 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel electrochemical sensing method was devised for the first time to detect plasma cortisol, a potential psychological stress biomarker, in human immunodeficiency virus (HIV)-positive subjects. A miniaturized potentiostat (reconfigured LMP91000 chip) interfaced with a microfluidic manifold containing a cortisol immunosensor was employed to demonstrate electrochemical cortisol sensing. This fully integrated and optimized electrochemical sensing device exhibited a wide cortisol-detection range from 10 pg/mL to 500 ng/mL, a low detection limit of 10 pg/mL, and sensitivity of 5.8 μA (pg mL)−1, with a regression coefficient of 0.995. This cortisol-selective sensing system was employed to estimate plasma cortisol in ten samples from HIV patients. The electrochemical cortisol-sensing performance was validated using an enzyme-linked immunosorbent assay technique. The results obtained using both methodologies were comparable within 2%–5% variation. The information related to psychological stress of HIV patients can be correlated with disease-progression parameters to optimize diagnosis, therapeutic, and personalized health monitoring.
Introduction
Progression of human immunodeficiency virus (HIV) infection leads to life-threatening diseases, such as acquired immunodeficiency syndrome (AIDS).Citation1–Citation3 Progress in HIV infection is characterized as a function of immune system impairments wherein various stress factors, such as cortisol, play a significant role in diseases progression and diagnosis.Citation1–Citation7 HIV infection activates the hypothalamic production and secretion of corticotropin-releasing hormone. This in turn causes the release of cytokine inflammatory response against viral infection, which stimulates the hypothalamus–pituitary–adrenal axis. This immunomodulation generates psychological stress that activates the corticotropic axis. HIV patients exhibit increased glucocorticoid-receptor expression and reduced substrate-binding affinity, which increases plasma cortisol and correlates with adrenocorticotropic hormone levels, affecting adrenal sufficiency.Citation1,Citation2 The hypothesis related to cortisol secretion and its relation with sensitized behavior in HIV-infected patients is illustrated in .Citation8
Figure 1 Schematic illustration of HPA axes in HIV progression. In cases of HIV, there is impaired adrenal reverse and more peripheral glucocorticoid excess.
Abbreviations: HPA, hypothalamus–pituitary–adrenal; HIV, human immunodeficiency virus; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; AIDS, acquired immunodeficiency syndrome.
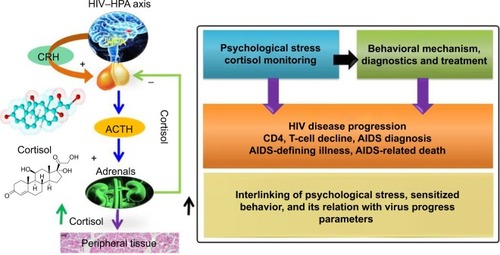
However, there is no clear evidence to prove the existence of a correlation between cortisol levels and HIV-infection stages.Citation9 Higher plasma cortisol levels have been observed more in untreated HIV-positive patients compared to treated patients.Citation5 Besides this, a negative correlation between CD4 cell count and plasma cortisol levels has also been reported.Citation2,Citation5,Citation8 It appears that cortisol contributes to suppression of beneficial activity of T-helper (Th)-1 cytokines in favor of Th2 cytokines (eg, IL-4, IL-6, and IL-10), and hence leads to greater HIV progression. Recently, many studies have reported the significance of cortisol detection and its correlation with different diseases.Citation3,Citation7,Citation10–Citation13 Therefore, diurnal cortisol detection in the laboratory and at the point of care (POC) has a great impact in monitoring sensitized/altered human behavior.Citation11,Citation12 Diurnal cortisol circadian rhythm is affected by schedule/activity and psychological/emotional stress of individuals. Cortisol abnormalities depress the immune system and lead to Cushing’s disease, Addison’s disease, and posttraumatic disorder.Citation11,Citation12 The enzyme-linked immunosorbent assay (ELISA) is the most reliable classical method to detect cortisol levels in patient samples. This method is limited to the laboratory, due to its requirements of longer measurement time, complex sample processing, expert man power, and sensing limitation to high concentrations (ng/mL or more). Therefore, cortisol detection in patients at the clinic or POC is in demand to understand diurnal behavioral patterns of individual subjects.Citation14
Due to its salient features, such as fast, selective, and sensitive detection, an electrochemical cortisol-immunosensing methodology is being rapidly adopted for cortisol detection at the pg/mL level.Citation11–Citation13 The introduction of nano/microelectrodes, nanostructured sensing materials, microelectronics, and miniaturized sensing transducers in sensor fabrication have been shown to improve device performance.Citation11,Citation12,Citation15–Citation19 The integration of a cortisol sensor with a microfluidic system and a miniaturized potentiostat (M-P) has been reported to monitor cortisol with reduced form factors.Citation11,Citation18,Citation20 Further, efforts are being made to develop these sensing systems at POC levels for personalized health monitoring.Citation14,Citation18,Citation21–Citation23 Electrochemical immunosensors based on self-assembled monolayers (SAMs) and nanostructures have been reported to detect cortisol, but their application in real sample analysis is currently limited.Citation16–Citation18 Therefore, there is an urgent need to establish an electrochemical sensing protocol for detecting cortisol in desired fluids, such as plasma, blood, saliva, urine, and interstitial fluid, of patients at the POC.
In this current research, we illustrate the integration of an electrochemical sensing device capable of performing cyclic voltammetry (CV) to detect plasma cortisol levels in HIV-positive patients. We validated the performance of the developed method by ELISA, and sensing performance was in correlation within 2%–5% variation. Therefore, in conclusion we say that this method can be adopted as a part of stress management, specifically for personalized health care monitoring of HIV-positive patients.
Materials and methods
Fabrication of electrochemical immunosensor
Dithiobis(succinimidyl propionate) (DTSP), sodium borohydride (NaBH4), monoclonal anticortisol antibody (anti-Cab), cortisol, and other chemicals were purchased from Sigma-Aldrich, and were used without any further purification. Phosphate-buffered saline (PBS) solution (10 mM, pH 7.4) was prepared by dissolving one PBS tablet in 200 mL of deionized (DI) water and used to prepare the anti-Cab (1 mg/mL) and cortisol concentrations.
The detailed experimental process related to electrochemical cortisol sensor fabrication was described in our previous publications in detail.Citation18,Citation24 We employed an electrochemically cleaned interdigitated gold microelectrode (IDE-Au; chamber volume ~5 μL, electrode width and electrode gap 10 μm), which were dispersed in a 2 mg/mL solution of DTSP (reduced using NaBH4 [10 mg/mL in DI water]) for 2 hours to prepare SAMs. The unbound DTSP particles on the electrode surface were washed with acetone, and final washing was done with DI water. For electrochemical immunosensor fabrication, 5 μL of anti-Cab (1 mg/mL) was covalently immobilized (for 2 hours) onto a DTSP-SAM/IDE-Au electrode via amide-bond formation between the amine group of antibodies and the reactive succinimidyl group of the DTSP. Anti-Cab/DTSP-SAM/IDE-Au bioelectrodes were washed using PBS (pH 7.4, 10 mM) to remove any unbound molecules. Ethanolamine (EA, 5 μL of 2 mg/mL) was immobilized on the anti-Cab/DTSP/IDE-Au bioelectrode for 10 minutes to bind the nonspecific site of the immunoelectrode, followed by washing using DI water. Finally, EA/anti-Cab/DTSP/IDE-Au immunoelectrodes were stored in a refrigerator at 4°C when not in use.
Design of LTCC-based microfluidic manifold
A low temperature co-fired ceramic (LTCC) microfluidic architecture was utilized, which consisted of two microchannels. Both channels were connected to a reaction chamber placed on top of a biosensor chip. These channels were used for sample-in and waste-out processes using a three-way solenoid fluidic valve. During sensing, the washing step is crucial to avoid contamination and nonspecific moieties from the sensor surface. To obtain optimum washing efficiency, a computational fluid dynamic approach via Comsol Multiphysics® software was used to characterize fluid-flow profiles. The details of the design optimization of the LTCC microfluidic manifold were published in our previous report.Citation18 The LTCC microfluidic chip consists of three layers of green tape (DuPont 951). The bottom layer is a reaction chamber (5 mm diameter and volume of 10 μL) that interfaces with the sensor. The middle layer has an inlet and outlet microchannels for the navigation of sample/buffer/waste. The top layer covers the microchannels and ports for incorporating fluidic connectors. Green tapes were patterned using a computer-controlled 10.6 μm CO2 laser with a spot size of 35 μm. Further, the patterned tape was aligned to be laminated using an isostatic hot press (Phi-Tulip) at 150°C for 15 minutes at 3,000 psi pressure. The aligned laminated green tape layers were fused to the LTCC through heating at 850°C in the presence of oxygen. Tygon tubing was connected at an inlet and outlet port of the microfluidic manifold via polydimethylsiloxane (Dow Corning). The fluids were introduced into the device via a programmable two-syringe pump network (New Era Pumps). A fabricated microfluidic device containing a biosensor chip was integrated with an acrylic fixture for portability. The final device was connected with an M-P for electrochemical measurement.Citation18
During measurement, 10 μL of each sample was introduced in the inlet channel at a flow rate of 10 μL/min. The sample was incubated on immunosensor electrodes (static condition) for 30 minutes for the completion of the antigen–antibody reaction. Further, the washing buffer solution (30 μL) was then introduced into the reaction chamber (10 μL/min for 3 minutes) to remove unbound moieties. For electrochemical experiments, 5 μL of measurement buffer (PBS, pH 7.4) containing 5 mM Fe(II)/Fe(III) as redox moieties was allowed to flow in the reaction chamber. Solenoid three-way valves (Lee) were utilized for the successful flow of samples and buffer.
Design of miniaturized potentiostat
A schematic illustration of the electrochemical cortisol-immunosensing protocol, preparation of the immunosensor, architecture of the LTCC-based microfluidic manifold, reconfigured M-P chip (LMP9100) for electrochemical measurement, and comparison of M-P-obtained CV curve of IDE with a conventional potentiostat is shown in . The details related to the preparation of the M-P have been explained in our previous work.Citation14 In brief, the LMP91000 board was procured from Texas Instruments and reconfigured to design an M-P capable of performing full-range CV measurement using a three-electrode system. For CV measurements using the LMP91000, an external central processing unit with SensorAFE Designer software was connected with a USB communication feature. A Beagle-Bone microcontroller unit was added to make the device portable. To enable the communication, the LMP91000EVM was powered and controlled by the microcontroller via the I2C interface.
Figure 2 Schematic illustration of the stepwise fabrication and integration of electrochemical cortisol immunosensor with LTCC microfluidic manifold and M-P to detect cortisol in HIV-infected patients.
Notes: A BeagleBone microcontroller is connected with the reconfigured LMP91000EVM to perform full-range CV using three-electrode systems. The obtained data is stored in the BeagleBone SD card and transferred to the display system via SSH. Reproduced from Cruz AFD, Norena N, Kaushik A, Bhansali S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens Bioelectron. 2014;62:249–254.Citation16 An electrochemical immunosensor is prepared via immobilizing anti-Cab onto an SAM-modified IDE-Au electrode. Stepwise schematic illustration of LTCC-based microfluidic chip. For the making of the manifold, green taps are cut according to design and aligned to fuse them on 780°C. A fully assembled LTCC microfluidic manifold integrated with a cortisol biosensor chip.Citation18 This fabricated immunosensor is integrated with an LTCC-based microfluidic manifold for the automation of sample and reconfigured M-P for full-range CV measurement. To the reconfigured LMP9100 chip, a two-wire jumper short was removed to allow for three-electrode-based electrochemical measurements. The J_MENB jumper short was moved to the far left to enable manual configuration of the LMP91000 chip. The ADC takes the analog output from the potentiostat chip and transfers it to the microcontroller unit via SPI using the I2C interface of the LMP91000.Citation14 This developed electrochemical immunosensing method is used to detect cortisol and plasma cortisol of patients. This sensing device can be used as an analytical tool for stress-management programs for obtaining bioinformatics needed to optimize therapeutics.
Abbreviations: LTCC, low temperature co-fired ceramic; M-P, miniaturized potentiostat; HIV, human immunodeficiency virus; CV, cyclic voltammetry; anti-Cab, anticortisol antibody; SAM, self-assembled monolayer; IDE, interdigitated electrode; ADC, analog digital converter; SPI, serial peripheral interface; ADC. analog to digital converter; SAM. self-assembled monolayer; SSH. secure shell.
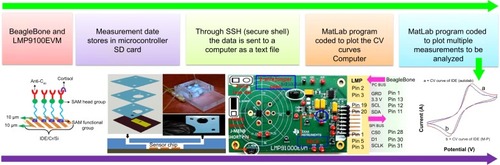
To achieve three-electrode-based electrochemical measurements, we removed the two-wire jumper pin and connected a J_MENB jumper to enable manual operation mode. A reconfigured LMP91000EVM generated an output voltage proportional to the current flowing through the working electrode. This voltage was provided by the transimpedance amplifier at the output stage. The acquisition of the signal response of the potentiostat was sent back to the microcontroller through the serial peripheral interface of the ADC161S626 analog digital converter (embedded on the LMP91000EVM). On applying optimized programming, the LMP91000EVM performed CV as a function of varying scan-rate potential in the range from −0.6 V to 0.6 V. The output voltage of the LMP91000EVM is proportional to the cell current. This can be obtained by plugging the output voltage of the LMP91000EVM in the transimpedance-amplifier transfer-function equation, taking into consideration the parameters set for the CV measurement using the equation:
The electrochemical performance of the Au-IDE based on CV using the M-P was comparable with the results obtained using a Metrohm Autolab potentiostat, thus validating M-P performance.Citation16
Electrochemical measurement
CV-based electrochemical measurements using the M-P at a scan rate of 50 mV/s in 5 μL of PBS (pH 7.4) containing 5 mM (Fe[CN]6)3−/4− as redox moieties in a potential range from −0.6 to 0.6 V were conducted to characterize sensor fabrication and to detect cortisol concentrations. To retain high biological activity of biomolecules, a pH of 7.4 for the PBS was chosen. This was attributed to the fact that this is the optimum pH selected for electrochemistry of immunosensors. An EA/anti-Cab/DTSP-SAM/IDE-Au electrochemical immunosensor was used to detect cortisol in plasma samples of HIV-positive patients.
Plasma sample collection of HIV patients
Blood donors were apprised of this study, and their consent was obtained consistent with the policies of Florida International University (FIU) and the National Institutes of Health (NIH). The study protocol was approved by the institutional review board of FIU. HIV-infected patients were recruited from the Borinquen Health Care Center, Miami. Patients were males and females. All vital clinical information, ie, race, sex, drug therapy, and CD4 levels of each available HIV patient (n=10), obtained from the hospital is summarized in . The identities of all the subjects recruited were kept confidential. Blood from the donors to be processed as plasma was directly drawn into blood-collection tubes containing the anticoagulant K2 ethylenediaminetetraacetic acid (BD; Fisher Scientific; catalog number 366643). Following collection, all plasma samples were centrifuged immediately for 15 minutes at 5,000 g at 4°C, and supernatants were carefully collected.
Table 1 Patient demographics
Plasma cortisol samples were stored at −20°C to maintain their biological activity. All the samples were defrosted to room temperature for further use to detect cortisol concentration using electrochemical immunosensors and ELISA. A cortisol (human) ELISA kit was procured form Abnova Assays, and a standard protocol was adopted to estimate plasma cortisol. In brief, 50 μL of 1:3 PBS diluted plasma cortisol samples of all patients were used for ELISA measurements.
Results and discussion
Electrochemical studies of cortisol sensor
The reconfigured LMP91000-based M-P chip was utilized to characterize electrochemical immunosensor fabrication and cortisol-sensing performance. The electrochemical response study of the IDE-Au electrode (curve a), DTSP-SAM/IDE-Au electrode (curve b), anti-Cab/DTSP-SAM/IDE-Au immunoelectrode (curve c), EA/anti-Cab/DTSP-SAM/IDE-Au immunoelectrode (curve d), and response change after adding cortisol on the immunosensor surface (curve e) is shown in . A detailed explanation of sensor fabrication, characterization, and optimization of operation parameters has been described in our previous publications.Citation18,Citation24 In brief, fabrication of DTSP-SAM onto electronically conducting IDE-Au hinders electron transport from electrolytes to the working electrode, due to the insulating nature of SAMs. This confirms the preparation of SAMs onto Au via thiol bonding. The electrochemical response-current magnitude of DTSP-SAM/IDE-Au further decreased after immobilizing the anti-Cab via amide bonding. The presence of insulating anti-Cab on the surface caused hindrance in electron transport, thus confirming immobilization. The nonbinding sites of the anti-Cab/DTSP-SAM/IDE-Au immunoelectrode were blocked using EA. This also decreased the electrochemical response current, due to a reduction in electron transport from electrolytes to the IDE. The scan-rate-dependent CV of electrodes and bioelectrodes was also studied. A linear relationship between the magnitude of the electrochemical response current and the square root of the scan rate was observed (data not shown). This proved that the electrode surface was a linear diffusion-driven process. A linear relationship was also observed between the scan rate and potential differences, revealing facile electron transport from electrolytes to the working electrode surface.
Figure 3 Electrochemical stepwise characterization of cortisol immunosensor and cortisol sensing calibration curve.
Notes: (A) CV response study of IDE-Au (curve a) electrode, DTSP-SAM/IDE-Au (curve b) electrode, anti-Cab/DTSP-SAM/IDE-Au immunoelectrode (curve c), EA/anti-Cab/DTSP-SAM/IDE-Au immunoelectrode (curve d), and response change after adding cortisol on sensor surface (curve e). (B) A calibration curve was obtained using an electrochemical cortisol sensor as a function of cortisol concentrations (10–500 pg/mL) on a logarithmic scale.
Abbreviations: CV, cyclic voltammetry; IDE, interdigitated electrode; DTSP, dithiobis(succinimidyl propionate); SAM, self-assembled monolayer; EA, ethanolamine; anti-Cab, anticortisol antibody; SD, standard deviation.
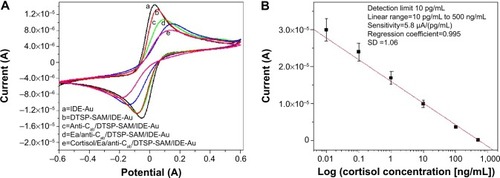
Electrochemical cortisol-immunosensing studies of the EA/anti-Cab/DTSP-SAM/IDE-Au sensor were performed as a function of cortisol concentration (10 pg/mL to 500 ng/mL) using M-P, as described in the Materials and methods section. Five microliters of each cortisol concentration was incubated on the sensor surface. An incubation time of 30 minutes was used to achieve optimum binding of the antibody and cortisol. Prior to electrochemical measurement, all immunoelectrodes were washed using 30 μL of PBS (pH 7.4) to remove the unbound cortisol. All the measurements were made in triplicate, and an average of these was used for cortisol-concentration estimation. A reduction in electrochemical response current was observed after the addition of each cortisol concentration. This was attributed to the insulating nature of the immunocomplex formed between anti-Cab and cortisol. This barrier layer inhibited the electron transport from electrolytes to the IDE-Au.
A calibration curve was plotted between the magnitude of current response and logarithm of cortisol concentration (). A linear-dependent relation was observed, which followed the equation:
The electrochemical cortisol immunosensor developed by us exhibited a wide linearity from 10 pg/mL to 500 ng/mL (on logarithmic scale), a low detection limit of 10 pg/mL, and a sensitivity of 5.8 μA (pg mL)−1, with a regression coefficient of 0.995. The studies related to the selectivity and stability of this immunosensor have been discussed in our previous publications.Citation18,Citation24 The electrochemical response current of immunosensors using prostate specific antigen (100 pg/mL), neuron-specific enolase (100 pg/mL), Epidermal growth factor receptor (100 pg/mL), bovine serum albumin + cortisol (100 pg/mL), and cortisol (100 pg/mL) exhibited maximum change in the case of cortisol only. A variation of electrochemical response of ~1%–2% in case of interferents and ~14% with respect to cortisol was observed. This confirmed the selectivity of our developed cortisol immunosensor (data not shown).Citation24 CV studies related to the shelf life of the EA/anti-Cab/DTSP-SAM/IDE-Au immunoelectrode at intervals of 1 week revealed that the electrochemical sensor exhibited a response within 5% variation and stability for 28 days (data not shown).Citation24
Electrochemical immunosensing of plasma cortisol
For plasma cortisol analysis, the EA/anti-Cab/DTSP-SAM/IDE-Au immunosensor was utilized for detecting plasma cortisol concentrations in HIV-positive patients. A plasma sample (5 μL) from each patient was incubated on the cortisol-immunosensor surface for 30 minutes. Further, the sensor surface was washed using 30 μL of PBS (pH 7.4) to remove unbound plasma particles. The magnitude of the electrochemical response current of the EA/anti-Cab/DTSP-SAM/IDE-Au immunosensor was found to change after the addition of each plasma sample. The established calibration curve () was used to estimate final cortisol concentration. All measurements were made in triplicate, and an average value was computed to estimate plasma cortisol concentration (). The obtained sensing performance as validated with ELISA technique is presented in .
Table 2 Plasma cortisol detection using ELISA and electrochemical measurement
Plasma cortisol detection using ELISA
The ELISA technique was performed using a 96-well titer plate with respect to six known concentrations (20, 50, 100, 200, 400, and 800 ng/mL) to establish a calibration curve (). A detection limit of 1.5 ng/mL, a detection range of 20–800 ng/mL, a sensitivity of 50 ng/mL (Iab, intensity of absorbance), along with a regression coefficient of 0.989 and a standard deviation of ±1.06, were achieved using an ELISA kit. The sensing-performance outcomes of both techniques are summarized in . The obtained calibration curve was used to measure unknown plasma cortisol concentration in HIV-positive patient samples. All measurements were made in triplicate, and an average value was utilized to estimate the final concentration, as shown in . A comparison of plasma cortisol level estimated using ELISA and electrochemical immunosensor is presented in .
Table 3 Summary of cortisol-sensing performance using ELISA and miniaturized electrochemical cortisol sensing device
Figure 4 ELISA calibration curve to estimate plasma cortisol concentration and comparisons of plasma cortisol concentrations of HIV positive patients estimated using ELISA and CV method.
Notes: (A) Calibration plot obtained using ELISA techniques to detect plasma cortisol concentrations in HIV-positive patient. (B) A comparison of plasma cortisol of cocaine using HIV patients using ELISA and CV methods.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; CV, cyclic voltammetry; B%/Bo. mean absorbance of standards/means absorbance of negative control; au, arbitrary unit.
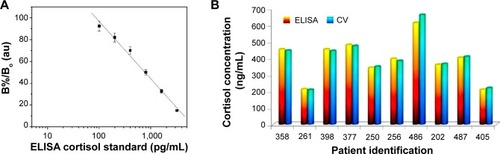
The transduction techniques, ie, mode of output response, of ELISA (IIab) and the electrochemical immunosensor (A) are different in nature of principle. Thus, obtained outcomes were in different magnitudes for each concentration. To establish a correlation for comparison between both techniques, the electrochemically detected cortisol concentrations were normalized by a factor of 2.4. Both techniques showed comparable results with 2%–5% variation, thus confirming the validation of the electrochemical cortisol-immunosensing protocol. However, in our previous study, a correlation factor of 2.2 was established to validate the results obtained using an electrochemical EA/anti-Cab/DTSP-SAM/IDE-Au immunosensor and ELISA to detect salivary cortisol.Citation24 This difference is due to the physiological variation of biological fluids, eg, saliva has free cortisol, and plasma also contains bound cortisol, ie, corticosteroid-binding globulin.
An established, well-correlated, and validated electrochemical immunosensing protocol enables the quantification of plasma cortisol, and has the potential to detect cortisol in other biologically relevant fluids, such as saliva and interstitial fluids.
Conclusion
In this work, we detected plasma cortisol levels in HIV-positive patient samples using an electrochemical immunosensing device. The investigated device, consisting of an M-P chip interfaced with an LTCC microfluidic manifold, demonstrated cortisol detection at the pg/mL level with a sensitivity of 5.8 μA (pg mL)−1. The plasma cortisol of HIV-positive patients was also estimated successfully using the ELISA technique. The obtained results were in good correlation (2%–5%) with those obtained using the investigated electrochemical sensor. This confirmed the validation of the developed method. Efforts are being made to coordinate with clinics and hospitals to increase the sample volume of HIV-positive patients. Higher sample volumes will be of use to understand the relation of diurnal cortisol variation of HIV patients with virus-infection progress. Such obtained information or findings can be correlated with clinical diagnostics of HIV patients for personalized health monitoring and to decide on therapeutics.
Acknowledgments
This work was supported by NIH grants RO1-DA027049, R21-MH 101025, RO1-MH085259, and RO1-DA 034547, and partially supported by NSF-NERC (1160483). The authors are also thankful to Dr Abhay Vasudev (Intel process engineer) for his help in system design and Prof. Dinesh Sood for scientific discussion.
Disclosure
The authors report no conflicts of interest in this work.
References
- AntoniMHStress management and psychoneuroimmunology in HIV infectionCNS Spectr200381405112627048
- BonsJMoreauLLefebvreHAdrenal disorders in human immunodeficiency virus (HIV) infected patientsAnn Endocrinol (Paris)2013745–650851424262982
- PattersonSMoranPEpelECortisol patterns are associated with T cell activation in HIVPloS One201387e6342923922644
- AounSRamosEHypertension in the HIV-infected patientCurr Hypertens Rep20002547848110995524
- BrownTTThe effects of HIV-1 infection on endocrine organsBest Pract Res Clin Endocrinol Metab201125340341321663835
- AbbottMKhooSHHammerMRWilkinsEGLPrevalence of cortisol deficiency in late HIV diseaseJ Infect1995311148522825
- DjamshidianAO’SullivanSSPapadopoulosASalivary cortisol levels in Parkinson’s disease and its correlation to risk behaviourJ Neurol Neurosurg Psychiatry201482101107111121478206
- GeorgeMMBhangooAHuman immune deficiency virus (HIV) infection and the hypothalamic pituitary adrenal axisRev Endocr Metab Disord201314210511223728720
- CrepazNPassinWFHerbstJHMeta-analysis of cognitive-behavioral interventions on HIV-positive persons’ mental health and immune functioningHealth Psychol200827141418230008
- DjamshidianAAverbeckBBLeesAJO’SullivanSSClinical aspects of impulsive compulsive behaviours in Parkinson’s diseaseJ Neurol Sci20113101–218318821839478
- KaushikAVasudevAAryaSKPashaSKBhansaliSRecent advances in cortisol sensing technologies for point-of-care applicationBiosens Bioelectron20145349951224212052
- SinghAKaushikAKumarRNairMBhansaliSElectrochemical sensing of cortisol: a recent updateAppl Biochem Biotechnol201417431115112624723204
- VabbinaPKKaushikAPokhrelNBhansaliSPalaNElectrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakesBiosens Bioelectron20156312413025064820
- CruzAFDNorenaNKaushikABhansaliSA low-cost miniaturized potentiostat for point-of-care diagnosisBiosens Bioelectron20146224925425016332
- AryaSKChornokurGVenugopalMBhansaliSAntibody functionalized interdigitated micro-electrode (IDmE) based impedimetric cortisol biosensorAnalyst201013581941194620589269
- AryaSKChornokurGVenugopalMBhansaliSDithiobis(succinimidyl propionate) modified gold microarray electrode based electrochemical immunosensor for ultrasensitive detection of cortisolBiosens Bioelectron201025102296230120382518
- KaushikAVasudevAAryaSKBhansaliSMediator and label free estimation of stress biomarker using electrophoretically deposited Ag@AgO-polyaniline hybrid nanocompositeBiosens Bioelectron201350354123831854
- VasudevAKaushikATomizawaYNorenaNBhansaliSAn LTCC-based microfluidic system for label-free, electrochemical detection of cortisolSens Actuators B Chem2013182139146
- LoncaricCTangYHoCParameswaranMAYuHZA USB-based electrochemical biosensor prototype for point-of-care diagnosisSens Actuators B Chem20121611908913
- SrinivasanVPamulaVKFairRBAn integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluidsLab Chip20044431031515269796
- SafaviehMAhmedMUSokulluENgABraescuLZourobMA simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detectionAnalyst2014139248248724300967
- TudosAJBesselinkGASchasfoortRBTrends in miniaturized total analysis systems for point-of-care testing in clinical chemistryLab Chip200112839515100865
- WeaverWKitturHDharMDi CarloDResearch highlights: micro-fluidic point-of-care diagnosticsLab Chip2014141219621965
- PashaSKKaushikAVasudevASnipesSABhansaliSElectrochemical immunosensing of saliva cortisolJ Electrochem Soc20141612B3077B3082
