Abstract
This report compares the effect of lipid and polymeric nanoparticles upon human neutrophils in the presence of cationic surfactants. Nanostructured lipid carriers and poly(lactic-co-glycolic) acid nanoparticles were manufactured as lipid and polymeric systems, respectively. Some cytotoxic and proinflammatory mediators such as lactate dehydrogenase (LDH), elastase, O2•−, and intracellular Ca2+ were examined. The nanoparticles showed a size of 170–225 nm. Incorporation of cetyltrimethylammonium bromide or soyaethyl morpholinium ethosulfate, the cationic surfactant, converted zeta potential from a negative to a positive charge. Nanoparticles without cationic surfactants revealed a negligible change on immune and inflammatory responses. Cationic surfactants in both nanoparticulate and free forms induced cell death and the release of mediators. Lipid nanoparticles generally demonstrated a greater response compared to polymeric nanoparticles. The neutrophil morphology observed by electron microscopy confirmed this trend. Cetyltrimethylammonium bromide as the coating material showed more significant activation of neutrophils than soyaethyl morpholinium ethosulfate. Confocal microscope imaging displayed a limited internalization of nanoparticles into neutrophils. It is proposed that cationic nanoparticles interact with the cell membrane, triggering membrane disruption and the following Ca2+ influx. The elevation of intracellular Ca2+ induces degranulation and oxidative stress. The consequence of these effects is cytotoxicity and cell death. Caution should be taken when selecting feasible nanoparticulate formulations and cationic additives for consideration of applicability and toxicity.
Introduction
The application of nanoparticles for biomedical use has increased during the past decades, with its incorporation into drug and gene delivery, bioimaging, and diagnosis.Citation1 The nanosystems for biomedicine can be categorized into three groups: metallic, lipid, and polymeric nanoparticles.Citation2 Among the three nanosystems, greater efforts have been made recently to develop lipid and polymeric nanoparticles in a clinical setting. Nanostructured lipid carriers (NLCs) are new-generation lipid nanoparticles prepared from both solid and liquid lipids. The core matrix of an NLC is basically soft. NLCs possess some advantages for therapy and diagnosis, including improved drug permeability, reduced adverse response, prolonged half-life, and drug/gene targeting.Citation3 Poly(lactic-co-glycolic) acid (PLGA) nanoparticles are frequently used polymeric nanocarriers with biodegradability and biocompatibility. PLGA is approved by the US Food and Drug Administration (FDA) for use in parenteral injections, implants, and drug delivery systems.Citation4 PLGA nanoparticles hold a hard matrix in the particulate core. They are widely employed for vaccinations, cancer therapy, and theranostics.Citation5 Surface coating exhibits an important role in the functionality of nanomedicine. Cationic components are usually incorporated into nanoparticles for the purpose of improving cell uptake, conjugation with genes, open tight junction, and increased drug loading.Citation6,Citation7 Such nanosystems are useful in the application of gene therapy, tumor targeting, brain delivery, and immunization.Citation8
A concern with nanosystems is the possibility of adverse effects on cells and organisms due to their smaller size and larger specific surface area compared to bulk materials. The cationic surface of nanoparticles further augments the toxicity,Citation9 resulting in limited applicability. Nanoparticles also influence the immunological response of cells. Polymorphonuclear neutrophils (PMNs) play an essential role in immunopathogenesis. Neutrophils participate in the communication networks that form the foundations of immunity. As one of the first cell types to arrive at sites of infection, PMNs secrete cytokines and chemokines that are critical in the unfolding of the inflammatory response and in establishing the correct environmental conditions for launch of the adaptive immune response.Citation10 PMNs are the most abundant leukocytes in circulation, representing about 65% of the total blood cells. Neutrophils are the first leukocytes to respond to foreign materials by producing proinflammatory mediators. Excessive activation of PMNs would cause tissue injury. Although some studies have evaluated the effect of metallic nanoparticles on neutrophils,Citation11,Citation12 few studies have examined the neutrophil response to lipid and polymeric nanoparticles. A detailed investigation of the role of cationic surfactants in nanosystems for cytotoxicity and inflammation of human neutrophils was justified in this study, with an objective to elucidate different effects between lipid and polymeric nanoparticles. Another objective was to examine whether different surfactants could affect the extent of neutrophil activation. The two cationic surfactants used in this work were cetyltrimethylammonium bromide (CTAB) and soyaethyl morpholinium ethosulfate (SME). Both are reported to be toxic to cells and organs, such as fibroblasts, the liver, and kidneys.Citation7,Citation13
The nanoparticles were characterized for size, the polydispersity index (PDI), and surface charge before testing neutrophil responses. To achieve the purpose of this study, cell viability, lactate dehydrogenase (LDH) release, degranulation, respiratory burst, and Ca2+ influx of PMNs in response to nanodispersions were detected. The cellular uptake and neutrophil morphology after incubation of nanoparticles were also determined to explore mechanisms related to immunomodulation and inflammation.
Materials and methods
Materials
Soybean oil, Pluronic® F68, CTAB, PLGA (50:50, molecular weight 30–60 kDa), and poly(vinyl alcohol) were provided by Sigma-Aldrich Co. (St Louis, MO, USA). Cetyl palmitate was purchased from Acros (Geel, Belgium). SME was supplied by Croda (East Yorkshire, UK). Soybean phosphatidylcholine (SPC) (Phospholipon® 80H) was from American Lecithin (Oxford, CT, USA). Hydroethidine (HE) and Fura-2 AM were purchased from Thermo Fisher Scientific (Waltham, MA, USA). 1,2-Bis(2-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid (BAPTA-AM) was from Calbiochem (Billerica, MA, USA). Rhodamine 800 was provided by AAT Bioquest (Sunnyvale, CA, USA).
Preparation of NLCs
Soybean oil (6% w/w of the final product), cetyl palmitate (6%), and SPC (1%) were blended as the lipid phase, while the aqueous phase was the mixture of water and Pluronic F68 (3.5%). CTAB or SME (2%) was incorporated in the lipid phase when preparing cationic NLCs at room temperature. Both phases were heated at 85°C for 15 minutes separately. The aqueous phase was mixed into the lipid phase and homogenized by a high-shear homogenizer for 20 minutes at 12,000 rpm. The mixture was further treated with a probe-type sonicator for 15 minutes to produce the final product.
Preparation of PLGA nanoparticles
Polymeric nanoparticles were manufactured based on a solvent extraction/evaporation technique. PLGA (0.3% w/w of the final product) was dissolved in 3 mL acetone as the organic phase. The aqueous phase was made up of poly(vinyl alcohol) (2.4%) and cationic surfactants (2%) in water. The aqueous phase was heated at 85°C for 15 minutes, and then treated by a probe-type sonicator for 5 minutes. After cooling, the organic phase was slowly pipetted into the aqueous phase. The mixture was stirred overnight to evaporate the organic solvent. Purification of nanoparticles was performed by ultracentrifugation (9,000 rpm for 30 minutes). Pellet resuspension was carried out by vortexing.
Size and zeta potential
The mean diameter (z-average) and zeta potential of nanoparticles were detected using a laser-scattering method (Zetasizer Nano ZS90; Malvern Instruments, Malvern, UK). The nanodispersions were diluted 100-fold with water before testing. The measurement was repeated three times per sample for three batches. The particle number of nanosystems was detected using the qNano particle counter (Izon, Christchurch, New Zealand).
Preparation of human neutrophils
Human PMNs were purified from healthy volunteers (20–30 years old) using a protocol approved by the institutional review board at Chang Gung Memorial Hospital (Kweishan, Taiwan). The volunteers provided written informed consent in order to participate. Neutrophils were isolated with sedimentation prior to centrifugation in a Ficoll-Hypaque gradient and hypotonic lysis of erythrocyte.Citation14
Cell viability
Survival of PMNs was measured with a water-soluble tetrazolium (WST) cell proliferation reagent (WST-1) (Hoffman-La Roche Ltd., Basel, Switzerland). Cells (1×10Citation6 cells/mL) were treated with various concentrations (1/3,000, 1/1,000, and 1/500 dilution of the nanodispersion) of nanoparticles for 15 minutes at 37°C. The treated medium was centrifuged at 200× g for 8 minutes. The supernatant was then removed. WST-1 reagent was added and, after 1 hour’s incubation at 37°C, the absorbance was measured at 450 nm.
LDH release
The LDH level was examined using a commercially available method (CytoTox 96® Cytotoxicity Assay; Promega Corporation, Fitchburg, WI, USA). PMNs (6×10Citation5 cells/mL) were equilibrated at 37°C for 2 minutes and then treated with nanoparticles at different concentrations for 15 minutes. The cytotoxicity was represented by LDH release in cell-free medium as a percentage of the total LDH released. The total LDH release was detected by lysing cells with 0.1% Triton X-100.
Elastase release
MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide was employed as the elastase substrate. After supplementation with the substrate (100 μM), neutrophils (6×10Citation5 cells/mL) were equilibrated at 37°C for 2 minutes and then treated with nanoparticles for 15 minutes. The absorbance at 405 nm was monitored for 15 minutes to determine the elastase level.
Intracellular superoxide anion (O2•−) production
HE can penetrate into cells and exhibit fluorescence at an excitation wavelength of 480 nm after intracellular oxidation by oxygen radicals. PMNs (2.5×10Citation6 cells/mL) were loaded with HE (10 μM) at 37°C for 5 minutes. PMNs were incubated with nanoparticles for 15 minutes at 37°C. After treatment, Hank’s balanced salt solution (HBSS) at 4°C was used to stop the reaction, and the fluorescence was monitored by flow cytometry. There were 10,000 cells analyzed on the flow cytometer per sample.
Intracellular Ca2+
PMNs (3×10Citation6 cells/mL) were loaded with Fura-2 AM (2 μM) at 37°C for 40 minutes. After being washed, neutrophils were resuspended in HBSS. Nanoparticles were added into the medium with neutrophils for 15 minutes at 37°C. The change in fluorescence was examined by a spectrofluorometer with continuous stirring. The excitation and emission wavelengths were set at 340/380 nm and 505 nm, respectively. The detailed procedure was described in a previous report.Citation15
Inhibition of Ca2+ and inflammatory mediators by BAPTA-AM
In another set of experiments, including determinations of LDH, elastase, O2•−, and Ca2+, BAPTA-AM was joined into neutrophil medium for suppressing Ca2+ homeostasis. BAPTA-AM (20 μM) was added into neutrophil suspensions in Ca2+-free solution before nanoparticle treatment. The other procedures were in accordance with the methods described in the earlier sections.
Cellular uptake examined by confocal laser scanning microscopy
PMNs (1.8×10Citation7 cells/mL) were equilibrated at 37°C for 2 minutes. Subsequently, rhodamine 800-loaded nanoparticles were added into neutrophil medium at 37°C for 5 minutes. The concentration of rhodamine 800 in nanodispersion was 0.1 mg/mL. HBSS was used to terminate the reaction at 4°C. The fluorescence inside the cells was imaged by confocal laser scanning microscopy (CLSM) as a measure of nanoparticle uptake into the cells.
Cell morphology examined by scanning electron microscopy
PMNs (1.8×10Citation7 cells/mL) were incubated with nanoparticles for 5 minutes. The suspension was centrifuged at 200× g at 4°C for 8 minutes. Glutaraldehyde (0.5%) was added to the neutrophils after supernatant removal. A cacodylate buffer with glutaraldehyde (3%) and paraformaldehyde (2%) was added to fix PMNs overnight, then postfixed in cacodylate buffer containing osmium tetraoxide. The cells were dehydrated by ethanol, dried, mounted on stubs, and sputter-coated with gold palladium alloy. The morphology of PMNs was imaged under scanning electron microscopy (SEM).
Statistical analysis
Experimental results were expressed as the mean ± standard error of the mean, and comparisons were made based on unpaired Student’s t-test or analysis of variance (ANOVA). A probability of <0.001, <0.01, or <0.05 was considered significant.
Results
Size and zeta potential
demonstrates physicochemical characterization of all nanoparticles tested. The lipid nanoparticles without cationic components (blank NLCs [N-b]) showed an average diameter of 255 nm. The size was greatly reduced, to 170 nm (P<0.05), by the incorporation of CTAB (CTAB-coated NLCs [N-c]). On the other hand, SME incorporation did not alter the mean diameter of NLCs (SME-coated NLCs [N-s]). With respect to polymeric nanoparticles, the average size of the formulation without cationic surfactant (blank PLGA nanoparticles [P-b]) was centered on 102 nm. The addition of CTAB (CTAB-coated PLGA nanoparticles [P-c]) and SME (SME-coated PLGA nanoparticles [P-s]) significantly increased (P<0.05) particulate size, to 246 and 220 nm, respectively. All formulations revealed a PDI of <0.2 except N-b (PDI =0.33). The nanodispersions with a PDI value of <0.2 achieved a relatively uniform size. The zeta potential of N-b was negative (−43 mV). CTAB and SME rendered NLCs positively charged (52 mV). The surface charge of unmodified PLGA nanoparticles (P-b) was slightly anionic (−6 mV). Both cationic surfactants provided positive charges to polymeric nanoparticles, with P-s showing a higher charge (39 mV) than CTAB-coated formulations (29 mV). These nanosystems displaying a variety of core materials and surface coatings were then subjected to PMNs for evaluation.
Table 1 The characterization of the lipid nanoparticles and polymeric nanoparticles by particle size, PDI, and zeta potential
Cell viability
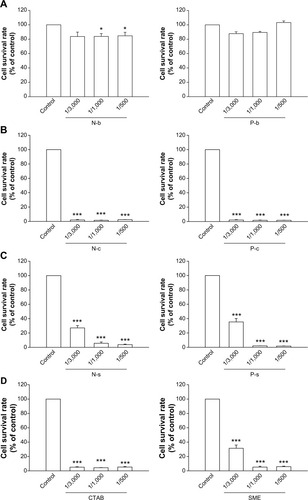
summarizes the survival rate of PMNs after incubation of nanoparticles. As shown in , NLCs without cationic compounds (N-b) slightly but significantly decreased neutrophil viability at higher concentrations (1/1,000 and 1/500). The P-b did not modify the viability of PMNs. N-c completely inhibited cell viability at all concentrations tested (). The cell viability was significantly increased in the group of N-s as compared to the CTAB group (). There was a relationship between N-s concentration and reduction in cell survival rate. Cell survival profiles of P-c and P-s were similar to those of lipid nanoparticles (). In order to examine the contribution of cationic surfactants to cytotoxicity of neutrophils, the cells were exposed to the solutions of corresponding surfactants in the same concentrations as those corresponding to nanosystems (). The results showed a complete inhibition of cell viability by CTAB. A lesser inhibition was observed for SME, especially at the lower concentration (1/3,000).
Figure 1 Effects of nanosystems and cationic surfactants at different concentrations on human neutrophil survival rate.
Notes: (A) Blank nanosystems; (B) CTAB-loaded nanosystems; (C) SME-loaded nanosystems; (D) free cationic surfactants. All data are expressed as the mean ± SEM (n=4). *P<0.05; ***P<0.001 compared to the control.
Abbreviations: CTAB, cetyltrimethylammonium bromide; N-b, blank NLCs; N-c, CTAB-coated NLCs; NLCs, nanostructured lipid carriers; N-s, SME-coated NLCs; P-b, blank PLGA nanoparticles; P-c, CTAB-coated PLGA nanoparticles; PLGA, poly(lactic-co-glycolic) acid; P-s, SME-coated PLGA nanoparticles; SEM, standard error of the mean; SME, soyaethyl morpholinium ethosulfate.
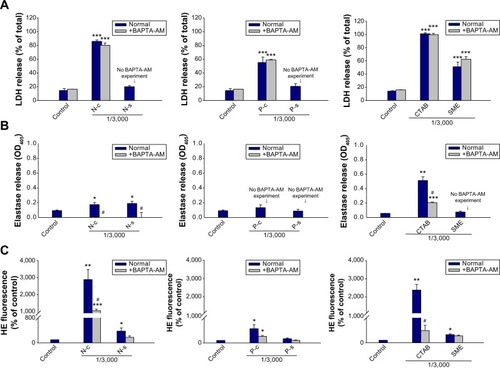
LDH release
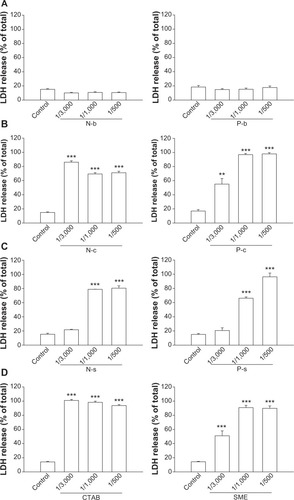
LDH leakage from PMNs reflects cell membrane disruption. shows LDH release in neutrophils treated with nanoparticles. Lipid and polymeric nanoparticles without cationic surfactants produced no effect on LDH (). Positively charged nanoparticles were cytotoxic within the concentrations tested, with CTAB showing greater LDH release than SME (). As the effect of cationic surfactants was examined, CTAB also increased LDH leakage to a greater extent than SME (). At the concentration of 1/3,000, PLGA nanoparticles were less toxic than NLCs according to the LDH level. Polymeric nanoparticles (P-c and P-s) stimulated LDH release in a concentration-dependent manner. It is worth noting that SME at a concentration of 1/3,000 had significantly enhanced LDH in free form, but not in the nanoparticulate form.
Figure 2 Effects of nanosystems and free cationic surfactants at different concentrations on LDH release from human neutrophils.
Notes: (A) Blank nanosystems; (B) CTAB-loaded nanosystems; (C) SME-loaded nanosystems; (D) free cationic surfactants. All data are expressed as the mean ± SEM. (n=4). **P<0.01; ***P<0.001 compared to the control.
Abbreviations: CTAB, cetyltrimethylammonium bromide; LDH, lactate dehydrogenase; N-b, blank NLCs; N-c, CTAB-coated NLCs; NLCs, nanostructured lipid carriers; N-s, SME-coated NLCs; P-b, blank PLGA nanoparticles; P-c, CTAB-coated PLGA nanoparticles; PLGA, poly(lactic-co-glycolic) acid; P-s, SME-coated PLGA nanoparticles; SEM, standard error of the mean; SME, soyaethyl morpholinium ethosulfate.
Elastase release
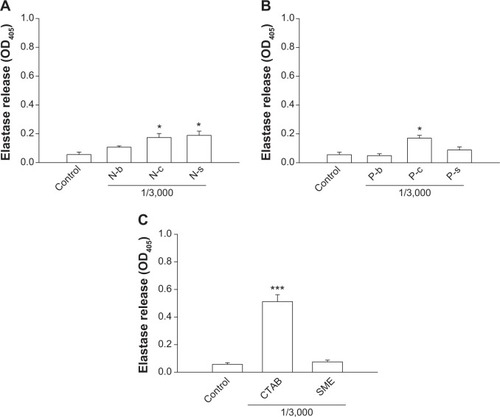
Neutrophil inflammation is a type of immune response in which the body reacts to foreign irritation. One indication is degranulation, which can be measured by elastase release. We evaluated elastase release from PMNs incubated with lipid and polymeric nanoparticles as depicted in . The nanoparticle concentration selected was 1/3,000. N-c and N-s but not N-b stimulated elastase release by PMNs (P<0.05) (). A threefold increase of elastase was detected for cationic NLCs as compared to the control group. Incubation of PMNs with P-c also induced a significant threefold increase (P<0.05) in elastase (). Nevertheless, this increase was not observed by P-s. As illustrated in , treating CTAB in the free form causes an exponential elevation in elastase release. An elevenfold increase in elastase was detected for free CTAB. This indicates that CTAB loading into nanoparticles could shield its influence on PMNs. Free SME did not cause neutrophil degranulation.
Figure 3 Effects of nanoparticles and cationic surfactants on elastase release from human neutrophils.
Notes: (A) Lipid nanoparticles; (B) polymeric nanoparticles; (C) cationic surfactants. All data are expressed as the mean ± SEM (n=4). *P<0.05; ***P<0.001 compared to the control.
Abbreviations: CTAB, cetyltrimethylammonium bromide; N-b, blank NLCs; N-c, CTAB-coated NLCs; NLCs, nanostructured lipid carriers; N-s, SME-coated NLCs; P-b, blank PLGA nanoparticles; P-c, CTAB-coated PLGA nanoparticles; PLGA, poly(lactic-co-glycolic) acid; P-s, SME-coated PLGA nanoparticles; SEM, standard error of the mean; SME, soyaethyl morpholinium ethosulfate.
O2•− production
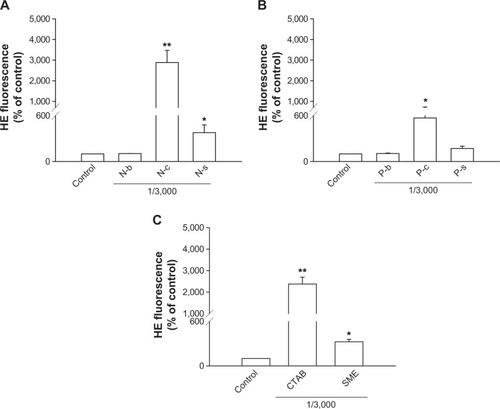
Another relevant aspect of neutrophil activation is the oxygen-dependent pathway that refers to respiratory burst. Whether this mechanism was involved in cationic nanoparticle-induced activation was explored by examining O2•− generation as shown in . The profiles of O2•− production induced by cationic surfactants in nanoparticulate and free forms were similar to those of elastase. NLCs containing CTAB and SME significantly increased O2•−, by 30- and fourfold, respectively (). In the group of polymeric nanoparticles, only CTAB-coated formulations induced an oxygen-dependent mechanism (). SME-coated polymeric nanosystems failed to alter basal O2•−. Both free CTAB and SME increased O2•− in PMNs, as shown in . Again, a greater response was revealed with CTAB than with SME.
Figure 4 Effects of nanoparticles and cationic surfactants on O2•− production of human neutrophils.
Notes: (A) Lipid nanoparticles; (B) polymeric nanoparticles; (C) cationic surfactants. All data are expressed as the mean ± SEM (n=4). *P<0.05; **P<0.01; compared to the control.
Abbreviations: CTAB, cetyltrimethylammonium bromide; HE, hydroethidine; N-b, blank NLCs; N-c, CTAB-coated NLCs; NLCs, nanostructured lipid carriers; N-s, SME-coated NLCs; P-b, blank PLGA nanoparticles; P-c, CTAB-coated PLGA nanoparticles; PLGA, poly(lactic-co-glycolic) acid; P-s, SME-coated PLGA nanoparticles; SEM, standard error of the mean; SME, soyaethyl morpholinium ethosulfate.
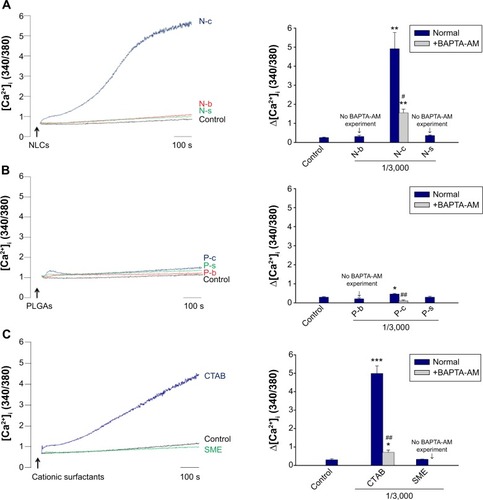
Intracellular Ca2+
The time course of Ca2+ in PMNs after incubation with nanoparticles and cationic surfactants is shown in . demonstrates the quantification of intracellular Ca2+. There was a correlation between Ca2+ and O2•−. Blank and SME-containing nanoparticles exhibited a comparable Ca2+ curve compared to the control group (). A great increase of Ca2+ was observed for CTAB-containing nanoparticles. NLCs with CTAB (ie, N-c) displayed a higher Ca2+ concentration in comparison with PLGA nanoparticles. Free CTAB but not SME evoked an immediate increase of Ca2+, as shown in .
Figure 5 The effect of nanoparticles and cationic surfactants on Ca2+ mobilization in human neutrophils.
Notes: Typical traces and intracellular Ca2+ of the effect of (A) lipid nanoparticles, (B) polymeric nanoparticles, and (C) cationic surfactants. Intracellular Ca2+ of human neutrophils treated with nanoparticles and cationic surfactants was determined with or without BAPTA-AM (20 μM). Some formulations were excluded in the BAPTA-AM experiment due to the lack of elevation of these mediators in the absence of BAPTA-AM. *P<0.05; **P<0.01; ***P<0.001 compared to the control. #P<0.05; ##P<0.01; compared to the corresponding control.
Abbreviations: BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid; CTAB, cetyltrimethylammonium bromide; N-b, blank NLCs; N-c, CTAB-coated NLCs; NLCs, nanostructured lipid carriers; N-s, SME-coated NLCs; P-b, blank PLGA nanoparticles; P-c, CTAB-coated PLGA nanoparticles; PLGA, poly(lactic-co-glycolic) acid; P-s, SME-coated PLGA nanoparticles; s, seconds; SME, soyaethyl morpholinium ethosulfate.
Inhibition of Ca2+ and inflammatory mediators by BAPTA-AM
Ca2+ influx is a main mechanism of neutrophil activation by inflammatory mediators. BAPTA-AM is a Ca2+ influx inhibitor that is used to reduce neutrophil-induced damage during the inflammatory process. PMNs were co-treated with BAPTA-AM to elucidate the role of Ca2+ on toxicity induced by nanoparticles. As demonstrated in , BAPTA-AM partially attenuates the increase in intracellular Ca2+ from N-c. The same phenomenon was observed for free CTAB in aqueous solution (). On the other hand, Ca2+ concentration in PMNs treated with P-c decreased to the baseline level with the addition of BAPTA-AM (). The effect of Ca2+ on the increase of LDH, elastase, and O2•− levels was studied by BAPTA-AM chelating. Some formulations were excluded in this experiment due to the lack of elevation of these mediators in the absence of BAPTA-AM (N-s and P-s for LDH release; P-c, P-s, and free SME for elastase release). shows that there was no inhibition in LDH production after exposure to BAPTA-AM. The chelator depleted elastase in cells treated with NLCs containing CTAB or SME (). BAPTA-AM also inhibited elastase of the free CTAB group. However, this inhibition did not reach the level of the control group. O2•− was suppressed when the cells were treated with BAPTA-AM (). This indicates the requirements of intracellular Ca2+ for O2•− generation in our study.
Figure 6 Effects of treatment of human neutrophils with nanoparticles and cationic surfactants with or without BAPTA-AM (20 μM).
Notes: (A) LDH release; (B) elastase release; (C) O2•− production. Some formulations were excluded in the BAPTA-AM experiment due to the lack of elevation of these mediators in the absence of BAPTA-AM. *P<0.05; **P<0.01; ***P<0.001 compared to the control. #P<0.05; compared to the corresponding control.
Abbreviations: BAPTA-AM, 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid; CTAB, cetyltrimethylammonium bromide; HE, hydroethidine; LDH, lactate dehydrogenase; N-c, CTAB-coated NLCs; NLCs, nanostructured lipid carriers; N-s, SME-coated NLCs; P-c, CTAB-coated PLGA nanoparticles; PLGA, poly(lactic-co-glycolic) acid; P-s, SME-coated PLGA nanoparticles; SME, soyaethyl morpholinium ethosulfate.
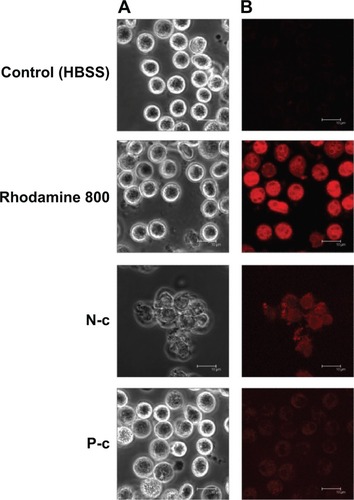
Cellular uptake examined by CLSM
PMNs may be responsive to ingesting nanoparticles, and then undergo immunomodulation and inflammatory activation. The effect of cell internalization for nanoparticle-induced neutrophil response was studied by CLSM. As observed in , the morphology of PMNs treated with free rhodamine 800 was similar to that of the untreated control. A greater degree of fluorescence was noted in the cells exposed to free rhodamine, indicating a cellular inclusion into the cytoplasm. Unlike free dye, NLCs and PLGA nanoparticles containing CTAB were poorly internalized by PMNs. The PMNs treated with N-c showed a disrupted morphology with aggregation. The size of some PMNs treated by CTAB polymeric nanoparticles was larger than that of the untreated PMNs. This may be due to the influx of extracellular water caused by membrane leakage.
Figure 7 Confocal laser scanning microscopy.
Notes: Cell morphology (A) and cellular uptake (B) of human neutrophils treated with free rhodamine 800 and N-c and P-c, and examined by confocal laser scanning microscopy.
Abbreviations: CTAB, cetyltrimethylammonium bromide; HBSS, Hank’s balanced salt solution; N-c, CTAB-coated nanostructured lipid carriers; P-c, CTAB-coated poly(lactic-co-glycolic) acid nanoparticles.
Cell morphology examined by SEM
The appearance of PMNs was further examined by SEM. As shown in , the control group displays a spherical morphology. The membranes of PMNs treated by N-c were deformed and damaged. The surface morphology was more complex than that of the control. The level of damage seemed to be less for PMNs treated with PLGA nanoparticles than for NLCs. Some pores were found in the cell membrane, possibly indicating the formation of micropores or leaks by PLGA nanoparticles.
Figure 8 The morphology of human neutrophils examined by scanning electron microscopy.
Notes: Human neutrophils were examined after treatment with (A) HBSS (control), (B) N-c, and (C) P-c.
Abbreviations: CTAB, cetyltrimethylammonium bromide; HBSS, Hank’s balanced salt solution; N-c, CTAB-coated nanostructured lipid carriers; P-c, CTAB-coated poly(lactic-co-glycolic) acid nanoparticles.

Discussion
The loading of cationic surfactants in nanosystems exhibited different effects on particulate size. The diameter of NLCs was not changed by SME incorporation, but it was significantly decreased by the addition of CTAB. This may indicate that CTAB had an emulsifying effect on lipid nanoparticles, contributing to size reduction. SME did not exert this effect on lipid nanoparticles. Contrary to lipid systems, both cationic surfactants increased the size of polymeric nanopar-ticles by >2-fold. The emulsifier system with a determined hydrophile–lipophile balance can stabilize nanoparticles with a certain polarity.Citation16 CTAB and SME may not fit this criterion, and thus did not show emulsifier activity on PLGA nanoparticles. The occupation of cationic surfactants in the interface led to the enlargement of a particulate dimension.
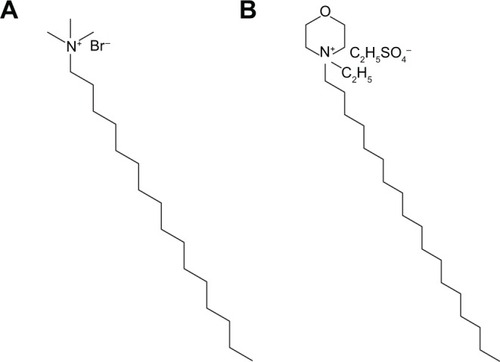
The typical adult has a blood volume of 4.7–5.0 L. The injection volume for the commonly used intravenous medications is between approximately 1 and 5 mL, which shows a dilution of 1/5,000–1/1,000 to the total blood volume. Our tested concentrations of the nanosystems were physiologically relevant to the medical treatment. Previous studiesCitation9,Citation17 have also demonstrated that cytotoxicity of cationic nanoparticles is a result of cell death. The results of cell viability showed that PLGA nanoparticles in the absence of cationic surfactants (P-b) exhibited no apparent cytotoxicity to PMNs. This is consistent with previous reports that PLGA nanoparticles show a negligible cytotoxicity to cells such as fibroblasts, macrophages, and lymphocytes.Citation18,Citation19 In the absence of cationic coating, lipid nanoparticles (N-b) at higher concentrations elicited slight cell death. SPC and soybean oil in NLCs may be the inducers of cytotoxicity.Citation20,Citation21 However, the N-b did not alter the release of other mediators from PMNs. This indicates that the adverse effect of SPC and soybean oil was minimal. CTAB and SME possess amino moieties in their structures (), which would be ionized in a physiological environment and cause a strong interaction to a negatively charged cell membrane. This adherence can result in cell lysis and death. Cell membrane damage is a characteristic of cytotoxicity mediated by necrosis.Citation22 Our results showed a relationship between LDH release and neutrophil death, suggesting that PMNs were disintegrated by cationic nanoparticles as evidenced by compromised membrane integrity.
Figure 9 Chemical structures of (A) cetyltrimethylammonium bromide and (B) soyaethyl morpholinium ethosulfate.
Elastase is packed in granules that are released upon activation.Citation23 CTAB in free form demonstrated greater elastase release than that in nanoparticulate form. This may be due to the immobilization of CTAB by the nanoparticulate surface possibly lessening the exposed probability to PMNs as compared to free CTAB molecules. Neutrophil-derived elastase exerts the ability to degrade the extracellular matrix component, resulting in tissue and organ injury.Citation24 Production of O2•−, a precursor of reactive oxygen species, is involved in the destruction of tissues surrounding activated neutrophils. Huang et alCitation25 have mentioned that both O2•− generation and LDH can reflect oxidative stress of PMNs. As was the case with degranulation, CTAB elicited a greater oxidative burst than SME. Bhattacharjee et alCitation26 also suggest that oxidative stress is a principal mechanism for cellular injury induced by cationic nanoparticles. The subsequent risk of respiratory burst elicited by nanoparticles should be concerned, since neutrophil-derived O2•− may promote carcinogenesis by genotoxic stress and genomic instability.Citation10 Moreover, O2•− and proinflammatory mediators trigger a systemic hypersensitivity reaction and shock/disseminated intravascular coagulation.Citation27
An earlier incident during the activation of PMNs is the elevation of intracellular Ca2+.Citation28,Citation29 The same as O2•− production, our results showed that CTAB and NLCs were key components generating intracellular Ca2+ in PMNs. BAPTA-AM as a chelator can directly suppress Ca2+ influx. It was found that BAPTA-AM inhibited Ca2+, elastase, and O2•− concentrations raised by cationic nanoparticles. According to the experimental profiles shown in this report, we can speculate possible pathways about cytotoxicity and inflammation elicited by nanosystems. Cationic nanoparticles are conceivably incorporated into lipid rafts in plasma membrane,Citation30 which are microdomains in the cell membrane with a high level of glycosphingolipids and protein receptors. Previous studiesCitation31,Citation32 indicate the adhesion of lipid and PLGA nanoparticles onto lipid rafts of the cell surface. Exposure of cationic nanoparticles thus compromised the membrane integrity, which allowed LDH elevation and the subsequent release of cellular contents due to leakage. Either Ca2+ or O2•− initiate membrane disruption pathways in response to nanoparticles.Citation25 The loss of membrane integrity leads to Ca2+ influx. O2•− is released from cytoplasm membrane and intracellular organs as the membrane is stimulated by nanoparticles.Citation33 The relationship between Ca2+ and O2•− has been investigated for human neutrophils. Ca2+ mobilization plays a role in regulating O2•− secretion by PMNs after triggering Ca2+ influx.Citation34 Another possibility is that oxidative stress induces oxidant damage, leading to intracellular Ca2+ influx and subsequent cell death.Citation35 Ca2+ and O2•− have been shown to play important roles in governing neutrophil degranulation. Ca2+ mobilization is necessary for the activation of the elastase pathway.Citation36 Oxidative stress can pump electrons into phagocytic vacuoles, causing a movement of compensating ions that activate elastase.Citation37 Cytotoxicity and apoptosis of neutrophils occur via the pathways of degranulation and respiratory burst.Citation38 The increase of elastase and O2•− by nanoparticles confirmed degranulation- and oxygen-dependent inductions of apoptosis.
Although Ca2+ influx may be inferred as a mechanism evoking death of PMNs, our results demonstrated a negligible change of LDH after treatment with BAPTA-AM. This suggests that cationic nanoparticles also developed a Ca2+-independent cell death. The level of some mediators was not completely inhibited to the normal range by BAPTA-AM. This is possibly due to insufficient inhibition concentration (20 μM) for chelation of Ca2+. Another practicability is the possible plenty increase of intracellular Ca2+ by the endoplasmic reticulum and Golgi inside the cells but not just extracellular influx.Citation39
Besides cell membrane interaction and fusion, nanoparticle internalization into PMNs may be another factor in cytotoxicity and inflammatory activation. Nanoparticles can be endocytosed by cells through pinocytosis and clathrin- and caveolin-mediated routes.Citation40 Based on CLSM imaging, rhodamine-loaded nanoparticles had a minimal effect to be internalized into PMNs as compared to free dye. It is possible for polymer chains in polymeric nanoparticles to form a steric barrier, thus avoiding the phagocytic process.Citation41,Citation42 Modification of lipid nanoparticles by Pluronic F68 also reduces cellular uptake by PMNs.Citation43 The membrane collapse by cationic nanosystems as observed by CLSM and SEM correlated well with the reduced neutrophil viability. The experimental result showed that neutrophil activation was not necessarily correlated to cellular uptake. Interaction between the cell membrane and nanoparticles was enough to trigger a biological effect.
It is generally accepted that nanoparticles presenting more positive charges can facilitate a biological effect more easily because of their affinity to negatively charged membrane. Lipid nanoparticles exhibited a higher positive charge than polymeric nanoparticles, leading to greater cytotoxicity to PMNs. The zeta potential of CTAB-containing formulations did not surpass that of SME-containing formulations, although CTAB exerted higher activation on PMNs. This indicates that the surface charge was not the sole factor regulating interaction between PMNs and cationic nanoparticles. N-c showed a smaller size and a larger total surface area than N-s; this resulted in a greater toxic effect on PMNs. However, this inference cannot explain the case of polymeric nanoparticles, since the size of CTAB-coated formulations was larger than that of SME-coated ones. Particulate size did not necessarily imply neutrophil activation. Neutrophil response to cationic nanoparticles was dependent upon the particulate types and surfactants selected. The particulate cores of lipid and PLGA nanosystems can be considered to be soft and rigid, respectively. High flexibility of nanoparticles can act as a positive factor for interaction and fusion with the plasma membrane.Citation44 This may be the reason why lipid nanoparticles displayed a greater neutrophil response than polymeric nanoparticles. We also determined the number of particles in N-c and PLGA nanoparticles using the qNano particle counter. The particle concentration of N-c and P-c was 1.5×10Citation12 and 1.1×10Citation11 particles/mL, respectively. The higher concentration of NLCs than PLGA nanoparticles was the possible reason for greater responses being exhibited.
The molecular structure of surfactants at the interface of nanoparticles influences the biophysical interaction with the cell membrane. To summarize the above results pertaining to cytotoxicity and immunomodulation, CTAB in free or nanoparticulate form was more cytotoxic to PMNs than SME. The quaternary ammonium group in the structures of CTAB and SME was the main moiety producing cytotoxicity. Both CTAB and SME possessed an alkyl chain to be entrapped into particulate cores, while the hydrophilic amino group was exposed outside the shells. As shown in , the N+ of CTAB in nanoparticles may directly interact with PMNs due to its location in the margin of the structure. Peetla and LabhasetwarCitation45 also reported that CTAB-coated polymeric nanoparticles strongly anchored onto the lipid domains of the endothelial cell membrane. On the other hand, the N+ in SME was enclosed in a morpholin six-membrane structure. The SME structure shielded the quaternary ammonium, resulting in difficulty contacting the plasma membrane.
Conclusion
Cationic additives in nanoparticles could trigger cell death and toxic responses. Inflammatory mediators of PMNs including elastase and intracellular Ca2+ were increased by cationic nanoparticles. These nanosystems also induced O2•− generation. The mechanisms of cytotoxicity or inflammation caused by cationic nanoparticles can be proposed based on experimental data in the present study. The lipid rafts in the plasma membrane are the targets of nanoparticles. The particles interact with the cell membrane, triggering membrane disruption and leakage. Intracellular Ca2+ is increased due to this disruption, leading to O2•− generation. Both Ca2+ and O2•− elevation can induce degranulation of PMNs. The cell death occurs because of the production of oxidative stress and excitation of inflammation. The nature of the particulate core and the type of cationic surfactants play key roles in the neutrophil response. Lipid nanoparticles generally revealed a greater response to PMNs than polymeric nanoparticles. Inflammatory activation raised by nanoparticulate coating with CTAB was more significant compared to SME coating due to the structural differences. The size and surface charge did not greatly affect the responses to PMNs. We demonstrated that cationic nanoparticles altered the immune response and homeostasis of PMNs. The mediators evaluated in this work may act as efficient markers for diagnosing nanoparticulate toxicity. The fact that nanoparticles exhibited some properties of immunomodulation and inflammation could be of help for designing clinically acceptable nanosystems.
Acknowledgments
The authors are grateful to the financial support of Chang Gung Memorial Hospital (grant number: EMRPD1D901 and CMRPD1B0332).
Disclosure
The authors report no conflicts of interest in this work.
References
- KimBYRutkaJTChanWCNanomedicineN Engl J Med2010363252434244321158659
- FangCLAl-SuwayehSAFangJYNanostructured lipid carriers (NLCs) for drug delivery and targetingRecent Pat Nanotechnol201371415522946628
- TsaiMJWuPCHuangYBBaicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targetingInt J Pharm2012423246147022193056
- SahHThomaLADesuHRSahEWoodGCConcepts and practices used to develop functional PLGA-based nanoparticulate systemsInt J Nanomedicine2013874776523459088
- DanhierFAnsorenaESilvaJMCocoRLe BretonAPréatVPLGA-based nanoparticles: an overview of biomedical applicationsJ Control Release2012161250552222353619
- RamadanALagarceFTessier-MarteauAOral fondaparinux: use of lipid nanocapsules as nanocarriers and in vivo pharmacokinetic studyInt J Nanomedicine201162941295122162653
- PanTLWangPWAl-SuwayehSAHuangYJFangJYToxicological effects of cationic nanobubbles on the liver and kidneys: biomarkers for predicting the riskFood Chem Toxicol201250113892390122809472
- LonezCVandenbrandenMRuysschaertJMCationic liposomal lipids: from gene carriers to cell signalingProg Lipid Res200847534034718424270
- ArisakaMNakamuraTYamadaANegishiYAramakiYInvolvement of protein kinase Cdelta in induction of apoptosis by cationic liposomes in macrophage-like RAW264.7 cellsFEBS Lett201058451016102020122929
- AmulicBCazaletCHayesGLMetzlerKDZychlinskyANeutrophil function: from mechanisms to diseaseAnn Rev Immunol20123045948922224774
- AbrikossovaNSkoglundCAhrénMBengtssonTUvdalKEffects of gadolinium oxide nanoparticles on the oxidative burst from human neutrophil granulocytesNanotechnology2012232727510122706406
- BabinKAntoineFGoncalvesDMGirardDTiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophilsToxicol Lett20132211576323726862
- AielloCAndreozziPLa MesaCRisuleoGBiological activity of SDS-CTAB cat-anionic vesicles in cultured cells and assessment of their cytotoxicity ending in apoptosisColloids Surf B Biointerfaces201078214915420347276
- HwangTLYehSHLeuYLChernCYHsuHCInhibition of superoxide anion and elastase release in human neutrophils by 3′-isoproxychalcone via a cAMP-dependent pathwayBr J Pharmacol20061481788716501579
- HwangTLWangCCKuoYHThe hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activationBiochem Pharmacol20108081190120020599799
- LinCHFangYPAl-SuwayehSAYangSYFangJYPercutaneous absorption and antibacterial activities of lipid nanocarriers loaded with dual drugs for acne treatmentBiol Pharm Bull201336227628623370356
- IwaokaSNakamuraTTakanoSTsuchiyaSAramakiYCationic liposomes induce apoptosis through p38 MAP kinase-caspase-8-Bid pathway in macrophage-like RAW264.7 cellsJ Leukoc Biol200679118419116275897
- de LimaRdo Espirito Santo PereiraAPortoRMFracetoLFEvaluation of cyto- and genotoxicity of poly(lactide-co-glycolide) nanoparticlesJournal of Polymers and the Environment2011191196202
- XiongSGeorgeSYuHSize influences the cytotoxicity of poly(lactic-co-glycolic acid) (PLGA) and titanium dioxide (TiO(2)) nanoparticlesArch Toxicol20138761075108622983807
- SillimanCCElziDJAmbrusoDRLysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calciumJ Leukoc Biol200373451152412660226
- Cury-BoaventuraMFGorjãoRde LimaTMToxicity of a soybean oil emulsion on human lymphocytes and neutrophilsJPEN J Parenter Enteral Nutr200630211512316517956
- LohJWYeohGSaundersMLimLYUptake and cytotoxicity of chitosan nanoparticles in human liver cellsToxicol Appl Pharmacol2010249214815720831879
- CaielliSBanchereauJPascualVNeutrophils come of age in chronic inflammationCurr Opin Immunol201224667167723127555
- WrightHLMootsRJBucknallRCEdwardsSWNeutrophil function in inflammation and inflammatory diseasesRheumatology (Oxford)20104991618163120338884
- HuangCCAronstamRSChenDRHuangYWOxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticlesToxicol In Vitro2010241455519755143
- BhattacharjeeSde HaanLHJEversNMRole of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cellsPart Fibre Toxicol201072520831820
- FlannaganRSCosíoGGrinsteinSAntimicrobial mechanisms of phagocytes and bacterial evasion strategiesNat Rev Microbiol20097535536619369951
- BurgosRAConejerosIHidalgoMAWerlingDHermosillaCCalcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovinesVet Immunol Immunopathol20111431–211021764141
- SalmonMDAhluwaliaJPharmacology of receptor operated calcium entry in human neutrophilsInt Immunopharmacol201111214514821081191
- ArisakaMTakanoKNegishiYArimaHAramakiYInvolvement of lipid rafts in macrophage apoptosis induced by cationic liposomesArch Biochem Biophys20115081727721315683
- SchroederALevinsCGCortezCLangerRAndersonDGLipid-based nanotherapeutics for siRNA deliveryJ Intern Med2010267192120059641
- GrabowskinNHillaireauHVergnaudJToxicity of surface-modified PLGA nanoparticles toward lung alveolar epithelial cellsInt J Pharm2013454268669423747506
- KumazawaRWatariFTakashiNTanimuraYUoMTotsukaYEffects of Ti ions and particles on neutrophil function and morphologyBiomaterials200223173757376412109701
- BréchardSTschirhartEJRegulation of superoxide production in neutrophils: role of calcium influxJ Leukoc Biol20088451223123718519744
- GeorgeSPokhrelSXiaTUse of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through ion dopingACS Nano200941152920043640
- El ZeinNBadranBSaribanEThe neuropeptide pituitary adenylate cyclase activating polypeptide modulates Ca2+ and pro-inflammatory functions in human monocytes through the G protein-coupled receptors VPAC-1 and formyl peptide receptor-like 1Cell Calcium200843327028417651798
- SegalAWHow neutrophils kill microbesAnnu Rev Immunol20052319722315771570
- FialkowLWangYDowneyGPReactive oxygen and nitrogen species as signaling molecules regulating neutrophil functionFree Radic Biol Med200742215316417189821
- BaronSStruyfSWuytackFContribution of intracellular Ca2+ stores to Ca2+ signaling during chemokinesis of human neutrophil granulocytesBiochim Biophys Acta2009179361041104919095014
- VasirJKLabhasetwarVBiodegradable nanoparticles for cytosolic delivery of therapeuticsAdv Drug Deliv Rev200759871872817683826
- MainardesRMGremiãoMPDBrunettiILDa FonsecaLMKhalilNMZidovudine-loaded PLA and PLA-PEG nanoparticles: influence of polymer type on phagocytic uptake by polymorphonuclear cellsJ Pharm Sci200998125726718425813
- LiuCWLinWJPolymeric nanoparticles conjugate a novel heptapeptide as an epidermal growth factor receptor-active targeting ligand for doxorubicinInt J Nanomedicine201274749476722973097
- GoncalvesDMde LizRGirardDActivation of neutrophils by nanoparticlesScientificWorldJournal2011111877188522125444
- VermaAStellacciFEffect of surface properties on nanoparticle-cell interactionsSmall201061122119844908
- PeetlaCLabhasetwarVEffect of molecular structure of cationic surfactants on biophysical interactions of surfactant-modified nanoparticles with a model membrane and cellular uptakeLangmuir20092542369237719161268
