Abstract
Nanoparticles (NPs) are advantageous for the delivery of diagnosis agents to brain tumors. In this study, we attempted to develop an l-cysteine coated FePt (FePt-Cys) NP as MRI/CT imaging contrast agent for the diagnosis of malignant gliomas. FePt-Cys NPs were synthesized through a co-reduction route, which was characterized by transmission electron microscopy, high-resolution transmission electron microscopy, powder X-ray diffraction, Fourier transform infrared spectroscopy, and dynamic light scattering. The MRI and CT imaging ability of FePt-Cys NPs was evaluated using different gliomas cells (C6, SGH44, U251) as the model. Furthermore, the biocompatibility of the as-synthesized FePt-Cys NPs was evaluated using three different cell lines (ECV304, L929, and HEK293) as the model. The results showed that FePt-Cys NPs displayed excellent biocompatibility and good MRI/CT imaging ability, thereby indicating promising potential as a dual MRI/CT contrast agent for the diagnosis of brain malignant gliomas.
Introduction
Malignant brain gliomas are the fourth most common cause of cancer death because of their extremely high rate of morbidity and mortality.Citation1 In the past decade, numerous novel strategies based on various nanomaterials have been developed to conquer the highly mortal brain tumors.Citation2–Citation5 Interesting topics in this field include the targeted and controlled delivery system of anticancer drugs;Citation6–Citation9 photodynamic, photothermal, and magnetothermal therapy of tumors;Citation10–Citation16 and MRI, CT, and fluorescent imaging of brain tumors.Citation17–Citation22 Among various nanomaterials, magnetic nanoparticles (NPs) such as superparamagnetic iron oxide NPs (SPIONs) are the most extensively studied. Applications of these SPIONs range from the drug delivery system to MRI contrast agent and magnetothermal therapy for brain gliomas.Citation23–Citation26 Thus, studying novel magnetic nanomaterials other than SPIONs for application in the diagnosis and therapy of brain gliomas is of great interest.
FePt NPs with chemically disordered face-centered cubic structure have been well studied because of their promising potential in biomedical applications, including as an anticancer agent and for hyperthermic ablation, magnetic separation of bacteria and biomolecules (such as DNA and proteins), and MRI/CT imaging, due to their excellent chemical stability and magnetic properties.Citation27–Citation29 In particular, the theoretical magnetic moment of FePt NPs is as high as ~1,000 emu/cc, which is much higher than that of iron oxide (300–400 emu/cc). Recently, FePt NPs coated with iron oxides, SiO2, and cysteamine have been demonstrated to have promising potential for application as MRI and CT contrast agents.Citation30–Citation33 Although good biocompatibility of SiO2 and iron oxides has been suggested, one of the crucial concerns is the possibility of introducing the exogenous coatings into various tissues and organs (especially the brain) when these nanomaterials are used as therapeutic and diagnostic reagents.Citation34–Citation38
In our previous work,Citation39 FePt NPs showed high activity in suppressing the proliferation of glioma cells depending on their surface coatings. Lipophilic FePt NPs coated with oleic acid/oleylamine significantly suppressed the proliferation of different gliomas cells (U251, U87, and H4) in time- and dose-dependent manners, but no obvious suppressing effect was observed when glioma cells were treated with l-cysteine (Cys)-coated FePt NPs under the same conditions. Furthermore, FePt NPs coated with either oleic acid/oleylamine or Cys were effectively uptaken by different glioma cells. Therefore, it is interesting to explore the potential of FePt-Cys NPs as a MRI/CT imaging agent for the diagnosis of malignant gliomas. In the present work, the use of Cys-coated FePt NPs as MRI/CT contrast agent was evaluated in three glioma cells (C6, SGH44, and U251). The biocompatibility of FePt-Cys NPs was assessed via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on different cells (ECV304, L929, and HEK293). The results demonstrated that FePt-Cys NPs showed favorable biocompatibility and strong signals for dual MR/CT contrast imaging. Therefore, FePt-Cys NPs can potentially be utilized as a dual MRI/CT contrast agent for the diagnosis of malignant brain gliomas.
Materials and methods
Materials
K2PtCl6, FeCl2·H2O, and NaBH4 were of analytical grade (Sinopharm Chemical Reagent Co., Ltd, Shanghai, People’s Republic of China) and were used without further purification. l-cysteine (Cys) was of biochemical grade. Deionized water (16 MΩ·cm) was supplied by a Nanopure water system (Thomas Scientific, Swedesboro, NJ, USA).
The glioma cell lines (C6, SGH44, and U251) and normal cell lines (ECV304, L929, and HEK293) were purchased from the China Center for Type Culture Collection (CCTCC, Wuhan, People’s Republic of China) and preserved in our laboratory.
Synthesis of Cys-coated FePt NPs
FePt-Cys NPs were synthesized according to our previous work.Citation39 In brief, 0.0183 g of Cys (0.15 mmol), 0.3651 g of K2PtCl6 (0.75 mmol), and 0.0497 g of FeCl2·H2O (0.25 mmol) was successively dissolved in 90 mL deionized water in N2 atmosphere under magnetic stirring. Subsequently, 0.1892 g of NaBH4 (5 mmol) was dissolved in another 10 mL of deionized water and added dropwise into the solution containing Cys and metal salts. The reaction was performed for 2 hours. The black precipitate was collected by adding 200 mL of ethanol and centrifuging at 9,000 rpm for 3 minutes. The product was dried in a vacuum at 30°C overnight.
Characterization of Cys-coated FePt NPs
The phase structure of the sample was identified by powder X-ray diffraction (XRD) on an X’Pert PRO diffractometer (PANalytical B.V., Almelo, the Netherlands) using Cu Kα radiation (λ=1.5406 Å). The surface information was analyzed using Fourier transform infrared spectroscopy (FT-IR) on a Nexus Fourier transform infrared spectrophotometer (Thermo Nicolet, Waltham, MA, USA). Atomic absorption spectrometry (GBC AVANTA M; GBC Scientific Equipment Pty Ltd., Melbourne, SA, Australia) was used to analyze the molar ratio of Fe/Pt in the sample. High-resolution transmission electron microscopy (HRTEM) (JEM-2100F; JEOL, Tokyo, Japan) was used to observe the morphology, the particle size, and detailed information on the lattice of the samples. The size of FePt NPs was measured using a dynamic light scattering instrument (Zetasizer Nano ZS90; Malvern Instruments, Malvern, UK). In order to assess the stability of FePt NPs in solution, the change of particle size was measured in different media (deionized H2O and cell culture media) in the period of 7 days.
Relaxivity measurements
In a typical measurement, the sample was dissolved in deionized water and diluted at an iron concentration range of 0–20 μg/mL. The samples were transferred to a 96-well plate, and T2 relaxation time was determined by using a whole-body magnetic resonance (MR) scanner (SignaHDx 3.0T; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA).
CT imaging capability
The as-synthesized FePt-Cys NPs were diluted in distilled water at a Pt concentration range of 0–40 mg/mL. Samples were transferred to a 96-well plate. Iohexol was used as the control. CT imaging ability of the FePt-Cys NPs was determined by using the IVIS Lumina XR system.
MRI and CT imaging of Cys-coated FePt NPs in cells
Different glioma cells (C6, SGH44, and U251) were used to evaluate the MRI and CT imaging ability of FePt-Cys NPs. These glioma cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and antibiotics penicillin (100 U/mL) and streptomycin (100 μg/mL) in a humidified atmosphere with 5% CO2 at 37°C.
In a typical MRI measure, glioma cells were incubated with FePt-Cys NPs dissolved in DMEM at different iron concentrations (0, 5, 10, 15, and 20 μg/mL) at 37°C for 3 hours. After that, the cells were washed with phosphate-buffered saline (0.1 M, pH =7.4) three times. Subsequently, the cells were dispersed and suspended in 300 μL of agarose gel (0.5%). The samples were then quickly transferred to a 96-well plate. MRI imaging was performed by using the 3.0-T whole-body MR scanner.
In a typical CT imaging measurement, glioma cells were incubated with FePt-Cys NPs dissolved in DMEM at different platinum concentrations (0, 2, 5, 10, and 20 mg/mL) at 37°C for 3 hours. After that, the cells were washed with phosphate-buffered saline (pH =7.4) three times. Subsequently, the cells were dispersed and resuspended in deionized water. The samples were then transferred to a black 96-well plate. CT images were acquired by using an IVIS Lumina XR system.
Biocompatibility studies
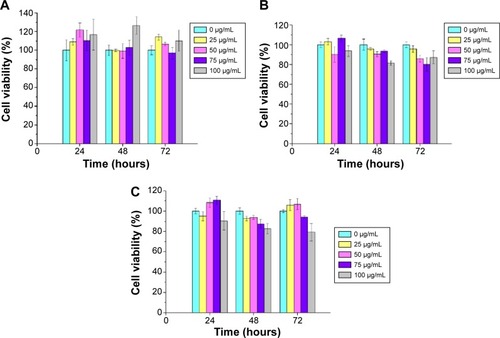
The cytotoxicity of FePt-Cys NPs was evaluated using different cell lines (ECV304, L929, and HEK293) via MTT assays. In brief, these cells were treated with FePt-Cys NPs at different concentrations (25, 50, 75, and 100 μg/mL Fe) and incubated for different times (24, 48, and 72 hours). The control well contained culture medium without the FePt-Cys NPs. Then, 20 μL of MTT (5 mg/mL) was added to each well and incubated for another 4 hours. Formazan crystal was dissolved in 150 μL of dimethyl sulfoxide. The absorbance of each well was measured by a microplate reader (1420 multilabel counter; Perkin Elmer Inc., Waltham, MA, USA) at 490 nm to determine the relative cell viability.
Results and discussion
Transmission electron microscopy (TEM) and HRTEM images of as-synthesized FePt-Cys NPs are shown in . The homogeneous NPs were obtained with slight aggregation (), which could be attributed to low surface protection. The size of as-synthesized FePt-Cys NPs obtained from TEM image was ~4.8±0.6 nm. The perfectly aligned lattice planes, as shown in the upper-right inset of , exhibited a well-crystallized structure. The interplanar spacing of ~0.22 nm obtained from HRTEM image can be ascribed to the adjacent (111) planes of FePt.
Figure 1 TEM (A) and HRTEM (B) images of as-synthesized FePt-Cys NPs.
Note: The arrows in the inset of B indicate the distance between the two adjacent planes.
Abbreviations: d, interplanar distance; FePt-Cys, l-cysteine coated FePt; HRTEM, high-resolution TEM; NPs, nanoparticles; TEM, transmission electron microscopy.

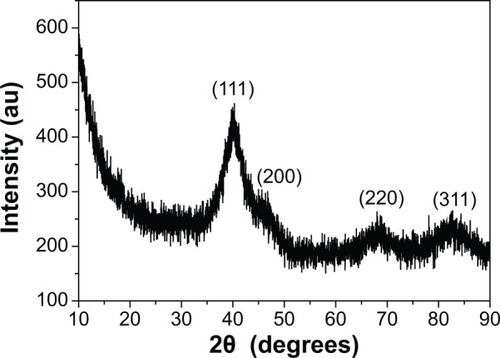
XRD pattern of as-synthesized FePt-Cys NPs is shown in . The diffraction peaks in XRD pattern were indexed to the (111), (200), (220), and (311) planes of FePt. The diffraction peaks derived from planes (111), (220), and (311) were significantly broadened, which could be attributed to the extremely small size of FePt-Cys NPs. The Fe/Pt molar ratio in FePt-Cys NPs was Fe28Pt72 according to the atomic absorption spectrometry measurement.
Figure 2 XRD pattern of FePt-Cys NPs.
Abbreviations: FePt-Cys, l-cysteine coated FePt; NPs, nanoparticles; XRD, powder X-ray diffraction.
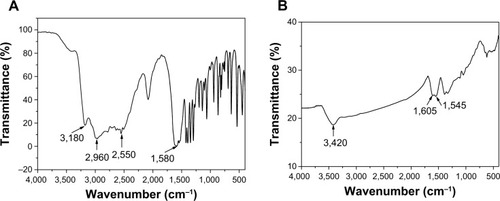
The surface information of FePt-Cys NPs was characterized by FT-IR spectra. shows the FT-IR spectra of pure Cys and FePt-Cys NPs. The absorption bands at 3,200–2,900 cm−1 and ~1,580 cm−1 in were designated as the stretching vibrations of NH3+ and COO−, respectively. These absorption bands were shifted to the higher wavenumbers of ~3,420 cm−1 and 1,605 cm−1 in FT-IR spectrum of FePt-Cys NPs (), respectively, because of the change in the dipole moment when Cys was adsorbed on the surface of NPs with high electron density.Citation40–Citation42 It is noteworthy that the absorption at ~2,550 cm−1 derived from the S–H stretching vibration of the Cys molecules disappeared in the FT-IR spectrum of FePt-Cys NPs (), indicating the interactions between Cys molecules and FePt NPs because of the high affinity of the mercapto group to the Pt atoms.
Figure 3 FT-IR spectra of pure Cys molecules (A) and FePt-Cys NPs (B).
Abbreviations: Cys, l-cysteine; FePt-Cys, l-cysteine coated FePt; FT-IR, Fourier transform infrared spectroscopy; NPs, nanoparticles.
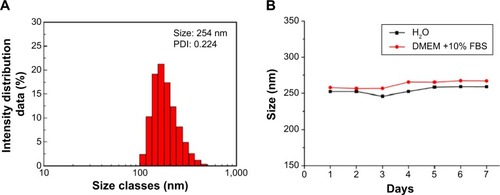
The size distribution and stability of FePt-Cys NPs dispersed in different media were measured. As shown in , the dynamic light scattering hydrodynamic size of FePt-Cys NPs in deionized H2O was~254 nm, which was larger than that obtained from TEM image (). The size of FePt-Cys NPs was slightly increased in a cell culture medium (DMEM +10% fetal bovine serum) compared with that in H2O. FePt-Cys NPs could be aggregated together in different media and the accuracy of hydrodynamic size measured by the light scattering method strongly depends on knowing the optical parameters of the particles (such as refractive index and light absorption characteristics).Citation43 The stability of FePt-Cys NPs in different media was studied by monitoring the change of hydrodynamic size in the period of 7 days. shows that the hydrodynamic size of FePt-Cys NPs was slightly increased from ~252.3 nm to 259.1 nm in H2O and from ~258.0 nm to 267.4 nm in cell culture media at the end of 7 days, respectively, implying a good stability of FePt-Cys NPs in different media.
Figure 4 Size distributions of FePt-Cys dispersed in different media for different times.
Notes: (A) Size distribution of FePt-Cys NPs dispersed in H2O. (B) Size changes of FePt-Cys NPs in H2O and cell culture media (DMEM +10% FBS) in the period of 7 days.
Abbreviations: DMEM, Dulbecco’s Modified Eagle’s Medium; FBS, fetal bovine serum; FePt-Cys, l-cysteine coated FePt; NPs, nanoparticles; PDI, polydispersity index.
The magnetic property of the as-synthesized FePt-Cys NPs was measured by vibrating sample magnetometer at room temperature. shows the characteristic superparamagnetic curve of FePt-Cys NPs. The saturated mass magnetization value was~16.1 emu/g Fe, which was approximate to that of cysteamine-coated FePt NPs (12.3 emu/g Fe), but was lower than those of Fe2O3−, SiO2−, and tetraethylene glycol-coated FePt NPs.Citation30–Citation33 The magnetic property of FePt NPs is not only strongly correlated with the degree of chemical ordering of the alloy, but also influenced by the composition and surface coating of the alloy.Citation44–Citation46 The surface iron sites are the primary contributors to the magnetization in FePt. The strong bonding interaction between the surfactant and the surface iron sites results in the decrease of magnetization. For example, theoretical calculations showed that the charge is transferred to the surface iron sites from oleylamine and lowers the atomic magnetization by about 60% at the iron site in oleylamine-coated FePt NPs.Citation44
Figure 5 Magnetization hysteresis loop of the as-synthesized FePt-Cys NPs.
Abbreviations: FePt-Cys, l-cysteine coated FePt; NPs, nanoparticles.
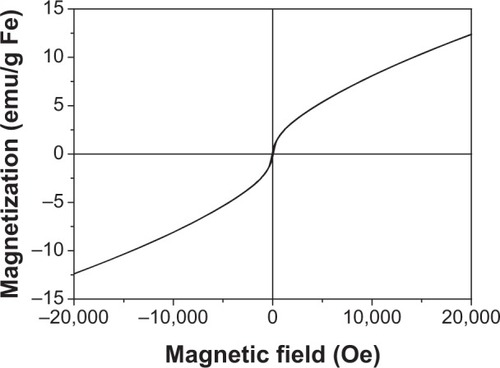
The T2 weighted image for FePt-Cys NPs was examined in a 3.0 T MR imager at various Fe concentrations. shows the T2 weighted images of FePt-Cys NPs in the Fe concentration range of 0–20 μg/mL in deionized water. A significant concentration-dependent inverse MR image contrast was observed. shows the linear correlation of the T2 relaxation rates (1/T2) against the iron concentration. The calculated relaxation rate (r2) value was~16.9 mM−1·s−1.
Figure 6 MR contrast imaging of FePt-Cys NPs.
Notes: (A) The T2-weighted MR images of FePt-Cys NPs at different Fe concentrations. (B) T2 relaxation rates (1/T2) plotted against the Fe concentration of FePt-Cys NPs.
Abbreviations: FePt-Cys, l-cysteine coated FePt; MR, magnetic resonance; NPs, nanoparticles; r2, relaxation rate.
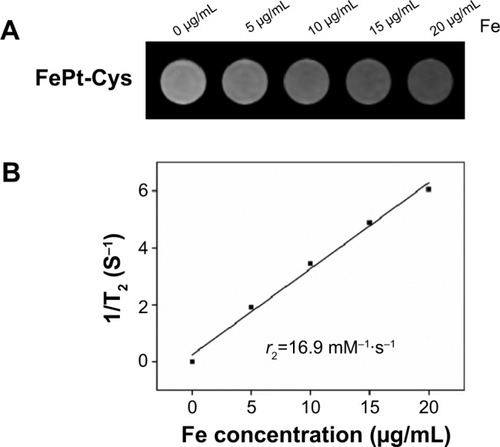
The CT imaging capability of FePt-Cys NPs was evaluated by using an IVIS Lumina XR system. FePt-Cys NPs were dissolved in deionized water at a Pt concentration range of 0–40 mg/mL, and were subsequently transferred to a black 96-well plate. Iohexol at an iodine concentration range of 0–80 mg/mL was used as the control. As shown in and B, the concentration-dependent enhancement of contrast effect was observed in both iohexol and FePt-Cys NPs. The calculated CT signal contrast intensities were plotted against the concentration of iohexol () and Pt (). The CT signal intensity of FePt-Cys NPs at a Pt concentration of 40 mg/mL was significantly similar to that of iohexol at an iodine concentration of 80 mg/mL. These results demonstrated the significantly enhanced CT contrast imaging of FePt-Cys NPs, which was due to the high X-ray absorption coefficient of Pt contained in FePt-Cys NPs.Citation32
Figure 7 CT imaging evaluation of different samples.
Notes: (A) The CT images of iohexol at different iodine concentrations; (B) The CT images of FePt-Cys NPs at different Pt concentrations; (C) The signal intensity of iohexol plotted against the concentration of iodine; (D) The signal intensity of FePt-Cys NPs plotted against the concentration of Pt.
Abbreviations: CT, computed tomography; FePt-Cys, l-cysteine coated FePt; NPs, nanoparticles.
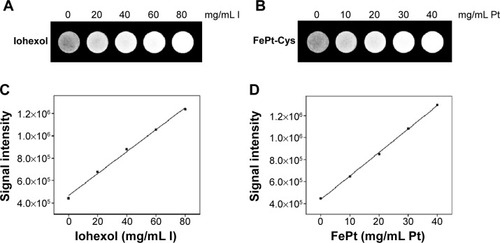
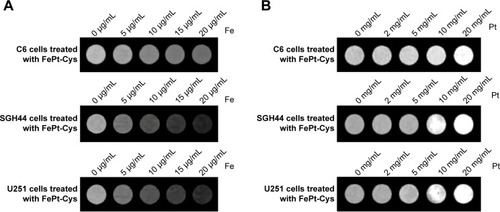
The MR imaging of FePt-Cys NPs was further evaluated using different glioma cells (C6, SGH44, and U251), as shown in . When FePt-Cys NPs were incubated with C6 glioma cells at different Fe concentrations, a gradual MR signal decay was observed with increasing of the Fe concentration from 0 μg/mL to 20 μg/mL. The Fe concentration-dependent MR signal decay was also observed on SGH44 cells and U251 cells. The CT imaging of FePt-Cys NPs was evaluated using three glioma cells (C6, SGH44, and U251) at different Pt concentrations. As shown in , a significant enhancement of the gray-scale signal can be observed with increasing Pt concentration on three glioma cells. These results suggested that FePt-Cys NPs could be used as MR and CT contrast agents in glioma cells.
Figure 8 Imaging of different gliomas cells (C6, SGH44, and U251) treated with FePt-Cys NPs.
Notes: (A) MR imaging of different gliomas cells at different Fe concentrations; (B) CT imaging of different gliomas cells at different Pt concentrations.
Abbreviations: CT, computed tomography; FePt-Cys, l-cysteine coated FePt; MR, magnetic resonance; NPs, nanoparticles.
In order to evaluate the biocompatibility of FePt-Cys NPs as an MRI/CT imaging agent, cytotoxicity was measured by MTT assay using different cells (ECV304, L929, and HEK293 cells) as the model. As shown in , these cells were treated with FePt-Cys NPs at different concentrations ranging from 25 μg/mL to 100 μg/mL for 72 hours. No significant differences in the viability of these cells were found between the FePt-Cys NP-treated group and the control group. For example, when ECV304 cells were treated with FePt-Cys NPs at a concentration of 100 μg/mL for 72 hours, the viability was ~110.2% compared with that of the control. Similarly, no significant decrease in cell viability was observed in L929 cells and HEK293 cells treated with FePt-Cys NPs at the same concentrations. These results indicated a good biocompatibility of FePt-Cys NPs, which is consistent with the results reported previously.Citation39
Conclusion
In summary, the water-soluble FePt-Cys NPs have promising potential as a dual MRI/CT contrast agent for malignant brain gliomas. The CT contrast imaging of FePt-Cys NPs was significantly more sensitive than that of the commonly used iohexol, even if the r2 value of FePt-Cys NPs was lower than that of FePt NPs coated by Fe2O3 and SiO2. Moreover, surface coating of FePt NPs with Cys not only provided good biocompatibility for the FePt-Cys NPs, but also prevented the introduction of unnecessary coatings (such as Fe2O3, and SiO2) that may damage brain tissue.Citation34–Citation38 Therefore, based on our preliminary results, FePt-Cys NPs could possibly be used as a dual MRI/CT contrast agent for the diagnosis of brain malignant gliomas. Further optimization of the composition of FePt-Cys NPs is required. In addition, more studies, especially in vivo studies using the animal as the model are needed to further evaluate this agent before it can be developed as a dual-mode imaging agent for clinical application.
Acknowledgments
We appreciate Ketao Mu and Wenzhen Zhu (Radiology Department of Tongji Hospital, Huazhong University of Science and Technology, Wuhan, People’s Republic of China) for great help in the MRI measurement. This work was financially supported by the Natural Science Foundation of China (No 30800256), the basic research project of Wuhan Science and Technology Bureau (No 2014060101010041, No 2014060202010120), the National Basic Research Program of China (973 Program, 2012CB932500), the Keygrant Project of Chinese Ministry of Education (No 313041), and the Fundamental Research Funds for the Central Universities (WUT: 2014-VII-028).
Disclosure
The authors report no conflicts of interest in this work.
References
- WenPYKesariSMalignant gliomas in adultsN Engl J Med2008359549250918669428
- OrringerDAKooYEChenTKopelmanRSagherOPhilbertMASmall solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapyClin Pharmacol Ther200985553153419242401
- RozhkovaEANanoscale materials for tackling brain cancer: recent progress and outlookAdv Mater20112324H136H15021506172
- LiMDengHPengHWangQFunctional nanoparticles in targeting gliomas diagnosis and therapiesJ Nanosci Nanotechnol201414141543224730272
- ChengYMorshedRAAuffingerBTobiasALLesniakMSMultifunctional nanoparticles for brain tumor imaging and therapyAdv Drug Deliv Rev201466425724060923
- SuZXingLChenYLactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomasMol Pharm20141161823183424779677
- ChungEJChengYMorshedRFibrin-binding, peptide amphiphile micelles for targeting glioblastomaBiomaterials20143541249125624211079
- YangZZLiJQWangZZDongDWQiXRTumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomasBiomaterials201435195226523924695093
- KangTGaoXHuQiNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomasBiomaterials201435144319433224565520
- YouJShaoRWeiXGuptaSLiCNear-infrared light triggers release of paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activitySmall2010691022103120394071
- SilvaACOliveiraTRMamaniJBApplication of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatmentInt J Nanomedicine2011659160321674016
- HainfeldJFSmilowitzHMO’ConnorMJDilmanianFASlatkinDNGold nanoparticle imaging and radiotherapy of brain tumors in miceNanomedicine (Lond)20138101601160923265347
- ChengYMorshedRChengSHNanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapySmall20139244123412923873826
- BechetDMordonSRGuilleminFBarberi-HeyobMAPhotodynamic therapy of malignant brain tumors: a complementary approach to conventional therapiesCancer Treat Rev201440222924122858248
- YiGQGuBChenLKThe safety and efficacy of magnetic nano-iron hyperthermia therapy on rat brain gliomasTumor Biol201435324452449
- MohamedMSVeeranarayananSPouloseACType 1 ribotoxin-curcin conjugated biogenic gold nanoparticles for a multimodal therapeutic approach towards brain cancerBiochim Biophys Acta2014184061657166924361614
- VeisehOSunCGunnJOptical and MRI multifunctional nanoprobe for targeting gliomasNano Lett2005561003100815943433
- ChertokBMoffatBADavidAEIron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumorsBiomaterials200829448749617964647
- RayAWangXLeeYKTargeted blue nanoparticles as photoacoustic contrast agent for brain tumor delineationNano Res201141111631173
- XiaoNGuWWangHDengYLShiXYeLT1-T2 dual-modal MRI of brain gliomas using PEGylated Gd-doped iron oxide nanoparticlesJ Colloid Interface Sci201441715916524407672
- NiDZhangJBuWDual-targeting upconversion nanoprobes across the blood–brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastomaACS Nano2014821231124224397730
- DengYWangHGuWHo3+ doped NaGdF4 nanoparticles as MRI/optical probes for brain gliomas imagingJournal of Materials Chemistry B2014215211529
- ShevtsovMANikolaevBPYakovlevaLYSuperparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumorsInt J Nanomedicine2014927328724421639
- ChertokBDavidAEYangVCBrain tumor targeting of magnetic nanoparticles for potential drug delivery: effect of administration route and magnetic field topographyJ Control Release2011155339339921763736
- XieHZhuYJiangWLactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivoBiomaterials201132249550220970851
- SunCFangCStephenZTumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticlesNanomedicine (Lond)20083449550518694312
- GaoJGuHXuBMultifunctional magnetic nanoparticles: design, synthesis, and biomedical applicationsAcc Chem Res20094281097110719476332
- FreyNAPengSChengKSunSHMagnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storageChem Soc Rev20093892532254219690734
- HoDSunXSunSMonodisperse magnetic nanoparticles for theranostic applicationsAcc Chem Res2011441087588221661754
- GaoJLiangGCheungJSMultifunctional yolk-shell nanoparticles: a potential MRI contrast and anticancer agentJ Am Chem Soc200813035118281183318681432
- ChenSWangLDuceSLEngineered biocompatible nanoparticles for in vivo imaging applicationJ Am Chem Soc201013242150221502920919679
- ChouSWShauYHWuPCYangYSShiehDBChenCCIn vitro and in vivo studies of FePt nanoparticles for dual modal CT/MRI molecular imagingJ Am Chem Soc201013238132701327820572667
- YangHZhangJTianQOne-pot synthesis of amphiphilic superparamagnetic FePt nanoparticles and magnetic resonance imaging in vitroJ Magn Magn Mater20103228973977
- KochFMöllerAMFrenzMPeilesUKuehni-BoghenborKMevissenMAn in vitro toxicity evaluation of gold-, PLLA- and PCL-coated silica nanoparticles in neuronal cells for nanoparticle-assisted laser-tissue solderingToxicol In Vitro201428599099824768613
- HouYLaiMChenXEffects of mesoporous SiO2, Fe3 O4, and TiO2 nanoparticles on the biological functions of endothelial cells in vitroJ Biomed Mater Res A201410261726173623776183
- BellusciMLa BarberaAPadellaFBiodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical processInt J Nanomedicine201491919192924790434
- SharmaHSMuresanuDFPatnaikRSharmaAExacerbation of brain pathology after partial restraint in hypertensive rats following SiO2 nanoparticles exposure at high ambient temperatureMol Neurobiol201348236837923832531
- WuJDingTSunJNeurotoxic potential of iron oxide nanoparticles in the rat brain striatum hippocampusNeurotoxicology20133424325322995439
- SunHChenXChenDInfluences of surface coatings and components of FePt nanoparticles on the suppression of gliomas cell proliferationInt J Nanomedicine201273295330722848161
- ZhangKYuYSunSFacile synthesis l-cysteine capped CdS:Eu quantum dots and their Hg2+ sensitive propertiesAppl Surface Sci2013276333339
- AryalSB K CRDharmarajNBhattaraiNKimCHKimHYSpectroscopic identification of S-Au interaction in cysteine capped gold nanoparticlesSpectrochim Acta A Mol Biomol Spectrosc200663116016315955726
- ChandraSSaleemHSebastianSSundaraganesanNThe spectroscopic (FT-IR, FT-Raman), NCA, first order hyperpolarizability, NBO analysis, HOMO and LUMO analysis of l-cysteine by ab inito HF and density functional methodSpectrochim Acta A Mol Biomol Spectrosc20117851515152421377921
- ZhuXMasonTGNanoparticle size distributions measured by optical adaptive-deconvolution passivated-gel electrophoresisJ Colloid Interface Sci2014435677425218049
- WuXWLiuCLiLJonesPChantrellRWWellerDNonmagnetic shell in surfactant-coated FePt nanoparticlesJ Appl Phys2004951168106812
- AslamMFuLLiSDravidVPSilica encapsulation and magnetic properties of FePt nanoparticlesJ Colloid Interface Sci200529044444915935370
- YuACCMizunoMSasakiYKondoHAtomic composition effect on the ordering of solution-phase synthesized FePt nanoparticle filmsAppl Phys Lett2004852562426244