Abstract
A rapid, green phytosynthesis of silver nanoparticles (AgNPs) using the aqueous extract of Helianthus tuberosus (sunroot tuber) was reported in this study. The morphology of the AgNPs was determined by transmission electron microscopy (TEM). Scanning electron microscopy–energy-dispersive spectroscopy (SEM–EDS) and X-ray powder diffraction (XRD) analysis confirmed the presence of AgNPs. Fourier transform infrared spectroscopy (FTIR) analysis revealed that biomolecules in the tuber extract were involved in the reduction and capping of AgNPs. The energy-dispersive spectroscopy (EDS) analysis of the AgNPs, using an energy range of 2–4 keV, confirmed the presence of elemental silver without any contamination. Further, the synthesized AgNPs were evaluated against phytopathogens such as Ralstonia solanacearum and Xanthomonas axonopodis. The AgNPs (1–4 mM) extensively reduced the growth rate of the phytopathogens. In addition, the cytotoxic effect of the synthesized AgNPs was analyzed using rat splenocytes. The cell viability was decreased according to the increasing concentration of AgNPs and 67% of cell death was observed at 100 μg/mL.
Introduction
Nanobiotechnology is a promising interdisciplinary field of biotechnology and nanoscience that offers novel nanoscale materials with interesting applications in different scientific fields such as medicine, biotechnology, chemistry, physics, and material sciences.Citation1,Citation2 Metallic nanoparticles are attractive due to their potential applications in novel technologies.Citation3 Among the metallic nanoparticles, silver nanoparticles (AgNPs) have attracted the attention of researchers in the field of science and technology due to their vast biological applications. Several studies have reported the synthesis of AgNPs by physicochemical methods.Citation4,Citation5 The chemical synthesis of AgNPs may lead to the presence of some toxic chemicals on the surface that may have adverse effects in its application. Also, the chemical used in the synthesis may pollute the environment.Citation6 Hence, there is an urgent need to develop eco-friendly biological methods of AgNPs synthesis instead of using toxic chemicals.
Nowadays, biological synthesis of AgNPs has been proposed as an emerging technology, and offers several advantages compared to conventional methods. Several studies reported AgNPs synthesis using biological materials, such as microorganisms, plant extracts, milk, and panchakavya.Citation7–Citation10 Phytosynthesis of AgNPs using plant materials has an edge over microbial mediated synthesis owing to their immediate and large-scale production.Citation11,Citation12 Thus, considerable attention is given to exploit the synthesis of AgNPs using plant-based products. Several studies reported on the synthesis of AgNPs using various plant extracts.Citation13–Citation15 It has been established that proteins present in the plant extracts were involved in the reduction of Ag+ ions to nanocrystallites. Helianthus tuberosus L., a perennial herb, is a species of sunflower native to eastern North America and widely cultivated across the temperate zone for its edible tuber. Extracts of H. tuberosus L. tubers are aperient, cholagogue, and diuretic and have long been used in folk medicine to treat stomach problems, diabetes, and rheumatism.Citation16,Citation17 However, to our knowledge, the sunroot (H. tuberosus L.) tuber extract has never been used for the synthesis of AgNPs.
In vitro cytotoxicity study is an important assay to evaluate the mechanisms of toxicity caused by nanoparticles. AgNP-induced toxicity is related with mitochondrial damage, oxidative stress, DNA damage, and induction of apoptopsis.Citation18 Previous studies reported the cytotoxicity of AgNPs against NIH 3T3 fibroblast cells, HeLa cells, human glioblastoma cells, and human breast cancer cells (MCF-7).Citation19–Citation22 However, to our knowledge, cytotoxicity of AgNPs in rat splenocytes have never been explored.
Plant disease control is an important requirement for agriculture in the 21st century. Microorganisms are associated with several devastating diseases in economically important crops worldwide. Phytopathogenic bacteria cause enormous problems in agriculture, resulting in severe economic losses, since plants are the main nutrient sources of these pathogens.Citation23 Ralstonia solanacearum and Xanthomonas axonopodis are the most extensively studied phytopathogens in potato (Solanum tuberosum), tobacco (Nicotiana tabacum), and tomato (Lycopersicon esculentum) plant systems. The increasing population of these phytopathogens causes degradation of the occluded xylem vessels and the death of the plants. Hence, the synthesis of AgNPs and their antibacterial properties are emerging as fields of great interest among researchers. Hence, the objectives of the present study were (i) to synthesize AgNPs using H. tuberosus tuber extract, (ii) to characterize the synthesized AgNPs, and (iii) to assess the cytotoxicity of AgNPs synthesis against freshly isolated rat splenocytes, and (iv) to evaluate the bactericidal activities of the synthesized AgNPs.
Materials and methods
Plant material
The dried tuber of H. tuberosus was purchased from a local shop in Iksan, South Korea. One kilogram of tuber powder was soaked in 2.5 L methanol for 78 hours with occasional stirring. The solvent was removed by using Rotovac below 70°C. The solvent-free aqueous extract was used for the synthesis of AgNPs.
Synthesis of AgNPs
Silver nitrate (AgNO3) was purchased from Sigma-Aldrich (St Louis, MO, USA) and the synthesis of AgNPs was carried out according to Lee et al.Citation8 Briefly, 4 mL of the extract was mixed with 96 mL of 1 mM AgNO3 solution and the resulting greenish white mixture was incubated for 8 hours in a rotary shaker (200 rpm) at 26°C. The reduction of Ag+ ions to Ag nanocrystals was monitored by the change in the color of the reaction mixture from greenish white to dark brown.
Characterization of AgNPs
The morphology of the synthesized AgNPs was examined using transmission electron microscopy (Bio-TEM) (H-7650; Hitachi Ltd., Tokyo, Japan). The elemental composition of the synthesized AgNPs was confirmed by scanning electron microscopy–energy-dispersive spectra (SEM–EDS) (JEOL-64000; Tokyo, Japan). The X-ray powder diffraction (XRD) was carried out using Rigaku X-ray diffracto-meter (Rigaku, Japan). The scanning was performed in the region of 2θ=30°−80° at 0.041°/min with a time constant of 2 seconds. The Fourier transform infrared spectrum (FTIR) of the AgNPs was obtained on a PerkinElmer FTIR spectrophotometer (Waltham, MA, USA) in the diffuse reflectance mode at a resolution of 4 cm−1 in KBr pellets.
Antibacterial activity of AgNPs
The phytopathogenic bacterial strains R. solanacearum and X. axonopodis were procured from the Korean Agriculture Culture Collection (KACC), South Korea. The freshly cultured bacterial strains from the Luria-Bertani (LB) agar plates were inoculated into LB broth and incubated at 37°C in a shaking incubator. After appropriate growth, the cultures were used for further experiments. The cultures were allowed to grow in 100 mL of LB broth containing the synthesized AgNPs at different concentrations in the range 1–4 mM. The optical density was measured every 4 hours to determine the growth of the bacteria using the Shimadzu UV-1800 spectrophotometer. The culture without AgNPs was used as a control.
Isolation and propagation of rat splenocytes
Adult (Sprague dawley, 8–12 week old) rats were purchased from Koatech, South Korea. The rats were maintained in a specific pathogen-free facility. Fresh splenocytes of the rat was obtained by teasing the spleen under aseptic conditions according to Lu et al.Citation24 Single-cell suspensions were prepared from rat spleen by pressing the tissues through a sterile wire mesh and washing the cells in Roswell Park Memorial Institute (RPMI) medium containing 1% antibiotic (Anti–Anti; Gibco, South Korea). The animal experiments were carried out in accordance with the institutional animal care and use committee at Chonbuk National University.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The isolated rat splenocytes were treated with series of 10–100 μg/mL of phytosynthesized AgNPs in 96-well tissue culture plates. The treated cells were incubated for 24 hours at 37°C with 5% CO2 for cytotoxicity analysis. The cells were then subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The stock concentration (5 mg/mL) of MTT, a yellow tetrazole, was prepared and 20 μL of MTT was added in each AgNP-treated well and incubated for 4 hours. Purple colored formazan crystals were observed in the bottom of the well and these crystals were dissolved with 200 μL of dimethyl sulfoxide (DMSO), and read at 595 nm18,Citation22 in a multi-well plate reader (Epoch microplate spectrophotometer; Biotek, Winooski, USA).
Results and discussion
Characterization studies
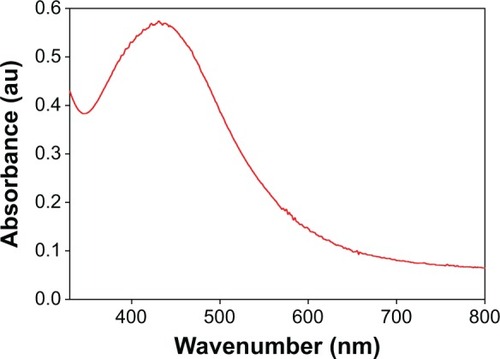
The addition of the tuber extract to 1 mM-solution of AgNO3 changed the color from greenish white to dark brown in about 1 hour. The intensity of the color increased after 8 hours of incubation and the reduction of pure Ag+ ions to Ag0 was monitored by measuring the UV–Vis spectrum of the reaction media (). The UV–Vis spectra of the silver surface plasmon resonance band occurs near 430 nm. Previous studies have reported the presence of AgNPs exhibiting yellowish brown color in solution due to the excitation of surface Plasmon vibrations.Citation25,Citation26 However, the color change was not observed in the control flask. The biomolecules present in the tuber extract may reduce the Ag ions present in the solution. This was supported by the results from FTIR studies where stretching vibrations of amines, alkanoids, and alkaloids were observed.
Figure 1 UV–Vis absorption spectrum of the AgNPs prepared from 1 mM AgNO3 solution.
Abbreviation: AgNPs, silver nanoparticles.
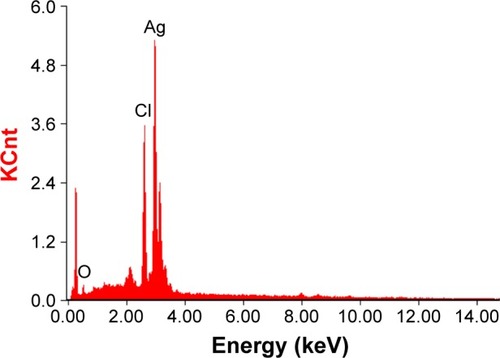
The morphology and size of the phytosynthesized AgNPs were observed by TEM images (). The particles were spherical in shape, monodisperse, and the size of the particles varied from 10–70 nm. To confirm the presence of Ag, the samples were analyzed in SEM–EDS, and the results are shown in . The results showed strong silver signals (3 keV), along with weak oxygen and carbon peaks, which might have originated from the tuber extract. The results are consistent with previous studies that reported the strong peak for AgNPs at 3 keV.Citation7,Citation8 The EDS quantitative analysis showed the presence of silver (100%) without any contaminants.
Figure 2 TEM image of AgNPs.
Abbreviations: TEM, transmission electron microscopy; AgNPs, silver nanoparticles.
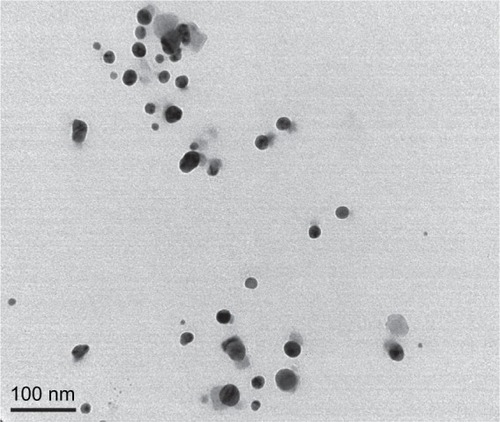
Figure 3 SEM–EDS spectrum of AgNPs. A strong peak at 3 keV confirms the presence of Ag.
Abbreviations: SEM–EDS, scanning electron microscopy–energy-dispersive spectroscopy; AgNPs, silver nanoparticles.
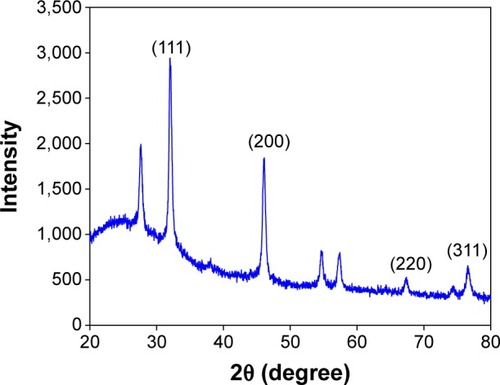
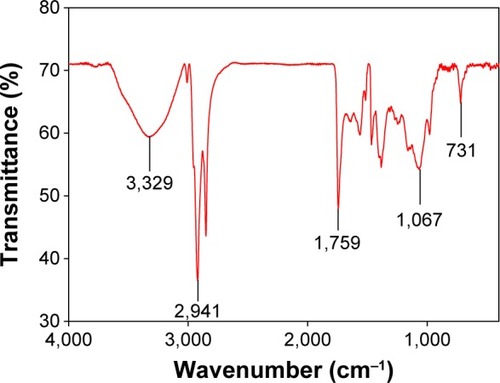
XRD was used for the identification of the crystal nature of the AgNPs. The XRD patterns of the synthesized AgNPs are shown in . The peaks at 2θ values of 38, 46, 67.4, and 78 correspond to 111, 200, 220, and 311, indicating the cubic nature of AgNPs. The results are in agreement with several studies that reported the cubic nature of biologically synthesized AgNPs.Citation27,Citation28 The FTIR spectrum of the AgNPs is shown in . Some pronounced peaks were observed at 3,329, 2,941, 1,759, 1,067, and 731 cm−1 in the 4,000–400 cm−1 region. The corresponding peaks were associated with the stretching vibrations of –C–O, C–H, C=C, CH2, and O–H, respectively. The absorbance peaks could be attributed to the phytochemicals present in the tuber extract, such as reducing sugars, flavonoids, saccharides, and proteins.Citation29 Pan et alCitation16 reported the presence of coumarins, unsaturated fatty acids, and phenols in the H. tuberosus extract.
Antibacterial activity
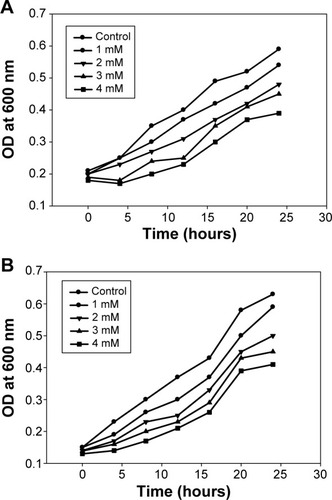
Numerous studies reported that biologically synthesized AgNPs have significant antibacterial and antifungal activities, and could be used for the treatment of bacterial and fungal diseases in biotic communities.Citation7,Citation8 However, the adverse effects of AgNPs need to be carefully evaluated before they could be used in antimicrobial products. Hence, phytosynthesized AgNPs were studied for antibacterial activity against phytopathogenic bacteria, namely, R. solanacearum and X. axonopodis. The bacterial growth measurements at different concentrations (1–4 mM) of the AgNPs were determined 0–24 hours at regular time intervals (). The observed results indicated that 4 mM concentrations of the AgNPs effectively encountered the bacterial population in the medium. The results are consistent with our previous study reporting the antibacterial activity of AgNPs against antibiotic resistant strains.Citation7
Cytotoxicity
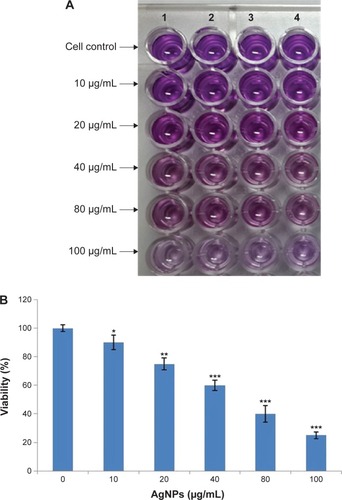
The cytotoxic potential of the biologically synthesized AgNPs was assessed using MTT assay. shows the viability of splenocytes with the increasing concentration (10–100 μg/mL) of AgNPs, and the reduction of color compared to untreated cells observed at 595 nm revealed the cytotoxicity. Thus, in vitro cytotoxicity of the AgNPs was evaluated against rat splenocytes at different concentrations (10, 20, 40, 80 and 100 μg/mL), and the results are shown in . The results clearly demonstrated that the splenocytes viability was directly proportional to the concentration of the AgNPs. The cell death (10%) was reported at 10 μg/mL and gradually increased according to the concentration and reached the maximum (67%) at 100 μg/mL. Hackenberg et alCitation30 reported that 10 μg/mL of AgNPs reduced human mesenchymal stem cells viability within 1 hour exposure. Vivek et alCitation22 reported that the cytotoxicity of AgNPs was significantly increased according to the concentration of nanoparticles. Moreover, several studies reported that AgNPs may induce reactive oxygen species and cause damage to cellular components leading to cell death.Citation31–Citation33
Conclusion
To the best of our knowledge, this is the first study to report the synthesis of AgNPs using H. tuberosus tuber extract. The proposed method is a simple, green, and cost-effective method for the synthesis of AgNPs without any harmful chemicals. The analytical results confirmed the presence of AgNPs without any contamination. The green synthesized AgNPs had a significant bactericidal property against phytopathogenic bacteria. The cytotoxicity of the synthesized AgNPs indicates that further studies are needed before using these nanoparticles in antimicrobial products.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A4A01011658).
Disclosure
The authors declare no conflict of interest in this work.
References
- RaveendranPFuJWallenSLCompletely “green” synthesis and stabilization of metal nanoparticlesJ Am Chem Soc2003125139401394114611213
- ProwTSmithJNGrebeRConstruction, gene delivery, and expression of DNA tethered nanoparticleMol Vis20061260661516760897
- DubeySPLahtinenMSarkkaHSillanpaaMBioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloidsColloids Surf B Biointerfaces2010801263320620889
- HuangHYangYPreparation of silver nanoparticles in inorganic clay suspensionsCompos Sci Technol20086829482953
- SastryMAhmadAKhanMIKumarRBiosynthesis of metal nanoparticles using fungi and actinomyceteCurr Sci200385162170
- SumanTYRadhika RajasreeSRKanchanaABeenaEBiosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extractColloids Surf B Biointerfaces2013106747823434694
- GovarthananMSelvankumarTManoharanKBiosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agentInt J Nanomedicine201491593159924741307
- LeeKJParkSHGovarthananMSynthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogensMater Lett2013105128131
- Ananda BabuSGurumallesh PrabuSSynthesis of AgNPs using the extract of Calotropis procera flower at room temperatureMater Lett20116516751677
- OtariSVPatilRMNadafNHGhoshSJPawarSHGreen biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus spMater Lett2012729294
- ShankerSSRaiAAhmedASastryMRapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf brothJ Colloid Interface Sci2004275249650215178278
- RamtekeCChakrabartiTSarangiBKPandeyR-ASynthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activityJ Chem2013201317
- ZayedMFEisaWHShabakaAAMalya parviflora extract assisted green synthesis of silver nanoparticlesSpectrochim Acta A201298423428
- ShankarSAhmadASastryMGeranium leaf assisted biosynthesis of silver nanoparticlesBiotechnol Prog20031961627163114656132
- ChandranSPChadudharyMPasrichaRAhamadASastryMSynthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extractBiotechnol Prog200622257758316599579
- PanLSindenMRKennedyAHChaiHWatsonLEGrahamTLBioactive constituents of Helianthus tuberosus (Jerusalem artichoke)Phytochem Lett2009211518
- TalipovaMLipids of Helianthus tuberosus LChem Nat Comp2001373213215
- SukirthaRPriyankaKAntonyJJCytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice modelProcess Biochem2012472273279
- MiuraNShinoharaYCytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cellsBiochem Biophys Res Commun200939073373719836347
- HsinYHChenCFHuangSShihTSLaiPSChuehPJThe apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cellsToxicol Lett200817913013918547751
- Asha RaniPVLow Kah MunGHandeMPValiyaveettilSCytotoxicity and genotoxicity of silver nanoparticles in human cellsACS Nano2009327929019236062
- VivekRThangamRMuthuchelianKGunasekaranPKaveriKKannanSGreen biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cellsProcess Biochem20124724052410
- PelegriniPBNoronhaEFMunizMAAn antifungal peptide from passion fruit (Passiflora edulis) seeds with similarities to 2S albumin proteinsBiochim Biophys Acta2006176461141114616766236
- LuLHsiehMOrissTBGeneration of DC from mouse spleen cell cultures in response to GM-CSF: immunophenotypic and functional analysesImmunology1995841271347890296
- VidhuVKAromalSSPhilipDGreen synthesis of silver nanoparticles using Macrotyloma uniorumSpectrochim Acta A Mol Biomol Spectrosc20118339239721920808
- Ashok KumarDPalanichamyVMohana RoopanSGreen synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activitySpectrochim Acta A Mol Biomol Spectrosc201412716817124632169
- ShaligramNSBuleMBhambureRBiosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain processProcess Biochem200944939943
- BalajiDSBasavarajaSDeshpandeRMaheshDBPrabhakarBKVenkataramanAExtracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungusColloids Surf B Biointerfaces2009681889218995994
- MuniyappanNNagarajanNSGreen synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activitiesProcess Biochem201449610541061
- HackenbergSScherzedAKesslerMSilver nanoparticle: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cellsToxicol Lett20112011273321145381
- JacobSJFinubJSNarayananASynthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell lineColloids Surf B Biointerfaces20129121221422119564
- PatelBShahVRBavadekarSAAnti-proliferative effects of carvacrol on human prostate cancer cell line, LNCaPFASEB J2012261037.5
- SankarRKarthikAPrabuAKarthikSShivashankariKSRavikumarVOriganum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activityColloids Surf B Biointerfaces2013108808423537829