?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Genistein is one of the most studied isoflavonoids with potential antitumor efficacy, but its poor water solubility limits its clinical application. Nanoparticles (NPs), especially biodegradable NPs, entrapping hydrophobic drugs have promising applications to improve the water solubility of hydrophobic drugs. In this work, TPGS-b-PCL copolymer was synthesized from ε-caprolactone initiated by d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) through ring-opening polymerization and characterized by Fourier transform infrared spectroscopy, proton nuclear magnetic resonance spectroscopy, gel permeation chromatography, and thermogravimetric analysis. The genistein-loaded NPs were prepared by a modified nanoprecipitation method and characterized in the aspects of particle size, surface charge, morphology, drug loading and encapsulation efficiency, in vitro drug release, and physical state of the entrapped drug. The TPGS-b-PCL NPs were found to have higher cellular uptake efficiency than PCL NPs. MTT and colony formation experiments indicated that genistein-loaded TPGS-b-PCL NPs achieved the highest level of cytotoxicity and tumor cell growth inhibition compared with pristine genistein and genistein-loaded PCL NPs. Furthermore, compared with pristine genistein and genistein-loaded PCL NPs, the genistein-loaded TPGS-b-PCL NPs at the same dose were more effective in inhibiting tumor growth in the subcutaneous HeLa xenograft tumor model in BALB/c nude mice. In conclusion, the results suggested that genistein-loaded biodegradable TPGS-b-PCL nanoparticles could enhance the anticancer effect of genistein both in vitro and in vivo, and may serve as a potential candidate in treating cervical cancer.
Introduction
Although great progress has been made in prevention, early diagnosis, and treatment, cancer will remain a leading human health problem, causing an increase in the percentage of morbidity and mortality both in developed and developing countries. Cervical carcinoma is mainly caused by human papillomavirus (HPV) and is the third most common cancer in women all over the world. Because of cancer screening tests and vaccine, cervical cancer has relatively low mortality in Western countries. However, it is still the leading cause of cancer death in women in developing nations.Citation1
Many kinds of natural compounds derived from traditional Chinese medicine have been confirmed to have antitumor properties and also have little or no toxicity compared with synthetic chemicals.Citation2 Flavonoids, including isoflavones, are natural polyphenolic compounds present ubiquitously in many plants and have antioxidant, anti-inflammatory, and antitumor properties.Citation3,Citation4 Genistein (4′,5,7-trihydroxyisoflavone, ) is one of the most abundant and best studied soy isoflavones and has received great attention for its many physiological functions, including potential antitumor activity.Citation5,Citation6 Recent studies have indicated that genistein could inhibit tumor cell growth and proliferation, arrest cell cycle at G2/M phase, suppress tumor migration, invasion, and angiogenesis, and induce apoptosis and autophagocytosis.Citation7–Citation11 However, the clinical use of genistein for cancer therapy was hindered by its poor water solubility and oral bioavailability. The emerging applications of nanotechnology-based cancer therapy provide a potent platform to improve poor water solubility and bioavailability of hydrophobic antitumor agents.Citation12,Citation13
Recently, nanoparticle (NP)-based anticancer drug delivery systems, especially drug formulations with biodegradable polymeric NPs, have attracted considerable attention for their numerous advantages such as high cellular uptake, enhanced permeability and retention effect, and reduced cancer cell drug resistance.Citation14–Citation17 Among the US Food and Drug Administration (FDA)-approved biodegradable polymers, poly(ε-caprolactone) (PCL) has gained considerable interest for drug delivery. However, its slow degradation rate and bad compatibility with soft tissues limit its application in nanomedicine. These drawbacks could be solved by copolymerizing PCL with other monomers, such as an amphiphilic water-soluble PEGylated derivative of natural vitamin E (d-α-tocopheryl polyethylene glycol 1000 succinate [TPGS]), which is also approved by the FDA.Citation18–Citation21 Other than being an excellent emulsifier, TPGS itself has been reported to have anticancer efficacy because of its function as an inhibitor of P-glycoprotein to surmount multidrug resistance in tumor cells.Citation22–Citation24
In this work, the TPGS-b-PCL copolymer was synthesized and characterized. The genistein-loaded NPs were prepared with a modified nanoprecipitation method instead of solvent extraction/evaporation method and characterized. Compared with the NPs prepared by the solvent extraction/evaporation method, the current NPs were much smaller. The novel nanoformulations had higher cellular uptake and could accumulate at the tumor site preferentially due to their enhanced permeability and retention effects. Furthermore, this kind of NPs used as drug carriers have other advantages, such as more reasonable pharmacokinetics and more desirable biodistribution as well as easy industry application.Citation15,Citation25 Also, the efficacy of genistein-loaded TPGS-b-PCL NPs on human cervical carcinoma was investigated both in vitro and in vivo, in close comparison with pristine genistein and genistein-loaded PCL NPs. These results suggest that genistein-loaded TPGS-b-PCL nanoformulation has potential application in human cervical cancer therapy.
Materials and methods
Materials
PCL (MW, 14,000 Da), ε-caprolactone (ε-CL), TPGS, stannous octoate (Sn(Oct)2), methanol (HPLC [high-performance liquid chromatography]-grade), genistein powder with about 98% purity (HPLC-grade), coumarin-6, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum were obtained from Gibco BRL (Invitrogen Co, Carlsbad, CA, USA). Human cervical carcinoma cell line HeLa cells were kept by our laboratory.
Synthesis and characterization of TPGS-b-PCL copolymer
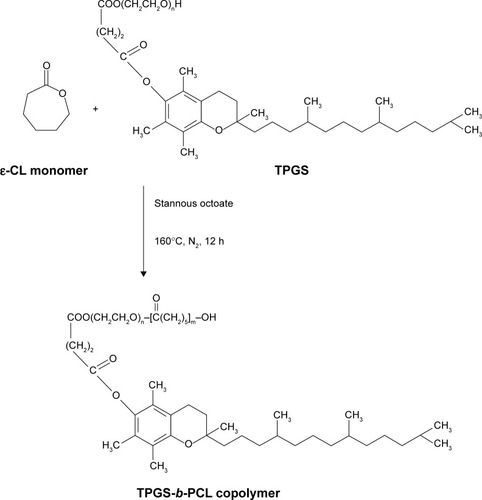
Synthesis of TPGS-b-PCL copolymer was carried out through ring-opening polymerization, as previously described.Citation18,Citation26 The reaction route scheme is shown in . In brief, ε-CL (1.63 g, 14.3 mmol), TPGS (0.15 g, 0.1 mmol), and catalyst Sn(Oct)2 (0.02 g, 8 μL) were added in a dried flask, which was connected to a vacuum system, evacuated, refilled with nitrogen gas, and sealed. Then the flask was heated in an oil bath at 160°C and the mixture was allowed to react overnight. After cooling to room temperature, the product was dissolved in dichloromethane and then precipitated in excess cold methanol to remove any impurity. Thereafter, the crude product was collected by filtration and washed twice with methanol to remove the oligomer and unreacted monomer. Finally, the synthetic TPGS-b-PCL copolymer was dried under vacuum at 40°C for 24 hours.
Figure 2 Schematic description of the synthesis of TPGS-b-PCL diblock copolymer.
Abbreviations: ε-CL, ε-caprolactone; h, hours; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
The molecular structure of TPGS-b-PCL copolymer was investigated using Fourier transform infrared (FTIR) spectrophotometer (Thermo Nicolet; Thermo Fisher Scientific, Waltham, MA, USA). The structure of synthesized TPGS-b-PCL copolymer was verified by a nuclear magnetic resonance (1H-NMR) spectrometer (Bruker ACF 300; Bruker Instruments Inc., Billerica, MA, USA) using CDCl3 as a solvent. Gel permeation chromatography (GPC) was carried out to determine the weight-averaged molecular weight and weight distribution of the copolymer. The thermal property of the TPGS-b-PCL copolymer was measured by thermogravimetric analysis (TGA; TGA 2050 thermogravimetric analyzer, PerkinElmer Inc., Waltham, MA, USA).
Formulation and characterization of genistein-loaded nanoparticles
Genistein-loaded PCL and TPGS-b-PCL NPs were prepared by a modified nanoprecipitation method, as previously described.Citation26,Citation27 In short, 10 mg of genistein and 100 mg of PCL polymer or TPGS-b-PCL copolymer were dissolved in 8 mL of acetone. Then the dissolved mixture was slowly added into 100 mL of 0.03% (w/v) TPGS aqueous solution using a 1 mL injector, under stirring. After stirring overnight at room temperature to completely remove acetone, the suspension was centrifuged at 20,000× g for 15 minutes and then washed three times to remove the emulsifier TPGS and unencapsulated genistein. Finally, the resulting NPs were dispersed in 10 mL of water and lyophilized for further use. The preparation of fluorescent coumarin-6-loaded PCL and TPGS-b-PCL NPs was by the same method except for 1 mg of coumarin-6 instead of genistein.
The particle size, size distribution, and zeta potential of NPs were determined by dynamic light scattering (Zetasizer Nano ZS90; Malvern Instruments, Malvern, UK). All measurements were taken at 25°C. The morphology of genistein-loaded TPGS-b-PCL NPs was observed by a field emission scanning electron microscopy (FESEM) using a JEOL JSM-6301F (JEOL, Tokyo, Japan) system operated at a 5.0 kV accelerating voltage and further examined by transmission electron microscopy (TEM; Tecnai G2 20, FEI Company, Hillsboro, OR, USA).
Drug loading (DL) and encapsulation efficiency (EE) of genistein-loaded PCL NPs and TPGS-b-PCL NPs were determined using HPLC (LC 1200, Agilent Technologies, Santa Clara, CA, USA) as follows. Briefly, 5 mg of lyophilized genistein-loaded NPs was dissolved in 100 μL of dichloromethane and then diluted with methanol. After passing through a 0.45 μm filter to remove precipitation, the clear solution was subjected to HPLC analysis using a reverse-phase C-18 column (150×4.6 mm; pore size, 5 μm; Agilent Technologies) at 25°C to determine the amount of encapsulated genistein at 262 nm. Methanol/water (60/40, v/v) was used as the mobile phase at a flow rate of 1 mL/min. The amount of genistein was calculated according to a standard genistein sample. The DL and EE of genistein in NPs were calculated as follows:
Differential scanning calorimetry (DSC Q2000 thermogravimetric analyzer, PerkinElmer Inc.) was carried out to determine the physical status of genistein inside the NPs. The pristine genistein, blank NPs, or genistein-loaded NPs were purged with dry nitrogen at a flow rate of 20 mL/min, and the temperature was raised at a rate of 10°C/min.
In vitro drug release profiles of genistein-loaded PCL and TPGS-b-PCL NPs were investigated by a dialysis method as described previously.Citation18,Citation28 In brief, 10 mg of lyophilized genistein-loaded NPs was resuspended in 1 mL of releasing buffer (PBS [phosphate-buffered saline] containing 0.1% w/v Tween-80) and transferred into a dialysis tube (MWCO =3.5 kDa and the dialysis area is 1 cm2). Subsequently, the dialysis tubes were immersed into a centrifuge tube containing 10 mL of releasing buffer and incubated at 37°C with gentle shaking (120× g). At designated time intervals, the entire release buffer was collected and replaced with prewarmed fresh release buffer. The released genistein was quantified using HPLC in terms of DL and EE.
Cell culture
Human cervical carcinoma cell line HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Cellular uptake of NPs
Because coumarin-6 could serve as fluorescence, it was encapsulated into PCL NPs and TPGS-b-PCL NPs to quantitatively and qualitatively evaluate the cellular uptake of NPs.Citation29–Citation31
For quantification of PCL NPs and TPGS-b-PCL NPs uptake by HeLa cells, the cells were seeded in a 96-well black culture plate at an initial density of 2×104 cells/well and cultured overnight. Then the medium was removed and incubated with fresh medium containing 100, 250, and 500 μg/mL coumarin-6-loaded PCL NPs or TPGS-b-PCL NPs for further 2 h. Subsequently, the cells were washed and lysed with 50 μL of 0.2 N NaOH (containing 0.5% Triton X-100), and the fluorescence intensity was determined by a microplate reader (GENios, Tecan, Switzerland) with excitation wave length at 430 nm and emission wave length at 485 nm.
For qualitative observation, HeLa cells were seeded onto coverslips in a six-well plate at a density of 2×105. After 24 hours of attachment, the medium was replaced with fresh medium containing coumarin-6-loaded NPs at a concentration of 250 μg/mL for 4 hours. Then the cells were washed three times with cold PBS, fixed by cold methanol for 20 minutes, and further washed twice with PBS, and the nuclei were counterstained with DAPI for 10 minutes. Finally, the cells were mounted on microscope slides using fluorescent mounting medium and imaged using a confocal laser scanning microscope (Olympus Fluoview FV-1000, Tokyo, Japan).
Cytotoxicity assay
The in vitro cytotoxicity assay of pristine genistein, genistein-loaded PCL NPs, and TPGS-b-PCL NPs were performed on HeLa cells using the MTT method. In brief, HeLa cells were plated in 96-well transparent plates at an initial density of 2×104 cells/well. After 24 hours of attachment, the medium was replaced with fresh medium containing genistein-loaded NP suspensions or pristine genistein at final concentrations of 5.0, 10.0, 20.0, and 40.0 μg/mL equivalent genistein or drug-free NP suspension with the same amount of NPs for 24, 48, and 72 hours. At the designed time intervals, the cell viability was measured using MTT assay standard protocol at 570 nm using a microplate reader (Bio-Rad Model 680; Bio-Rad Laboratories Inc., Hertfordshire, UK). IC50, the drug concentration at which 50% of cell growth is inhibited, was measured at final concentrations of 5.0, 10.0, 20.0, 30.0, 40.0, 60.0, and 80.0 μg/mL equivalent genistein for 24, 48, and 72 hours.
Colony formation analysis
HeLa cells were cultured in a six-well plate at an initial density of 500 cells/well and were allowed to attach for 24 hours. After preincubation, the medium was incubated with fresh medium or fresh medium containing genistein-loaded PCL or TPGS-b-PCL NP suspensions or pristine genistein at final concentrations of 10 μg/mL equivalent genistein or drug-free NP suspension with the same amount of NPs for 48 hours. Then, the cells were washed three times with prewarmed PBS and recultured in fresh DMEM for another 10 days. Finally, the cells were stained with crystal violet and images were captured using a digital camera.
In vivo anticancer effect
All animal studies were carried out according to the protocols approved by the Administrative Committee on Animal Research in Graduate School at Shenzhen, Tsinghua University. Five-week-old female BALB/c nude mice were purchased from the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Science. They were subcutaneously implanted with 7.5×106 HeLa cells/mouse/100 μL at the right flank region. After inoculation of the HeLa cells, the subcutaneous tumor in each mouse was closely monitored. The tumor volume can be calculated from the formula: Tumor volume (mm3) =0.524× (length) × (width)Citation2. When the tumor was touchable, the mice were divided into three groups (five mice per group) and the in vivo antitumor studies were performed. The HeLa tumor-bearing female BALB/c nude mice were intraperitoneally injected with saline (as a control), the genistein-loaded PCL and TPGS-b-PCL nanoformulations, or pristine genistein, at a dose of 50 mg/kg equivalent genistein every other day. Animal body weights and tumor volumes were measured every other day. After treatment for 16 days, the animals were humanely killed by cervical decapitation and tumors were excised and weighted. The terminal tumor weight (in milligram) was used to evaluate the anticancer activity of genistein-loaded TPGS-b-PCL NPs.
Statistical analysis
Data were expressed as the mean value ± standard deviation (SD). Statistical analysis was done with one-way analysis of variance (ANOVA) using the SPSS software. P- values < 0.05 were considered to be statistically significant.
Results and discussion
Synthesis and characterization of TPGS-b-PCL copolymer
The TPGS-b-PCL copolymer used in this study was prepared through the ring-opening polymerization of ε-CL initiated by TPGS by using (Sn(Oct)2) as the catalyst. schematically illustrates the general synthetic route.
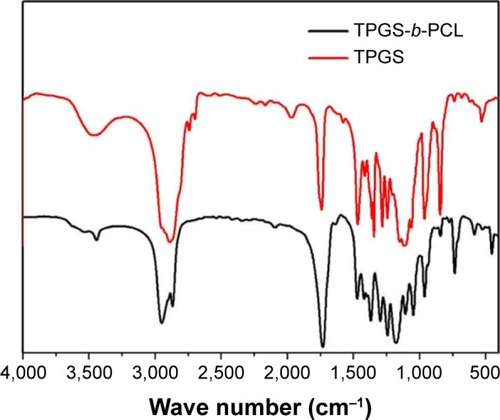
The FTIR of the TPGS-b-PCL copolymer and TPGS are presented in . The carbonyl band of TPGS appears at 1,739 cm−1. For the synthesized diblock copolymer, the carbonyl band is shifted to about 1,730 cm−1, which is different from the carbonyl bands of PCL at 1,725–1,726 cm−1.Citation18,Citation32 In the TPGS-b-PCL spectrum, the bands in the range 2,867–2,949 cm−1 are assigned to –CH2 stretching band of PCL, and the CH2 stretching band of TPGS at 2,880 cm−1 was not observed because of overlapping with that of TPGS-b-PCL copolymer. The absorption band at about 3,442 cm−1 is due to the terminal hydroxyl group and that at 1,045–1,297 cm−1 is attributed to the C–O stretching. Of those, the absorption band at 1,242 cm−1 is assigned to asymmetric COC stretching. The band at about 1,297 cm−1 has been used for the investigation of the crystallinity change in PCL.
Figure 3 FTIR spectra of TPGS and TPGS-b-PCL copolymer.
Abbreviations: FTIR, Fourier transform infrared; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
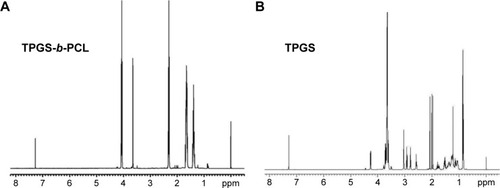
1H-NMR was carried out to determine the TPGS content, molecular weight, and structure of the obtained TPGS-b-PCL copolymer, and the results are presented in . Peaks at 1.39, 1.65, 2.29–2.33, and 4.06 ppm are assigned to –CH2 protons in PCL units.Citation18,Citation32 The peak at 3.65 ppm was assigned to the –CH2 protons of poly(ethylene oxide) part of TPGS. The lower peaks in the aliphatic region belong to various moieties of vitamin E tails. The number-averaged molecular weight (Mn) of the TPGS-b-PCL copolymer could be calculated by using the ratio between the peak areas at 3.65 and 4.06 ppm. The Mn of the TPGS-b-PCL copolymer was determined to be 18,473. The ratio of ε-CL and TPGS molecular mass which were integrated into the TPGS-b-PCL copolymer is 91.81% and 8.19%.
Figure 4 Typical 1H-NMR spectra of TPGS-b-PCL copolymer (A) and TPGS (B).
Abbreviations: 1H-NMR, proton nuclear magnetic resonance; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
In the GPC analysis (), a monomodal pattern was observed, indicating that the polymer product is a pure copolymer not contaminated by PCL and TPGS. The peak for TPGS appeared at 28.3 minutes. Instead, the peak of the TPGS-b-PCL copolymer shifted to 17.9 minutes. The polydispersity of the copolymer molecular weight was about 1.08. The Mn calculated from the GPC chromatograph was 20,791, which is in good agreement with the results of 1H-NMR studies.
Figure 5 Characterization of TPGS-b-PCL copolymer.
Notes: (A) Typical gel permeation chromatograms of TPGS and TPGS-b-PCL diblock copolymer; (B) thermogravimetric profiles of TPGS and TPGS-b-PCL copolymer.
Abbreviations: min, minutes; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
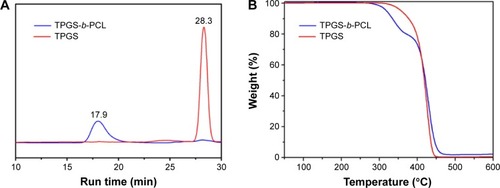
To investigate the thermal properties of the synthesized copolymer, TGA was performed. As shown in , there are two typical stages of weight loss for TPGS-b-PCL diblock copolymer, whereas the TPGS monomer has only a single step of weight loss. Each turning point marked the combustion of a new component in the copolymer.Citation18,Citation32
Preparation and characterization of genistein-loaded PCL and TPGS-b-PCL NPs
Genistein was encapsulated by PCL and TPGS-b-PCL to improve the drug’s poor water solubility. As described previously,Citation26,Citation27 genistein-loaded PCL and TPGS-b-PCL NPs were prepared by a modified nanoprecipitation method using acetone as the solvent and TPGS as the emulsifier. Then the NPs were characterized in detail.
Particle size, size distribution, and zeta potential
As reported previously, the NP size and surface chemistry influence absorption, biodistribution, and pharmacokinetics of the drug.Citation33–Citation35 As shown in , the size, size distribution, and zeta potential of NPs were measured using the dynamic light scattering. The mean particle size of genistein-loaded TPGS-b-PCL NPs was 181.83 nm, which was much smaller than genistein-loaded PCL NPs (225.80 nm). Moreover, the size distribution of TPGS-b-PCL nanoformulation (polydispersity index [PDI] =0.255) was a little narrower than that of PCL NPs (PDI =0.291). This is because TPGS is an excellent emulsifier and the TPGS in the TPGS-b-PCL copolymer may play a self-emulsifying function, reducing surface energy of the particles to resist coalescence and flocculation of the particles.Citation36,Citation37 Zeta potential represents the surface charge of the NP and is a crucial parameter reflecting the stability of NP suspensions. The high magnitude of zeta potential suggests strong electrostatic repellent interactions between NPs and thus sufficient dispersion stability.Citation31,Citation38 As shown in , the zeta potentials of genistein-loaded PCL and TPGS-b-PCL formulations were −11.29 and −14.70 mV, respectively. Compared with positive surface charge, the negative surface charge of both NPs not only indicated the high stability of NP suspensions but also suggested less toxicity to normal cells.
Table 1 Characterization of nanoparticles
Drug loading and encapsulation efficiency
The DL and EE, detected by HPLC, are shown in . The percentage of genistein loaded in the PCL and TPGS-b-PCL NPs were 8.21% and 8.69%, respectively. And the encapsulation of the PCL NPs was 90.27% and that of the TPGS-b-PCL NPs was 95.56% (n=5). It could be seen that TPGS-b-PCL NPs achieved a higher drug EE.
Surface morphology
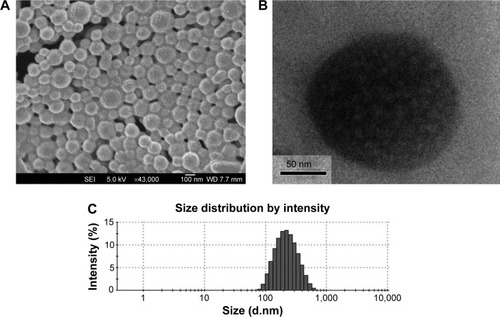
A high-resolution image of genistein-loaded TPGS-b-PCL NPs was obtained using FESEM and TEM. As shown in (FESEM image) and 6B (TEM image), the genistein-loaded TPGS-b-PCL NPs were in moderate uniformity with a nearly spherical shape and smooth surface. Moreover, the particle size was around 170 nm, which was slightly smaller than that obtained from the dynamic light scattering method () because of lack of hydration shell when particle size was determined by FESEM and TEM.
Figure 6 FESEM image (A), TEM image (B), and DLS spectra (C) of genistein-loaded TPGS-b-PCL NPs.
Abbreviations: DLS, dynamic light scattering; FESEM, field emission scanning electron microscopic; NPs, nanoparticles; PCL, poly(ε-caprolactone); TEM, transmission electron microscopic; TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
Physical state of genistein in the NPs
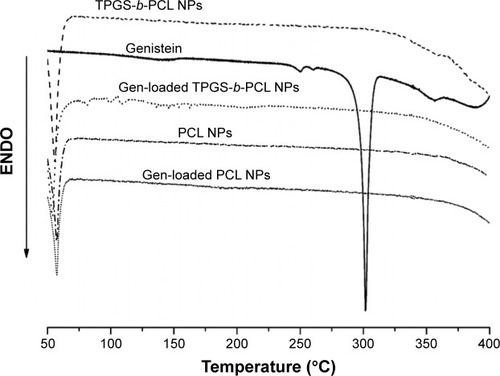
To investigate the physical state of genistein in the NPs, DSC analysis was carried out using pure genistein, genistein-loaded PCL NPs, and genistein-loaded TPGS-b-PCL NPs. As shown in , the DSC curve of pure genistein indicated a crystalline state with a melting endothermic peak at 301.7°C. However, when genistein was encapsulated into PCL and TPGS-b-PCL NPs, such a peak disappeared, implying that genistein was essentially in an amorphous or disordered crystalline phase after having been encapsulated by both NPs.
Figure 7 DSC thermograms of the pure genistein, blank PCL NPs, blank TPGS-b-PCL NPs, and genistein-loaded PCL NPs, and TPGS-b-PCL NPs.
Abbreviations: DSC, differential scanning calorimetry; ENDO, endotherm; NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
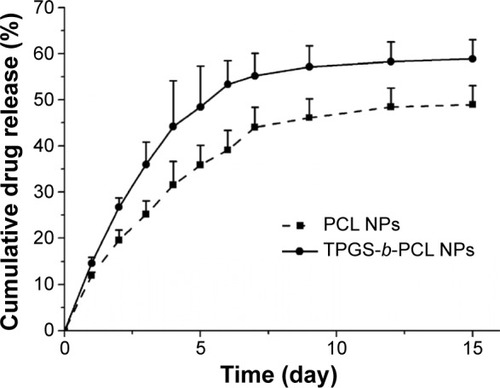
In vitro drug release of genistein from PCL NPs and TPGS-b-PCL NPs
illustrates the in vitro drug release profiles of genistein-loaded PCL NPs and TPGS-b-PCL NPs in PBS buffer (pH 7.4) supplemented with 0.1% w/v Tween 80 in the first 15 days, which exhibited biphasic release patterns. It could be observed from that the release of genistein from the drug-loaded PCL NPs and TPGS-b-PCL NPs showed an initial burst of 11.97% and 14.58%, respectively, in the 1st day. In the following days, the cumulative release of genistein persistently increased. After 15 days’ incubation, the cumulative release of genistein was 48.95% for PCL NPs and 58.84% for TPGS-b-PCL NPs. It could be easily concluded that TPGS-b-PCL NPs exhibited a much faster drug release than did PCL NPs. This is probably because of the hydrophilic part of the TPGS, which promotes the uptake and permeation of PBS buffer into the core of NPs to facilitate drug release.
In vitro cellular uptake of NPs
The internalization of PCL and TPGS-b-PCL NPs into cells was determined by measuring the cellular uptake of NPs in vitro. A fluorescent marker coumarin-6-loaded PCL NPs and TPGS-b-PCL NPs were used to quantitatively and qualitatively investigate in vitro cellular uptake of NPs. Human cervical carcinoma cell line HeLa was chosen. After incubating HeLa cells with 100, 250, and 500 μg/mL coumarin-6-loaded PCL NPs or TPGS-b-PCL NPs for 2 hours, the cellular uptake efficiency of both NPs was measured. As shown in , it could be concluded that the cellular uptake efficiency of NPs decreased with the increasing concentration of NPs, which indicated a saturated and limited capability of cellular uptake of the nanoparticles. Moreover, the TPGS-b-PCL NPs exhibited 1.25-, 1.22-, 1.28-fold higher cellular uptake efficiency than did PCL NPs at the concentrations of 100, 250, and 500 μg/mL, respectively.
Figure 9 Cellular uptake of coumarin-6-loaded PCL NPs and TPGS-b-PCL NPs after incubated with HeLa cells.
Notes: (A) Uptake efficiency of coumarin-6-loaded PCL NPs and TPGS-b-PCL NPs by HeLa cells (*P<0.05); (B) confocal laser scanning microscopic images of HeLa cells after incubation with the coumarin-6-loaded TPGS-b-PCL NPs. Scale bar 10 μm.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
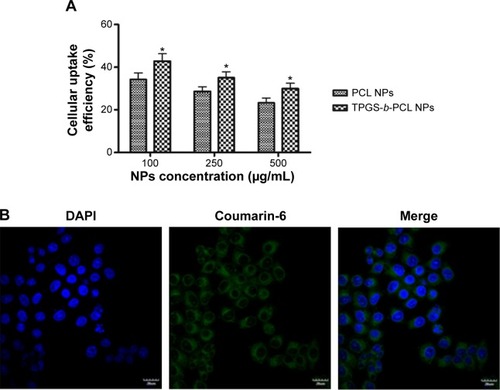
The cellular uptake of NPs was further visualized by confocal laser scanning microscopy. shows the confocal images of HeLa cells after 4 h of incubation with 250 μg/mL coumarin-6-loaded TPGS-b-PCL NPs. The image in column 1 was from DAPI channel (blue) representing the nucleus; the image in column 2, obtained from fluorescein isothiocyanate channel (green), was green fluorescence representing coumarin-6; and the image in column 3 was the merged channels of fluorescein isothiocyanate and DAPI. It could be easily seen from column 3 that most internalized coumarin-6-loaded NPs were in the cytoplasm, although some NPs went into the nucleus, especially into the nucleolus.
In vitro cytotoxicity and cell colony formation analysis
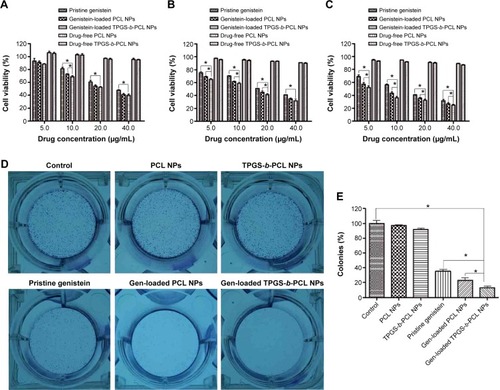
HeLa cells were used to evaluate the cytotoxicity of pristine genistein, genistein-loaded PCL and TPGS-b-PCL NPs, and drug-free PCL and TPGS-b-PCL NPs by the MTT method. After 24, 48, and 72 hours incubation with pristine genistein or genistein-loaded PCL NPs and TPGS-b-PCL NPs suspension at 5.0, 10.0, 20.0, and 40.0 μg/mL equivalent genistein concentrations or drug-free NP suspension with the same amount of NPs, the cell viabilities are shown in . It could be concluded that 1) the placebo NPs of PCL and TPGS-b-PCL are biocompatible since they show little decrease in cellular viability of HeLa cells; 2) the cytotoxicity of the three genistein formulations were concentration- and incubation time-dependent; 3) the nanoformulations of genistein showed greater cytotoxicity than pristine genistein; 4) compared with pristine genistein and genistein-loaded PCL NPs, the genistein-loaded TPGS-b-PCL NPs displayed significant advantages after 48 and 72 h of incubation. It could be seen from and C that the cell viability of HeLa decreased from 50.81% for pristine genistein, 44.85% for genistein-loaded PCL NPs, to 41.44% for genistein-loaded TPGS-b-PCL NPs (a 9.37% and 3.41% more effective in vitro therapeutic effect) after 48 hours of incubation and from 40.98% for pristine genistein, 35.38% for genistein-loaded PCL NPs, to 32.00% for genistein-loaded TPGS-b-PCL NPs (a 8.98% and 3.38% more effective in vitro therapeutic effect) after 72 hours of incubation at the drug concentration of 20.0 μg/mL. Because of the cumulative release of genistein by NPs, both genistein-loaded PCL NPs and TPGS-b-PCL NPs exhibited a much higher cytotoxicity than did pristine genistein. Moreover, due to the degradation of TPGS-b-PCL NPs releasing TPGS, which has anticancer activity according to a previous study, genistein-loaded TPGS-b-PCL NPs showed a better antitumor effect than did genistein-loaded PCL NPs.
Figure 10 The anticancer activity of pristine genistein and genistein-loaded NPs on HeLa cervical cancer cells in vitro.
Notes: (A–C) The inhibitory effect of pristine genistein, genistein-loaded NPs, and drug-free NPs on the proliferation of HeLa cells at 24 h (A), 48 h (B), and 72 h (C), determined by MTT assay (*P<0.05); (D) the inhibitory effect of pristine genistein, genistein-loaded NPs, and drug-free NPs on the colony formation of HeLa cells; (E) colonies were counted and the data are expressed as a percentage of control cells (*P<0.05).
Abbreviations: NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate; h, hours; MTT, 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
The antitumor effects of the three genistein formulations were further quantificated by the IC50 values from . summarizes the IC50 values of HeLa cells incubated with pristine genistein, genistein-loaded PCL NPs and TPGS-b-PCL NPs at final concentrations of 5.0, 10.0, 20.0, 30.0, 40.0, 60.0, and 80.0 μg/mL equivalent genistein for 24, 48, and 72 hours. It was shown that the IC50 values for HeLa cells were 31.3, 23.9, and 13.6 μg/mL for pristine genistein, 26.1, 16.5, and 7.4 μg/mL for genistein-loaded PCL NPs, and 24.3, 13.6, and 5.0 μg/mL for genistein-loaded TPGS-b-PCL NPs after treatment for 24, 48, and 72 hours, respectively. That is, drugs encapsulated by the NPs exhibited higher cytotoxicity. Furthermore, we could conclude that the in vitro antitumor effects of the three genistein formulations were in the following order: genistein-loaded TPGS-b-PCL NPs > genistein-loaded PCL NPs > pristine genistein.
Table 2 IC50 values of pristine genistein, genistein-loaded PCL NPs, and genistein-loaded TPGS-b-PCL NPs on human cervical carcinoma cell line HeLa after 24, 48, and 72 hours of incubation
In order to further examine the cytotoxicity and growth inhibitory effects of the three genistein formulations on HeLa cells, colony formation assay was carried out. As shown in , all the genistein formulations significantly impaired the cell colony formation, with genistein-loaded TPGS-b-PCL NPs having the strongest effect, while the drug-free NPs showing no obvious effect. This result is consistent with that of the cytotoxicity assay.
Anticancer efficacy of genistein in vivo
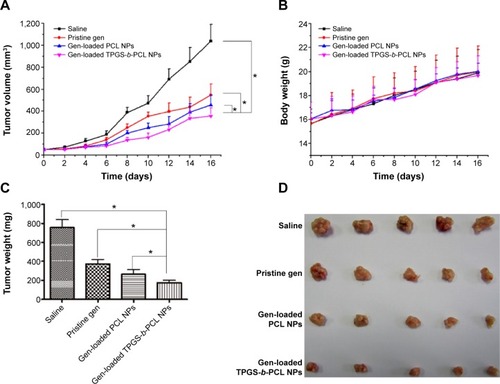
To compare the antitumor activity of genistein-loaded TPGS-b-PCL nanoformulation with that of the pristine genistein in vivo, HeLa cells were subcutaneously inoculated into the right flank of female BALB/c nude mice. When the tumor xenografts were touchable, mice were divided to three groups (n=5 for each group) and injected intraperitoneally every 2 days with saline, pristine genistein, genistein-loaded PCL NPs, or genistein-loaded TPGS-b-PCL NPs at the genistein dose of 50 mg/kg. As shown in , all the three genistein formulations significantly decreased the growth of HeLa tumor in vivo, with genistein-loaded TPGS-b-PCL NPs being more effective in comparison with pristine genistein and genistein-loaded PCL NPs. Moreover, all the three genistein formulations were well tolerated without significant impact on the mice body weight (). After treatment for 16 days, the mice were humanely killed and tumors were excised and weighted. As shown in , compared with saline group, the tumor weight in all the three genistein formulations-treated groups was significantly reduced, and the mice treated with genistein-loaded TPGS- b-PCL NPs exhibited the lightest tumor weight. Furthermore, the images of tumors from each group also indicated the advantages of genistein-loaded TPGS-b-PCL NPs versus pristine genistein and genistein-loaded PCL NPs in repressing tumor growth (). All animal care and procedures for animal experiments were with approval from the Administrative Committee on Animal Research in Tsinghua University.
Figure 11 Antitumor effect of genistein formulated in TPGS-b-PCL in comparison with pristine genistein and genistein-loaded PCL NPs (n=5).
Notes: (A) Tumor growth curve of the BALB/c nude mice bearing HeLa cells xenograft after administration (*P<0.05); (B) body weight after administration; (C) weight of the tumor from each group taken out from the sacrificed mice at the end of the study (*P<0.05); (D) image of tumor from each group taken out from the sacrificed mice at the end of the study.
Abbreviations: NPs, nanoparticles; PCL, poly(ε-caprolactone); TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
Conclusion
In this research, biodegradable TPGS-b-PCL NPs entrapping genistein were prepared using a modified nanoprecipitation method to treat HeLa cervical carcinoma in vitro and in vivo. The obtained genistein-loaded TPGS-b-PCL NPs showed smaller size, higher DL and EE, faster drug release rate, and higher cellular uptake efficiency and cytotoxicity than genistein-loaded PCL NPs. All of these could be attributed to the introduction of the TPGS component into the PCL polymer, which made the PCL polymer more suitable for drug delivery. Compared with pristine genistein, the genistein-loaded TPGS-b-PCL NPs were more effective in the suppression of HeLa cell growth both in vitro and in vivo. Furthermore, both the genistein formulations did not show any significant effect on the mice body weight. Therefore, the prepared genistein-loaded TPGS-b-PCL NPs are an excellent low-toxic aqueous formulation of genistein with improved anticancer activity, which may have potential application in cervical cancer therapy.
Acknowledgments
The authors are grateful for financial support from the National Natural Science Foundation of China (Numbers 31270019, 51203085), China Postdoctoral Science Foundation (Number 2012M520242), Natural Science Foundation of Guangdong Province (Numbers S2012040006820, S2012010010046), Science, Technology and Innovation Commission of Shenzhen Municipality (Numbers JCYJ20120616213729920, KQC201105310021A, JCYJ20120614191936420, JC201005270308A), and Program for New Century Excellent Talents in University (NCET-11-0275).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin201161699021296855
- Li-WeberMTargeting apoptosis pathways in cancer by Chinese medicineCancer Lett201333230431220685036
- BuerCSIminNDjordjevicMAFlavonoids: new roles for old moleculesJ Integr Plant Biol2010529811120074144
- SpagnuoloCRussoMBilottoSTedescoILarattaBRussoGLDietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemiaAnn N Y Acad Sci201212599510322758641
- LiQSLiCYLiZLZhuHLGenistein and its Synthetic Analogs as Anticancer AgentsAnticancer Agents Med Chem20121227128122043996
- UllahMFAhmadAZubairHSoy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen speciesMol Nutr Food Res20115555355921462322
- HussainAHarishGPrabhuSAInhibitory effect of genistein on the invasive potential of human cervical cancer cells via modulation of matrix metalloproteinase-9 and tissue inhibitiors of matrix metalloproteinase-1 expressionCancer Epidemiol201236e387e39322884883
- LiHQLuoYQiaoCHThe mechanisms of anticancer agents by genistein and synthetic derivatives of isoflavoneMini Rev Med Chem20121235036222303948
- OuyangGYaoLRuanKSongGMaoYBaoSGenistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathwaysCell Biol Int2009331237124419732843
- QiWWeberCRWaslandKSavkovicSDGenistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activityBMC Cancer20111121921639915
- KimSHLeeSCSongYSInvolvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cellsAnn N Y Acad Sci2009117119620119723056
- GuoGFuSZhouLPreparation of curcumin loaded poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) nanofibers and their in vitro antitumor activity against Glioma 9L cellsNanoscale201133825383221847493
- LiXZhangZLiJSunSWengYChenHDiclofenac/biodegradable polymer micelles for ocular applicationsNanoscale201244667467322732776
- ParrottMCDeSimoneJMRelieving PEGylationNat Chem20124131422169865
- TomasinaJLheureuxSGauduchonPRaultSMalzert-FreonANanocarriers for the targeted treatment of ovarian cancersBiomaterials2013341073110123174141
- MiYLiuXZhaoJDingJFengSSMultimodality treatment of cancer with herceptin conjugated, thermomagnetic iron oxides and docetaxel loaded nanoparticles of biodegradable polymersBiomaterials2012337519752922809649
- MeiLZhangZZhaoLPharmaceutical nanotechnology for oral delivery of anticancer drugsAdv Drug Deliv Rev20136588089023220325
- HuangLChenHZhengYNanoformulation of d-α-tocopheryl polyethylene glycol 1000 succinate-b-poly (ε-caprolactone-ran-glycolide) diblock copolymer for breast cancer therapyIntegr Biol (Camb)20113993100221938302
- GaoXWangBWeiXAnticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancerNanoscale201247021703023044718
- GongCDengSWuQImproving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micellesBiomaterials2013341413143223164423
- ZhangZMeiLFengSSVitamin E D-α-tocopheryl polyethylene glycol 1000 succinate-based nanomedicineNanomedicine (London)2012716451647
- YoukHJLeeEChoiMKEnhanced anticancer efficacy of α-tocopheryl succinate by conjugation with polyethylene glycolJ Control Release2005107435216081183
- CollnotEMBaldesCSchaeferUFEdgarKJWempeMFLehrCMVitamin E TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of accessMol Pharm2010764265120205474
- GuoYLuoJTanSOtienoBOZhangZThe applications of Vitamin E TPGS in drug deliveryEur J Pharm Sci20134917518623485439
- AcharyaSSahooSKPLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effectAdv Drug Deliv Rev20116317018320965219
- ZengXTaoWMeiLHuangLTanCFengSSCholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancerBiomaterials2013346058606723694904
- ValenciaPMHanewich-HollatzMHGaoWEffects of ligands with different water solubilities on self-assembly and properties of targeted nanoparticlesBiomaterials2011326226623321658757
- GouMMenKShiHCurcumin-loaded biodegradable polymeric micelles
- YanFZhangCZhengYThe effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicityNanomedicine2010617017819447200
- MiYLiuYFengSSFormulation of docetaxel by folic acid-conjugated d-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS 2k) micelles for targeted and synergistic chemotherapyBiomaterials2011324058406621396707
- MiYZhaoJFengSSTargeted co-delivery of docetaxel, cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancerJ Control Release201316918519223403395
- ZhangYTangLSunLA novel paclitaxel-loaded poly (ε-caprolactone)/poloxamer 188 blend nanoparticle overcoming multidrug resistance for cancer treatmentActa Biomater201062045205219969111
- AlbaneseATangPSChanWCThe effect of nanoparticle size, shape, and surface chemistry on biological systemsAnnu Rev Biomed Eng20121411622524388
- PetrosRADeSimoneJMStrategies in the design of nanoparticles for therapeutic applicationsNat Rev Drug Discov2010961562720616808
- WalkeyCDOlsenJBGuoHEmiliAChanWCNanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptakeJ Am Chem Soc20121342139214722191645
- KulkarniSAFengSSEffects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug deliveryPharm Res201330102512252223314933
- Van EerdenbrughBVermantJMartensJAA screening study of surface stabilization during the production of drug nanocrystalsJ Pharm Sci2009982091210318803265
- de PlanqueMRAghdaeiSRooseTMorganHElectrophysiological characterization of membrane disruption by nanoparticlesACS Nano201153599360621517083