Abstract
Neurodegenerative causes of blindness and deafness possess a major challenge in their clinical management as proper treatment guidelines have not yet been found. Brain-derived neurotrophic factor (BDNF) has been established as a promising therapy against neurodegenerative disorders including hearing and visual loss. Unfortunately, the blood–retinal barrier and blood–cochlear barrier, which have a comparable structure to the blood–brain barrier prevent molecules of larger sizes (such as BDNF) from exiting the circulation and reaching the targeted cells. Anatomical features of the eye and ear allow use of local administration, bypassing histo-hematic barriers. This paper focuses on highlighting a variety of strategies proposed for the local administration of the BDNF, like direct delivery, viral gene therapy, and cell-based therapy, which have been shown to successfully improve development, survival, and function of spiral and retinal ganglion cells. The similarities and controversies for BDNF treatment of posterior eye diseases and inner ear diseases have been analyzed and compared. In this review, we also focus on the possibility of translation of this knowledge into clinical practice. And finally, we suggest that using nanoparticulate drug-delivery systems may substantially contribute to the development of clinically viable techniques for BDNF delivery into the cochlea or posterior eye segment, which, ultimately, can lead to a long-term or permanent rescue of auditory and optic neurons from degeneration.
Introduction
Secreted protein brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family. This signaling molecule has been well-documented for its ability to regulate neuronal plasticity, cell growth, proliferation, cell survival, and long-term memory.Citation1 Being involved in pathogenesis of neurodegenerative diseases, BDNF has become a major approach in drug development for the treatment of Alzheimer’s disease,Citation2 Parkinson’s disease,Citation3 Huntington’s disease,Citation4 Rett syndrome,Citation5 stroke,Citation6 traumatic brain injury,Citation7 depression,Citation8 drug abuse,Citation9 schizophrenia,Citation10 amniotic lateral sclerosis,Citation11 and multiple sclerosis.Citation12 The crucial limitation pertaining to the use of BDNF in the treatment of central nervous system (CNS) disease is its inability to cross the blood–brain barrier (BBB). In fact, numerous recent reviews have described modern accomplishments in various approaches directed to surmount the BBB, including the modulation of tight junctions or transport systems, or the exploitation of colloid drug-delivery nanosystems.Citation13–Citation15
Furthermore, the retinaCitation16 and the inner ear,Citation17 like the brain, also have their own blood barriers with similar structure and function to the BBB. Those barriers also constrain the usage of BDNF as a treatment for blindness and deafness caused by neurodegeneration of retinal or spiral ganglion cells, respectively. Fortunately, specific anatomical construction of these sensory organs offers the possibility for local drug delivery that can avoid the barriers. Eventually, the main approaches of BDNF delivery to the posterior segment of the eye or the inner ear appear to be similar and include direct delivery of the pure protein (intravitreous injection or implantation of the osmotic minipump to the scala tympani), viral gene, and cell-based therapy. Ultimately, this knowledge should be translated to patients, however, regardless of success in preclinical studies, BDNF has yet to prove its potential as a neurorestorative in clinics. In this review, some important points concerning the structure and mechanism of action of the BDNF are discussed. Besides, all published methods of BDNF delivery to the posterior eye segment and to the inner ear are highlighted, and additionally, these methods are analyzed and compared. Finally, future prospects in BDNF delivery that may benefit the potential treatment of blindness and deafness among patients are suggested.
BDNF
BDNF structure
BDNF belongs to the neurotrophin family of proteins, and it is the most specific in the biological action family of growth factors. Apart from BDNF, the other group members are: nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). The first neurotrophin, named NGF, was identified by Rita Levi-Montalcini in 1952Citation18 as “unknown tumoral factor”, which influenced nerve growth in mouse sarcoma and chick embryo. Thirty years later, Barde et alCitation19 in their study on the survival and the growth of nerve fibers in neuronal cell culture, purified, 2 μg of protein BDNF with a molecular weight (MW) of 12,300 Da from 3 kg of porcine brain. Later, the 252 amino acid (AA) residues in the sequence of BDNF were identified.Citation20 The three-dimensional molecular structure of BDNF appears to be a homodimer with noncovalent bonds, where each monomer consists of three pairs of anti-parallel β-sheets connected to four loops of β-strands containing 3 disulfide bonds with cysteine knot motif.Citation21 Besides, the crystallographic BDNF structure is shown in protein databases as heterodimers with NT-3 (code 1B8M) and NT-4 (code 1BND).Citation22,Citation23 The BDNF human gene is mapped on chromosome 11 between 11p13 and 11p14.Citation24,Citation25 Apparently, BDNF synthesis occurs in the CNS,Citation20 as well as in the peripheral tissues, including the liver, muscles, pancreas, colon, intestine, lungs, bladder,Citation26 and placenta.Citation27 It is well known that BDNF synthesis always follows a precursor molecule (pro-BDNF) synthesis, which is the 35,000 Da protein with its own CNS activity.Citation28 The polypeptide pro-BDNF that consists of 247 AA residues should be cleaved to form the mature protein with a MW 14,000 Da and 119 AA residues.Citation29 In fact, there are two existing pathways for BDNF to mature, namely the intracellular and pericellular pathways. The furin, which is located in the Golgi apparatus, plays a crucial role in the intracellular processing,Citation30 while the pericellular pathway involves serine protease tissue plasmin, which is synthesized from plasminogen activated by the tissue plasminogen activator.Citation31 Furthermore, it is important to note that the absence of cleavage leads to the accumulation of pro-BDNF that can act in a manner opposite to BDNF. In addition, studies have shown that pro-BDNF binding with p75 receptor induces neuronal apoptosis,Citation32 whereas binding with sortilin results in a more stable form of pro-BDNF and the activation of intracellular enzyme furin.Citation33 Additionally, the Val66Met (valine amino-acid is substituted by the methionine in 66th codon) genetic polymorphism of the pro-BDNF leads to the inability of the pro-BDNF to be bound with sortilin, resulting in a decrease in the production of mature protein that subsequently leads to numerous CNS disorders.Citation34
BDNF receptors
BDNF is a ligand to three different receptors, namely tropomyosin related kinase B (TrkB), p75 neurotrophin receptor (p75NTR), and sortilin. It is well known that TrkB belongs to a large group of tyrosine-kinase receptors, and BDNF as well as NT-4 are the only ligands for this receptor.Citation21 The human TrkB is a transmembrane glycoprotein type I that consists of 792 AA residues. Its extracellular domain comprises of three tandem leucinerich motifs, bordered by two cysteine clusters, and trailed by two immunoglobulin (Ig)-like domains, Ig1 and Ig2,Citation35 where Ig2, the closest to cell membrane, is the binding site for BDNF.Citation36 The receptor is capable of being in a dynamic equilibrium between monomeric and dimeric states and regulates the activity of further intracellular biochemical cascades. Moreover, binding with the ligand results in the conversion of the receptor’s monomeric structure into the dimeric form, which is accompanied by autophosphorylation of the intracellular domain.Citation37
Basically, the TrkB receptor has three core isoforms in the human brain, specifically a full-length, catalytic form (TrkB. FL), and two isoforms that lack a tyrosine kinase domain called truncated forms, namely, TrkB.T and TrkB.Shc. The truncated forms are synthesized by an alternative splicing of the primary gene and are independently regulated.Citation38 Furthermore, the TrkB.FL is seen to be expressed in the brain cortex, the hippocampus, the thalamus, the choroid plexus, granule cell layer of the cerebellum, the brainstem, the spinal cord, and the retina.Citation39 It initiates the survival of neuronal cells and the differentiation and plasticity of synaptic signals, whereas truncated TrkB are capable of inhibiting all these processes when their heterodimerization with the activated TrkB.FL occurs.Citation40 Additionally, a study has shown that the relationship between levels of the TrkB.FL and the truncated isoforms influences the cellular response to BDNF.Citation41 Notably, BDNF binds with TrkB.T, becoming immobilized and unable to bind with TrkB.FL, thus reducing BDNF-signaling.Citation42 Also, the formation of TrkB.T and TrkB.FL heterodimers affects the signaling by acting as a dominant-negative inhibitor.Citation43 Therefore, the maximal activity of TrkB is possible only in the case of homodimerization of TrkB.FL.
Another receptor, p75NTR, appears to be the 16th member of tumor necrosis factor (TNF) receptor superfamily.Citation44 The precursors of all the four neurotrophins, such as pro-NGF, pro-BDNF, pro-NT-3, and pro-NT-4 are the ligands to p75NTR. Mature neurotrophins may also be the ligands to p75NTR, but with substantially lower affinity.Citation45 The p75NTR structure has an extracellular stalk domain, a single transmembrane domain, and a cytoplasmic domain.Citation46 The regulation of cell survival, cell cycle, axonal growth, and myelin formation have been considered to be the main functions of the p75NTR.Citation47,Citation48 It can cooperate with other receptors to form heterodimeric complexes. For instance, the interaction with the TrkB has been shown to increase the affinity of BDNF to its binding site,Citation49 thus enhancing the cell survival. On the other hand, the interface with sortilin leads to the initiation of the apoptosis by pro-BDNF.Citation50 Sortilin, as mentioned above, is another receptor that interacts with BDNF. It belongs to type-I transmembrane vacuolar protein-sorting 10 protein (Vps10p) domain containing receptors family.Citation51 Sortilin operates as a coreceptor that regulates TrkB or p75NTR expression in response to a pro-neurotrophin and initiates the apoptotic cascade.Citation50 Also, it has been recently shown that the interaction of sortilin with Huntingtin-associated protein-1 causes the activation of intracellular protease furin, which, in turn, cleaves the pro-BDNF to a mature form.Citation33
BDNF-mediated signal transduction
TrkB receptor
Binding of BDNF to TrkB with picomolar affinity () induces the dimerization of the receptor and the autophosphorylation of the tyrosine residues in the juxtamembrane domain that creates the binding or docking sites for such molecules as Src-homology 2 domain containing transforming protein (Shc), Src-homology phosphatase 2 (Shp2), growth factor receptor-binding protein 2 (Grb2), and phospholipase Cγ (PLCγ) 1.Citation52 Further interaction of these molecules initiates the activation of various signaling cascades, which have been described in numerous reviews elsewhere.Citation53–Citation55 Here, this will be discussed superficially, in order to give a general overview of BDNF’s action.
Figure 1 A general scheme, illustrating BDNF-TrkB signaling through three main pathways in the neuronal cell (NC).
Notes: PLCγ pathway results in activation of neuronal plasticity and long-term memory formation via PKC, TRPC, and transcriptional factor CREB. Also PLCγ activates inositol trisphosphate receptor (IP3R) via IP3 to release intracellular calcium (Ca2+) from sarcoplasmatic reticulum (SR). PI3K-Akt pathway activates CREB and causes further downstream activation of mTOR, which stimulates protein synthesis in neuronal dendrites leading to cell growth, proliferation, and synaptic plasticity. Also, PI3K-Akt pathway blocks Bax protein causing antiapoptotic action. Mitogen-activated protein kinases (MAPK) cascade results in activation of transcriptional factor CREB with further stimulation of cell growth, differentiation, protection, releasing of neurotransmitters, and memory formation.
Abbreviations: BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; PLCγ, phospholipase Cγ; PKC, protein-kinase C; TRPC, transient receptors potential channels; CREB, cyclic adenosine monophosphate response element-binding protein; Shc, Src-homology 2 domain containing transforming protein; Grb-2, growth factor receptor-binding protein 2; IP3, inositol triphosphate; PIP2, phosphatidylinositol 4,5-biphosphate; DAG, diacylglycerol; CaMKII, Ca2+/calmodulin-dependent protein kinase 2; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; PDK1, phosphoinositide-dependent kinase-1; Bax, B-cell lymphoma 2 associated X protein; mTOR, mammalian target of rapamycin; SOS, son of sevenless; Ras, rat sarcoma; GTP, guanosine triphosphate; B-Raf, rapid accelerated fibrosarcoma B; ERK, extracellular signal-regulated kinases.
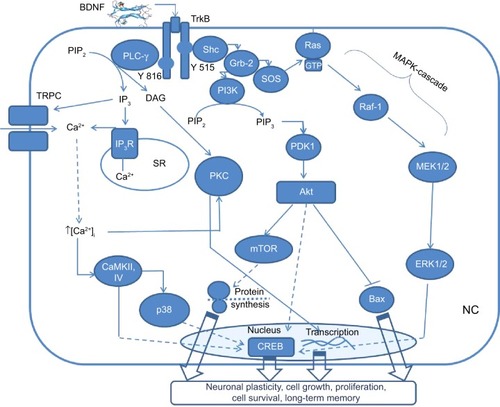
PLCγ pathway () is mainly essential for synaptic plasticity.Citation55 Phosphorylation of the tyrosine residue at position 816 causes PLCγ to bind to this site in the juxtamembrane domain. Then, PLCγ can hydrolyze phosphatidylinositol 4,5-biphosphate (PIP2) to form diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 binds to the calcium (Ca2+) ion channel of the endoplasmic reticulum, releasing Ca2+ into the cytoplasm. High intracellular Ca2+ initiates protein-kinase C (PKC) activation by DAG as well as activation of membrane transient receptors potential channels (TRPC) that, in turn, induce dendritic remodeling.Citation56 Furthermore, released Ca2+ stimulates Ca2+/calmodulin-dependent protein kinase 2 (CaMKII) or CAMKIV capable of phosphorylating the activating Ser-133 site of cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) either directly or via activation of downstream kinases p38 of mitogen-activated protein kinases (MAPK) superfamily.Citation57 The CREB is well-documented as a transcriptional factor involved in the neuronal plasticity, learning and memory, outgrowth of neuronal processes, induction of neurotrophic and neuroprotectant cellular programs, and regulation of circadian rhythms.Citation58
Phosphoinositide 3-kinase (PI3K) pathway () is mostly crucial for the survival and proliferation of neuronal cells.Citation59 Upon phosphorylation of the tyrosine domain at position 515, the adaptor protein Shc binds to this site and is able to interact with Grb2-associated-binding protein 1 (GAB1) that forms a docking site for PI3K (). Then active PI3K binds to PIP2 and converts it to PIP3 leading to the activation of Akt, also known as protein kinase B (PKB), through phosphoinositide-dependent kinase-1 (PDK1) interaction. The proto-oncoprotein Akt binds with B-cell lymphoma 2 (Bcl-2) associated X protein (Bax)Citation60 increasing its proportion in the Bcl-2/Bax ratio leading to the antiapoptotic effect. Another signaling cascade of Akt involves the activation of mammalian target of rapamycin (mTOR), a high MW serine-threonine protein kinase that promotes protein synthesis in neuronal dendrites, initiating neurocyte growth, proliferation, and synaptic plasticity.Citation61 Additionally, a recent study showed that the BDNF/mTOR pathway is also involved in the stabilization of memory traces, which is crucial for the long-term memory (LTM) formation.Citation62
MAPK pathway is known to be important in neuronal cell growth, differentiation, protection, and release of neurotransmitters.Citation63 The activation of this pathway involves recruitment of Shc with further interaction with Grb2. The formation of Grb 2/son of sevenless (SOS) complex results in the initiation of rat sarcoma (Ras) protein by the conversion of guanosine diphosphate to guanosine triphosphate (GTP) (). Active Ras turns out to be able to bind the effector protein kinase rapid accelerated fibrosarcoma B (B-Raf), which, in turn, phosphorylates mitogen-activated protein kinase kinase (MEK) 1/2 leading to the activation of extracellular signal-regulated kinases (ERK) 1/2. Finally, the activated ERK 1/2 influences the previously mentioned transcriptional factor CREB.Citation64
p75NTR and sortilin
As described earlier in the “BDNF receptors” section, BDNF has a 1,000-fold lower affinity to pan-neurotrophin receptor p75 that interacts with TrkB enhancing its response to BDNF, thus promoting the cell survival.Citation65 The opposite action of p75NTR as a cell death receptor occurs when it forms the receptor complex with sortilin.Citation50 Beside this, p75NTR causes axon degeneration when it forms a triple complex with neurite outgrowth inhibitory protein receptor (NogoR) and leucine-rich repeat and Ig domain containing NogoR interacting protein 1 (LINGO-1). This complex promotes the suppression of axon growth involving three myelin-associated glycoproteins, specifically Nogo, myelin-associated glycoprotein, and oligodendrocyte-myelin glycoprotein.Citation66 The signaling cascades, which follow the binding BDNF or pro-BDNF to p75NTR have been shown to promote the neurite outgrowth, proliferation, and apoptosis.Citation67 All these pathways are set off after the binding of adaptor proteins to activated p75NTR, including neurotrophin receptor-interacting factor (NRIF), p75NTR interacting protein (NRAGE), p75NTR-associated death executor (NADE, NRH2),Citation67 and TNF receptor-associated factors (TRAF) proteins.Citation68
Jun-kinases signaling pathway () results in the activation of phosphoprotein p53 and apoptosis. In response to BDNF, p75NTR promotes Jun-kinases signaling pathway via interactions with NRAGE, TRAF6, and NRIF, stimulating the stress kinase c-Jun N-terminal kinase (JNK). The activation of Jun-kinases cascade involves the initiation of cell division control protein 42 (Cdc42), which, in turn, activates apoptosis signal regulated kinase 1 (ASK1). Additionally, a Jun-kinases kinase named MKK7 is shown to be a link between ASK1 and JNK.Citation69 JNK activation induces the phosphorylation of transcription factor c-Jun and tumor suppressor p53,Citation70 resulting in upregulation of proapoptotic genes and caspasesCitation71 that ultimately causes cell death.Citation72,Citation73
Figure 2 An overall scheme illustrating p75NTR signaling.
Notes: Heterodimerization with TrkB in response to BDNF action causes phosphorylation of IκB resulting in liberating of NF-κB, which after translocation into the nucleus promotes gene transcription to support neuron survival. Oppositely, heterodimerization with sortilin in response to pro-BDNF induces activation of Jun-kinase signaling pathway resulting in activation of proapoptotic genes and caspases causing cell death. Signaling molecule ceramide takes part in both NF-κB and Jun-kinases signaling cascades.
Abbreviations: BDNF, brain-derived neurotrophic factor; p75NTR, p75 neurotrophin receptor; TrkB, tropomyosin related kinase B; NRIF, neurotrophin receptor-interacting factor; NRAGE, p75NTR interacting protein; TRAF, tumor necrosis factor receptor-associated factor; Cdc42, cell division control protein 42; ASK1, apoptosis signal regulated kinase 1; JNK, c-Jun N-terminal kinase; IRAK, interleukin-1 receptor-associated kinase; aPKC, atypical protein kinase C; IκB, kappa light polypeptide gene enhancer in B-cells inhibitor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; MKK7, mitogen-activated protein kinase kinase 7.
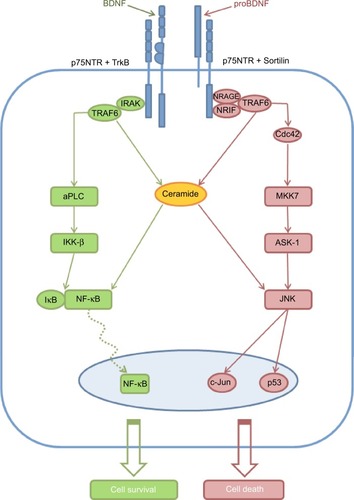
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway () in contrast to the previous one is proven to be an antiapoptotic.Citation74 Binding of the neurotrophin promotes the interaction between TRAF6 proteins and p75NTR cytoplasmic domain. Interleukin-1 receptor-associated kinase (IRAK) also binds to p75NTR forming a complex with TRAF6 that results in the activation of atypical PKC, which, in turn, phosphorylates nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB) kinase-β (IKK-β).Citation55 IKK-β-mediated phosphorylation of IκB results in the translocation of NF-κB into the nucleus, where genes, induced by NF-κB, promote cell survival or inhibition of the apoptosis.Citation75
The signaling molecule ceramide () is a result of the activation of the sphingomyelin cycle.Citation76 The role of the ceramide in neuronal cell signaling has yet been fully understood.Citation77 But, it is well-known that ceramide influences Jun-kinases and NF-κB signaling pathways, promoting both apoptotic and prosurvival pathways.Citation55
BDNF as a therapeutic option for blindness
In the year 2010, there were 32.4 million blind people and 191 million vision-impaired people worldwide.Citation78 Diabetic retinopathy, age-related macular degeneration, and glaucoma are recognized to be the main diseases, which can lead to irreversible blindness.Citation79 Various stimuli, like high intraocular pressure (IOP), blockage of axonal flow, and retinal ischemia may cause cellular damage in the retina.Citation80 Glaucoma,Citation81 ischemia,Citation82 age-related macular degeneration,Citation83 diabetic retinopathy,Citation84 and optic neuritisCitation85 are the potential contributors to retinal ganglion cells (RGCs) neurodegeneration and subsequent loss. It has been studied that survival of RGCs was enhanced by BDNF in vitro.Citation86 Moreover, at the retina, BDNF receptor, TrkB, has been found to be expressed by a number of cell types, namely, a subset of cone photoreceptors,Citation87 amacrine cells,Citation88 Muller glia,Citation89 and RGCs.Citation90 Therefore, in recent years, BDNF appears to be a major therapeutic strategy in vivo for ocular or systemic diseases that may lead to cellular damage in the retina. A variety of approaches associated with the delivery of BDNF to the posterior eye segment have been presented in . BRB, which has similar features with the BBB,Citation91 limits the movements of BDNF after systemic and periocular administration to the retina. On the other hand, anatomical features of the eye are known to circumvent the need for systemic administration by using relatively straightforward direct access to the retina, like intravitreal injection. This anatomical advantage avoids pharmacological challenges faced by BDNF delivery into the CNS, such as BBB penetration and degradation in the circulation.
Table 1 Different approaches of application of BDNF in the treatment of posterior eye segment degenerative diseases
Meanwhile, axotomy, as an animal model, has been extensively used to discover the survival of RGCsCitation92 and BDNF signal transduction pathways.Citation93,Citation94 This model has been reported to be performed in rats,Citation92,Citation95,Citation96 cats,Citation97 mice,Citation98 and hamsters.Citation99 Apart from this, other animal models, used to establish the BDNF activity in the retina, include light damage,Citation100 elevated intraocular pressure,Citation101 potassium cyanide-induced retinal damage,Citation102 N-methyl-D-aspartate (NMDA) excitotoxicity,Citation103 optic nerve trauma,Citation97 anterior ischemic optic neuropathy,Citation104 exploiting the dystrophic royal college of surgeons (RCS) rats strainCitation105 and investigation of the normal retina development in young rats.Citation106
Direct BDNF delivery to the posterior eye segment
Pure BDNF
The first intravitreal injection of pure recombinant BDNF was made to the axotomized rats in doses of 0.1–0.15 μg/rat/eye.Citation107 It had shown substantial increase in survival of injured cells 3 weeks after lesion in comparison to control. However, it had been discovered that the single injections could only temporarily rescue RGCs from neuronal death (). Five weeks after injury, the level of surviving cells did not increase very much, showing the transient effect of BDNF single injection. Later, the multiple injections, as a simple and logical way to surmount this limitation, were demonstrated by Mansour-Robaey et al.Citation92 Importantly, this study has detected the optimal dose, which is 5 μg of BDNF/one rat’s eye. As it turned out, the size of the animals is critical for dosing of BDNF, thus for mice, rats, young rats, and hamsters, the dose range was from 0.1 to 5 μg/eye/animal, whereas for cats, with the size of eye and vitreal volume comparable to those of primates, 30 μg was chosen as an optimal dose (). Even though the volume of vitreal chamber in cat’s eye is around 3 mL, while rat’s eye is 60-fold smaller (0.05 mL), the concentration of drug at the site of action appeared approximately the same (~0.01 μg BDNF/μL of vitreal volume).Citation97 Moreover, LaVail et alCitation108 compared the effectiveness of the intravitreal injection of the BDNF in concentrations of 1, 2, 5, and 10 mg/mL for both mice and rats, but the difference in volume of the injected solution was just 2 fold (0.5 and 1 μL, respectively), while mice have in 6.6-fold smaller vitreal chamber than rats.
Although intravitreal injection of BDNF have been shown as a good strategy for the treatment of various ocular and systemic diseases, some important limitations have been observed. Chen and WeberCitation97 displayed that at some doses, the increased levels of BDNF had resulted in less neuronal survival. They also revealed that continuous application of BDNF intravitreally led to a decrease in the TrkB.FL receptors in rat retina, but not the “truncated” forms.Citation109 They concluded that BDNF limits its own long-term neuroprotective efficacy by initiating downregulation of its TrkB receptor. Consequently, it is possible to assume that BDNF acts in a dose-dependent manner, however, multiple applications do not inevitably have an additive effect on RGCs survival. Furthermore, the safety profile of intravitreal injections, which depends on the surgical technique, the risk of cataract and retinal ischemia, as well as endophthalmitis, appears to be the major limitation of multiple injections.Citation110 Therefore, in order to overcome difficulties with BDNF short half-life, dosing, and undesirable effects of multiple injections, other methods of BDNF delivery have been explored for long-term maintenance. One of them was a proposal to use hyaluronic acid aqueous solution of BDNF instead of conventional phosphate buffered saline or Hank’s balanced salt solutions. BDNF was dissolved in 0.5% hyaluronic acid aqueous solution, resulting in a long-acting 1 mg/mL BDNF solution with sustained release of BDNF for ~1 week.Citation111 In this study, long-acting BDNF improved the differentiation of the hippocampus-derived neural stem cells embedded into the developing rat retina in comparison to BDNF dissolved in Dulbecco’s phosphate buffered saline.
Combinations with BDNF
The usage of combinations of BDNF with other growth factors or biological molecules () has been proven to prolong half-life,Citation112 increase effectiveness,Citation113,Citation114 enhance sprouting,Citation115 and reduce side effects.Citation116 Of interest, Weber et alCitation117 have demonstrated that BDNF improves the survival of RGCs after optic nerve injury, as well as appearing to be a key factor in the protection of structure and visual function of ganglion cells. In this study, cat’s eye with a crushed nerve was treated by BDNF (90 μg) intravitreally, while an infusion cannula was connected to an osmotic minipump to deliver the BDNF (100 μL of 0.3 μg/μL solution) into the visual thalamus. Such a combination substantially enriched the survival of RGCs and preserved central visual function.
Viral mediated BDNF delivery
To achieve stable and long-lasting transduction of retinal cells, researchers have utilized gene therapy by using viral vectors (). Among them, adeno-associated virus (AAV) was considered to be the most appropriate viral vector for gene therapy due to its sustained expressionCitation118 and safety.Citation119 Furthermore, a research had shown that intravitreal injection of adenovirus (Ad) vector containing BDNF had resulted in the transduction of the retinal Muller cells, which then began to secrete Ad-mediated BDNF.Citation120 Muller cells are essential for the maintenance and function of RGCs, moreover, they span across the entire sickness of the retina. Meanwhile, Di Polo et alCitation121 reported a persistent expression of Ad-mediated BDNF by transduced Muller cells which had promoted survival of axotomized RGCs, but not for long-term. Next, Gauthier et alCitation122 showed that BDNF gene transfer by AAV to Muller cells had served as a promising strategy to protect photoreceptors from light-induced retinal degeneration. Another study that explored the possibility of using recombinant AAV serotype 2 (AAV2) to deliver BDNF, revealed that this virus was able to provide efficient transduction of ganglion cells rather than Muller cells.Citation123 Furthermore, Martin et alCitation124 suggested the incorporation of woodchuck hepatitis posttranscriptional regulatory element (WPRE) in AAV-BDNF to facilitate transfection of neurons in rats’ RGCs. It has also been reported that electroporation was utilized to deliver BDNF gene into the RGCs.Citation125 Such transport may decrease unwanted effects of the intravitreal injection technique, and BDNF cDNA can be delivered into RGCs without detectable damage to the RGCs. Although cells transduced by Ad show a more sustained release of BDNF within the posterior eye segment in comparison with a single intravitreal administration of pure BDNF protein, the onset of gene expression appears to be the major limitation for the usage of viral vectors.Citation126 On the other hand, the combination of gene therapy with intravitreal injection of BDNF can compensate for the slow onset of viral-mediated expression. One study found that the combination of BDNF with AAV-BDNF had increased amount of survived RGCs by up to 97.4%, which was greater than using AAV-BDNF alone.Citation127
Cell-based BDNF delivery
The employment of stem cells (SCs) to transfer specific genes has been proposed as an alternative to the viral gene delivery approach ().Citation105,Citation128–Citation130 The essential benefits of SCs are their extensive survival and the possibility to create cell and stem cell-based delivery systems for diverse therapeutics.Citation131 It has been shown that bone marrow stem cells (BMSCs) transplanted into the subretinal space can express BDNF, thus executing trophic and protective effects on light-damaged rats’ retinas.Citation128 In addition, one study established a positive effect of usage of retinal-pigment epithelial cells carrying vectors transduced with the gene of a BDNF that were transplanted into the subretinal space of rats. This vector, which included a tetracycline-responsive element (TRE) and the cDNA of BDNF enabled upregulation of the expression of BDNF through the exposure of Tet-BDNF-RPE cells by topically applied doxycycline.Citation132 Another study suggested that the usage of genetically modified Schwann cell lines would enhance the release of several growth factors.Citation105 The combination of BMSCs with BDNF has been shown to promote differentiation of primitive cells derived from the bone marrow to the glial cells and neurons.Citation133 Park et alCitation129 applied a retroviral vector carrying rats’ BDNF cDNA to transduce rat bone marrow mesenchymal stem cells (rMSCs). After intravitreal or subretinal injection of modified rMSCs, the incorporation of the cells into the retina with further production of BDNF was detected. It is important to note that these methods present complications of subretinal injection technique, such as retinal folding and splitting, as well as rejection, accidental tumor growth, proliferative vitreoretinopathy, and choroidal neovascularization.Citation134
Nanoparticulate delivery
Ophthalmic drug delivery via nanoparticulate systems has the potential of being a great discovery as such systems accomplish the four important criteria of ophthalmic drug delivery, namely, improved drug permeation, low toxicity, controlled sustained drug release, and selective drug targeting.Citation135 Despite numerous promising results of using liposomes, solid lipid nanoparticles, polymeric nanoparticles, polymeric nanomicelles, and nanoemulsions for the treatment of posterior eye segment, the application of BDNF has not been published yet.Citation136 On the other hand, a similar approach has been reported where intravitreal injection of the gelatin nanoparticles (NPs) with basic fibroblast growth factor (bFGF) was used for the treatment of photoreceptor degeneration in dystrophic RCS rats.Citation137
BDNF as a therapeutic option for deafness
Hearing loss has affected over 360 million people worldwide,Citation138 causing substantial burden for the economy, and has a high impact on quality of life and causes emotional distress.Citation139 The existing conventional therapy for patients with injured hair cells (HCs) leading to hearing loss is a cochlear implant (CI) auditory prosthesis. CI electrodes directly stimulate spiral ganglion cells (SGCs) soma and probably their central axons, delivering partial restoration of sensory organ function in patients.Citation140 In such cases, the maximal preservation of the population of SGCs becomes essential because severe SGC degeneration constrains the effectiveness of hearing rehabilitation by a CI. BDNF has been shown to take part in both the development and the maintenance of SGCs.Citation141 Besides, BDNF has also been well-documented to be expressed by HCs, as well as by supporting cells of the organ of Corti.Citation142 Obviously, the damage of HCs or supporting cells in the auditory epithelium leads to a decrease in BDNF expression, causing degenerative changes in SGCs.Citation143 Therefore, BDNF has a major place within different therapeutic approaches of deafness treatment (). However, systemic application of BDNF as a treatment for hearing loss was not very effective as it has short half-life, poor pharmacokinetics, and high risk of unwanted effects of high dose.
Table 2 Different approaches of application of BDNF in the treatment of inner ear degenerative diseases
Furthermore, BCB is well-known to be critical in restricting the passage of large molecular weight substances into perilymph. The protein concentration in perilymph is ~1/20 that of blood.Citation17 Of interest, protein penetration is about ten times higher than that of cerebrospinal fluid and four times higher than that of aqueous humor.Citation144 Based on this, the delivery of pure BDNF from the systemic circulation into the inner ear should be easier than into the retina or brain, however, it still remains a challenge. In addition, the anatomical features do not allow large proteins to easily bypass this barrier using local direct injection, like intravitreal injection, as the organ of Corti is located inside the dense otic capsule of the temporal bone thus, limiting straight access. Direct drug delivery to patients using intratympanic delivery or diffusion through the round window membrane (RWM) or via cochleostomy, across the stapes footplate, or the endolymphatic sac seems to be a challenging task.Citation145 Beside this, there are two advantages of such anatomical structures: first, the peri- and endolymphatic fluids provide a means for the distribution of BDNF throughout the whole cochlea and, second, BDNF delivered to the inner ear will not reach the systemic circulation, therefore, reducing adverse reactions to the organism.Citation146
Many experimental models have been presented to cause hearing loss. Traditionally, the combination of aminoglycosides and diuretics is considered to be a classical one.Citation147 Systemic administration of gentamicin, kanamycin or neomycin, and furosemide or etacrynic acid has been widely used by different researchers for producing loss of SGCs (). Besides this, other experimental models of deafness include, intraperitoneal injection of cisplatin,Citation148 noise exposure,Citation149 or exploiting of deaf Pou4f3 mutant mice.Citation143 Although guinea pigs were established to be the most suitable animals for the experimental deafness, cats, rats, chinchillas, mice, and pigeons were also used ().
Direct BDNF delivery into the inner ear
Pure BDNF
One of the most popular approaches to deliver BDNF into the inner ear is the implantation of osmotic minipump into scala tympani (). The main benefit of the mini-osmotic pump is that it allows regulated duration of the infusion of therapeutics from days to weeks, whereas injections offer discrete infusions and are followed by latent periods. Additionally, the opportunity to put in new full drug reservoir instead of consumed one by a minor surgery may provide long-lasting delivery of more than 2 weeks.Citation150 Technically, direct delivery of pure BDNF protein is carried out by exploiting implanted osmotic minipump with drug primed cannulas that release of 0.5 or 0.25 μL/h of human recombinant BDNF. The overall dose of BDNF appeared to be important when using experimental animals with different sizes. The total effective dose range for guinea pigs was 10–16.8 μg/ear, for rats was 1.35 μg, and for cats was 37.5 μg (). Of interest, Radeloff and SmoldersCitation151 investigated the effectiveness of the treatment in deaf pigeons using a very small dose of 0.42 μg BDNF/ear. They revealed no reduction in the auditory brain stem responses (ABR) threshold, thus concluding that BDNF was not a limiting factor during HC regeneration and reinnervation. Nonetheless, the analysis of the corresponding doses, used for other animals showed that the application of such small amount of drug was the main reason for the unsuccessful result rather than other factors. Shoji et alCitation149 also showed that 0.144 and 1.44 μg BDNF were not efficacious in the protection of the sensory epithelium in guinea pigs with acoustic trauma. The authors admitted that they had used five times less dose than other researchers. However, they had compared BDNF with NT-3 action and had assumed that BDNF did not have any specific action of sensory epithelium. Meanwhile, another study claimed that BDNF had no protective effect on HCs at 15 days (total 9 μg of BDNF) and 60 days (36 μg), but some effects at 30 days (18 μg).Citation152 One more study demonstrated that direct placement of 0.05 μg of BDNF into the inner ear after cochleostomy did not improve or treat hearing loss after cisplastin-induced deafening.Citation148 At the same time, the positive effect of 8.4 ng BDNF on SGCs survival in deaf guinea pigs has remained controversial in the context of dose-dependent manner of BDNF action.Citation153 As an alternative approach cochleostomy was performed. The posterior part of the tympanic membrane was separated, then the underlying cochlea was opened into the scala tympani just anterior to the round window using a small diameter cutting drill and, finally, pure BDNF was placed there.Citation148 Due to lack of therapeutic effectiveness,Citation154 this method needs further investigation to establish an effective scheme of BDNF administration.
Combinations with BDNF
One of the main limitations of using osmotic minipumps is the transient protection of SGCs.Citation152 Indeed, the survival effects of BDNF on neuronal cells were not observed after the treatment phase.Citation155 This phenomenon has been widely confirmed by other studies in different neural systems, indicating that the survival effects of BDNF, as well as other growth factors only last so long as the treatment itself.Citation156 To overcome this limitation or to increase the effectiveness, the usage of several combinations of the BDNF with other growth factors or electrical stimulation (ES) was suggested (). Some studies have established that a chronic depolarization of the auditory nerve via ES provided a trophic effect on SGCs without the contact of HCs,Citation157,Citation158 however, these findings turned out to be controversial.Citation159 The effectiveness of the combination of BDNF with ES on SGCs survival also appeared to be not so universal (). This inconsistency displayed substantial methodological differences among these studies. The selection of animals can also be crucial for such studies: catsCitation160 and ratsCitation161 are possibly more sensitive to ES than guinea pigs.Citation162 For future translation to patients, it is critical to note that continued chronic ES after cessation of BDNF delivery had significantly reduced the rate of SGCs loss.Citation163 In addition, there was also evidence about the protective effects on the SGCs that employed other neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF),Citation164 fibroblast growth factor (FGF),Citation165 and ciliary neurotrophic factor (CNTF).Citation166 The combination of BDNF and any one of the other growth factors has been reported to be more effective than either agent alone in protecting SGCs and in decreasing ABR threshold (). Thus, the combination of BDNF with other agents may be promising as a potential therapeutic agent to promote the survival of SGCs in the auditory system. However, mini-osmotic pump appears to be inappropriate for use in patients due to fixed delivery period and high risk of bacterial contamination related to the surgical technique of inserting the pump into the cochlea and cannula clogging.Citation167
Viral mediated BDNF delivery
Viral vectors are known to be a potent method for gene delivery, which can be successfully applied for the treatment of the inner ear. Several types of viruses have been created for inner ear transfection: Ad, AAV, lentivirus, herpes simplex virus (HSV), vaccinia virus, and Sendai virus.Citation168 It was considered that gene delivery via cochleostomy into the basal turn of the scala media was more effective than via RWM due to the existence of tight junctions between the scala tympani and scala media.Citation169 HSV, as a gene transfer vector is identified to be a promising carrier because of its ability to infect several cell types, including quiescent and proliferating cells. HSV with BDNF had successfully infected HCs and neurons, and this saved 94.7% of the original population of SGCs after aminoglycoside ototoxic injuries.Citation170 Additionally, Ad vectors can also be beneficial, basically, due to their possibility to infect different cell types effectively and to provide relatively fast beginning of gene production following infection. The first successful transfection by Ad of retinal Muller cells in vivo, which then were able to secrete BDNF, was made by Di Polo et al.Citation121 Later, the same methodology was used for gene delivery into the inner ear. Rejali et alCitation171 demonstrated that Ad.BDNF transfected guinea pig fibroblast cells could release BDNF in vitro. The administration of Ad.BDNF into the cochlea following aminoglycoside deafening had been reported to enhance SGC survival rate at 21 days,Citation169 28 days,Citation172 and 48 daysCitation171 postinoculation, and to decrease psychophysical as well as ABR thresholds.Citation173 Recently, it was also found that Ad.BDNF had improved the auditory nerve survival and peripheral sprouting in Pou4f3 mutant mouse ears.Citation143 However, adenovirus gene expression appears to be transitory, hence high levels of transgenes cannot be persistent over a long period. Moreover, another limitation of Ad vectors is the activation of immune responses.Citation174 One more effective viral vector, AAV, had shown different cell tropisms at different serotypes.Citation175 Among them, AAV2 may easily transduce HCs and SGCs in vitro,Citation175 although, in vivo, it was revealed that this vector can only infect spiral limbus, spiral ligament, and spiral ganglion cells, following its delivery across the RWM.Citation176 Besides, a recent paper had demonstrated a successful transfection of the basilar membrane area (epithelial and mesothelial cells) by AAV2 carrying the BDNF gene.Citation177 Moreover, AAV is considered to be one of the most promising gene therapy vectors for human clinical trials.Citation168 However, there are some limitations for AAV vectors, which include their inability to perform effective gene transduction in patients with antibodies against AAV or those with AAV infection in the past, for instance, in childhood when it could be very common. Additionally, the risk of virus toxicity due to the application of viral vectors continues to be a major restriction for its clinical use.
Biodegradable materials for sustained BDNF release
In order to achieve sustained delivery of BDNF, other approaches were tested in vivo. A promising advance is the development of extracochlear application systems based on the ability of BDNF to diffuse across the RMW. Biodegradable hydrogel infiltrated with BDNFCitation178 has been successfully applied as a sustained-release carrier. With that, Ito et alCitation178 demonstrated significantly higher BDNF concentration in the guinea pigs’ cochlear perilymph in the hydrogel group in comparison to either control animals or the group that received an injection through the RWM. These data provided factual evidence for the diffusion of BDNF across the guinea pig’s round window. As a result, after 1 week, animals implanted with BDNF-containing collagen hydrogels had lower ABR thresholds and higher density of surviving SGCs from the basal up to the apical cochlear turns, moreover, the perilymph level of BDNF was over 100 times higher than control animals treated with saline.Citation178 Another option for sustained BDNF release to the cochlea was a gelatin sponge (Gelfoam®, Pharmacia and Upjohn, Bridgewater, NJ, USA) placed onto RMW.Citation179 Of interest, Havenith et alCitation179 had used a 10-fold less dose of BDNF than Ito et al.Citation178 Anyway, both studies are very significant, as they showed the possibility of usage of biodegradable carriers that contributed to low risk of cell toxicity. Nowadays, for the treatment of hearing loss, among all growth factors, only recombinant human insulin-like growth factor-1 (IGF-1) combined with gelatin hydrogel has been approved for clinical trials. It has finished Phase I/IIa clinical trial on the safety and efficacy of local IGF-1 application via the RWM using gelatin hydrogel for patients with acute sensorineural hearing loss.Citation180
Cell-based BDNF delivery
Apart from other approaches, Schwann cells or fibroblasts have been suggested as cell therapy methods. These types of cells have been tested both in vitro and in vivo.Citation181 The disadvantages include difficulties with the retrieval of cells and the potential to spread viral particles intracranially.Citation181 One study had suggested the creation of an electrode, which can secrete BDNF, using the ex vivo gene transfer pattern employing Ad containing BDNF gene insert. The proposed electrode was coated with fibroblasts transduced with the BDNF transgene. Then, the electrode with encased BDNF was transplanted into the scala tympani in a hydrogel scaffold on a CI to increase the survival of SGCs.Citation171 Another study that combined cell-based gene transfer with alginate technology, had demonstrated the survival effects on auditory neurons of encapsulated BDNF-expressing Schwann cells.Citation182 Such transplanted cells, encapsulated in a biocompatible matrix, would be protected against strong immune responses, and this would allow the patients to avoid using toxic immunodepressants, thus minimizing the associated risk of transplant rejection.
Without a doubt, osmotic minipump, gene therapy, stem cell therapy, and cochleostomy are hopeful steps toward successful treatment of the degeneration of SGCs in the inner ear and the loss of hearing. However, it is difficult to predict how these technologies can be translated into clinical practice. Moreover, BDNF is documented to act in dose-dependent manner, while accurate control of the dose, for some approaches, has remained to be a challenge.
Nanoparticulate delivery
NP delivery to the inner ear is a new promising strategy for targeting compounds to the cochlea in a sustained and controlled manner. Poly(D,L-lactide-co-glycolide acid) PLGA, a biodegradable and nontoxic polymer approved by US Food and Drug Administration for human use, has been established as the best nanoparticulate drug carrier.Citation183 Furthermore, recent work had demonstrated that systemic application of PLGA nanoparticles loaded with rhodamine resulted in the appearance of NPs in the liver, cochlea, and kidney, however, the placement of NPs into the RMW caused substantially higher accumulation of rhodamine in the scala tympani.Citation184 Another study had shown a wide distribution of PLGA throughout all turns of chinchilla cochlea when NPs were placed upon the RWM for a duration of 40 minutes.Citation185 Although delivery of BDNF to the inner ear using NPs has not been studied yet, either for systemic or for intratympanic administration, we assume that PLGA nanoparticles are a very promising approach for the inner ear drug targeting.
Similarities, controversies, and future prospects
Hearing or vision loss is not only a prominent burden to the health care system, but also a ground for the worse quality of life associated with isolation and decreased socializing and, as a consequence, leads to an uprising of symptoms of depression on individuals.Citation186,Citation187 In both cases, the neurodegenerative disorder of neurons of sensory organs is a crucial causal factor. Together with this, BDNF is considered to be a promising and an effective protein that is able to protect and increase the survival of RGCs as well as SGCs.
Besides, systemic delivery with oral, intravenous, and intramuscular routes are still thought as the most appropriate methods of drug administration, and they are currently recognized as the first line tactic in the treatment of the inner ear or eye posterior segment disorders in clinic. On the other hand, the availability of drug in the posterior eye segment or scala tympani following systemic administration is limited by the presence of BRB or BCB, which are almost nonpermeable to large proteins, like BDNF (14 kDa). A variety of efforts related to the delivery of pure BDNF to the target organs has been documented in many research papers. For retinal diseases, single or multiple intravitreal injections are known as the most convenient methods, while for the inner ear, implantation of the osmotic minipump is the most applicable. It turned out that establishing the dose could be a challenging task which, fairly frequently, was not so universal. As mentioned earlier, 5 μg of BDNF for one rat’s eye was as an optimal dose, whereas 30 μg BDNF was chosen as the optimal dose for cats. As for the ear, studies have shown that the total effective dose range for guinea pigs was 10–16.8 μg/ear, for rats was 1.35 μg, and for cats was 37.5 μg. Even if the doses look comparable, it would be incorrect to compare them for some obvious reasons, for instance, intravitreal injection is mostly single dosing, whereas osmotic minipump delivers the drug over a span of 2 weeks. Also, of note is the fact that the volume of human eye is ~4 mL, while the volume of cochlea is lesser, ~70–80 μL.Citation188 Overall, it has been explicitly clear that for both eye and ear, the BDNF has a dose-dependent manner of action. Eventually, it has been shown, that increasing the dose may lead to lack of effectiveness of BDNF neuroprotection,Citation97 which is possibly due to negative feedback-mediated downregulation of TrkB receptors.Citation189 Additionally, overdosing of BDNF enhances the excitability of the neurons that contribute to the initiation of seizures,Citation190 thus, it may be epileptogenic.Citation191 Furthermore, BDNF is found to cause an increase in the formation of free radicals, specifically nitric oxide (NO), hence boosting necrotic cell death.Citation116 To improve its effectiveness, reduce adverse effects, or to overcome the limitations, like transient action, it has been suggested that BDNF should be used in combinations. Mixtures of BDNF with other growth factors led to the enhancement of neuronal survival. Of interest, CNTF was exploited for both retina and inner ear, and it showed positive effect for immediate and delayed after-deafness treatment,Citation192 while for retina treatment, no additive effect was observed.Citation114 Another study had proposed the usage of LINGO-1 antagonist in combination with BDNF in rats with progressive neuropathy secondary to high intraocular pressure. LINGO-1 antagonist prevented the negative regulation of activity for TrkB receptors during interaction with BDNF and showed long-term protection for RGCs.Citation112 Besides, the combination with free radical scavenger S-PBNCitation101,Citation116 or NO synthase inhibitor l-NAME substantially reduced level of NO and improved RGCs survival in comparison to BDNF alone.Citation93,Citation116 As for deafened guinea pigs, ES was used in combination with BDNF to prolong survival of SGCs.Citation163,Citation193 Further, medicinal forms with sustained release appear to be another approach directed to overcome the transient effect of BDNF. Biodegradable polymer drug-delivery systems, like biodegradable hydrogel or Gelfoam® with BDNF, have been successfully applied for sustained delivery into the inner ear.Citation179 For the treatment of retinal disease, there is no documentation about BDNF delivery into the eye using biodegradable polymers. However, one such application had been tested in models of glaucoma, but by exploiting NGF. This showed that biodegradable PLGA microspheres, mixed with bioactive molecules with subsequent degradation of the material in vivo, had resulted in slow, sustained NGF release.Citation134 Alternatively, local drug delivery method, which employs viral gene, has been widely used for both eye and inner ear treatments. Furthermore, first, Ad vector was established by Dr Adriana Di Polo for BDNF therapy of axotomized rats.Citation121 Of interest, this adenoviral vector was later given by Dr Di Polo as a gift to the researchers who investigated the possibility of using gene therapy in deaf guinea pigs.Citation171,Citation172 Among the different viral vectors, AAV is the most promising to be translated to patients due to its safety,Citation194 different cell tropisms, and sustained expression of BDNF.Citation118 Recent advances in cell-based therapy have met the needs in long-term stable delivery of BDNF. Schwann cells, which are capable of expressing BDNF, have been effectively transplanted into the subretinal space of RCS rat strainsCitation105 and to the guinea pig’s cochlea.Citation182 However, viral vector and cell-based therapy are still limited by surgical technique and human immune system. As a promising solution to protect cells from rejection and tumorigenicity, cell transplantation and gene transfer, combined with encapsulation technologies, has been suggested.Citation182 BDNF-expressing Schwann cells with alginate encapsulation technology have been applied to deaf guinea pigs and this showed survival effect on auditory neurons. Although BDNF has not been used with such strategy for the treatment of posterior eye segment, data about a Phase I clinical trial exploiting immortalized human retinal pigmented epithelial cells overexpressing CNTF for the treatment of retinitis pigmentosa have been published.Citation195
A major challenge that has been raised is how to translate to patients the delivery of BDNF into these organs. Some drawbacks may be associated with the technique of administration itself. For instance, it was demonstrated that surgical manipulation on the patient’s ear like cochlear implantation has led to a significant risk of deafness.Citation196 At the same time, although repeated intravitreal injections of growth factors has become usual in many retinal clinics, other less invasive routes of administration capable of stable long-term delivery are of interest.Citation134 In future, the delivery of BDNF to the inner ear or to the posterior segment of the eye via nanotechnology-based products should be investigated more extensively. To date, some successes in the delivery of various therapeutics into the CNS have been widely recognized. Many studies on the different approaches to overcome the BBB are based on the knowledge of transport mechanisms of diverse biological molecules across the BBB. In fact, lots of techniques have been established to facilitate the penetration of NPs into the brain, namely carrier-mediated transport, receptor-mediated transcytosis, adsorptive-mediated transcytosis and cell-mediated transcytosis.Citation15 A comprehensive understanding of BRB and BCB physiology and matching them with the accumulated knowledge on BBB function should play a key role in the search for new strategies and will elucidate the potential of using NPs for the treatment of degenerative disorders of the retina and inner ear. It is possible to expect that targeted BDNF delivery that employs NPs should assist to bypass the limitations related to surgical intrusion into the sensory organ and simultaneously provide therapeutically effective concentration of molecule at the site of action and avoid unwanted systemic effects.
Conclusion
Currently, various therapeutic strategies to treat inner ear and posterior eye segment diseases are limited by poor systemic delivery of BDNF. Although local delivery into the eye or inner ear provides a high concentration of BDNF with reduced systemic adverse reactions, anatomic features of these sensory organs require surgical intervention to achieve this delivery, which may lead to severe complications when translated to patients. It would be excellent to apply knowledge about BBB permeation to create a clinically feasible technique for BDNF delivery into the inner ear or posterior eye segment exploiting systemic administration. We assume that promising BDNF-containing drug for the treatment of inner ear and retina disorders should be designed as safe and effective medicine based on nanoparticulate drug delivery systems. This can make possible the long-lasting rescue of auditory and optic neurons from degenerative effects, substantially improving the quality of life of deaf or blind people.
Acknowledgments
The authors would like to thank the National Defence University of Malaysia for fully supporting this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- AllenSJWatsonJJShoemarkDKBaruaNUPatelNKGDNF, NGF and BDNF as therapeutic options for neurodegenerationPharmacol Ther2013138215517523348013
- NagaharaAHMerrillDACoppolaGNeuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s diseaseNat Med200915333133719198615
- LevivierMPrzedborskiSBencsicsCKangUJIntrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s diseaseJ Neurosci19951512781078208613721
- BemelmansAPHorellouPPradierLBrunetIColinPMalletJBrain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transferHum Gene Ther199910182987299710609659
- LiWPozzo-MillerLBDNF deregulation in Rett syndromeNeuropharmacology201476Pt C73774623597512
- SchabitzWRSchwabSSprangerMHackeWIntraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in ratsJ Cereb Blood Flow Metab19971755005069183287
- BlahaGRRaghupathiRSaatmanKEMcIntoshTKBrain-derived neurotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficitsNeuroscience200099348349311029540
- HoshawBAMalbergJELuckiICentral administration of IGF-I and BDNF leads to long-lasting antidepressant-like effectsBrain Res200510371–220420815777771
- OtisJMFitzgeraldMKMuellerDInfralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preferenceJ Neurosci201434176057606424760865
- KoevaYASivkovSTAkabalievVHBrain-derived neurotrophic factor and its serum levels in schizophrenic patientsFolia Med20145612023
- KalraSGengeAArnoldDLA prospective, randomized, placebo-controlled evaluation of corticoneuronal response to intrathecal BDNF therapy in ALS using magnetic resonance spectroscopy: feasibility and resultsAmyotroph Lateral Scler Other Motor Neuron Disord200341222612745614
- MakarTKBeverCTSinghISBrain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicleJ Neuroimmunol20092101–2405119361871
- ChenYLiuLModern methods for delivery of drugs across the blood–brain barrierAdv Drug Deliv Rev201264764066522154620
- PardridgeWMDrug transport across the blood–brain barrierJ Cereb Blood Flow Metab201232111959197222929442
- AlyautdinRKhalinINafeezaMIHaronMHKuznetsovDNanoscale drug delivery systems and the blood–brain barrierInt J Nanomedicine2014979581124550672
- MannermaaEVellonenKSUrttiADrug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokineticsAdv Drug Deliv Rev200658111136116317081648
- JuhnSKRybakLPFowlksWLTransport characteristics of the blood–perilymph barrierAm J Otolaryngol1982363923966297328
- Levi-MontalciniRThe nerve growth factor 35 years laterScience19872374819115411623306916
- BardeYAEdgarDThoenenHPurification of a new neurotrophic factor from mammalian brainEMBO J1982155495537188352
- LeibrockJLottspeichFHohnAMolecular cloning and expression of brain-derived neurotrophic factorNature198934162381491522779653
- PattarawarapanMBurgessKMolecular basis of neurotrophin-receptor interactionsJ Med Chem200346255277529114640536
- RobinsonRCRadziejewskiCSpraggonGThe structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding siteProtein Sci19998122589259710631974
- RobinsonRCRadziejewskiCStuartDIJonesEYStructure of the brain-derived neurotrophic factor/neurotrophin 3 heterodimerBiochemistry19953413413941467703225
- MaisonpierrePCLe BeauMMEspinosaR3rdHuman and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizationsGenomics19911035585681889806
- HansonIMSeawrightAvan HeyningenVThe human BDNF gene maps between FSHB and HVBS1 at the boundary of 11p13-p14Genomics1992134133113331505967
- LommatzschMBraunAMannsfeldtAAbundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functionsAm J Pathol199915541183119310514401
- GarcesMFSanchezETorres-SierraALBrain-derived neurotrophic factor is expressed in rat and human placenta and its serum levels are similarly regulated throughout pregnancy in both speciesClin Endocrinol2014811141151
- LessmannVGottmannKMalcangioMNeurotrophin secretion: current facts and future prospectsProg Neurobiol200369534137412787574
- NegroATavellaAGrandiCSkaperSDProduction and characterization of recombinant rat brain-derived neurotrophic factor and neurotrophin-3 from insect cellsJ Neurochem19946224714788294909
- SeidahNGBenjannetSPareekSChretienMMurphyRACellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertasesFEBS Lett199637932472508603699
- GrayKEllisVActivation of pro-BDNF by the pericellular serine protease plasminFEBS Lett2008582690791018291105
- TengHKTengKKLeeRProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilinJ Neurosci200525225455546315930396
- YangMLimYLiXZhongJHZhouXFPrecursor of brain-derived neurotrophic factor (proBDNF) forms a complex with Huntingtin-associated protein-1 (HAP1) and sortilin that modulates proBDNF trafficking, degradation, and processingJ Biol Chem201128618162721628421357693
- EganMFKojimaMCallicottJHThe BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal functionCell2003112225726912553913
- UltschMHWiesmannCSimmonsLCCrystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkCJ Mol Biol1999290114915910388563
- NaylorRLRobertsonAGAllenSJA discrete domain of the human TrkB receptor defines the binding sites for BDNF and NT-4Biochem Biophys Res Commun2002291350150711855816
- MassaSMYangTXieYSmall molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodentsJ Clin Invest201012051774178520407211
- MiddlemasDSLindbergRAHunterTTrkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptorsMol Cell Biol19911111431531846020
- BarbacidMThe Trk family of neurotrophin receptorsJ Neurobiol19942511138614037852993
- WongJRothmondDAWebsterMJWeickertCSIncreases in two truncated TrkB isoforms in the prefrontal cortex of people with schizophreniaSchizophr Bull201339113014021795612
- ArevaloJCWuSHNeurotrophin signaling: many exciting surprises!Cell Mol Life Sci200663131523153716699811
- BiffoSOffenhauserNCarterBDBardeY-ASelective binding and internalisation by truncated receptors restrict the availability of BDNF during developmentDevelopment19951218246124707671810
- EideFFViningEREideBLZangKWangXYReichardtLFNaturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signalingJ Neurosci19961610312331298627351
- GrussHJDowerSKThe TNF ligand superfamily and its relevance for human diseasesCytokines Mol Ther199512751059384666
- FradeJMBardeYANerve growth factor: two receptors, multiple functionsBioessays19982021371459631659
- TomelliniELagadecCPolakowskaRLe BourhisXRole of p75 neurotrophin receptor in stem cell biology: more than just a markerCell Mol Life Sci201471132467248124481864
- BarkerPAp75NTR is positively promiscuous: novel partners and new insightsNeuron200442452953315157416
- SkeldalSMatusicaDNykjaerACoulsonEJProteolytic processing of the p75 neurotrophin receptor: a prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR)Bioessays201133861462521717487
- HempsteadBLMartin-ZancaDKaplanDRParadaLFChaoMVHigh-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptorNature199135063206786831850821
- NykjaerALeeRTengKKSortilin is essential for proNGF-induced neuronal cell deathNature2004427697784384814985763
- JacobsenLMadsenPJacobsenCNielsenMSGliemannJPetersenCMActivation and functional characterization of the mosaic receptor SorLA/LR11J Biol Chem200127625227882279611294867
- AtwalJKMassieBMillerFDKaplanDRThe TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinaseNeuron200027226527710985347
- CowansageKKLeDouxJEMonfilsM-HBrain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticityCurr Mol Pharmacol201031122920030625
- ChaoMVNeurotrophins and their receptors: a convergence point for many signalling pathwaysNature Reviews Neuroscience200344299309
- ReichardtLFNeurotrophin-regulated signalling pathwaysPhilos Trans R Soc Lond B Biol Sci200636114731545156416939974
- AmaralMDPozzo-MillerLTRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formationJ Neurosci200727195179518917494704
- BlanquetPRMarianiJDererPA calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampusNeuroscience2003118247749012699783
- TarditoDPerezJTiraboschiEMusazziLRacagniGPopoliMSignaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overviewPharmacol Rev200658111513416507885
- ZhengWHQuirionRComparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survivalJ Neurochem200489484485215140184
- Perez-NavarroEGavaldaNGratacosEAlberchJBrain-derived neurotrophic factor prevents changes in Bcl-2 family members and caspase-3 activation induced by excitotoxicity in the striatumJ Neurochem200592367869115659237
- TakeiNInamuraNKawamuraMBrain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendritesJ Neurosci200424449760976915525761
- SlipczukLBekinschteinPKatcheCCammarotaMIzquierdoIMedinaJHBDNF activates mTOR to regulate GluR1 expression required for memory formationPloS One200946e600719547753
- NumakawaTSuzukiSKumamaruEAdachiNRichardsMKunugiHBDNF function and intracellular signaling in neuronsHistol Histopathol201025223725820017110
- HaoYCresonTZhangLMood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesisJ Neurosci200424296590659915269271
- BibelMHoppeEBardeYABiochemical and functional interactions between the neurotrophin receptors trk and p75NTREMBO J19991836166229927421
- MiSLeeXShaoZLINGO-1 is a component of the Nogo-66 receptor/p75 signaling complexNat Neurosci20047322122814966521
- ChaoMVRajagopalRLeeFSNeurotrophin signalling in health and diseaseClin Sci2006110216717316411893
- ZottiTVitoPStiloRThe seventh ring: exploring TRAF7 functionsJ Cell Physiol201222731280128422105767
- KanamotoTMotaMTakedaKRole of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neuronsMol Cell Biol200020119620410594022
- DhanasekaranDNReddyEPJNK signaling in apoptosisOncogene200827486245625118931691
- MacLachlanTKEl-DeiryWSApoptotic threshold is lowered by p53 transactivation of caspase-6Proc Natl Acad Sci U S A200299149492949712089322
- MiyashitaTReedJCTumor suppressor p53 is a direct transcriptional activator of the human bax geneCell19958022932997834749
- BogoyevitchMAKobeBUses for JNK: the many and varied substrates of the c-Jun N-terminal kinasesMicrobiol Mol Biol Rev20067041061109517158707
- KarinMLinANF-κB at the crossroads of life and deathNat Immunol20023322122711875461
- GentryJJBarkerPACarterBDThe p75 neurotrophin receptor: multiple interactors and numerous functionsProg Brain Res2004146253914699954
- DobrowskyRTWernerMHCastellinoAMChaoMVHannunYAActivation of the sphingomyelin cycle through the low-affinity neurotrophin receptorScience19942655178159615998079174
- LewinGRCarterBDNeurotrophic Factors – Handbook of Experimental Pharmacology220Berlin, HeidelbergSpringer-Verlag2014129
- JonasJBBourneRRWhiteRAVisual impairment and blindness due to macular diseases globally: a systematic review and meta-analysisAm J Ophthalmol2014158480881524973605
- BourneRRJonasJBFlaxmanSRPrevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010Br J Ophthalmol201498562963824665132
- NickellsRWZackDJApoptosis in ocular disease: a molecular overviewOphthalmic Genet19961741451659010866
- QuigleyHANeuronal death in glaucomaProg Retin Eye Res199918139579920498
- BernsteinSLGuoYKelmanSEFlowerRWJohnsonMAFunctional and cellular responses in a novel rodent model of anterior ischemic optic neuropathyInvest Ophthalmol Vis Sci200344104153416214507856
- MedeirosNECurcioCAPreservation of ganglion cell layer neurons in age-related macular degenerationInvest Ophthalmol Vis Sci200142379580311222543
- KernTSBarberAJRetinal ganglion cells in diabetesJ Physiol2008586Pt 184401440818565995
- ShindlerKSGuanYVenturaEBennettJRostamiARetinal ganglion cell loss induced by acute optic neuritis in a relapsing model of multiple sclerosisMult Scler200612552653217086896
- JohnsonJEBardeYASchwabMThoenenHBrain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cellsJ Neurosci1986610303130382876066
- Di PoloAChengLBrayGMAguayoAJColocalization of TrkB and brain-derived neurotrophic factor proteins in green-red–sensitive cone outer segmentsInvest Ophthalmol Vis Sci200041124014402111053307
- CellerinoAKohlerKBrain-derived neurotrophic factor/neurotrophin-4 receptor TrkB is localized on ganglion cells and dopaminergic amacrine cells in the vertebrate retinaJ Comp Neurol199738611491609303531
- RohrerBKorenbrotJILaVailMMReichardtLFXuBRole of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retinaJ Neurosci199919208919893010516311
- JelsmaTNFriedmanHHBerkelaarMBrayGMAguayoAJDifferent forms of the neurotrophin receptor trkB mRNA predominate in rat retina and optic nerveJ Neurobiol1993249120712148409978
- SteuerHJaworskiAElgerBFunctional characterization and comparison of the outer blood-retina barrier and the blood–brain barrierInvest Ophthalmol Vis Sci20054631047105315728564
- Mansour-RobaeySClarkeDBWangYCBrayGMAguayoJEffects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cellsProc Natl Acad Sci U S A1994915163216368127857
- KlockerNKermerPWeishauptJHLabesMAnkerholdRBahrMBrain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signalingJ Neurosci200020186962696710995840
- NakazawaTTamaiMMoriNBrain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathwaysInvest Ophthalmol Vis Sci200243103319332612356841
- WeibelDCadelliDSchwabMERegeneration of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitorsBrain Res19946421–22592668032887
- WeibelDKreutzbergGWSchwabMEBrain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerveBrain Res199567922492547543356
- ChenHWeberAJBDNF enhances retinal ganglion cell survival in cats with optic nerve damageInvest Ophthalmol Vis Sci200142596697411274073
- Galindo-RomeroCValiente-SorianoFJJimenez-LopezMEffect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microgliaInvest Ophthalmol Vis Sci201354297498523307961
- ZhangCWLuQYouSWCNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injuryInvest Ophthalmol Vis Sci20054641497150315790921
- LaVailMMGorrinGMProtection from light damage by ocular pigmentation: analysis using experimental chimeras and translocation miceExp Eye Res19874468778893653278
- KoMLHuDNRitchRSharmaSCThe combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucomaInvest Ophthalmol Vis Sci200041102967297110967052
- IkedaKTaniharaHHondaYTatsunoTNoguchiHNakayamaCBDNF attenuates retinal cell death caused by chemically induced hypoxia in ratsInvest Ophthalmol Vis Sci19994092130214010440270
- KidoNTaniharaHHonjoMNeuroprotective effects of brain-derived neurotrophic factor in eyes with NMDA-induced neuronal deathBrain Res20008841–2596711082487
- TomitaMAdachiYYamadaHBone marrow-derived stem cells can differentiate into retinal cells in injured rat retinaStem Cells200220427928312110696
- LawrenceJMKeeganDJMuirEMTransplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons ratsInvest Ophthalmol Vis Sci200445126727414691183
- CellerinoAPinzon-DuarteGCarrollPKohlerKBrain-derived neurotrophic factor modulates the development of the dopaminergic network in the rodent retinaJ Neurosci1998189335133629547243
- MeyJThanosSIntravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivoBrain Res199360223043178448673
- LaVailMMYasumuraDMatthesMTProtection of mouse photoreceptors by survival factors in retinal degenerationsInvest Ophthalmol Vis Sci19983935926029501871
- ChenHWeberAJBrain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult ratsBrain Res2004101119910615140649
- MarticorenaJRomanoVGomez-UllaFSterile endophthalmitis after intravitreal injectionsMediators Inflamm2012201292812322973075
- SuzukiTOotoSAkagiTEffects of prolonged delivery of brain-derived neurotrophic factor on the fate of neural stem cells transplanted into the developing rat retinaBiochem Biophys Res Commun2003309484384713679050
- FuQLLiXYipHKCombined effect of brain-derived neurotrophic factor and LINGO-1 fusion protein on long-term survival of retinal ganglion cells in chronic glaucomaNeuroscience2009162237538219422885
- KoeberlePDBallAKNeurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factorNeuroscience2002110355556711906793
- WatanabeMTokitaYKatoMFukudaYIntravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retinaNeuroscience2003116373374212573715
- TropeaDCaleoMMaffeiLSynergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult ratsJ Neurosci200323187034704412904464
- KlockerNCellerinoABahrMFree radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells in vivoJ Neurosci1998183103810469437024
- WeberAJViswanathanSRamanathanCHarmanCDCombined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injuryInvest Ophthalmol Vis Sci201051132733419710411
- GuyJQiXMuzyczkaNHauswirthWWReporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerveArch Ophthalmol1999117792993710408459
- XiaoXLiJMcCownTJSamulskiRJGene transfer by adeno-associated virus vectors into the central nervous systemExp Neurol199714411131249126160
- BennettJWilsonJSunDForbesBMaguireAAdenovirus vector-mediated in vivo gene transfer into adult murine retinaInvest Ophthalmol Vis Sci1994355253525428163342
- Di PoloAAignerLJDunnRJBrayGMAguayoAJProlonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cellsProc Natl Acad Sci U S A1998957397839839520478
- GauthierRJolySPernetVLachapellePDi PoloABrain-derived neurotrophic factor gene delivery to muller glia preserves structure and function of light-damaged photoreceptorsInvest Ophthalmol Vis Sci20054693383339216123443
- SchuettaufFVorwerkCNaskarRAdeno-associated viruses containing bFGF or BDNF are neuroprotective against excitotoxicityCurr Eye Res200429637938615764082
- MartinKRQuigleyHAZackDJGene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma modelInvest Ophthalmol Vis Sci200344104357436514507880
- MoXYokoyamaAOshitariTRescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult ratsInvest Ophthalmol Vis Sci20024372401240512091443
- FerrariFKSamulskiTShenkTSamulskiRJSecond-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectorsJ Virol1996705322732348627803
- RenRLiYLiuZLiuKHeSLong-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressureInvest Ophthalmol Vis Sci20125321003101122247466
- ZhangYWangWEffects of bone marrow mesenchymal stem cell transplantation on light-damaged retinaInvest Ophthalmol Vis Sci20105173742374820207980
- ParkHYKimJHSun KimHParkCKStem cell-based delivery of brain-derived neurotrophic factor gene in the rat retinaBrain Res20121469102322750585
- ZhouX-MYuanH-PWuD-LStudy of brain-derived neurotrophic factor gene transgenic neural stem cells in the rat retinaChin Med J (Engl)200912214164219719965
- RothJCCurielDTPereboevaLCell vehicle targeting strategiesGene Ther2008151071672918369326
- AbeTWakusawaRSetoHAsaiNSaitoTNishidaKTopical doxycycline can induce expression of BDNF in transduced retinal pigment epithelial cells transplanted into the subretinal spaceInvest Ophthalmol Vis Sci20084983631363918660427
- Goldenberg-CohenNAvraham-LubinBCSadikovTAskenasyNEffect of coadministration of neuronal growth factors on neuroglial differentiation of bone marrow-derived stem cells in the ischemic retinaInvest Ophthalmol Vis Sci201455150251224370836
- JohnsonTVBullNDMartinKRNeurotrophic factor delivery as a protective treatment for glaucomaExp Eye Res201193219620320685205
- EdelhauserHFRowe-RendlemanCLRobinsonMROphthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applicationsInvest Ophthalmol Vis Sci201051115403542020980702
- KaurIPKakkarSNanotherapy for posterior eye diseasesJ Control Release201419310011224862316
- SakaiTKunoNTakamatsuFProlonged protective effect of basic fibroblast growth factor-impregnated nanoparticles in royal college of surgeons ratsInvest Ophthalmol Vis Sci20074873381338717591912
- WHODeafness and Hearing LossSwitzerland, Geneva: WHO2014 Available from: http://www.who.int/mediacentre/factsheets/fs300/en/Accessed October 2, 2014
- FellingerJHolzingerDGerichJGoldbergDMental distress and quality of life in the hard of hearingActa Psychiatr Scand2007115324324517302625
- WilsonBSDormanMFCochlear implants: a remarkable past and a brilliant futureHear Res20082421–232118616994
- FritzschBPirvolaUYlikoskiJMaking and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implicationsCell Tissue Res1999295336938210022958
- ErnforsPLeeKFJaenischRMice lacking brain-derived neurotrophic factor develop with sensory deficitsNature199436864671471508139657
- FukuiHWongHTBeyerLABDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant miceSci Rep2012283823150788
- JuhnSKHaugenJStevenLSome chemical parameters of serum, cerebrospinal fluid, perilymph, and aqueous humor of the chinchillaLab Anim Sci19742446916954369187
- StaeckerHBroughDEPraetoriusMBakerKDrug delivery to the inner ear using gene therapyOtolaryngol Clin North Am20043751091110815474113
- BudenzCLPfingstBERaphaelYThe use of neurotrophin therapy in the inner ear to augment cochlear implantation outcomesAnat Rec (Hoboken)2012295111896190823044834
- JohnssonLGHawkinsJEJrStrial atrophy in clinical and experimental deafnessLaryngoscope1972827110511255078636
- MeenEBlakleyBQuddusiTBrain-derived nerve growth factor in the treatment of sensorineural hearing lossLaryngoscope200911981590159319479743
- ShojiFMillerALMitchellAYamasobaTAltschulerRAMillerJMDifferential protective effects of neurotrophins in the attenuation of noise-induced hair cell lossHear Res20001461–213414210913890
- HendricksJLChikarJACrumlingMARaphaelYMartinDCLocalized cell and drug delivery for auditory prosthesesHear Res20082421–211713118573323
- RadeloffASmoldersJWBrain-derived neurotrophic factor treatment does not improve functional recovery after hair cell regeneration in the pigeonActa Otolaryngol2006126545245916698693
- RuanRSLeongSKMarkIYeohKHEffects of BDNF and NT-3 on hair cell survival in guinea pig cochlea damaged by kanamycin treatmentNeuroreport199910102067207110424676
- MillerJMChiDHO’KeeffeLJKruszkaPRaphaelYAltschulerRANeurotrophins can enhance spiral ganglion cell survival after inner hair cell lossInt J Dev Neurosci1997154–56316439263039
- MeenEBlakleyBQuddusiTDoes intracochlear brain-derived nerve growth factor improve auditory brainstem click thresholds in sensorineural hearing loss?J Otolaryngol Head Neck Surg201039323223520470666
- GillespieLNClarkGMBartlettPFMarzellaPLBDNF-induced survival of auditory neurons in vivo: cessation of treatment leads to accelerated loss of survival effectsJ Neurosci Res200371678579012605404
- MonteroCNHeftiFRescue of lesioned septal cholinergic neurons by nerve growth factor: specificity and requirement for chronic treatmentJ Neurosci198888298629992842469
- LeakePAHradekGTRebscherSJSnyderRLChronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened catsHear Res19915422512711938628
- MitchellAMillerJMFingerPAHellerJWRaphaelYAltschulerRAEffects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pigHear Res19971051–230439083802
- ShepherdRKMatsushimaJMartinRLClarkGMCochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittensHear Res1994811–21501667737922
- LeakePAStakhovskayaOHetheringtonARebscherSJBonhamBEffects of brain-derived neurotrophic factor (BDNF) and electrical stimulation on survival and function of cochlear spiral ganglion neurons in deafened, developing catsJ Assoc Res Otolaryngol201314218721123392612
- SongBNLiYXHanDMDelayed electrical stimulation and BDNF application following induced deafness in ratsActa Otolaryngol2009129214215418607918
- LandryTGWiseAKFallonJBShepherdRKSpiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatmentHear Res20112821–230331321762764
- ShepherdRKCocoAEppSBNeurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing lossHear Res20082421–210010918243608
- MaruyamaJMillerJMUlfendahlMGlial cell line-derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafnessNeurobiol Dis2008291142117870569
- GlueckertRBitscheMMillerJMDeafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factorJ Comp Neurol200850741602162118220258
- HartnickCJStaeckerHMalgrangeBNeurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neuronsJ Neurobiol19963022462548738753
- PettingillLNRichardsonRTWiseAKO’LearySJShepherdRKNeurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory systemIEEE Trans Biomed Eng2007546 Pt 11138114817551571
- SacheliRDelacroixLVandenackervekenPNguyenLMalgrangeBGene transfer in inner ear cells: a challenging raceGene Ther201320323724722739386
- WiseAKHumeCRFlynnBOEffects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochleaMol Ther20101861111112220216530
- StaeckerHGabaizadehRFederoffHVan De WaterTRBrain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell lossOtolaryngol Head Neck Surg199811917139674508
- RejaliDLeeVAAbrashkinKAHumayunNSwiderskiDLRaphaelYCochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neuronsHear Res20072281–218018717416474
- NakaizumiTKawamotoKMinodaRRaphaelYAdenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damageAudiol Neurootol20049313514315084818
- ChikarJAColesaDJSwiderskiDLDi PoloARaphaelYPfingstBEOver-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neuronsHear Res20082451–2243418768155
- HoltJRJohnsDCWangSFunctional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectorsJ Neurophysiol19998141881188810200223
- StoneIMLurieDIKelleyMWPoulsenDJAdeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochleaMol Ther200511684384815922954
- DuanMLBordetTMezzinaMKahnAUlfendahlMAdenoviral and adeno-associated viral vector mediated gene transfer in the guinea pig cochleaNeuroreport200213101295129912151790
- ShibataSBCortezSRBeyerLATransgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleaeExp Neurol2010223246447220109446
- ItoJEndoTNakagawaTKitaTKimTSIguchiFA new method for drug application to the inner earORL J Otorhinolaryngol Relat Spec200567527227516374059
- HavenithSVersnelHAgterbergMJSpiral ganglion cell survival after round window membrane application of brain-derived neurotrophic factor using gelfoam as carrierHear Res20112721–216817720969940
- SakamotoTNakagawaTHorieRTInner ear drug delivery system from the clinical point of viewActa Otolaryngol Suppl201056310110420879828
- GillespieLNShepherdRKClinical application of neurotrophic factors: the potential for primary auditory neuron protectionEur J Neurosci20052292123213316262651
- PettingillLNWiseAKGeaneyMSShepherdRKEnhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pigPloS One201164e1873321525998
- Di ToroRBettiVSpampinatoSBiocompatibility and integrin-mediated adhesion of human osteoblasts to poly (DL-lactide-co-glycolide) copolymersEur J Pharm Sci200421216116914757487
- TamuraTKitaTNakagawaTDrug delivery to the cochlea using PLGA nanoparticlesLaryngoscope2005115112000200516319613
- GeXJacksonRLLiuJDistribution of PLGA nanoparticles in chinchilla cochleaeOtolaryngol Head Neck Surg2007137461962317903580
- ArlingerSNegative consequences of uncorrected hearing loss – a reviewInt J Audiol200342Suppl 22S172S2012918624
- HorowitzAThe prevalence and consequences of vision impairment in later lifeTop Geriatr Rehabil2004203185195
- KirkECGosselin-IldariADCochlear labyrinth volume and hearing abilities in primatesAnat Rec (Hoboken)2009292676577619462443
- FrankLWiegandSJSiuciakJALindsayRMRudgeJSEffects of BDNF infusion on the regulation of TrkB protein and message in adult rat brainExp Neurol1997145162709184109
- ScharfmanHEGoodmanJHSollasALCrollSDSpontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factorExp Neurol2002174220121411922662
- BinderDKCrollSDGallCMScharfmanHEBDNF and epilepsy: too much of a good thing?Trends Neurosci2001241475311163887
- ShinoharaTBredbergGUlfendahlMNeurotrophic factor intervention restores auditory function in deafened animalsProc Natl Acad Sci U S A20029931657166011818566
- ShepherdRKCocoAEppSBCrookJMChronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing lossJ Comp Neurol2005486214515815844207
- XiaoXLiJSamulskiRJEfficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vectorJ Virol19967011809881088892935
- SievingPACarusoRCTaoWCiliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implantsProc Natl Acad Sci U S A2006103103896390116505355
- AdunkaOUnkelbachMHMackMHambekMGstoettnerWKieferJCochlear implantation via the round window membrane minimizes trauma to cochlear structures: a histologically controlled insertion studyActa Otolaryngol2004124780781215370564
- UnokiKLaVailMMProtection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factorInvest Ophthalmol Vis Sci19943539079158125754
- SawaiHClarkeDBKittlerovaPBrayGMAguayoAJBrain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cellsJ Neurosci19961612388738948656282
- Peinado-RamonPSalvadorMVillegas-PerezMPVidal-SanzMEffects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo studyInvest Ophthalmol Vis Sci19963744895008595949
- CellerinoAArango-GonzalezBAKohlerKEffects of brain-derived neurotrophic factor on the development of NADPH-diaphorase/nitric oxide synthase-positive amacrine cells in the rodent retinaEur J Neurosci19991182824283410457179
- KurokawaTKataiNShibukiHBDNF diminishes caspase-2 but not c-Jun immunoreactivity of neurons in retinal ganglion cell layer after transient ischemiaInvest Ophthalmol Vis Sci199940123006301110549664
- ChenHWeberAJExpression of glial fibrillary acidic protein and glutamine synthetase by Müller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factorGlia200238211512511948805
- OtaTHaraHMiyawakiNBrain-derived neurotrophic factor inhibits changes in soma-size of retinal ganglion cells following optic nerve axotomy in ratsJ Ocul Pharmacol Ther200218324124912099545
- Krueger-NaugAEmsleyJMyersTCurrieRClarkeDAdministration of brain-derived neurotrophic factor suppresses the expression of heat shock protein 27 in rat retinal ganglion cells following axotomyNeuroscience20031161495812535937
- LeeEJSongMCKimHJBrain-derived neurotrophic factor modulates the dopaminergic network in the rat retina after axotomyCell Tissue Res2005322219119916075211
- ChenRYinXBPengCXLiGLEffect of brain-derived neurotrophic factor on c-jun expression in the rd mouse retinaInt J Ophthalmol20125326627122773970
- YanQWangJMathesonCRUrichJLGlial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF)J Neurobiol199938338239010022580
- WeberAJHarmanCDBDNF treatment and extended recovery from optic nerve trauma in the catInvest Ophthalmol Vis Sci201354106594660423989190
- WangJMSunNXHuiNFanYZFengHXZhaoSPEffects of rAAV-mediated rhBDNF gene transfection on BDNF gene expression in the retina of a rabbit model of acute high intraocular pressureNan Fang Yi Ke Da Xue Xue Bao2009291122012204 Chinese19923066
- WanCLiuNNLiuLMCaiNChenLEffect of adenovirus-mediated brain derived neurotrophic factor in early retinal neuropathy of diabetes in ratsInt J Ophthalmol20103214514822553539
- McGuinnessSLShepherdRKExogenous BDNF rescues rat spiral ganglion neurons in vivoOtol Neurotol20052651064107216151360
- AgterbergMJVersnelHde GrootJCSmoorenburgGFAlbersFWKlisSFMorphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigsHear Res20082441–2253418692557
- SongBNLiYXHanDMEffects of delayed brain-derived neurotrophic factor application on cochlear pathology and auditory physiology in ratsChin Med J2008121131189119618710637
- AgterbergMJVersnelHvan DijkLMde GrootJCKlisSFEnhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigsJ Assoc Res Otolaryngol200910335536719365690
- SlyDJHampsonAJMinterRLBrain-derived neurotrophic factor modulates auditory function in the hearing cochleaJ Assoc Res Otolaryngol201213111622086147
- LeakePAHradekGTHetheringtonAMStakhovskayaOBrain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing catsJ Comp Neurol201151981526154521452221
- WaaijerLKlisSFRamekersDVan DeurzenMHHendriksenFGGrolmanWThe peripheral processes of spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigsOtol Neurotol201334357057823449444
- van LoonMCRamekersDAgterbergMJSpiral ganglion cell morphology in guinea pigs after deafening and neurotrophic treatmentHear Res2013298172623361189
- YamagataTMillerJMUlfendahlMDelayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigsJ Neurosci Res2004781758615372491
- WiseAKRichardsonRHardmanJClarkGO’LearySResprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3J Comp Neurol2005487214716515880560
- MillerJMLe PrellCGPrieskornDMWysNLAltschulerRADelayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factorJ Neurosci Res20078591959196917492794
- LalwaniAKHanJJCasteleinCMCarvalhoGJMhatreANIn vitro and in vivo assessment of the ability of adeno-associated virus–brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insultLaryngoscope200211281325133412172239
- LiLShuiQXLiXNeuroprotective effects of brain-derived neurotrophic factor (BDNF) on hearing in experimental pneumococcal meningitisJ Child Neurol2005201515615791923
