Abstract
It has been demonstrated that tumoral cells have a higher uptake of ascorbic acid compared to normal cells. This differential characteristic can be used as a way to improve the specificity of antitumoral compounds if combined with polymeric drug delivery systems. The aim of this study was to prepare, characterize and evaluate the antitumoral activity of poly- D,L-(lactide-co-glycolide) 50:50 loading the antitumoral compound violacein and capped with L-ascorbic acid. Nanoparticles were prepared using the nanoprecipitation method and morphologically characterized by scanning electron microscopy (SEM). The average diameter and Zeta potential were determined by photon correlation spectroscopy method (PCS), and assays were carried out to determine the content of ascorbic acid and in vitro drug release kinetics. The antitumoral activity of this system was also evaluated against HL-60 cells by tetrazolium reduction assay. Nanoparticles with size distribution between 300–400 nm and strong negative outer surface (−40 mV) were obtained by this method. Analysis of ascorbic acid content showed that this compound was mainly localized on the external surface of nanoparticles. Violacein loading efficiency was determined as 32% ± 1% and this drug was gradually released from nanoparticles at different rates depending on the composition of the release media. In addition, this system was observed to be 2 × more efficient as an antitumoral compared with free violacein.
Keywords:
Introduction
Research on antitumoral therapies have been broadly studied in the last few years in order to create systems targeted to tumoral cells.Citation1,Citation2 In this context, differential characteristics between tumoral and normal cells have been exploited as a promising way to improve antitumoral activities of new and currently used drugs. Some examples are antiangiogenic compounds,Citation3 drugs that block activity of abnormal expressed signaling proteins such as Ras and Akt,Citation4 cytokines-based therapies,Citation5 DNA- or protein-based vaccines against specific tumoral markers,Citation6 and tyrosine-kinase inhibitors.Citation2
Another strategy that can be employed in this context is the obtention of drug carriers containing antitumoral drugs and with their external surface recovered (capped) with a specific substrate or antibody.Citation7 In this approach, the complex–drug vehicle can reach specifically tumoral cells using differential characteristics between malignant and normal cells as targeting marker. Such capping can be obtained by covalent binding of substrates to the external surface moieties of the drug carriers, self-assembly based on hydrophobicity or hydrophilicity, electrostatic interactions or simple deposition/adsorption of the capping agent on the external surface of the system.
One remarkable characteristic and common to several tumoral lineages is their high uptake and accumulation of ascorbic acid due to a high expression and activity of glucose transporter proteins (GLUTs), which are membrane or cytosolic proteins involved with internalization of both glucose and ascorbic acid.Citation8 The pathway by which tumoral cells obtain ascorbic acid is summarized by the stromal oxidation mechanism, in which reactive oxygen species (ROS) generated by cell metabolism promote oxidation of extracellular ascorbic acid to dehydroascorbate anion. This oxidized form is transportable via GLUTs and, once in the intracellular environment, the dehydroascorbate anion is further reduced to ascorbic acid. Since the reduced form of ascorbic acid is not transportable through the cell membrane, this compound stays trapped into the cell.Citation9 Although this characteristic gives tumoral cells a high resistance against oxidative stress induced by chemotherapeutic compounds, it can be used as a way to improve the specificity of drugs if combined with drug delivery systems.
Drug-delivery systems have been broadly studied in nanomedicine as a way to improve the effects of several drugs against different biological models.Citation10,Citation11 Between the examples already described, there are first and second generation liposomes,Citation12 metallic nanoparticles,Citation11 magnetic nanoparticles,Citation13 dendrimersCitation14,Citation15 and polymeric nano and microparticles.Citation16,Citation17 Those systems have many advantages when compared with conventional drug therapies, such as increased bioavailability of drugs via protection of drugs against oxido-reduction and enzymatic reactions, improvement of solubility of hydrophobic drugs in biological fluids, biocompatibility of matrixes and sustained release of compounds for long-term therapies.Citation16 The possibility of capping the drug carriers with important molecules is also an important feature of those systems. Among the examples already described, there are capping of nanoparticles with stealth agents to avoid capture of the system by phagocytes and probing molecules for diagnostic aims. In addition, cellular substrates or antibodies can be used to target the system specifically to a type of cell, thereby reducing harmful effects of drugs on nontargeted cells.Citation16,Citation18 The combination of targeting properties of polymeric drug delivery systems with knowledge of differential biochemical features between normal and tumoral cells can be an interesting way to improve the pharmacological effects of already known or new antitumoral compounds.
One antitumoral compound has been isolated from several environmental bacteria such as Pseudoalteromonas luteoviolacea and Chromobacterium violaceum.Citation19,Citation20 This compound, violacein, has been shown to have several biological activities such as antitumoral, antibacterial, antiviral, antiparasitic, antioxidant, fungicidal and leishmanicide activities.Citation20 Its antitumoral activity is marked by multitarget cytotoxicity mechanisms. It is known that violacein is a potent apoptosis inducer in leukemic HL-60 lineage by tumoral necrosis factor 1, caspase-8 and MAP-kinase p38 activation.Citation21 This drug is also capable of inducing cell death by generalized oxidative stress in colorectal tumoral lineage HT-29Citation22 and direct enzyme modulation in lymphocytes.Citation23 Despite its therapeutic potential, violacein exhibits poor water-solubility, which restricts its use in vivo. Also, due to its broad spectra of biological activities, this compound has significant toxicity against the host. Thus, systems that allow to improve the hydrosolubility of violacein and to target this compound to a specific kind of cell can be useful to further exploit its pharmacological potential.
The aim of this work was the preparation, characterization and determination of antitumoral activity of poly-D,L-(lactide-co-glycolide) nanoparticles containing violacein and capped with ascorbic acid in order to improve the antitumoral activity of this drug. Specifically, the antitumoral activity of drug carriers capped with ascorbic acid and loading violacein as compared with the free form of violacein and its complex with drug carriers noncapped with ascorbic acid.
Experiment
Materials
Surfactants polyoxyethylenesorbitan monopalmitate (Tween 40, M.W. = 1,277 kDa), polyoxyethylene-polyoxypropylene (Pluronic F68, M.W. = 8,400 kDa), polymer poly-D,L-(lactide-co-glycolide) 50:50 (PLGA 50:50, M.W. = 40,000–75,000 kDa), dimethylsulfoxide (DMSO), 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) and antibiotics were purchased from Sigma-Aldrich (St. Louis, MO, USA). Violacein ([3-(1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ilydene)-1,3-dihydro-2H-indol-2-one]) was extracted and purified from Chromobacterium violaceum CCT 3468, as previously described.Citation24 The purity of violacein, assessed by nuclear magnetic resonance (NMR) and ultraviolet-visible spectroscopy (UV-vis), was greater than 99%. All organic solvents in PA grade used to extract and purify violacein were obtained from Synth (Sao Paulo, Brazil). Cell culture media RPMI 1640, heat-inactivated fetal bovine serum (FBS) and L-glutamine were obtained from Cultilab (Campinas, Brazil) and used as received.
Methods
Nanoparticles preparation
Nanoparticles were prepared by following the nanoprecipitation method.Citation25,Citation26 Briefly, PLGA 50:50 and violacein were dissolved in acetone at concentrations of 1.2% and 0.5% (W/V). respectively. This solution was added into an aqueous phase containing surfactant Tween 40 and L-ascorbic acid at 1.2% (w/v) of each. The mixture was magnetically stirred until the complete evaporation of the organic solvent and centrifuged two times (10,000 rpm for 30 min) in Mili-Q water. The final pellet was freeze-dried and submitted to further analyses. Four groups of preparations were analyzed: NP (nanoparticles prepared without violacein and without ascorbic acid), NP-AA (nanoparticles prepared without violacein and with ascorbic acid), NP-violacein (nanoparticles prepared with violacein and without ascorbic acid) and NP-AA-violacein (nanoparticles prepared with violacein and with ascorbic acid).
Characterization of nanoparticles
Average diameter and Zeta potential analysis
The average size and charge of the external surface (Zeta potential) were determined by photon correlation spectroscopy method in Mili-Q water using a Malvern ZetaSizer Nano Series (Malvern, United Kingdom), according to manufacturer’s specifications. The samples were serially diluted to avoid the interference of Tyndall effect in the measurements and all the presented data had polydispersity indexes lower than 0.05.
Morphological analysis
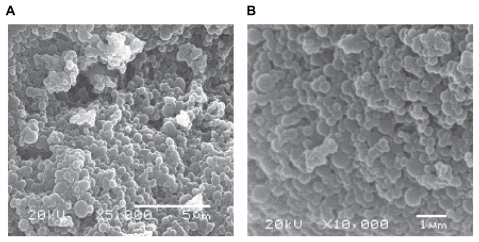
The final powder was submitted to morphological analysis using a JSM-6360LV JEOL scanning electron microscope. All samples were analyzed at an electron tension of 20 kV.
Drug loading efficiency and polymer recovery
Total amount of violacein (ɛ575 in ethanol = 3.13 × 10−2 mL.μg−1.cm−1) was determined by UV-Vis spectroscopy (Hitachi U-200) at wavelength of 575 nm.Citation27 The particles were dispersed in absolute ethanol, incubated for one hour at room temperature to extract the violacein and centrifuged at 10,000 rpm for 30 min to separate the drug from the reminiscent matrix. Polymer recovery was calculated by the expression: (total mass – total mass of recovered violacein)/(amount of polymer used in the preparation).
Ascorbic acid content analysis
To verify the final amount of ascorbic acid in the nanoparticles and whether this compound was localized on external surface of nanoparticles, the system was submitted to an ascorbic acid release kinetics assay. The samples were dissolved in phosphate-buffered saline (PBS; pH 7.4), incubated at 200 rpm and 37 °C. At different times, the solution was centrifuged for five minutes at 10,000 rpm and the supernatant was submitted to titration by the iodimetric methodCitation28 to determine the amount of ascorbic acid released in the respective time. As a control for dissolution of free ascorbic acid, a mass of this compound in powder (equivalent to the total content of this substance recovered from NP-AA system) was put into the release solution and submitted to the same analyses described above. Data was normalized as percentage of total ascorbic acid released after an interval of time (amount of ascorbic acid detected in a determined time/total content of ascorbic acid recovered from NP-AA).
Violacein release kinetics assay
In vitro release kinetics of violacein loaded in the final powder was carried out in a solution of PBS and 10% Pluronic F68 (w/v) or ethanol (v/v) at final pH of 7.4. These solutions, containing the nanoparticles, were incubated at 200 rpm and 37 °C. Periodically, the solutions were centrifuged and the supernatant was analyzed by UV-Vis spectroscopy at 575 nm to determine the total amount of violacein released. After each measurement, 1 ml of fresh release media was added to the experiments to avoid violacein saturation of the solution. As a control, free violacein solubilization rates were measured. Data was normalized as percentage of total violacein released after an interval of time (amount of violacein detected in a determined time/total violacein loaded in nanoparticles).
Cytotoxicity assays
Cell cultures
Human promyelocytic leukemia HL-60 cells, obtained from the Cell Culture Collection of the Universidade Federal do Rio de Janeiro (UFRJ), were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U.mL−1 of penicillin, 100 μg.mL−1 of streptomycin, and L-glutamine at 37 °C in a humidified atmosphere containing 5% CO2. Cells were used in the exponential growth phase at passage 15–30.
To assess cell viability, the cells were seeded (3.105 cells.mL−1) in 96 well plates and incubated with different concentrations of free or nanoparticle-loaded violacein for 72 hours, as previously described.Citation29 In all experiments with encapsulated violacein, the final concentration of nanoparticles was maintained constant by addition of a complementary amount of blank. Cell viability was determined by MTT reduction assay and the inhibitory concentration for 50% of the cells (IC50) was standardized as a toxicity measurement. Final concentrations of DMSO, needed to dissolve free violacein, never exceeded 0.2% (v/v). Controls were exposed to an equivalent concentration of DMSO and considered as 100% of viability.Citation26 In order to verify whether free ascorbic acid has any buffering effect in free violacein-mediated toxicity, cells were exposed to free violacein at IC50 values and a concentration of ascorbic acid equivalent to the total amount of this compound recovered from NP-AA. Also, in line with previous literature,Citation30 no decrease in the viability of HL-60 cells was observed when the cells were exposed to free ascorbic acid in concentrations below 10 μM (data not shown).
Tetrazolium reduction assay (MTT reduction)
The growth rate of HL-60 cells following treatments with free or encapsulated violacein was determined by MTT reduction assay.Citation26 Briefly, 0.1 ml of serum-free medium containing MTT (1 mg.mL−1) was added to each well. After four hours of incubation, the supernatant was removed and the blue formazan product obtained was dissolved with stirring in 0.1 ml of ethanol for 15 minutes on a microplate shaker. The absorbance at wavelength 570 nm was then obtained with a UV-vis spectrophotometer. No bleed-through effect (sum of absorbance values of two compounds caused by overlapping absorption wavelengths) was observed in the range of treatment concentrations analyzed.
Statistical analyses
All experiments were performed at least three times with four replicates. The IC50 values were calculated after expressing the results as a percentage of the controls and were determined graphically from the dose-response curves using the computer software package, Origin (version 6.0; OriginLab Corporation, Northampton, MA, USA). Probabilities of P < 0.05 were considered significant.
Results
Morphological analyses
The system obtained by this methodology presented a regular and smooth spherical shape, as observed in scanning electron microscopy (SEM) analyses (). No significant differences were observed between nanoparticles prepared with or without ascorbic acid (data not shown).
Drug loading efficiency and polymer recovery
Total loaded violacein rates for NP-violacein and NP-AA-violacein were determined to be 32% ± 1%, while polymer recovery varied between 75%–78%.
Analysis of ascorbic acid content
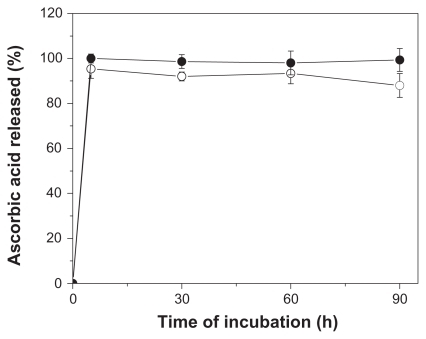
The total content of ascorbic acid reminescent in NP-AA and NP-AA-violacein were determined as 1.29 ± 0.2 and 1.31 ± 0.3 μmol per mg of particles. To further characterize the localization of this compound, a release kinetics assay was performed. As seen in , ascorbic acid in PLGA nanoparticles exhibited a burst releasing behavior at five minutes of incubation, which is very similar to the dissolution profile of free ascorbic acid under the described conditions.
Average diameter and Zeta potential
By this approach, particles with a nanometric size varying between 300 and 550 nm and a strong negative Zeta potential were obtained (). Also, the addition of ascorbic acid to the preparation increased significantly the negative charge of the external surface by 25–30 mV.
Table 1 Size distribution and Zeta potential of the obtained systems
Violacein release kinetics
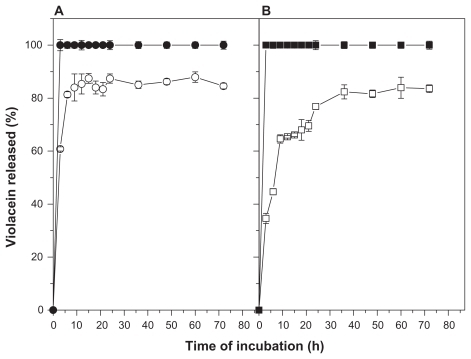
No differences of the dissolution profile of free violacein in surfactant-buffer or ethanol-buffer solutions were observed (). In terms of violacein release kinetics of nanoparticles, an initial burst of release in three hours of analysis was observed (about 40% and 60% for surfactant-buffer and ethanol- buffer solutions, respectively), followed by a gradual release until 36 hours and reaching a cumulative release of 80% of the initial content at 72 hours of analysis (). Moreover, a significant difference was observed in the release kinetics profile of this system depending on the composition of the analysis solution. The release rates in ethanol-buffer media was observed to be faster compared to the surfactant-buffer solution.
Figure 3 Release kinetics profile free or NP-AA-violacein in ethanol-buffer ethanol-buffer (A) or surfactant-buffer (B) solutions.
Notes: (●) free violacein in ethanol-PBS solution; (○) NP-AA-violacein in ethanol-PBS solution; (▪) free violacein in Tween-PBS solution (□) NP-AA-violacein in Tween-PBS solution.
Abbreviations: NP-AA, nanopatide-asconbic acid; PBS, Phosphate buffered saline.
Toxicity against HL-60 cells
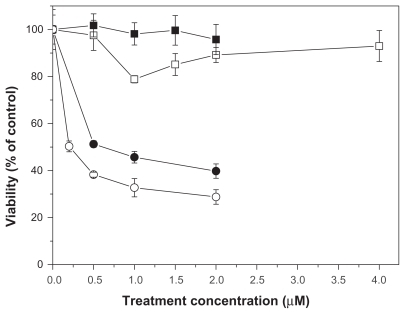
The results of cytotoxicity assays of free or NP-AA-violacein against HL-60 cells are shown in . IC50 values for free violacein and NP-AA-violacein were 0.5 μM and 0.2 μM, respectively. As a similar curve for the free violacein, NP-violacein showed an IC50 value of 0.4 μM (curve not shown). When the cells were treated with both free violacein and 2 μM of free ascorbic acid, a buffering effect of free violacein toxicity was observed, since IC50 values were not reached until 4.5 μM of drug exposure.
Discussion
Previous works have shown the broad spectra of biological activities of violacein and the multi-targeting toxicity mechanism triggered by this drug,Citation20 which reduces the rising of strains or cell lineages resistant to this compound. However, there are several hindrances to use violacein as a therapeutic compound. The most concerning is its poor solubility in water and biological fluids. For example, Lopes and colleaguesCitation31 evaluated the antiplasmodial activity of violacein in vivo and, even though an elimination of 90% of a lethal Plasmodium sp. strain was obtained, it was not possible to completely eliminate the parasite with free violacein due to its solubilization limit. In addition, violacein exerts toxic effects on normal cells. Due to this, strategies to guide this compound specifically to target cells are ongoing.
Herein, the characteristic of differential uptake of ascorbic acid between tumoral and normal cells was used to obtain a system with increased antitumoral activity. The methodology described here resulted in regular and smoothed shaped nanoparticles at a concentration of 1.2% of a nonpolymeric surfactant, which is the minimal amount of tensioactive necessary to stabilize the preparation. Using tensioactive concentrations lower than 1% resulted in preparations with poor stability and presented a rate of polymer recovery lower than 50% with significant macroscopic coagulation of polymer (data not shown). Such phenomena can be explained by the high ionic force of the aqueous phase of the system due to the high concentration of ascorbic acid used. Such high concentrations promote a salting-out effect on preparations with less than 1% of nonpolymeric surfactants or with the use of polymeric surfactants such as Pluronic F68 in the aqueous phase. Indeed, there was observed an incompatibility of polymeric surfactants with this preparation (data not shown).
Evaluating the average size distribution, nanoparticles with two main peaks of size distribution were obtained for all systems described as follows: 330 and 410 for NP, 290 and 487 for NP-violacein, 330 and 576 for NP-AA, 300 and 400 for NP-AA-violacein. This effect could be explained by the freeze-drying process, which promotes aggregation of vicinal nanoparticles. This consequently increases the average diameter of nanoparticle preparations and frequency of aggregates.Citation32–Citation34 However, the fact that nanoparticles with strong negative Zeta potential were obtained implies a better stability in aqueous solution due to the electrostatic repulsion between particles. This facilitates the dispersion in water of those systems. Although nanoparticles based in PLGA often have a negatively charged surface as consequence of ionizable carboxyl-terminal moieties of this polymer, a significant difference was observed between particles prepared with or without ascorbic acid. Since Zeta potential is affected by adsorption or binding of compounds to the external surface of colloidal particles, this difference indicates the presence of a reminiscent amount of ascorbic acid on the external surface of the system. Ascorbic acid has two ionizable hydroxyl groups at carbons 3 and 4 with pKa 4.2 and 11.6, respectively. Once the Zeta potential measurements were carried out at pH 7, the ionization of carbon 3 (leading to ascorbate anion formation) can explain the increased negative charge observed. The analysis of ascorbic acid content reinforces this observation since the release profile of ascorbic acid from the nanoparticles is marked by a massive release burst with five minutes of incubation. This suggests a weak adsorption of this compound on the external surface of the system.
In terms of violacein release kinetics of nanoparticles, it can be observed an initial burst of release at three hours of analysis can be observed followed by a gradual release until 72 hours. Such behavior suggests the system has violacein either in its external surface or mixed with the outer surface of the matrix (being, therefore, easily released in short time incubations) and an internal content of drug that is released gradually, reaching a release of 80% of the initial content at 72 hours of analysis. Also, a significant difference in release rates was observed between surfactant-buffer and ethanol-buffer solutions, with a faster drug release rate in the second media. This can be explained by the difference in the molecular weight of both compounds since ethanol is a low molecular weight compound and highly diffusible through the polymeric matrix, allowing it to bring out the violacein trapped in the inner side of the nanoparticles, while the surfactant with high molecular weight is unable to reach the inner side of nanoparticles. Thus, the media surfactant-buffer promotes diffusion but not arrest of the drug, mimicking the physiological situation where the particles are surrounded by high molecular weight compounds such as proteins.
In cytotoxicity assays, a significant difference of activity between nanoparticles-loaded and free violacein, being NP-violacein and NP-AA-violacein 1.25 and 2.5 times more efficient than free violacein respectively was observed. The increased biological activity of violacein loaded in nanoparticles has been demonstrated in previous works either with tumoral cellsCitation26 or Staphylococci strainsCitation27 and the addition of derivatives of ascorbic acid on external surface of nanoparticles had been shown to increase the efficiency of dehydrocrotonin loaded in PLGA nanoparticles with a protein mediated uptake mechanism and a shunt of cell death mechanism to receptor-mediated pathways.Citation29 However, an unexpected observation was reached, since free ascorbic acid exhibits a buffering effect in violacein-mediated toxicity, while the addition of ascorbic acid on the external surface of PLGA nanoparticles loading violacein actually promotes an improvement of the toxicity of this compound. This suggests a differential cell death mechanism between free violacein and NP-AA-violacein. Chen and colleaguesCitation35 demonstrated that, although ascorbic acid has mainly antioxidant properties in cell environment, it can act as a generator of reactive species of oxygen if it gets concentrated in the external tumor microenvironment. Therefore, one explanation for the observed fact is that the increasing in external concentration of ascorbic acid bound to NP-AA-violacein promotes generation of ROS in the external environment, while the high uptake of NP-AA-violacein complex promotes significant internal oxidative damage, leading to internal and external cell damage. Since ROS are necessary to promote the internalization of ascorbic acid, a positive feedback mechanism of internalization of NP-AA-violacein could be involved in the increased toxicity observed. Further studies should provide insights about the death mechanism triggered by the presented system.
Current drug delivery systems targeted to tumoral cells often use either peptides or proteins, such as transferrin and epithelial growth factor receptor (EGFR), or small molecules such as folate.Citation16,Citation36 All the cited molecules have been used in vitro or in vivo with promising results and high specificity. However, the efficiency of targeting depends on the lineage analyzed, since uptake of tranferrin, EGFR and folate broadly varies between tumoral cells. Specifically, transferrin and EGFR are useful for solid tumors,Citation36 whereas folate can be used for both solid and nonsolid tumors. It has been shown that uptake of folate-targeted drug delivery systems is decreased in tumoral models which have lower density of folate receptors, such as HeLa and MCF-7 cells.Citation37 Thus, the spectra of uses of those molecules are restricted depending on the cell model studied. On the other hand, the role of ascorbic acid in cancer metabolism is well described for several tumoral lineages and its high uptake is a characteristic common to most solid or nonsolid tumors analyzed,Citation38–Citation40 which makes it an interesting molecule to target a broader spectra of tumors. In addition, it has been reported that overexpression of GLUT proteins is a marker of very aggressive hepatocellular carcinomas.Citation41 In this context, the proposed system can be used to target drugs with multi-toxicity mechanisms to tumors refractory to current chemotherapies.
Although ascorbic acid is a molecule susceptible to oxidation, the oxidized form of this molecule (dehydroascorbate anion) can also target drug carriers, since the internalization of ascorbic acid requires its oxidation to dehydroascorbate by cellular ROS. However, efforts to create drug carriers with derivatives of ascorbic acid to avoid undesirable oxidation processes and to improve the shelf-life of ascorbic acid on nanoparticles are being carried out and showed promising results with polymeric nanoparticles carrying another antitumoral compound, dehydrocrotonin.Citation29 Complementary studies on toxicity of such complexes against normal cells and in vivo evaluations should be carried out to further characterize the pharmacological potential of this system.
Conclusions
The presented system could successfully load violacein and be capped with ascorbic acid, with good physical-chemical parameters of stability and a significant improvement of antitumoral activity of the complex. No buffering or antagonist effects on violacein activity were observed when ascorbic acid was used as a capping agent in the system. However, further characterization of the cell mechanism induced by this system and in vivo assays should be carried out. This preparation could be used as a selective way to administer higher doses of violacein in vitro and in vivo in order to improve its biological activity and to reduce side effects.
Acknowledgments
Financial support from CNPq, FAPESP and Brazilian Nanobiotechnology Network and Brazilian Nanocosmetics Network (MCT/CNPq), the suggestions of Dr. Marcelo M M de Azevedo and language reviewing by Ms Meena Kathiresan and Ms Zornitsa Stoyanova are acknowledged. The authors report no conflicts of interest in this work.
References
- SofouSSurface-active liposomes for targeted cancer therapyNanomedicine2007271172417976032
- SavonaMTalpazMGetting to the stem chronic myeloid leukaemiaNature Rev Cancer2008834135018385684
- NarayanaAKellyPGolfinosJAntiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survivalJ Neurosurg200911017318018834263
- PaternotSRogerPPCombined inhibition of MEK and mammalian target of rapamycin abolishes phosphorylation of cyclin-dependent kinase 4 in glioblastoma cell lines and prevents their proliferationCancer Res2009694577458119458076
- EgilmezNKKilincMOGuTControlled-release particulate cytokine adjuvants for cancer therapyEndocr Metab Immune Disord Drug Targets2007726627018220947
- NemunaitisJJGene immunotherapy for non-small cell lung cancerMethods Mol Biol200954249951419565919
- Douziech-EyrollesLMarchaisHHervéKNanovectors for anticancer agents based on superparamagnetic iron oxide nanoparticlesInt J Nanomedicine2007254155018203422
- CiampiRVivaldiARomeiCExpression analysis of facilitative glucose transporters (GLUTs) in human thyroid carcinoma cell lines and primary tumorsMol Cell Endocrinol2008291576218571834
- AgusDBVeraJCGoldeDWStromal cell oxidation: a mechanism by which tumors obtain vitamin CCancer Res1999184555455810493506
- TorchilinVPMicellar nanocarriers: pharmaceutical perspectivesPharm Res20072411617109211
- MarcatoPDDuránNNew aspects of nanopharmaceutical delivery systemsJ Nanosci Nanotechnol200882216222918572633
- Har-elYEKatoYIntracellular delivery of nanocarriers for cancer therapyCurr Nanosci20073329338
- ArrueboMFernández-PachecoRIbarraMRSantamaríaJMagnetic nanoparticles for drug deliveryNanotoday200722232
- BharaliDJKhalilMGurbuzMSimoneTMMousaSANanoparticles and cancer therapy: A concise review with emphasis on dendrimersInt J Nanomedicine200941719421366
- TekadeRKKumarPVJainNKDendrimers in oncology: An expanding horizonChem Rev2009109498719099452
- NairLSLaurencinCTBiodegradable polymers as biomaterialsProgr Polym Sci200632762798
- JainKKRecent advances in nanoocologyTechnol Cancer Res Treat2008711318198919
- MundargiRCBabuVRRangaswamyVPatelPAminabhaviTMNano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivativesJ Control Release200812519320918083265
- DuránNMenckCFChromobacterium violaceum: a review of pharmacological and industrial perspectivesCrit Rev Microbiol20012720122211596879
- DuránNJustoGZFerreiraCVMeloPSCordiLMartinsDViolacein: properties and biological activitiesBiotechnol Appl Biochem20074812713317927569
- FerreiraCVBosCLVersteegHHJustoGZDuránNPeppelenboschMPMolecular mechanism of violacein-mediated human leukemia cell deathBlood20041041459146415130948
- de CarvalhoDDCostaFTMDuránNHaunMCytotoxic activity of violacein in human colon cancer cellsToxicol In Vitro2006201514152116889929
- BrombergNJustoGZHaunMDuránNFerreiraCVViolacein cytotoxicity on human blood lymphocytes and effect on phosphatasesJ Enzyme Inhib Med Chem20052044945416335052
- RettoriDDuránNProduction, extraction and purification of violacein: an antibiotic pigment produced byChromobacterium violaceumWorld J Microb Biotechnol199814685688
- ItaliaJLYahyaMMSinghDRavi KumarMNBiodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous FungizonePharm Res2009261324133119214716
- MeloPSDe AzevedoMMFrugilloLAnazettiMCMarcatoPDDuránNNanocytotoxicity: violacein and violacein-loaded poly(D,L-lactide-co-glycolide) nanoparticles acting on human leukemic cellsJ Biomed Nanotechnol2009519220120055097
- MartinsDCostaFTMBrocchiMEvaluation of the antibacterial activity of poly-(D,L-lactide-co-glycolide) nanoparticles containing violaceinJ Nanoparticle Res2009 Submission number: NANO2280
- SuntornsukLGritsanapunWNilkamhankSPaochomAQuantitation of vitamin C content in herbal juice using direct titrationJ Pharm Biomed Anal2002288495512039626
- FrungilloLMartinsDTeixeiraSAnazettiMCMelo PdaSDuránNTargeted antitumoral dehydrocrotonin nanoparticls with L-ascorbic acid 6-stearateJ Pharm Sci2009984796480719367621
- ArranzNHazaAIGarcíaADelgadoMERafterJMoralesPInhibition by vitamin C of apoptosis induced by N-nitrosamines in HepG2 and HL-60 cellsJ Appl Toxicol20082878879618344201
- LopesSCBlancoYCJustoGZViolacein extracted from Chromobacterium violaceum inhibits Plasmodium growth in vitro andin vivoAntimicrob Agents Chemother2009532149215219273690
- CabaneBBlanchonSNevesCRecombination of nanometric vesicles during freeze-dryingLangmuir2006221982199016489779
- LeeJChengYCritical freezing rate in freeze drying nanocrystal dispersionsJ Control Release200611118519216430987
- HolzerMVogelVMänteleWSchwartzDHaaseWLangerKPhysico-chemical characterisation of PLGA nanoparticles after freeze-drying and storageEur J Pharm Biopharm20097242843719462479
- ChenQEspeyMGSunAYAscorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluidin vivoProc Natl Acad Sci U S A20071048749875417502596
- YuBZhaoXLeeLJLeeRJTargeted delivery systems for oligonucleotide therapeuticsAAPS J20091195203
- ChenHAhnRVan den BosscheJThompsonDHO’HalloranTVFolate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxideMol Cancer Ther200981955196319567824
- MachedaMLRogersSBestJDMolecular and cellular regulation of glucose transporter (GLUT) proteins in cancerJ Cell Physiol200520265466215389572
- AirleyREMobasheriAHypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeuticsChemotherapy20075323325617595539
- FonteynePCasneufVPauwelsPExpression of hexokinases and glucose transporters in treated and untreated oesophageal adenocarcinomaHistol Histopathol20092497197719554504
- DaskalowKPfanderDWeichertWDistinct temporospatial expression patterns of glycolysis-related proteins in human hepatocellular carcinomaHistochem Cell Biol2009132213119350262