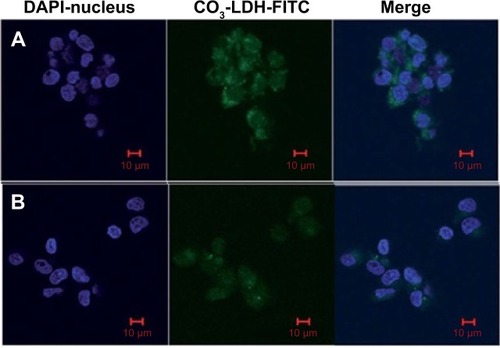
shows a confocal image depicting the cytoplasm in green fluorescence after incubation of 6.25 mg·mL−1 of CO3 LDH-FITC for 2.5 hours. Some of the particles have also entered the nucleus, as can be seen in green fluorescence in .Citation162 The free FITC anions showed very weak green fluorescence at a much higher concentration of 6.25 mg·mL−1 compared to CO3 LDH-FITC. The lower free FITC cytoplasm entrance can be attributed to the mechanism of entrance: free FITC entrance takes place due to diffusion and not by endocytosis.Citation162
Figure 10 Confocal microscopic images of intracellular localization in NSC 34 cells.
Notes: (A) 6.25 mg·mL−1 CO3 layered double hydroxide (LDH)–fluorescein isothiocyanate (FITC), incubated for 2.5 hours; (B) free 6.25 mg·mL−1 FITC anions incubated for 4 hours. Reproduced from Li SD, Li JH, Wang CL, et al. Cellular uptake and gene delivery using layered double hydroxide nanoparticles. J Mater Chem B. 2013:61–68, with permission of The Royal Society of Chemistry.Citation162
Layered double hydroxides in plasmid DNA delivery
Hu et al modified the surface of LDHs and studied the effect of surface change on the transfected properties of LDHs. They tailored the LDH surface by fabricating it with 2-(dimethylamino) ethyl methacrylate, and designed a series of surface-modified LDHs for gene delivery.Citation163 The surface-modified LDHs showed better tendency to conjugate with plasmid DNA and a higher level of gene delivery in different cell lines, including COS7 and HepG2, compared to free LDHs.Citation163
Layered double hydroxides as gene-protective biomaterial
Wu et al investigated the suitability of LDHs as a gene-protective agent in the adverse environment of a Cd2+/Pb2+ solution.Citation164 They intercalated DNA into LDHs and subjected both unprotected DNA and LDHs-DNA separately to the Cd2+/Pb2+ solution. The study revealed that the unprotected DNA structure was damaged, while LDH-protected DNA remained unaffected.Citation164 Previously, Swadling et al also proved the higher stability of DNA after intercalation by molecular dynamic simulation.Citation165 Zhang et al studied the structural stability of ribonucleic acid (RNA) before and after intercalation into LDHs by molecular simulation, and their study revealed that RNA had higher stability in LDHs compared to its free form.Citation166
Codelivery of 5-flourouracil and siRNAs by intercalation in layered double hydroxides
The emergence of multidrug-resistant cancer is one of the major drawbacks of longer chemotherapy duration for cancer.Citation167,Citation168 High-concentration doses are administered to multidrug-resistant cancer patients, causing adverse side effects to healthy tissue.Citation169 A useful strategy being applied is the coadministration of two different types of anticancer drugs, specifically 5-FU and small interfering RNAs (siRNAs), which have been in use for over 40 years.Citation170–Citation172 The sustained and simultaneous codelivery of the 5-FU and siRNAs will improve the therapeutic outcome against cancer.
Li et al designed a novel anticancer formulation by co-intercalation of 5-FU and siRNAs into LDHs for the effective cancer treatment.Citation173 The LDH-based sustained codelivery of 5-FU and siRNAs markedly enhanced anticancer activity in comparison to single treatment of either of the two against three cancer cell lines, namely human breast (MCF-7), osteosarcoma (U2OS), and colorectal (HCT-116).Citation173 This codelivery formulation has the strong potential to overcome the emergence of drug-resistant cancer.
Transfection of siRNA into cytoplasm by layered double hydroxides
LDHs are suitable vectors to facilitate the uptake and transfection of siRNA, due to their ability to dissolve in an endosomal environment (acidic condition) and buffer the environment.Citation158 Ladewig et al designed a siRNA-delivery system based on LDHs, and successfully transfected siRNA into the cytoplasm, because of their tremendous ability of escape endosomal environment.Citation161 The cellular uptake study revealed that siRNA LDHs successfully entered HEK293T cells; however, free siRNA failed to enter HEK293T cells. The developed formulation was found to be highly biocompatible with human embryonic kidney cells (HEK 293T) at very high concentrations (up to 0.200 mg·mL−1).Citation161
Many other studies have been conducted on LDHs for their application in gene therapy, such as Ladewig et al (transfected plasmid DNA LDHs into different cells, namely HEK 293T, NIH 3T3, COS-7, and CHO-K1),Citation161 Xu et al (supercoiled pEF-eGFP plasmid LDHs transfected to HEK 293T cells),Citation174 Hu et al (intercalated mononucleotides and DNA). summarizes the biomedical applications of inorganic nanolayers.Citation175
Table 1 Biomedical application of layered double hydroxides (LDHs)
Sustained release from inorganic nanolayers
The key parameter in designing drug-delivery systems is their tendency to release the active agents (drugs/genes/biomolecules, etc) in a sustained manner at the target site. The tendency of inorganic nanolayers (LDHs/LHS) to release the drug in a desired manner makes them superior to other drug-delivery systems. The drug release from inorganic nanolayers (LDHs/LHS) can be tuned for the desired applications, such as for delivery of the drug in a two-phase manner, initially fast followed by a slower release.Citation37,Citation176
The ability of inorganic nanolayers to protect, release in the desired manner, and release at the target site would avoid the physicochemical degradation of the drug, with a reduction in adverse effects and dosing concentration and frequency, and in general would improve the bioavailability of the drug. All of these characteristics would improve patients’ compliance with treatment, particularly in the management of cancer, TB, and Parkinson’s disease, whose drugs have a lot of side effects.Citation130,Citation177–Citation179 Here, we discuss the effect of different parameters, such as pH, types of anions present in the release media, and mechanisms involved in drug release from LDHs.
Release in phosphate-buffered saline of pH 7.4 and pH 4.8
In vitro release studies are conducted in various solutions simulating different routes of the delivery. In some cases, the release is carried out in PBS of pH 7.4 to check the suitability of the drug-delivery system for intravenous application, as PBS 7.4 simulates blood.Citation23 The condition of PBS 7.4 is most commonly applied to evaluate the release behavior of any newly designed drug-delivery system, and the release of different drugs from LDHs/LHS have been conducted in PBS 7.4. The drug release in PBS 7.4 from LDHs/LHS has been found to be highly sustained compared to acidic pH conditions.Citation22,Citation24,Citation37 The in vitro release is also most commonly conducted in PBS 4.8 to mimic the release in lysosomal conditions. Lysosomes are membrane-enclosed organelles present in the cell, and function as the digestive system of the cell.Citation180 A great deal of in vitro release studies of LDHs have revealed that LDH release in PBS at pH 4.8 is sustained, but relatively faster compared to pH 7.4.Citation22,Citation23,Citation37,Citation39,Citation41
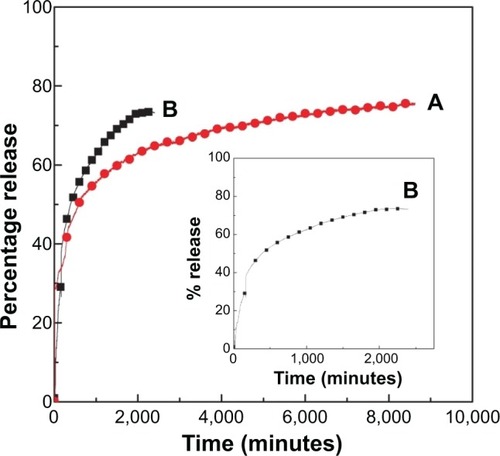
Kura et al intercalated the antiparkinsonian drug levodopa into LDHs and conducted in vitro release in PBS pH 7.4, wherein the drug release was found to be sustained until 8,500 minutes.Citation178 The release of levodopa in PBS solution of pH 4.8 was completed in 2,400 minutes; this release was still considered sustained, but was relatively faster than the release at pH 7.4. shows the release of levodopa from LDHs in PBS of pH 7.4 and pH 4.8.Citation178
Figure 11 Release profiles of levodopa from the nanocomposite at pH 7.4 (A) and pH 4.8 (B).
Notes: Inset shows the release profiles of levodopa from the nanocomposite at pH 4.8 from 0 to 2,000 minutes. Reproduced with permission from Kura AU, Hussein Al Ali SH, Hussein MZ, Fakurazi S, Arulselvan P. Development of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agent. Int J Nanomedicine. 2013;8:1103–1110.Citation178
The release at pH 7.4 is for the evaluation of the oral route PBS, with pH 6.8 being used to mimic the intestinal environment, and pH 4.8 is used as the simulator for a lysosomal environment. Rives et al comprehensively reviewed release studies on LDHs.Citation7,Citation8 Rojas et al studied the drug release from LDHs using ibuprofen as a model drug in an intestinal-simulated PBS solution of pH 6.8 and a gastric-simulated solution (HCl in 0.05 mol·L−1 NaCl) of pH 1.2.Citation176 They found the release at pH 6.8 was relatively similar with PBS 4.8 lysosomal pH. However, release in the gastric environment at pH 1.2 was much faster, such that the complete release took only about 200 minutes.Citation176 The dissimilar pattern of drug release from LDHs in unlike conditions is due to different release mechanisms. The drug can be released in two different ways: 1) an ion-exchange mechanism and 2) weathering mechanisms. The drug is released by an ion-exchange mechanism under basic or neutral PBS medium, whereas under acidic conditions the drug is released by both ion exchange and by the weathering of inorganic nanolayers.Citation181,Citation182
Biocompatibility of layered double hydroxides
The most important characteristic of the ideal material for drug-delivery systems is biocompatibility with the human body, cells, and tissues. Biocompatibility can be evaluated by two means: in vitro and in vivo cytotoxic studies. In the in vitro studies, the compounds of interest are treated with different cell lines, and their viability is determined by different assays. Many cytotoxic studies are carried out over LDHs to evaluate their biocompatibility.
In vitro environment
Qin et al intercalated antitumor drug VP16 into LDHs and evaluated its antitumor effect, as well as its cytocompatibility with the human GES-1 cell line. They found that free VP16 was toxic to GES-1. On the other hand, LDH-intercalated VP16 did not show any cytotoxicity, and the therapeutic effect was maintained against the tumor cell lines MKN45 and SGC-7901.Citation128
Hussein Al Ali et al developed a formulation for the inhibition of angiotensin-converting enzyme by intercalating perindopril erbumine (PE) into LDHs. The study revealed that PE LDHs showed better enzyme-inhibition activity against angiotensin-converting enzyme, and the PE LDHs were found to be highly biocompatible with a human liver cell line.Citation24 Xu et al designed a gene-delivery system based on supercoiled pEF-eGFP plasmid and LDHs, and evaluated its cytocompatibility against HEK 293T cells. They found that pEF-eGFP LDHs were highly biocompatible with HEK 293T cells, even at the highest concentration of 500 μg/mL.Citation174 The protein gene LDHs have been reported to be biocompatible with kidney (Vero3) cells of monkeys by Masarudin et al, and Ramli et al reported ZnLH–salicylic acid biocompatibility with kidney (Vero3) cells of monkeys.Citation183,Citation184
Marcato et al fabricated AgNPbio with Mg-Al LDHs (LDH-AgNPbio), and evaluated their biocompatibility against a permanent lung fibroblast cell line (V79).Citation146 The developed nanohybrid LDH-AgNPbio was found to be highly biocompatible with the permanent lung fibroblast cell line (V79).Citation146 Saifullah et al developed an anti-TB nanocomposite formulation based on PAS and ZnLHs and Zn/AL LDHs. The cytotoxic study revealed that the anti-TB nanocomposites were highly biocompatible with human normal lung cells (MRC-5) and fibroblast cells (3T3).Citation23,Citation37 Barahuie et al designed an anticancer nanohybrid by intercalating PA into Mg/Al LDHs, and found the nanohybrid possessed strong anticancer activity against MCF-7 human breast cancer and human cervical cancer cell lines. At the same time, the nanohybrid did not show any cytotoxicity against fibroblast cells (3T3).Citation122 Mohsin et al designed a sunscreen protection formulation that was able to cover the whole UV regions of A, B, and C by cointercalating the UV absorber CA, benzophenone-4, and Eusolex 232. The cytotoxic study of the UV-protection LDH formulation was found to be biocompatible with HDF cells.Citation101 Hussein et al designed nanohybrid-based EA into ZnLHs and evaluated the cytotoxicity of the nanohybrid EA ZnLHs against the normal cell lines 3T3 and MCF-10A. The EA ZnLHs were found to be highly biocompatible with these normal cells, and at the same time EA ZnLHs were effective in killing two human cancer cell lines (breast cancer MCF-7 and liver cancer HepG2).Citation185
Kura et al studied the toxicity of a nanohybrid based on the antiparkinsonian drug levodopa and Zn/Al LDHs on the PC12 cell model. They found the nanohybrid did not induce any toxicity to the PC12 cells at a concentration of 100 μg/mL; however, levodopa in the free form was found to be extremely toxic, causing 80% cell death at 100 μg/mL.Citation178
In vivo environment
Clay minerals have been applied in pharmaceutical products. Hydrotalcite is used as an antacid and antipepsin agent under the brand names Almax, Bemolan, and Talcid. It is also used in cosmetic products, such as dentifrices and deodorants, as an excipient.Citation186,Citation187
Indomethacin LDH in vivo biocompatibility
Indomethacin is an important anti-inflammatory drug applied for the treatment of ulcers, but it has gastrointestinal side effects. Del Arco et al intercalated indomethacin into Mg/Al LDHs and carried out a comparative pharmacological study of free indomethacin and Mg/Al LDH–indomethacin on Swiss mice of both sexes.Citation187 They found Mg/Al LDHs were able to reduce side effects and improve therapeutic outcomes against ulcers, and were much better compared to free indomethacin.Citation187
Ketoprofen LDH in vivo biocompatibility
Ketoprofen (Ket; 2-[3-benzoylphenyl]-propionic acid) is antipyretic, analgesic, and a nonsteroidal anti-inflammatory drug that is being applied for the cure of rheumatoid arthritis, osteoarthritis, and other chronic musculoskeletal conditions.Citation188,Citation189 Its application is limited by the side effects associated with it, such as peptic ulceration, anorexia, and bleeding.Citation190,Citation191 Silion et al intercalated Ket into Mg/Al LDHs and Zn/Al LDHs and conducted an in vivo comparative biocompatibility study with free Ket in mice. Their study revealed that Ket LDHs reduced the ulcerogenic effect to a greater extent compared to free Ket.Citation192
Acetylsalicylic acid–dextran-coated LDH in vivo biocompatibility
Dong et al designed drug-delivery systems based on dextran-coated LDHs for acetylsalicylic acid, and conducted a pharmacokinetic study with rabbits as the model animal.Citation160 They found the LDH-based delivery system increased the bioavailability and half-life and reduced the side effects of acetylsalicylic acid.Citation160 summarizes the in vitro cytotoxic studies of inorganic nanolayers.
Table 2 In vitro biocompatibility studies of layered double hydroxides (LDHs) toward various cell lines
LDH nanoparticle in vivo biocompatibility
Yu et al evaluated acute oral toxicity and kinetic behaviors of LDH NPs by using mice as the model animal. They found that LDH NPs did not cause any abnormal behaviors, mortality, symptoms, body-weight loss, or any symptoms at the very high concentration of 2,000 mg/kg for 14 days. Furthermore, their study revealed that LDH NPs did not cause any injury to the liver or kidneys.Citation193 Flesken-Nikitin et al evaluated the toxicity of Mg/Al LDH NPs and used different routes in mice, namely subcutaneous, intraperitoneal, and intravenous injections. The LDH NPs showed only minor in vivo toxicity, and their results were highly encouraging, with a high percentage of mouse survival.Citation194
Kura et al recently evaluated the subacute oral toxicity of levodopa–Zn/Al LDHs in rats of 5–500 mg/kg body weight. The study revealed that there was no significant change in body-weight gain, water intake, or percentage of survival between groups of rats treated with nanocomposites and the control group. In addition, the liver, spleen, and brain histology was similar with the control group.Citation195
In vivo biocompatibility is the most important characteristic of any material considered for biomedical application. The aforementioned in vivo biocompatibility studies are highly encouraging, but there is still need for further in vivo studies on LDHs in order to establish that they are safe for biomedical applications. summarizes the in vivo cytotoxic studies of Inorganic nanolayers.
Table 3 In vivo biocompatibility of layered double hydroxides (LDHs) using animal models
Conclusion
There are varieties of materials being studied for biomedical and other applications. However, inorganic nanolayers are unique and very useful in many aspects, such as their easy preparation and proven in vitro and in vivo biocompatibility. Their tendency to intercalate a variety of anions, such as inorganic and organic acids, organic dyes, pharmaceutical drugs, and biopolymers, and being able to be fabricated with other metallic materials like silver and ceramics either by intercalation or by surface interactions, resulting in fabulous material with numerous applications, makes them all the more advantageous.
The development of drug-delivery science is an important avenue in modern biomedical research. Inorganic nanolayers have been continuously exploited for the delivery of a number of drugs, and have been found to be the best material to be applied as a delivery system. For a material to be suitable for drug delivery, it should meet some important criteria: 1) it should be biocompatible with normal cells and tissues, 2) have a tendency to encapsulate/intercalate different pharmaceutical drugs, 3) be easy to prepare, 4) be cost-effective, 5) be able to release the drug in a sustained manner, 6) be able to deliver the drug at the disease site, and 7) be biodegradable and easily excreted from the body after releasing the drugs. This review has categorically proved the ability of nanolayers to meet these criteria, and hence these are an ideally suitable biomaterial to be applied for drug-delivery purposes.
Gene therapy is another revolutionary modern technique of biomedical science in which defective genes are replaced by healthy genes. Previously, viruses were being employed as gene carriers, but due to the risk of adverse health effects, scientists are looking for nonviral gene carriers. Inorganic nanolayers have been applied as nonviral gene carriers recently in gene delivery. Our review has shown that inorganic nanolayers have successfully transfected genes. Their positive surface charge assists them to cross the negatively charged cell membrane. Gene delivery is also one of the most important characteristics of nanolayers along with drug delivery.
The diagnosis of disease is another important research area of modern biomedical science. Previously, people needed to submit samples from the human body (such as blood, urine, and bone marrow) and wait for hours to days to get the reports. In modern medical diagnosis, a shorter time to reach diagnosis can be the difference between life and death. Biosensing technology has made it possible to diagnose diseases in a matter of seconds. Most importantly, a layman can easily understand the output of the results by eliminating the need of a laboratory expert to interpret the results.
Recently, inorganic nanolayers have been applied in biosensors, and are considered to be a suitable material, as they can easily intercalate the biosensing receptor biomolecules, are highly biocompatible, and provide a high surface area to facilitate faster electron transfer during biosensing activity. The application in the biosensors enhances the versatility of the inorganic nanolayers and further widens horizons for biomedical science. Inorganic nanolayers have the tendency to perform dual functions as a biosensor and drug-delivery system. This simultaneous dual-function characteristic has made it possible to identify the disease site and deliver the drug there. This has opened up a new research dimension in biomedical sciences.
The diagnosis of disease and therapy is collectively called theranostic science, and this has been facilitated by the use of inorganic nanolayers, which are fabulous biomaterials applied in theranostic science.
Bioimaging is also an important area of biomedical science that can be applied in the diagnosis of disease, monitoring the disease condition, and applying to the study of normal human physiology and anatomy. Most recently, inorganic nanolayers have been applied in bioimaging science, and results suggest that there are many fascinating biomaterial applications to be applied in bioimaging science. Inorganic nanolayers are suitable for bioimaging science, because of their proven high biocompatibility, ability to be intercalated with fluorescent organic material that can glow, and ability to be fabricated with metals that can confer the glow to them. Furthermore, the positively charged surface of the inorganic nanolayers would assist in internalization inside the cell. Only a few studies have been carried out recently on bioimaging applications of inorganic nanolayers. The initial results of these are highly encouraging.
The application of inorganic nanolayers in biosensors, drug delivery, gene delivery, and bioimaging biomaterial will enable us to diagnose the disease, release the drug at the target site, and help in monitoring therapeutic progress. It can be concluded that inorganic nanolayers are an excellent biomaterial for various biomedical applications, such as cosmetics, drug delivery, biosensing, and bioimaging technology.
Acknowledgments
Funding for this research was provided by the Higher Education Commission of Malaysia under the Commonwealth Scholarship and Fellowship Plan (KPT.B.600-6/3, Vol 68) to Bullo Saifullah and a Fundamental Research Grant Scheme (FRGS/2/2013/SG06/UPM/01/1 with vote number 5524467) to Mohd Zobir Hussein.
Disclosure
The authors report no conflicts of interest in this work.
References
- VaccariALayered double hydroxides: present and future: V. Rives (Ed.), Nova Science Publishers, Inc., New York, 2001, IX+439 pp., ISBN 1-59033-060-9Appl Clay Sci2001227576
- DuanXEEvansDGLayered Double Hydroxides (Structure and Bonding)BerlinSpringer2006
- CarilloACGriegoDAHydroxides: Synthesis, Types and Applications (Chemical Engineering Methods and Technology)Hauppauge (NY)Nova Science2012
- Del HoyoCLayered double hydroxides and human health: an overviewAppl Clay Sci200736103121
- KhanAIRagavanAFongBRecent developments in the use of layered double hydroxides as host materials for the storage and triggered release of functional anionsInd Eng Chem Res2009481019610205
- WangQO’HareDRecent advances in the synthesis and application of layered double hydroxide (LDH) nanosheetsChem Rev20121124124415522452296
- RivesVdel ArcoMMartínCIntercalation of drugs in layered double hydroxides and their controlled release: a reviewAppl Clay Sci201488–89239269
- RivesVdel ArcoMMartínCLayered double hydroxides as drug carriers and for controlled release of non-steroidal antiinflammatory drugs (NSAIDs): a reviewJ Control Release2013169283923583707
- WangRMikoryakCLiSCytotoxicity screening of single-walled carbon nanotubes: detection and removal of cytotoxic contaminants from carboxylated carbon nanotubesMol Pharm201181351136121688794
- DuanXLuJEvansDGAssembly chemistry of anion-intercalated layered materialsXuRPangWHuoQModern Inorganic Synthetic ChemistryAmsterdamElsevier2011375404
- ArizagaGGSatyanarayanaKGWypychFLayered hydroxide salts: synthesis, properties and potential applicationsSolid State Ionics200717811431162
- BrindleyGWBrownGCrystal Structure of Clay Minerals and their X-ray IdentificationLondonMineralogical Society1980
- KleinCManual of MineralogyHoboken (NJ)John Wiley & Sons1993
- CattiMFerrarisGHullSPaveseAStatic compression and H disorder in brucite, Mg(OH)2, to 11 GPa: a powder neutron diffraction studyPhys Chem Miner199522200206
- CavaniFTrifiròFVaccariAHydrotalcite-type anionic clays: preparation, properties and applicationsCatal Today199111173301
- FrondelCConstitution and polymorphism of the pyroaurite and sjörgrenite groupsJ Mineral Soc Am1915619
- WangQO’HareDRecent advances in the synthesis and application of layered double hydroxide (LDH) nanosheetsChem Rev20121124124415522452296
- RivesVLayered Double Hydroxides: Present and FutureHauppauge (NY)Nova Science2001
- RabenauAThe role of hydrothermal synthesis in preparative chemistryAngew Chem Int Ed Engl19852410261040
- TrobajoCEspinaAJaimezEKhainakovSAGarciaJRHydrothermal synthesis of iron(III) phosphates in the presence of ureaDalton Trans2000787790
- Morel-DesrosiersNPissonJIsraëliYTaviot-GuehoCBesseJPMorelJPIntercalation of dicarboxylate anions into a Zn-Al-Cl layered double hydroxide: microcalorimetric determination of the enthalpies of anion exchangeJ Mater Chem20031325822585
- Hussein-Al-AliSHAl-QubaisiMHusseinMZIsmailMZainalZHakimMNIn vitro inhibition of histamine release behavior of cetirizine intercalated into Zn/Al- and Mg/Al-layered double hydroxidesInt J Mol Sci2012135899591622754339
- SaifullahBHusseinMZHussein-Al-AliSHArulselvanPFakuraziSAntituberculosis nanodelivery system with controlled-release properties based on para-amino salicylate-zinc aluminum-layered double-hydroxide nanocompositesDrug Des Devel Ther2013713651375
- Hussein Al AliSHAl-QubaisiMHusseinMZIsmailMZainalZHakimMNControlled release and angiotensin-converting enzyme inhibition properties of an antihypertensive drug based on a perindopril erbumine-layered double hydroxide nanocompositeInt J Nanomedicine201272129214122619549
- BishDLAnion exchange in takovite: application to other hydroxide mineralsBull Miner1980103170175
- EricksonKLBostromTEFrostRLA study of structural memory effects in synthetic hydrotalcites using environmental SEMMater Lett200559226229
- StanimirovaTSKirovGDinolovaEMechanism of hydrotalcite regenerationJ Mater Sci Lett200120453455
- RochaJdel ArcoMRivesVA UlibarriMReconstruction of layered double hydroxides from calcined precursors: a powder XRD and 27Al MAS NMR studyJ Mater Chem1999924992503
- KatahiraSYasueKInagakiMIntercalation of E-caprolactam ions into organic hostsJ Mater Chem19991411781180
- AloisiGGCostantinoUEliseiFLatteriniLNataliCNocchettiMPreparation and photo-physical characterisation of nanocomposites obtained by intercalation and co-intercalation of organic chromophores into hydrotalcite-like compoundsJ Mater Chem20021233163323
- LerouxFTaviot-GuehoCFine tuning between organic and inorganic host structure: new trends in layered double hydroxide hybrid assembliesJ Mater Chem20051536283642
- YouYZhaoHVanceGFHybrid organic-inorganic derivatives of layered double hydroxides and dodecylbenzenesulfonate: preparation and adsorption characteristicsJ Mater Chem200212907912
- AisawaSHiraharaHIshiyamaKOgasawaraWUmetsuYNaritaESugar-anionic clay composite materials: intercalation of pentoses in layered double hydroxideJ Solid State Chem2003174342348
- ZhaoYXiaoFJiaoQHydrothermal synthesis of Ni/Al layered double hydroxide nanorodsJ Nanotechnol20112011646409
- WangQTangSVLesterEO’HareDSynthesis of ultrafine layered double hydroxide (LDHs) nanoplates using a continuous-flow hydrothermal reactorNanoscale2013511411723128781
- OgawaMAsaiSHydrothermal synthesis of layered double hydroxide-deoxycholate intercalation compoundsChem Mater20001232533255
- SaifullahBHusseinMZHussein-Al-AliSHArulselvanPFakuraziSSustained release formulation of an anti-tuberculosis drug based on para-amino salicylic acid-zinc layered hydroxide nanocompositeChem Cent J201377223601852
- SaifullahBArulselvanPEl ZowalatyMEDevelopment of a highly biocompatible antituberculosis nanodelivery formulation based on para-aminosalicylic acid-zinc layered hydroxide nanocompositesScientific World Journal2014201440146025050392
- Hussein Al AliSHAl-QubaisiMHusseinMZZainalZHakimMNPreparation of hippurate-zinc layered hydroxide nanohybrid and its synergistic effect with tamoxifen on HepG2 cell linesInt J Nanomedicine201163099311122163163
- BiswickTJonesWPacułaASerwickaESynthesis, characterisation and anion exchange properties of copper, magnesium, zinc and nickel hydroxy nitratesJ Solid State Chem20061794955
- HusseinMZAl AliSHZainalZHakimMNDevelopment of antiproliferative nanohybrid compound with controlled release property using ellagic acid as the active agentInt J Nanomedicine201161373138321796241
- MohsinSMHusseinMZSarijoSHFakuraziSArulselvanPHinTYSynthesis of (cinnamate-zinc layered hydroxide) intercalation compound for sunscreen applicationChem Cent J201372623383738
- KovandaFJirátováKSupported layered double hydroxide-related mixed oxides and their application in the total oxidation of volatile organic compoundsAppl Clay Sci201153305316
- BallarinBMignaniAScavettaESynthesis route to supported gold nanoparticle layered double hydroxides as efficient catalysts in the electrooxidation of methanolLangmuir201228150651507423025480
- ZhouCHBeltraminiJNLinCXXuZPLuGQTanksaleASelective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalystsCatal Sci Technol20111111122
- SelsBVosDDBuntinxMPierardFKirsch-De MesmaekerAJacobsPLayered double hydroxides exchanged with tungstate as biomimetic catalysts for mild oxidative brominationNature1999400855857
- GongMLiYWangHAn advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidationJ Am Chem Soc20131358452845523701670
- XuZPZhangJAdebajoMOZhangHZhouCCatalytic applications of layered double hydroxides and derivativesAppl Clay Sci201153139150
- OmwomaSChenWTsunashimaRSongYFRecent advances on polyoxometalates intercalated layered double hydroxides: from synthetic approaches to functional material applicationsCoord Chem Rev2014258–2595871
- YangRGaoYWangJWangQLayered double hydroxide (LDH) derived catalysts for simultaneous catalytic removal of soot and NOxDalton Trans201443103171032724492318
- ChaaraDPavlovicIBrunaFUlibarriMADraouiKBarrigaCRemoval of nitrophenol pesticides from aqueous solutions by layered double hydroxides and their calcined productsAppl Clay Sci201050292298
- WangZPChenMXShangguanWFSimultaneous catalytic removal of NOx and diesel soot over Cu-containing hydrotalcite derived catalystsActa Physicochim Sin2009257985
- LiuSWuXLinYLiMWengDActive oxygen-assisted NO-NO2 recycling and decomposition of surface oxygenated species on diesel soot with Pt/Ce0.6Zr0.4O2 catalystChin J Catal201435407415
- ZhangZZhangYWangZGaoXCatalytic performance and mechanism of potassium-promoted Mg-Al hydrotalcite mixed oxides for soot combustion with O2J Catal20102711221
- ZhangZMouZYuPZhangYNiXDiesel soot combustion on potassium promoted hydrotalcite-based mixed oxide catalystsCatal Commun2007816211624
- LiLDYuJJHaoZPXuZPNovel Ru-Mg-Al-O catalyst derived from hydrotalcite-like compound for NO storage/decomposition/reductionJ Phys Chem C20071111055210559
- KurodaYMiyamotoYHibinoMYamaguchiKMizunoNTripodal ligand-stabilized layered double hydroxide nanoparticles with highly exchangeable CO32−Chem Mater20132522912296
- YouYWVanceGFZhaoHTSelenium adsorption on Mg-Al and Zn-Al layered double hydroxidesAppl Clay Sci2001201325
- YouYWZhaoHTVanceGFRemoval of arsenite from aqueous solutions by anionic claysEnviron Technol2001221447145711873880
- GoswameeRLSenguptaPBhattacharyyaKGDuttaDKAdsorption of Cr(VI) in layered double hydroxidesAppl Clay Sci1998132134
- PshinkoGNLayered double hydroxides as effective adsorbents for U(VI) and toxic heavy metals removal from aqueous mediaJ Chem20132013347178
- LvLHeJWeiMEvansDGDuanXFactors influencing the removal of fluoride from aqueous solution by calcined Mg Al CO3 layered double hydroxidesJ Hazard Mater200613311912816343753
- WangHChenJCaiYJiJLiuLTengHHDefluoridation of drinking water by Mg/Al hydrotalcite-like compounds and their calcined productsAppl Clay Sci2007355966
- ZhaoHNagyKLDodecyl sulfate hydrotalcite nanocomposites for trapping chlorinated organic pollutants in waterJ Colloid Interface Sci200427461362415144837
- LinYFangQChenBMetal composition of layered double hydroxides (LDHs) regulating ClO−4 adsorption to calcined LDHs via the memory effect and hydrogen bondingJ Environ Sci201426493501
- JinSFallgrenPApplications of Layered Double Hydroxides in Removing Oxyanions from Oil Refining and Coal Mining WastewaterMorgantown (WV)National Energy Technology Laboratory2006
- KamedaTTsuchiyaYYamazakiTYoshiokaTPreparation of Mg-Al layered double hydroxides intercalated with alkyl sulfates and investigation of their capacity to take up N,N-dimethylaniline from aqueous solutionsSolid State Sci20091120602064
- ChuangYHTzouYMWangMKLiuCHChiangPNRemoval of 2-chlorophenol from aqueous solution by Mg/Al layered double hydroxide (LDH) and modified LDHInd Eng Chem Res20084738133819
- XueXGuQPanGNanocage structure derived from sulfonated β-cyclodextrin intercalated layered double hydroxides and selective adsorption for phenol compoundsInorg Chem2014531521152924422491
- El HassanEHLakraimiMBadreddineMLegrouriACherkaouiOBerrahoMRemoval of Remazol Blue 19 from wastewater by zinc aluminium chloride-layered double hydroxidesAppl Water Sci20133431438
- WatsonKFarréMJKnightNStrategies for the removal of halides from drinking water sources, and their applicability in disinfection by-product minimisation: a critical reviewJ Environ Manag2012110276298
- CheethamGMaSteitzTAInsights into transcription: structure and function of single-subunit DNA-dependent RNA polymerasesCurr Opin Struct Biol20001011712310679468
- YangQZChangYYZhaoHZPreparation and antibacterial activity of lysozyme and layered double hydroxide nanocompositesWater Res2013476712671824053938
- FengYTangPXiJJiangYLiDLayered double hydroxides as flame retardant and thermal stabilizer for polymersRecent Pat Nanotechnol2012623123722747723
- GaoYWangJHuangLSynthesis of highly efficient flame retardant high-density polyethylene nanocomposites with inorgano-layered double hydroxides as nanofiller using solvent mixing methodACS Appl Mater Interfaces201465094510424597470
- WangZHanEKeWInfluence of nano-LDHs on char formation and fire-resistant properties of flame-retardant coatingProg Org Coat2005532937
- DingYYXuLHuGSPerformance of halogen-free flame retardant EVA/MH/LDH composites with nano-LDHs and MHChin Sci Bull20115638783883
- BasharDBGünterBRainerSJosefBSignificance of aspect ratio on efficiency of layered double hydroxide flame retardantsWilkieCAMorganABNelsonGLFire and Polymers VI: New Advances in Flame Retardant Chemistry and ScienceWashingtonAmerican Chemical Society2012407425
- CostaFRWagenknechtUHeinrichGLDPE/MgeAl layered double hydroxide nanocomposite: thermal and flammability propertiesPolym Degrad Stab20079218131823
- MoustyCSensors and biosensors based on clay-modified electrodes – new trendsAppl Clay Sci200427159177
- YinHZhouYLiuTAmperometric nitrite biosensor based on a gold electrode modified with cytochrome C on Nafion and Cu-Mg-Al layered double hydroxidesMicrochim Acta2010171385392
- ZhangLJChenYCZhangAMLuCHighly selective sensing of hydrogen peroxide based on cobalt-ethylenediaminetetraacetate complex intercalated layered double hydroxide-enhanced luminol chemiluminescenceSens Actuators B Chem2014193752758
- LinJMShanXHanaokaSYamadaMLuminol chemiluminescence in unbuffered solutions with a cobalt(II)-ethanolamine complex immobilized on resin as catalyst and its application to analysisAnal Chem2001735043505111721898
- GiokasDLVlessidisAGEvmiridisNPOn-line selective detection of antioxidants free-radical scavenging activity based on Co(II)/EDTA-induced luminol chemiluminescence by flow injection analysisAnal Chim Acta2007589596517397653
- LiuLMJiangLPLiuFLuGYAbdel-HalimESZhuJJHemoglobin/DNA/layered double hydroxide composites for biosensing applicationsAnal Methods2013535653571
- GongJGuanZSongDBiosensor based on acetylcholinesterase immobilized onto layered double hydroxides for flow injection/amperometric detection of organophosphate pesticidesBiosens Bioelectron20133932032322868055
- WangZLiuFLuCChemiluminescence flow biosensor for glucose using Mg-Al carbonate layered double hydroxides as catalysts and buffer solutionsBiosens Bioelectron20123828428822770831
- ColombariMBallarinBCarpaniIGlucose biosensors based on electrodes modified with ferrocene derivatives intercalated into Mg/Al layered double hydroxidesElectroanalysis20071923212327
- LouJLuYZhanTGuoYSunWRuanCApplication of an ionic liquid-functionalized Mg2Al layered double hydroxide for the electrochemical myoglobin biosensorIonics20142014711479
- MirvishSSRole of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOCCancer Lett19959317487600541
- AmineAPalleschiGPhosphate, nitrate, and sulfate biosensorsAnal Lett200437119
- AnifantakiETouloupakisEGhanotakisDFAlliinase immobilization in calcium alginate beads and layered double hydroxides matricesJ Food Biochem2012361220
- MansouriHForanoCPrévotVJaffrezic-RenaultNBen Haj AmaraALayered double hydroxides/trypsin based conductometric biosensorsSensor Lett20097888895
- ChoyJHKwakSYJeongYJParkJSInorganic layered double hydroxide as non-viral vectorsAngew Chem Int Ed20003940414045
- ChenCYeeLKGongHZhangYXuRA facile synthesis of strong near infrared fluorescent layered double hydroxide nanovehicles with an anticancer drug for tumor optical imaging and therapyNanoscale201354314432023558400
- LiDZhangYTYuMGuoJChaudharyDWangCCCancer therapy and fluorescence imaging using the active release of doxorubicin from MSPs/Ni-LDH folate targeting nanoparticlesBiomaterials2013347913792223886730
- TanakaMAisawaSHidetoshiHIntracellular release of fluorescein anion from layered double hydroxide nanoparticles indicating endosomal escapeIOP Conf Ser Mater Sci Eng201347012006
- MusumeciAWMortimerGMButlerMKXuZPMinchinRFMartinDJFluorescent layered double hydroxide nanoparticles for biological studiesAppl Clay Sci201048271279
- PosatiTBellezzaFCipicianiASynthesis and characterization of luminescent nanoclaysCryst Growth Des20101028472850
- WangXZhouSWulFabrication of Fe3+ doped Mg/Al layered double hydroxides and their application in UV light-shielding coatingsJ Mater Chem C2014257525758
- MohsinSMHusseinMZSarijoSHFakuraziSArulselvanPTaufiq-YapYHCharacterisation and cytotoxicity assessment of UV absorbers-intercalated zinc/aluminium-layered double hydroxides on dermal fibroblast cellsSci Adv Mater20146648658
- MohsinSMHusseinMZSarijoSHFakuraziSArulselvanPTaufiq-YapYHOptimization of UV absorptivity of layered double hydroxide by intercalating organic UV-absorbent moleculesJ Biomed Nanotechnol2014101490150025016649
- LiSFShenYMLiuDBFanLHWuKKEncapsulation of 2,4-dihydroxybenzophenone into dodecylbenzenesulfonate modified layered double hydroxide for UV absorption propertiesBull Korean Chem Soc201435392396
- de MartelCFerlayJFranceschiSGlobal burden of cancers attributable to infections in 2008: a review and synthetic analysisLancet Oncol20121360761522575588
- International Agency for Research on CancerWorld Cancer Report 2014GenevaWHO2014
- CouffignalALLapeyre-MestreMBonhommeCBugatRMontastrucJLInternational Agency for Research on CancerAdverse effects of anticancer drugs: apropos of a pharmacovigilance study at a specialized oncology institutionTherapie200055635641 French11201979
- RemeshAToxicities of anticancer drugs and its managementInt J Basic Clin Pharmacol20121212
- StorteckySSuterTMInsights into cardiovascular side-effects of modern anticancer therapeuticsCurr Opin Oncol20102231231720535072
- VergèsBWalterTCariouBEndocrine side effects of anti-cancer drugs: effects of anti-cancer targeted therapies on lipid and glucose metabolismEur J Endocrinol2014170R43R5524154684
- No authors listedCancer multidrug resistanceNat Biotechnol200018IT18IT2011221708
- GottesmanMMMechanisms of cancer drug resistanceAnnu Rev Med20025361562711818492
- No authors listedReview of Gilman AG, Goodman LS, Gilman A. ‘The Pharmacological Basis of Therapeutics’Psychol Med198111433
- OhJMParkMKimSTJungJYKangYGChoyJHEfficient delivery of anticancer drug MTX through MTX-LDH nanohybrid systemJ Phys Chem Solids20066710241027
- ZhangXQZengMGLiSPLiXDMethotrexate intercalated layered double hydroxides with different particle sizes: structural study and controlled release propertiesColloids Surf B Biointerfaces20141179810624632036
- OliveiraRVOnoratoJMSilukDWalkoCMLindleyCWainerIWEnantioselective liquid chromatography mass spectrometry assay for the determination of ifosfamide and identification of the N-dechloroethylated metabolites of ifosfamide in human plasmaJ Pharm Biomed Anal20074529530317855037
- ZhangJTianQYung ChanSMetabolism and transport of oxazaphosphorines and the clinical implicationsDrug Metab Rev20053761170316393888
- NieHQHouWGSynthesis and characterization of ifosfamide intercalated layered double hydroxidesJ Dispers Sci Technol201133339345
- Garcia-CarboneroRSupkoJGCurrent perspectives on the clinical experience, pharmacology, and continued development of the camptothecinsClin Cancer Res2002864166111895891
- KehrerDFSoepenbergOLoosWJVerweijJSparreboomAModulation of camptothecin analogs in the treatment of cancer: a reviewAnticancer Drugs2001128910511261892
- WuXLiHSongSZhangRHouWFacile synthesis of camptothecin intercalated layered double hydroxide nanohybrids via a coassembly routeInt J Pharm201345445346123820133
- YinMCLinCCWuHCTsaoSMHsuCKApoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: potential mechanisms of actionJ Agric Food Chem2009576468647319601677
- BarahuieFHusseinMZHussein-Al-AliSHArulselvanPFakuraziSZainalZPreparation and controlled-release studies of a protocatechuic acid-magnesium/aluminum-layered double hydroxide nanocompositeInt J Nanomedicine201381975198723737666
- DrewinkoBBarlogieBSurvival and cycle-progression delay of human lymphoma cells in vitro exposed to VP-16-213Cancer Treat Rep197660129513061016966
- HainsworthJDGrecoFAEtoposide: twenty years laterAnn Oncol199563253417619747
- SlevinMLLow-dose oral etoposide: a new role for an old drug?J Clin Oncol19908160716092170588
- LowisSPNewellDREtoposide for the treatment of paediatric tumours: what is the best way to give it?Eur J Cancer199613229122979038612
- YouBTranchandBGirardPEtoposide pharmacokinetics and survival in patients with small cell lung cancer: a multicentre studyLung Cancer20086226127218442869
- QinLWangMZhuRYouSZhouPWangSThe in vitro sustained release profile and antitumor effect of etoposide-layered double hydroxide nanohybridsInt J Nanomedicine201382053206423737669
- LatipAFHusseinMZStanslasJWongCCAdnanRRelease behavior and toxicity profiles towards A549 cell lines of ciprofloxacin from its layered zinc hydroxide intercalation compoundChem Cent J2013711923849189
- KimHJRyuKKangJHChoiAJKimTIOhJMAnticancer activity of ferulic acid-inorganic nanohybrids synthesized via two different hybridization routes, reconstruction and exfoliation-reassemblyScientific World Journal2013201342196724453848
- HasanSAl AliHAl-QubaisiMControlled-release formulation of antihistamine based on cetirizine zinc-layered hydroxide nanocomposites and its effect on histamine release from basophilic leukemia (RBL-2H3) cellsInt J Nanomedicine201273351336322848164
- JinLLiuQSunZNiXWeiMPreparation of 5-fluorouracil/-cyclodextrin complex intercalated in layered double hydroxide and the controlled drug release propertiesInd Eng Chem Res2010491117611181
- PanDZhangHZhangTDuanXA novel organic inorganic microhybrids containing anticancer agent doxifluridine and layered double hydroxides: structure and controlled release propertiesChem Eng Sci20106537623771
- HesseDBadarMBleichALayered double hydroxides as efficient drug delivery system of ciprofloxacin in the middle ear: an animal study in rabbitsJ Mater Sci Mater Med20132412913623053799
- Hamilton-MillerJMBrumfittWMethenamine and its salts as urinary tract antiseptics: variables affecting the antibacterial activity of formaldehyde, mandelic acid, and hippuric acid in vitroInvest Urol19771428729113049
- Hussein Al AliSHAl-QubaisiMEl ZowalatyMHusseinMZIsmailMAntimicrobial activity of hippurate nanocomposite and its cytotoxicity effect in combination with cytarabine against HL-60J Nanomater20132013843647
- WangYZhangDBaoQWuJWanYControlled drug release characteristics and enhanced antibacterial effect of graphene oxide-drug intercalated layered double hydroxide hybrid filmsJ Mater Chem2012222310623113
- WangMHuQLiangDIntercalation of Ga3+-salicylidene-amino acid Schiff base complexes into layered double hydroxides: synthesis, characterization, acid resistant property, in vitro release kinetics and antimicrobial activityAppl Clay Sci20138384182190
- ValarezoETammaroLGonzálezSMalagónOVittoriaVFabrication and sustained release properties of poly(ε-caprolactone) electrospun fibers loaded with layered double hydroxide nanoparticles intercalated with amoxicillinAppl Clay Sci201372104109
- RyuSJJungHOhJMLeeJKChoyJHLayered double hydroxide as novel antibacterial drug delivery systemJ Phys Chem Solids201071685688
- ZhaoYWangCJGaoWSynthesis and antimicrobial activity of ZnTi-layered double hydroxide nanosheetsJ Mater Chem B2013159885994
- MishraGDashBPandeySMohantyPPAntibacterial actions of silver nanoparticles incorporated Zn Al layered double hydroxide and its spinelJ of Environ Chem Eng2013111241130
- DuránNMarcatoPDAlvesOLSouzaGIEspositoEMechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strainsJ Nanobiotechnology20053816014167
- NandaASaravananMBiosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSENanomedicine2009545245619523420
- HadrupNLamHROral toxicity of silver ions, silver nanoparticles and colloidal silver – a reviewRegul Toxicol Pharmacol2014681724231525
- MarcatoPDParizottoNVMartinezDSNew hybrid material based on layered double hydroxides and biogenic silver nanoparticles: antimicrobial activity and cytotoxic effectJ Braz Chem Soc201324266272
- CarjaGKameshimaYNakajimaADrancaCOkadaKNanosized silver-anionic clay matrix as nanostructured ensembles with antimicrobial activityInt J Antimicrob Agents20093453453919786342
- ZhangWDZhengYMXuYSPreparation and antibacterial property of waterborne polyurethane/Zn Al layered double hydroxides/ZnO nanocompositesJ Nanosci Nanotechnol20131340941623646747
- SharmaSKMohanAExtrapulmonary tuberculosisIndian J Med Res200412031635315520485
- World Health OrganizationGlobal Tuberculosis Report 2013GenevaWHO2013
- SumartojoEWhen tuberculosis treatment fails. A social behavioral account of patient adherenceAm Rev Respir Dis1993147131113208484650
- SaifullahBArulselvanPEl ZowalatyMEDevelopment of a highly biocompatible antituberculosis nanodelivery formulation based on para-aminosalicylic acid-zinc layered hydroxide nanocompositesScientific World Journal2014201440146025050392
- SaifullahBEl ZowalatyMEArulselvanPAntimycobacterial, antimicrobial, and biocompatibility properties of para-aminosalicylic acid with zinc layered hydroxide and Zn/Al layered double hydroxide nanocompositesDrug Des Devel Ther2014810291036
- SaifullahBArulselvanPEl ZowalatyMEDevelopment of a biocompatible nanodelivery system for tuberculosis drugs based on isoniazid-Mg/Al layered double hydroxideInt J Nanomedicine201494749476225336952
- SaifullahBEl ZowalatyMEArulselvanPAnti-tuberculosis nanodelivery formulation with high efficacy and biocompatibility based on isoniazid-Zn/Al layered double hydroxide nanocompositesJ Biomed Nanotechnol2014
- NielsenTOHuman germline gene therapyMcgill J Med19973126132
- Lister Hill National Center for Biomedical CommunicationsHandbook: Help Me Understand GeneticsWashingtonUS Department of Health and Human Services2014
- LadewigKXuZPLuGQLayered double hydroxide nanoparticles in gene and drug deliveryExpert Opin Drug Deliv2009690792219686052
- OhJMChoiSJKimSTChoyJHCellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: enhanced efficacy due to clathrin-mediated endocytosisBioconjug Chem2006171411141717105218
- DongLEGouGJiaoLCharacterization of a dextran-coated layered double hydroxide acetylsalicylic acid delivery system and its pharmacokinetics in rabbitActa Pharm Sin B20133400407
- LadewigKNiebertMXuZPGrayPPLuGQControlled preparation of layered double hydroxide nanoparticles and their application as gene delivery vehiclesAppl Clay Sci201048280289
- LiSDLiJHWangCLCellular uptake and gene delivery using layered double hydroxide nanoparticlesJ Mater Chem B20136168
- HuHXiuKMXuSLYangWTXuFJFunctionalized layered double hydroxide nanoparticles conjugated with disulfide-linked polycation brushes for advanced gene deliveryBioconjug Chem20132496897823682934
- WuPXLiWZhuYJTJTangYNZhuNWGuoCLThe protective effect of layered double hydroxide against damage to DNA induced by heavy metalsAppl Clay Sci20141007683
- SwadlingJBCoveneyPVGreenwellHCStability of free and mineral-protected nucleic acids: implications for the RNA worldGeochim Cosmochim Acta201283360378
- ZhangHOuyangDMurthyVWongYXuZSmithSCHydrotalcite intercalated siRNA: computational characterization of the interlayer environmentPharmaceutics2012429631324300233
- PeerDKarpJMHongSFarokhzadOCMargalitRLangerRNanocarriers as an emerging platform for cancer therapyNat Nanotechnol2007275176018654426
- GilletJPGottesmanMMechanisms of multidrug resistance in cancerZhouJMulti-drug Resistance in CancerNew YorkHumana20104776
- SaadMGarbuzenkoOBMinkoTCo-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancerNanomedicine2008376177619025451
- LongleyDBHarkinDPJohnstonPG5-Fluorouracil: mechanisms of action and clinical strategiesNat Rev Cancer2003333033812724731
- KhanMOngZYWiradharmaNAttiaABYangYYAdvanced materials for co-delivery of drugs and genes in cancer therapyAdv Healthc Mater2012137339223184770
- FanHHuQDXuFJLiangWQTangGPYangWTIn vivo treatment of tumors using host-guest conjugated nanoparticles functionalized with doxorubicin and therapeutic gene pTRAILBiomaterials2012331428143622079004
- LiLGuWChenJChenWXuZPCo-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticlesBiomaterials2014353331333924456604
- XuZPWalkerTLLiuKLCooperHMLuGQBartlettPFLayered double hydroxide nanoparticles as cellular delivery vectors of supercoiled plasmid DNAInt J Nanomedicine2007216317417722544
- HuRChenYYZhangLMSynthesis and characterization of in situ photogelable polysaccharide derivative for drug deliveryInt J Pharm201039397104
- RojasRPalenaMCJimenez-KairuzAFManzoRHGiacomelliCEModeling drug release from a layered double hydroxide ibuprofen complexAppl Clay Sci201262–631520
- SaifullahBHusseinMZHussein Al AliSHControlled-release approaches towards the chemotherapy of tuberculosisInt J Nanomedicine201275451546323091386
- KuraAUHussein Al AliSHHusseinMZFakuraziSArulselvanPDevelopment of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agentInt J Nanomedicine201381103111023524513
- Hussein Al AliSHAl-QubaisiMHusseinMZIsmailMBulloSHippuric acid nanocomposite enhances doxorubicin and oxaliplatin-induced cytotoxicity in MDA-MB231, MCF-7 and Caco2 cell linesDrug Des Devel Ther201372531
- CooperGMHausmanREThe Cell: A Molecular Approach2nd edSunderland (MA)Sinauer Associates2000
- XiaSJNiZMXuQHuBXHuJLayered double hydroxides as supports for intercalation and sustained release of antihypertensive drugsJ Solid State Chem200818126102619
- WangZWangEGaoLXuLSynthesis and properties of Mg2Al layered double hydroxides containing 5-fluorouracilJ Solid State Chem2005178736741
- MasarudinMJYusoffKRahimRAHusseinMZSuccessful transfer of plasmid DNA into in vitro cells transfected with an inorganic plasmid-Mg/Al-LDH nanobiocomposite material as a vector for gene expressionNanotechnology20092004560219417322
- RamliMHusseinMZYusoffKPreparation and characterization of an anti-inflammatory agent based on a zinc-layered hydroxide-salicylate nanohybrid and its effect on viability of Vero-3 cellsInt J Nanomedicine2013829730623345976
- Hussein-Al-AliSHAl-QubaisiMEl ZowalatyMIsmailMHusseinMZCytotoxicity and antimicrobial activity studies of an ellagic acid-zinc layered hydroxide intercalation compoundAdv Sci Eng Med2013515041513
- OokuboAOoiKHayashiHHydrotalcites as potential adsorbents of intestinal phosphateJ Pharm Sci199281113911401447721
- Del ArcoMCebaderaEGutiérrezSMg/Al layered double hydroxides with intercalated indomethacin: synthesis, characterization, and pharmacological studyJ Pharm Sci2004931649165815124221
- HeynemanCALawless-LidayCWallGCOral versus topical NSAIDs in rheumatic diseases: a comparisonDrugs20006055557411030467
- GriffinMREpidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injuryAm J Med199810441S42S
- KantorTGKetoprofen: a review of its pharmacologic and clinical propertiesPharmacotherapy19866931033526298
- PolissonRNonsteroidal anti-inflammatory drugs: practical and theoretical considerations in their selectionAm J Med199610031S36S8604725
- SilionMHritcuDJabaIMIn vitro and in vivo behavior of ketoprofen intercalated into layered double hydroxidesJ Mater Sci Mater Med2010213009301820820886
- YuJChungHEChoiSJAcute oral toxicity and kinetic behaviors of inorganic layered nanoparticlesJournal of Nanomaterials20132013628381
- Flesken-NikitinAToshkovINaskarJToxicity and biomedical imaging of layered nanohybrids in the mouseToxicol Pathol20073580681217943654
- KuraAUCheahPSHusseinMZToxicity evaluation of zinc aluminium levodopa nanocomposite via oral route in repeated dose studyNanoscale Res Lett2014926124948886