Abstract
To enhance the therapeutic effects of chemotherapy on malignant melanoma, paclitaxel (PTX)-loaded methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) nanoparticles (MPEG-b-PCL NPs) that had their surfaces modified with polydopamine (PTX-loaded MPEG-b-PCL NPs@PDA) were prepared as drug vehicles. The block copolymer MPEG-b-PCL was synthesized by ring-opening polymerization and characterized by proton nuclear magnetic resonance spectroscopy and gel permeation chromatography. The PTX-loaded NPs were prepared by a modified nanoprecipitation technique. The PTX-loaded NPs and PTX-loaded NPs@PDA were characterized in terms of size and size distribution, zeta potential, surface morphology, drug encapsulation efficiency, and drug release. Confocal laser scanning microscopy showed that coumarin-6-loaded NPs@PDA could be internalized by human melanoma cell line A875 cells. The cellular uptake efficiency of NPs was greatly enhanced after PDA modification. The antitumor efficacy of the PTX-loaded NPs@PDA was investigated in vitro by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and in vivo by a xenograft tumor model. The PTX-loaded NPs@PDA could significantly inhibit tumor growth compared to Taxol® and precursor PTX-loaded NPs. All the results suggested that the PTX-loaded MPEG-b-PCL NPs that had their surfaces modified with PDA are promising nanocarriers for malignant melanoma therapy.
Introduction
Malignant melanoma is the mainly aggressive form of skin cancer, causing the most number of skin cancer deaths.Citation1 According to the National Cancer Institute database of the USA, melanoma is one of the most prevalent types of cancer, with >65,000 new cases and 8,650 deaths from malignant melanoma in the USA alone.Citation2 During the past two decades, the incidence and the mortality rate of melanoma are increasing at a faster rate than for most other types of cancer.Citation3 Primary melanoma is a good candidate for surgical therapy, but once metastasis occurs, the therapeutic effect of surgical removal therapy is very poor, although various chemotherapeutic drugs and radiotherapy may have been adopted. Hence, enhancing the clinical therapeutic efficacy of chemotherapy for malignant melanoma is very important. There are some reports in literature that paclitaxel (PTX) has significant effects in the treatment of malignant melanoma.Citation1,Citation4–Citation6 However, the extremely poor water-soluble property, high toxicity, and low bioavailability of PTX have greatly hindered its therapeutic effects and limited its clinical application.Citation7 Taxol® is one of the formulations of PTX that can be used in the therapy of malignant melanoma. Unfortunately, Cremophor-EL, which is an adjuvant used in Taxol®, causes serious side effects and leads to hypersensitivity, cardiotoxicity, and nephrotoxicity reactions in many patients. To reduce the side effects, many efforts have been devoted to replace the Cremophor-EL-based vehicle for PTX delivery.Citation8
Recently, new strategies for malignant melanoma therapy based on anticancer drug-loaded nanocarrier formulations have emerged. Nanoparticles (NPs) represent promising drug vehicles, especially for specific delivery of anticancer agents to the tumor site, which could enhance the clinical effectiveness and reduce systemic toxic side effects.Citation9–Citation11 The NPs used as drug vehicles have many important advantages, such as high encapsulation efficiency (EE) and drug-loading capacity, minor drug leakage, sustained release, and excellent feasibility of routes of administration.Citation12,Citation13 Many chemotherapeutic drugs, such as docetaxel, puerarin, PTX, and doxorubicin, can be delivered by using NPs.Citation14–Citation16 The preparation techniques of polymeric NPs include nanoprecipitation, dialysis, solvent evaporation, and multiple emulsion preparation.Citation13,Citation17,Citation18
Biodegradable copolymers have the potential to be developed as polymeric NPs for drug delivery. Poly(ε-caprolactone) (PCL) is used for applications in the field of drug delivery and tissue engineering. PCL has attracted considerable interest because of its excellent permeability to drugs, noncytotoxicity, thermal properties, and biocom-patibility. However, it is well known that PCL is extremely hydrophobic and its degradation is too slow to meet the needs for drug delivery.Citation19–Citation21 Moreover, the PCL NPs could be rapidly removed in the liver and captured by the reticuloendothelial system (RES) when they are injected into the blood stream.Citation22 A large number of approaches for prolonging the circulation of NPs in blood have been reported. In particular, introduction of the flexible, hydrophilic poly(ethylene glycol) (PEG) can help the NPs to escape uptake by the mononuclear phagocytic system (MPS) and RES, resulting in prolonged blood circulation time.Citation22–Citation24 Although PEG has many advantages such as biocompatibility, antigenicity, and lack of toxicity, as well as the fact that PEGylation increases circulation longevity, it has been reported that PEGylated NPs do not completely avoid cumulative uptake by MPS cells, and they cannot mostly eliminate complement system activation for their corresponding naked NPs.Citation25,Citation26 Kaneda et alCitation27 reported that PEG showed a rather high urinary excretion and peripheral distribution volume. Besides, the molecular structure of PEG as a general polymeric modifier does also not readily allow the addition of new functions along its chain. To surmount these challenges, we use a simple and versatile surface modification method based on polydopamine (PDA).
PDA, a mimic of the specialized adhesive foot protein Mefp-5 (mytilus edulis foot protein-5) secreted by mussels, can modify almost all the drug-loaded polymeric NPs.Citation28 PDA coating displays excellent biocompatibility and low cytotoxicity, making it a versatile platform for applications, including biomolecule immobilization, molecular surface imprinting for protein recognition, cells adhesion, and encapsulation.Citation29–Citation31 Furthermore, PDA is also a major pigment of naturally occurring eumelanin.Citation32 PDA displays a lot of striking properties of naturally occurring eumelanin in terms of electrical, optical, and magnetic properties. Another valuable advantage of PDA lies in its chemical structure that possesses many functional groups such as amine, catechol, and imine. These functional groups could serve as the starting points for covalent modification with the targeting ligand for drug delivery. Due to these benefits, PDA has been rapidly changed into a wide range of applications across the biological, chemical, and nanomedicine fields. PDA coating prepared by oxidative self-polymerization of dopamine on solid or liquid templates showed negligible toxicity and exhibited promising applications for drug delivery.Citation33,Citation34
In this study, to enhance the therapeutic efficacy and minimize the side effects of malignant melanoma treatment, we prepared novel PTX-loaded methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) NPs (PTX-loaded MPEG-b-PCL NPs) and used surface modification with PDA as drug carrier. The surface-modified NPs were characterized and the antitumor effect was evaluated both in vitro and in vivo. The results were closely compared with the current PTX clinical formulation Taxol®.
Materials and methods
Materials
Stannous octoate (Sn(Oct)2), ε-caprolactone (ε-CL), meth oxy PEG (MPEG, molecular weight Mn=2,000 Da), d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS), and dopamine hydrochloride were purchased from Sigma-Aldrich (St Louis, MO, USA). PTX was purchased from Melone Pharmaceutical Co, Ltd (Dalian, People’s Republic of China). Acetonitrile and methanol were purchased from EM Science (ChromAR, high-performance liquid chromatography (HPLC) grade, Mallinckrodt Baker, NJ, USA). All other chemicals were of the highest quality commercially available and were used as received. Human melanoma cell line A875 cells were provided by Shanghai Maisha Biotech (Shanghai, People’s Republic of China).
Synthesis of block copolymer MPEG-b-PCL
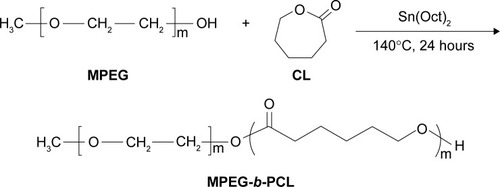
Copolymer MPEG-b-PCL was synthesized from ε-CL and MPEG in the presence of Sn(Oct)2 as a catalyst by ring-opening copolymerization, as presented in . Briefly, ε-CL (9.1 g, 80 mmol), MPEG (2.0 g, 1 mmol), and Sn(Oct)2 (0.1 mol% of monomer ε-CL) were added in a glass tube, which was connected to a vacuum system. Then an exhausting–refilling process with nitrogen was repeated three times. The tube was sealed and heated to 140°C in oil bath for 24 hours. After the reaction tube was cooled to room temperature, the resulting product was dissolved in dichloromethane, then precipitated in excess cold anhydrous ether, and washed with methanol. The final product was designated MPEG-b-PCL and vacuum dried at 40°C for 24 hours.
Characterization of copolymer MPEG-b-PCL
Proton nuclear magnetic resonance (1H-NMR; Bruker AMX500) was used to confirm the structure of the prepared copolymer MPEG-b-PCL; CDCl3 was used as a solvent. Gel permeation chromatography (GPC, Waters 150C) was used to obtain the molecular weight and molecular weight distribution, which were estimated using polystyrene standards. The eluent was tetrahydrofuran with 1 mL/min flow rate.
Formulation of PTX-loaded MPEG-b-PCL NPs and PTX-loaded MPEG-b-PCL NPs@PDA
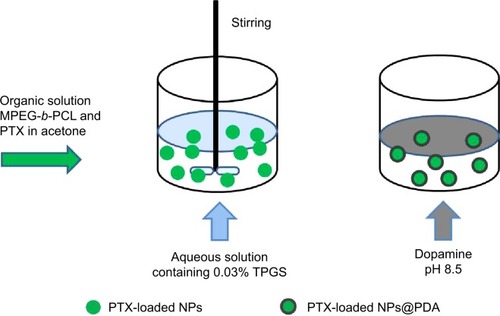
PTX-loaded MPEG-b-PCL NPs were prepared by a modified nanoprecipitation method.Citation13,Citation35 Briefly, 20 mg anticancer drug PTX powder and 100 mg of copolymer MPEG-b-PCL were dissolved in 10 mL of acetone by vortexing and sonication. This mixture was added dropwise into 100 mL of 0.03% TPGS aqueous solution under stirring. The resulting suspension was then stirred uncovered overnight to remove acetone fully. The NP suspension was centrifuged at 15,000 rpm for 20 minutes and then washed three times to remove the emulsifier TPGS and unencapsulated drug PTX. Finally, the dispersed solution was lyophilized 2 days for further surface modification. Fluorescent coumarin-6 (C6)-loaded MPEG-b-PCL NPs were prepared by the same procedure, except that PTX was replaced by C6.
Surface modification of NPs was carried out by the oxidative self-polymerization of dopamine.Citation29,Citation36 In brief, 50 mg lyophilized NPs were dispersed ultrasonically in 50 mL Tris-HCl buffer (pH 8.5, 10 mM) and then 20 mg dopamine hydrochloride was added. The mixture was magnetically stirred for 6 hours in the dark. The oxidative self-polymerized solid PDA-coated PTX-loaded MPEG-b-PCL NPs (designated PTX-loaded MPEG-b-PCL NPs@PDA) were centrifuged (15,000 rpm, 10 minutes) and washed with water to remove the unpolymerized dopamine. PTX-loaded MPEG-b-PCL NPs@PDA was lyophilized 2 days for further use.
Characterization of NPs
Size and zeta potential
The particle size and size distribution were measured by Malvern Mastersizer 2000 (Malvern Instruments Ltd, Malvern, UK). Before measurement, the freshly prepared particles were diluted and the concentration was 1 mg/mL. The measurements were performed in triplicate.
Surface morphology
The surface morphology of NPs was examined by field-emission scanning electron microscopy (FESEM) using a JEOL JSM-6700F system operated at a 5.0 kV accelerating voltage. To prepare samples for FESEM, the particles were fixed on the stub by a double-sided sticky tape and then coated with platinum layer by JFC-1300 automatic fine platinum coater (JEOL, Tokyo, Japan) for 60 seconds.
Drug loading and encapsulation efficiency
The drug loading content (LC) and EE of NPs were obtained by HPLC (LC 1200; Agilent Technologies, Santa Clara, CA, USA) according to the reported procedures.Citation37,Citation38 In summary, NPs (5 mg) were dissolved in 1 mL dichloromethane under vigorous vortexing. This solution was transferred to 5 mL of mobile phase consisting of deionized water and acetonitrile (50:50, v/v). A nitrogen stream was introduced for about 15 minutes to evaporate the dichloromethane, and then a clear solution was obtained for HPLC analysis. A reverse-phase C18 column (250 mm ×4.6 mm; Agilent Technologies) was used. The flow rate of mobile phase was 1 mL/min. The column effluent was detected at λmax of 227 nm with an ultraviolet-visible detector. The drug EE was calculated as the ratio between the amount of PTX encapsulated in the NPs and that fed into the process. The data were obtained as the average of three measurements.
In vitro drug release
The dialysis method was used to analyze the drug release in vitro.Citation39 Briefly, NPs (5 mg) were dispersed in 2 mL of phosphate-buffered saline (PBS, pH =7.4). The suspension was put into a regenerated cellulose dialysis bag (molecular weight cutoff =3,500 Da; Spectra/Por 6, Spectrum Laboratories, Inc., CA, USA). The closed bag was then put into a centrifuge tube and immersed into 15 mL of release medium PBS. The tube was transferred into an orbital water bath and shaken at 120 rpm at 37°C. An aliquot (10 mL) of release medium was taken at various time intervals for HPLC analysis and replaced with fresh PBS solution. The analysis method was similar to the measurement of LC and EE. Each batch of experiments was performed three times. The accumulative release of drug from PTX-loaded NPs and PTX-loaded NPs@PDA was plotted against time.
Cellular uptake of NPs
Human melanoma cell line A875 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. A875 cells, at the initial density of 1×105 cells per well, were plated in 96-well plates. The culture was kept in humidified atmosphere containing 95% air and 5% CO2 at 37°C. The cells were incubated with 100 μg/mL C6-loaded NPs and C6-loaded NPs@PDA at 37°C for 2 hours, washed with cold PBS three times, and then fixed with 4% paraformaldehyde for 20 minutes. Thereafter, cells were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Fluka, Buchs, Switzerland) for 10 minutes and washed twice with PBS. The cells were observed by a confocal laser scanning microscope (Olympus Fluoview FV-1000, Olympus Optical Co, Tokyo, Japan) with imaging software. The images of the cells were captured with differential interference contrast channel; the images of C6-loaded NPs and the nuclei of DAPI-stained cells were recorded with following channels: blue channel (DAPI) excited at 340 nm and green channel (C6) excited at 488 nm.Citation10 For quantitative analysis, the A875 cells at an initial density of 1×104 cells per well were plated in 96-well plates and left overnight. The cells were equilibrated with Hank’s buffered salt solution at 37°C for 1 hour before C6-loaded NPs and C6-loaded NPs@PDA were added at concentrations of 50 μg/mL, 100 μg/mL, and 200 μg/mL. After incubation for 2 hours, the medium was removed and the wells were washed three times with 50 μL cold PBS solution. Then, 50 μL 0.5% Triton X-100 in 0.2 mol/L sodium hydroxide was added into each sample well to lyse the cells.Citation13
In vitro cytotoxicity of PTX-loaded NPs and PTX-loaded NPs@PDA
A875 cells were seeded in 96-well plates at the density of 5×103 cells per well and incubated 24 hours to allow cell adherence. The cells were incubated with the PTX-loaded MPEG-b-PCL NPs, PTX-loaded MPEG-b-PCL NPs@PDA suspension, and Taxol® at equivalent drug concentrations ranging from 0.25 μg/mL to 25 μg/mL for 12 hours, 24 hours, and 48 hours. The PTX-free MPEG-b-PCL NPs@PDA had the same amount of NPs. Before harvest, the culture media were replaced with fresh media containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL) and incubated for additional 4 hours. MTT was aspirated off and dimethyl sulfoxide was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a microplate reader (Bio-Rad Model 680, UK). Untreated cells were taken as control with 100% viability, and cells without addition of MTT were used as blank to calibrate the spectrophotometer to zero absorbance.Citation40
In vivo antitumor efficacy and side effect analysis
Animal experiments were approved by the Administrative Committee on Animal Research (Southern Medical University, Guangzhou, People’s Republic of China). The mice were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences. The tumor growth inhibitory activities of PTX-loaded MPEG-b-PCL NPs@PDA were evaluated with A875 cell xenograft-bearing nude mice model. Human melanoma A875 cells (2×106 cells per mouse) were inoculated subcutaneously to mice at the right axilla. After inoculation of A875 cells, the tumor growth in each mouse was closely observed. The tumor volume was monitored with a caliper every 2 days until the 20th day. Tumor volume can be calculated from the following formula: V = d2 × D/2, where d and D are the shortest and the longest diameters of the tumor in millimeters, respectively.Citation13,Citation41 When the tumor volume reached around 100 mm3 (designated as the 0 day), treatments were carried out. The mice were randomly divided into four groups (each group has five animals; n=5). Physiological saline was used in the control experiment. PTX (Taxol®; 10 mg/kg), PTX-loaded NPs, and PTX-loaded NPs@PDA in PBS were injected via the caudal vein on days 0, 4, 8, 12, and 16. Animals were closely observed for clinical signs and behavior changes. Furthermore, to estimate the side effects of the PTX-loaded NPs and the NPs having surface modification with PDA (NPs@PDA), body weight of the animals was recorded during the treatment period.
Statistical methodology
All results are reported as the mean ± standard error of the mean of three independent experiments. Student’s t-test statistical analysis was used, and probability (P) less than 0.05 was considered statistically significant.
Results and discussion
Characterization of block copolymer MPEG-b-PCL
The biodegradable block copolymer MPEG-b-PCL was prepared by the ring-opening polymerization of monomer ε-CL using MPEG as the initiator and Sn(Oct)2 as the catalyst in bulk at 140°C for 24 hours. To confirm the formation of copolymer MPEG-b-PCL, 1H-NMR in CDCl3 was used, as shown in , with following results: peak a (δ=4.06 parts per million [ppm], CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), b (δ=2.32 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), c (δ=1.62–1.67 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), and d (δ=1.38 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–). Peak e is the solvent CDCl3. The peak f at 3.65 ppm was assigned to the (–CH2–CH2) protons of the initiator MPEG. Similar results were reported by Wan et alCitation42 and Knop et al.Citation43 The molecular weight of MPEG-b-PCL was calculated from the peak areas integral ratio of 4.06 ppm and 3.65 ppm; the molecular weight Mn of the copolymer MPEG-b-PCL was determined by 1H-NMR as 12,150 Da. The molecular weight and polydispersity index (PDI) of copolymer MPEG-b-PCL, which were evaluated by GPC, are 11,893 Da and 1.19, respectively. As shown, the molecular weights detected from GPC and 1H-NMR could confirm each other.
Figure 2 Typical 1H-NMR spectra of block copolymer MPEG-b-PCL.
Notes: 1H-NMR in CDCl3 was used, with following results: peak a (δ=4.06 parts per million [ppm], CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), b (δ=2.32 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), c (δ=1.62–1.67 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), and d (δ=1.38 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–). Peak e is the solvent CDCl3. The peak f at 3.65 ppm was assigned to the (–CH2–CH2) protons of the initiator MPEG.
Abbreviations: 1H-NMR, proton nuclear magnetic resonance; MPEG, methoxy poly(ethylene glycol); MPEG-b-PCL, methoxy poly(ethylene glycol)-b-poly(ε-caprolactone); ppm, parts per million.
![Figure 2 Typical 1H-NMR spectra of block copolymer MPEG-b-PCL.Notes: 1H-NMR in CDCl3 was used, with following results: peak a (δ=4.06 parts per million [ppm], CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), b (δ=2.32 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), c (δ=1.62–1.67 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–), and d (δ=1.38 ppm, CL repeating unit: –CO–CH2–CH2–CH2–CH2–CH2–O–). Peak e is the solvent CDCl3. The peak f at 3.65 ppm was assigned to the (–CH2–CH2) protons of the initiator MPEG.Abbreviations: 1H-NMR, proton nuclear magnetic resonance; MPEG, methoxy poly(ethylene glycol); MPEG-b-PCL, methoxy poly(ethylene glycol)-b-poly(ε-caprolactone); ppm, parts per million.](/cms/asset/9c762ad7-9f68-46bc-8364-2d98c3f57f65/dijn_a_79605_f0002_c.jpg)
Preparation and characterization of NPs
PTX-loaded MPEG-b-PCL NPs were prepared by a modified nanoprecipitation method; acetone was selected as an acceptable solvent. Nanoprecipitation provides a mild, facile, and low-energy input method for the formulation of polymeric NPs. The preparation technique of NPs is shown in . The copolymer MPEG-b-PCL and drug PTX could be fully dissolved in acetone to generate a homogeneous and clear solution. This mixture phase was injected into a stirred aqueous solution containing 0.03% TPGS as a surfactant. Copolymer deposition on the interface between the water and the organic solvent acetone, caused by fast diffusion of the solvent, leads to the instantaneous formation of an NP suspension.Citation13,Citation44 The surface modification of NPs was carried out by the oxidative self-polymerization of dopamine in Tris-HCl buffer (pH 8.5). Polymerized dopamine is known to bind tightly on solid surfaces via covalent and noncovalent interactions, forming a durable layer that serves as an intermediate for ligand incorporation.Citation45
Figure 3 Schematic representation of the preparation techniques of PTX-loaded NPs and PTX-loaded NPs@PDA.
Abbreviations: NP, nanoparticle; PDA, polydopamine; PTX, paclitaxel; PTX-loaded NPs@PDA, PTX-loaded methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) (MPEG-b-PCL) NPs that had their surfaces modified with PDA; TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate.
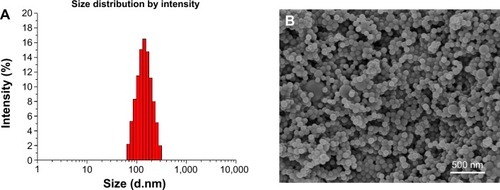
The size and size distribution of the PTX-loaded MPEG-b-PCL NPs and PTX-loaded MPEG-b-PCL NPs@PDA in this study are displayed in . Physicochemical characteristics, such as surface properties and particle size, play an important role in the drug release, cellular uptake, and cytotoxicity of these NPs, as well as their in vivo biodistribution and pharmacokinetics. Thus, the physicochemical properties of NPs affect the clinical application of the anticancer drug.Citation38,Citation46,Citation47 The size of NPs was 120–140 nm in diameter, which may result in highest cellular uptake by endocytosis and inhibit elimination by the RES, as well as promoting accumulation in tumor vasculature because of the enhanced permeability and retention effect on passive tumor targeting.Citation48,Citation49 The size of the PTX-loaded MPEG-b-PCL NPs@PDA was larger than that of the nonmodified PTX-loaded MPEG-b-PCL NPs; this could be ascribed to the coating of PDA on PTX-loaded NPs. The PDIs of PTX-loaded NPs and PTX-loaded NPs@PDA were 0.136 and 0.121, respectively. The PDI is rather narrow, which is advantageous for cancer therapy. The size distribution of the PTX-loaded MPEG-b-PCL NPs@PDA obtained from dynamic light scattering (DLS) is presented in . As can be seen from , PTX-loaded NPs and PTX-loaded NPs@PDA have similar drug LC and EE values. Furthermore, the drug LC of PTX in NPs@PDA could reach about 16.8%, providing satisfactorily efficient drug delivery system.
Table 1 Characterization of PTX-loaded NPs and PTX-loaded NPs@PDA
Figure 4 (A) DLS size distribution and (B) FESEM images of PTX-loaded MPEG-b-PCL NPs@PDA.
Abbreviations: DLS, dynamic light scattering; FESEM, field-emission scanning electron microscopy; NP, nanoparticle; PDA, polydopamine; PTX, paclitaxel; PTX-loaded MPEG-b-PCL NPs@PDA, PTX-loaded methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) NPs that had their surfaces modified with PDA.
The zeta potential also reflects the stability of NPs in vivo through electrostatic repulsion. As shown in , the zeta potentials of PTX-loaded NPs and PTX-loaded NPs@PDA range from −21.4 mV to 10.9 mV. The negative surface charge of the PTX-loaded NPs resulted from the ionized carboxyl groups of PCL segments. Zeta potential of PTX-loaded NPs@PDA was positive, which could enhance mucosal uptake due to the anionic nature of the mucous layer.Citation50
The surface morphology of the PTX-loaded MPEG-b-PCL NPs@PDA was observed by FESEM. shows the FESEM image for NPs@PDA. The NPs seemed to be about 100 nm in size and had smooth surface within the resolution level used. However, the particle size evaluated from FESEM was smaller than that from DLS analysis. This could be attributed to the fact that dry NPs were prone to shrinking and collapsing.Citation13,Citation51
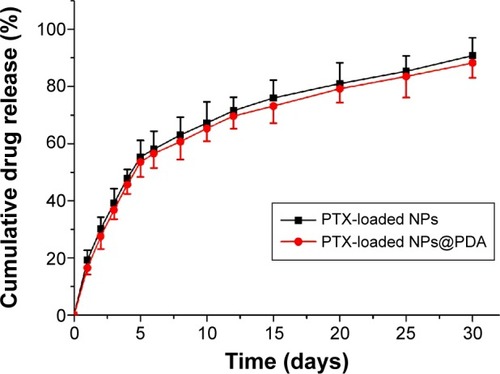
The in vitro drug release profiles of the PTX-loaded NPs and PTX-loaded NPs@PDA in the first 30 days are shown in . As can be seen from , similar drug release curves of PTX-loaded NPs and PTX-loaded NPs@PDA were observed at the same time. The results suggested that the chemotherapeutic drug PTX of PDA-modified NPs was almost totally preserved during the preparation procedure. The NPs showed steady, continuous release patterns, with initial burst releases. The drug release from PTX-loaded NPs@PDA was found to be 53.6% and 88.2% of the encapsulated drug in the first 5 days and after 30 days, respectively. The PTX release behaviors could be ascribed to the following three important factors. First, the drug was hardly entrapped in the polymeric core and just located beneath the periphery of NPs. Second, the drug was dispersed amorphously or in the solid solution state in the NPs, which accelerated drug release because amorphous PTX dissolves much faster than its crystalline form.Citation52 Third, sustained release mainly resulted from the diffusion of drug that was well encapsulated in the hydrophobic inner shell of NPs.
Figure 5 The in vitro drug release profile of PTX-loaded NPs and PTX-loaded NPs@PDA.
Note: Data represent mean ± SEM (n=3).
Abbreviations: NP, nanoparticle; PDA, polydopamine; PTX, paclitaxel; PTX-loaded NPs@PDA, PTX-loaded NPs that had their surfaces modified with PDA; SEM, standard error of the mean.
Cellular uptake of C6-loaded NPs and NPs@PDA
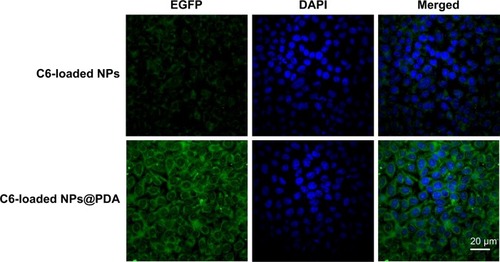
Generally speaking, the therapeutic effects of the drug-loaded NPs depend on internalization and sustained release of the NPs by the cancer cells. C6, a fluorescence molecule, has been widely used as a probe for marking NPs in cellular uptake investigation.Citation53,Citation54 C6 was used for replacing the PTX in the NPs to observe and analyze cellular uptake of drug-loaded NPs. presented confocal laser scanning microscopy images of the melanoma cell line A875 cells after 24-hour incubation with the C6-loaded NPs and C6-loaded NPs@PDA suspension in DMEM at 100 μg/mL NP concentration. The images were obtained from (A) enhanced green fluorescent protein channel (green), (B) DAPI channel (blue), and (C) the merger of the two channels. The C6-loaded NPs and C6-loaded NPs@PDA (green) were densely dispersed around the blue nuclei (stained by DAPI), implying that the fluorescent NPs had been internalized into the A875 cells. Furthermore, the fluorescence of C6-loaded NPs@PDA in the cytoplasm was much brighter than that of C6-loaded NPs. Thus, NPs could be more efficiently internalized into the A875 cell to deliver drug after surface modification with PDA. The results could be attributed to the facilitated internalization of cationic NPs (modified with PDA) by the negatively charged cell membranes.Citation34
Figure 6 CLSM images of A875 cells after 2-hour incubation with C6-loaded NPs and C6-loaded NPs@PDA.
Notes: The NPs and NPs@PDA were green (EGFP channel) and the cells were blue (stained by DAPI).
Abbreviations: C6, coumarin-6; CLSM, confocal laser scanning microscopy; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; EGFP, enhanced green fluorescent protein; NP, nanoparticle; NPs@PDA, NPs that had their surfaces modified with PDA; PDA, polydopamine.
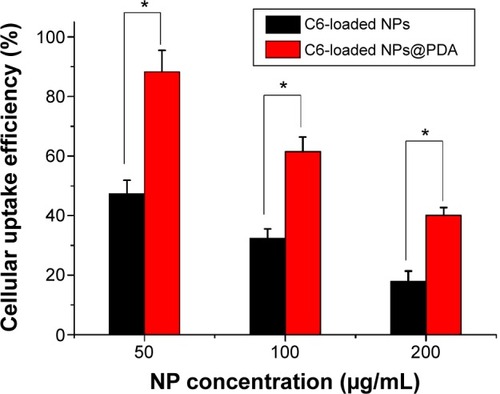
The cellular uptake efficiency of the C6-loaded NPs and C6-loaded NPs@PDA was evaluated and is shown in . As shown in , the cellular uptake efficiency of both C6-loaded NPs and NPs@PDA decreased with increase in the incubated NP concentration from 50 μg/mL to 200 μg/mL. The cellular uptake efficiency of C6-loaded NPs@PDA was 1.87-, 1.90-, and 2.24-fold higher than that of C6-loaded NPs at the incubated NP concentrations of 50 μg/mL, 100 μg/mL, and 200 μg/mL, respectively. Therefore, we strongly believe that the surface PDA could enhance the cellular uptake efficiency of NPs, which is very useful for malignant melanoma therapy.
Figure 7 The cellular uptake efficiency of C6-loaded NPs and C6-loaded NPs@PDA at different NP concentrations.
Note: Data represent mean ± SEM (n=3; *P<0.05).
Abbreviations: C6, coumarin-6; NP, nanoparticle; NPs@PDA, NPs that had their surfaces modified with PDA; PDA, polydopamine; SEM, standard error of the mean.
In vitro cell viability of DTX-loaded NPs
Human melanoma cell line A875 cells were used to investigate the in vitro cytotoxicity of PTX-loaded NPs@PDA and to prove that the drug that was encapsulated in the NPs remained bioactive.Citation13 According to the plasma levels of PTX achievable in human beings, the cells were treated with PTX-loaded MPEG-b-PCL NPs, NPs@PDA, and Taxol® at equivalent PTX concentrations of 0.25 μg/mL, 2.5 μg/mL, and 25 μg/mL for 12 hours, 24 hours, and 48 hours. The percentage of cell viability was determined by the MTT method (). As shown in , we can arrive at four conclusions. First, the cytotoxicity of the three PTX formulations increased with increasing drug dose and incubation time. Second, the cytotox-icity of the three formulations is very similar in the first 12 hours of incubation. However, after 24 hours and 48 hours of incubation, the PTX-loaded NPs@PDA showed significantly higher cytotoxicity than Taxol®. Third, the surface-modified PTX-loaded NPs@PDA are most destructive to A875 cells. Fourth, the synthesized copolymer MPEG-b-PCL and PDA seemed to be nontoxic in cell culture because no significant cytotoxicity was found for the drug-free NPs at various concentrations.
Figure 8 Viability of A875 cells after incubation with PTX-loaded NPs and PTX-loaded NPs@PDA in comparison with viability after treatment with Taxol® at the same PTX dose and drug-free NPs@PDA with the same amount of nanoparticles.
Notes: Incubation for (A) 12 hours; (B) 24 hours; and (C) 48 hours. Data represent mean ± SEM (n=3; *P<0.05, **P<0.01, ***P<0.001).
Abbreviations: NP, nanoparticle; NPs@PDA, NPs that had their surfaces modified with PDA; PDA, polydopamine; PTX, paclitaxel; SEM, standard error of the mean.
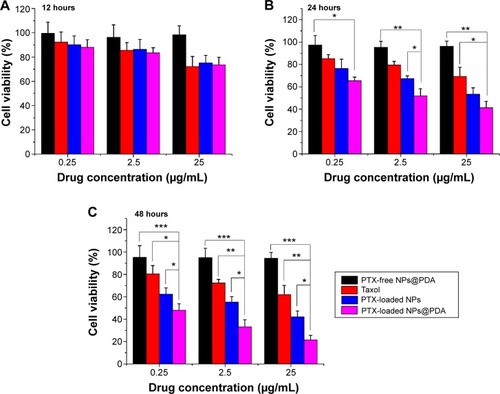
After 12 hours of incubation at a drug concentration of 2.5 μg/mL, the cell viability was 85.61% for Taxol®, 86.32% for PTX-loaded NPs, and 83.45% for PTX-loaded NPs@PDA. However, after 24 hours and 48 hours of incubation, the cytotoxicity of PTX-loaded NPs@PDA against A875 cells was, respectively, 27.48% and 39.17% higher than that of Taxol®. The PTX-loaded NPs@PDA showed better cytotoxicity efficacy against A875 cells than PTX-loaded NPs and commercial Taxol®; this could be attributed to the special physicochemical properties of PDA-modified PTX-loaded NPs. The PDA can improve the cellular uptake efficiency of PTX-loaded NPs due to its surface charge and biocompatibility.Citation32,Citation55
In vivo antitumor efficacy and side effect analysis
Due to the satisfactory in vitro cytotoxicity against melanoma cells A875, PTX-loaded MPEG-b-PCL NPs@PDA can be used as drug vehicles for treating melanoma. The antitumor efficacy of the PTX-loaded NPs and the NPs having surfaces modified with PDA was investigated in human melanoma cell line A875 cell-bearing nude mice; the clinical drug Taxol® and physiological saline were used for comparison. The drug was injected into the mice every 4 days for five consecutive cycles, and the tumor volumes and weights of the mice were recorded every 2 days.
shows the tumor growths of the mice after intravenous injection with PTX-loaded NPs, PTX-loaded NPs@PDA, Taxol®, and physiological saline after 20 days’ treatment. The tumor size of the saline group increased obviously. However, tumor growths in the groups treated with Taxol®, PTX-loaded NPs, and PTX-loaded NPs @PDA were significantly inhibited. The melanoma tumor inhibitory effects were in the following order: PTX-loaded NPs@PDA > PTX-loaded NPs > Taxol® > saline. The results of the antitumor efficacy experiment demonstrated that PTX-loaded NPs having surfaces modified with PDA could be released from the NPs effectively and that the released PTX maintained its bioactivity for melanoma treatment.
Figure 9 Tumor growth curve and weights of nude mice bearing A875 cell xenografts.
Notes: (A) Tumor growth curve of nude mice bearing A875 cell xenografts after injection with PTX-loaded NPs, PTX-loaded NPs@PDA, Taxol®, and saline; (B) weights of the nude mice bearing A875 cell xenografts after injection with PTX-loaded NPs, PTX-loaded NPs@PDA, Taxol®, and saline. Data represent mean ± SEM (n=5; *P<0.05, **P<0.01).
Abbreviations: NP, nanoparticle; NPs@PDA, NPs that had their surfaces modified with PDA; PDA, polydopamine; PTX, paclitaxel; SEM, standard error of the mean.
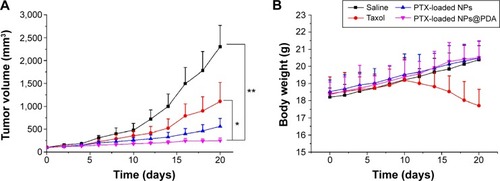
Minimizing the side effects of drug-loaded NPs is one of the crucial concerns while developing novel drug delivery vehicles.Citation38 Safety profiles of PTX formulations were evaluated by analyzing the changes in the body weights of animals. shows the body weight variations of mice in all groups; body weight loss was only found at the end of Taxol® therapy. However, the body weight of the groups injected with PTX-loaded NPs and PTX-loaded NPs@PDA grew steadily. Meanwhile, the mice treated with PTX-loaded NPs@PDA remained healthy and vigorous throughout the therapy period, but the mice treated with Taxol® showed weakened vitality. These observations demonstrated that the side effects in mice injected with PTX-loaded NPs@PDA were fewer than those after administration of commercial Taxol®. In other words, the PTX-loaded MPEG-b-PCL NPs having surface modification with PDA could retain the pharmacological activity of PTX and they significantly inhibited tumor growth compared to the effect of Taxol® at the same dose.
Conclusion
In this study, a novel type of PTX-loaded MPEG-b-PCL NPs, which possessed surface modification with PDA, were prepared for malignant melanoma treatment. The PTX-loaded NPs were manufactured by a modified nanoprecipitation method and subsequent surface modification with PDA. The hydrodynamic size of PTX-loaded NPs@PDA is about 140 nm and it has smooth surface. The PTX-loaded NPs@PDA and precursor PTX-loaded NPs had similar drug LC, EE, and drug release profiles. The drug-loaded NPs@PDA possessed higher cellular uptake activity and showed greater cytotoxicity than precursor PTX-loaded NPs. The in vitro and in vivo antitumor effects of the PTX-loaded NPs@PDA were obviously superior to those of PTX-loaded NPs and Taxol®. In short, the novel PTX-loaded NPs@PDA is capable of eliciting high therapeutic effects and low side effects during malignant melanoma treatment.
Acknowledgments
This research was supported by the Guangdong Natural Science Foundation (2014A030313758), Doctoral Fund of Ministry of Education of China (20120002120020), and Science, Technology & Innovation Commission of Shenzhen Municipality (Numbers JCYJ20120616213411826 and JCYJ20140417115840285).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhangWShiYChenYHaoJShaXFangXThe potential of pluronic polymeric micelles encapsulated with paclitaxel for the treatment of melanoma using subcutaneous and pulmonary metastatic mice modelsBiomaterials2011325934594421596432
- AgarwalaSSO’DaySJCurrent and future adjuvant immunotherapies for melanoma: blockade of cytotoxic T-lymphocyte antigen-4 as a novel approachCancer Treat Rev20113713314220638184
- DingBYWuXFanWAnti-DR5 monoclonal antibody-mediated DTIC-loaded nanoparticles combining chemotherapy and immunotherapy for malignant melanoma: target formulation development and in vitro anticancer activityInt J Nanomedicine201161991200521976975
- GogasHBafaloukosDBedikianAYThe role of taxanes in the treatment of metastatic melanomaMelanoma Res20041441542015457099
- BedikianAYPlagerCPapadopoulosNEtonOEllerhorstJSmithTPhase II evaluation of paclitaxel by short intravenous infusion in metastatic melanomaMelanoma Res200414636615091196
- HeWMaXYangXZhaoYQiuJHangHA role for the arginine methylation of Rad9 in checkpoint control and cellular sensitivity to DNA damageNucleic Acids Res2011394719472721321020
- VideiraMAlmeidaAJFabraAPreclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effectNanomedicine201281208121522206945
- XuZHGuWWHuangJIn vitro and in vivo evaluation of actively targetable nanoparticles for paclitaxel deliveryInt J Pharm200528836136815620876
- XiaoXZengXZhangXEffects of Caryota mitis profilin-loaded PLGA nanoparticles in a murine model of allergic asthmaInt J Nanomedicine201384553456224376349
- ZhuHChenHZengXCo-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistanceBiomaterials2014352391240024360574
- DianzaniCZaraGPMainaGDrug delivery nanoparticles in skin cancersBiomed Res Int2014201489598625101298
- ChoKJWangXNieSMChenZShinDMTherapeutic nanoparticles for drug delivery in cancerClin Cancer Res2008141310131618316549
- ZengXTaoWMeiLHuangLTanCFengSSCholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancerBiomaterials2013346058606723694904
- TaoWZengXLiuTDocetaxel-loaded nanoparticles based on star-shaped mannitol-core PLGA-TPGS diblock copolymer for breast cancer therapyActa Biomater201398910892023816645
- ZengXChenHZhengYEnhanced adsorption of puerarin onto a novel hydrophilic and polar modified post-crosslinked resin from aqueous solutionJ Colloid Interface Sci201238516617322878002
- HuangPSongHWangWIntegrin-targeted zwitterionic polymeric nanoparticles with acid-induced disassembly property for enhanced drug accumulation and release in tumorBiomacromolecules2014153128313825054812
- BarbaAADalmoroAd’AmoreMLambertiGIn vitro dissolution of pH sensitive microparticles for colon-specific drug deliveryPharm Dev Technol2013181399140623066945
- BarbaAADalmoroAd’AmoreMVascelloCLambertiGBiocompatible nano-micro-particles by solvent evaporation from multiple emulsions techniqueJ Mater Sci20144951605170
- TaoWZengXWZhangJXSynthesis of cholic acid-core poly(epsilon-caprolactone-ran-lactide)-b-poly(ethylene glycol) 1000 random copolymer as a chemotherapeutic nanocarrier for liver cancer treatmentBiomater Sci2014212621274
- LaleSVAswathyRGAravindAKumarDSKoulVAS1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA-PCL-pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapyBiomacromolecules2014151737175224689987
- TamboliVMishraGPMitraAKNovel pentablock copolymer (PLA-PCL-PEG-PCL-PLA)-based nanoparticles for controlled drug delivery: effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticlesColloid Polym Sci20132911235124523626400
- HeWZhaoYZhangCRad9 plays an important role in DNA mismatch repair through physical interaction with MLH1Nucleic Acids Res2008366406641718842633
- ZhangWPSunJLiuYPEG-stabilized bilayer nanodisks as carriers for doxorubicin deliveryMol Pharm2014113279329024754897
- SannaVPalaNSechiMTargeted therapy using nanotechnology: focus on cancerInt J Nanomedicine2014946748324531078
- MadaniFBessodesMLakroufAVauthierCSchermanDChaumeilJCPEGylation of microspheres for therapeutic embolization: preparation, characterization and biological performance evaluationBiomaterials2007281198120817113637
- ZhuZSLiYLiXLPaclitaxel-loaded poly(N-vinylpyrrolidone)-b-poly(epsilon-caprolactone) nanoparticles: preparation and antitumor activity in vivoJ Control Release201014243844619896997
- KanedaYTsutsumiYYoshiokaYThe use of PVP as a polymeric carrier to improve the plasma half-life of drugsBiomaterials2004253259326614980420
- LeeHDellatoreSMMillerWMMessersmithPBMussel-inspired surface chemistry for multifunctional coatingsScience200731842643017947576
- ZhengQSLinTRWuHYMussel-inspired polydopamine coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled releaseInt J Pharm2014463222624393764
- LiFMengJQYeJFYangBTianQDengCHSurface modification of PES ultrafiltration membrane by polydopamine coating and poly(ethylene glycol) grafting: morphology, stability, and anti-foulingDesalination2014344422430
- YangKLeeJSKimJPolydopamine-mediated surface modification of scaffold materials for human neural stem cell engineeringBiomaterials20123381868187
- LiuYLAiKLLuLHPolydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fieldsChem Rev20141145057511524517847
- PostmaAYanYWangYJZelikinANTjiptoECarusoFSelf-polymerization of dopamine as a versatile and robust technique to prepare polymer capsulesChem Mater20092130423044
- ChengFFZhangJJXuFHuLHAbdel-HalimESZhuJJpH-sensitive polydopamine nanocapsules for cell imaging and drug delivery based on folate receptor targetingJ Biomed Nanotechnol201391155116323909129
- WangZZengXMaYAntitumor efficiency of D-alpha-tocopheryl polyethylene glycol 1000 succinate-b-poly(epsilon-caprolactone-ran-lactide) nanoparticle-based delivery of docetaxel in mice bearing cervical cancerJ Biomed Nanotechnol2014101509151925016651
- ParkJBrustTFLeeHJLeeSCWattsVJYeoYPolydopamine-based simple and versatile surface modification of polymeric nano drug carriersACS Nano201483347335624628245
- ZhangYQTangLSunLLA novel paclitaxel-loaded poly(epsilon-caprolactone)/Poloxamer 188 blend nanoparticle overcoming multidrug resistance for cancer treatmentActa Biomater201062045205219969111
- ZhaoTJChenHZDongYCPaclitaxel-loaded poly(glycolide-co-epsilon-caprolactone)-b-D-alpha-tocopheryl polyethylene glycol 2000 succinate nanoparticles for lung cancer therapyInt J Nanomedicine201381947195723696703
- MaYDHuangLQSongCXZengXWLiuGMeiLNanoparticle formulation of poly(epsilon-caprolactone-co-lactide)-D-alpha-tocopheryl polyethylene glycol 1000 succinate random copolymer for cervical cancer treatmentPolymer20105159525959
- ZhangXDongYZengXThe effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatmentBiomaterials2014351932194324315578
- ZhangXZengXLiangXThe chemotherapeutic potential of PEG-b-PLGA copolymer micelles that combine chloroquine as autophagy inhibitor and docetaxel as an anti-cancer drugBiomaterials2014359144915425109439
- WanYMGanZHLiZBEffects of the surface charge on the stability of PEG-b-PCL micelles: simulation of the interactions between charged micelles and plasma componentsPolym Chem2014517201727
- KnopKStumpfSSchubertUSDrugs as matrix to detect their own drug delivery system of PEG-b-PCL block copolymers in matrix-assisted laser desorption/ionization time-of-flight mass spectrometryRapid Commun Mass Spectrom2013272201221223996394
- DalmoroALambertiGTitomanlioGBarbaAAd’AmoreMEnteric micro-particles for targeted oral drug deliveryAAPS Pharm Sci Tech20101115001507
- LeeHSchererNFMessersmithPBSingle-molecule mechanics of mussel adhesionProc Natl Acad Sci U S A2006103129991300316920796
- ZengXTaoWWangZDocetaxel-loaded nanoparticles of dendritic amphiphilic block copolymer H40-PLA-b-TPGS for cancer treatmentPart Part Syst Charact201532112122
- YanFZhangCZhengYThe effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicityNanomedicine2010617017819447200
- RakhmatullinRMKurkinINPavlovVVSemashkoVVEPR, optical, and dielectric spectroscopy of Er-doped cerium dioxide nanoparticlesPhys Status Solidi B201425115451551
- AcharyaSSahooSKPLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effectAdv Drug Deliv Rev20116317018320965219
- HariharanSBhardwajVBalaISitterbergJBakowskyURavi KumarMNDesign of estradiol loaded PLGA nanoparticulate formulations: a potential oral delivery system for hormone therapyPharm Res20062318419516267632
- LiuTTSeiffertSThieleJAbateARWeitzDARichteringWNon-coalescence of oppositely charged droplets in pH-sensitive emulsionsProc Natl Acad Sci U S A201210938438922203968
- ZhangLYSunMJGuoRChitosan surface-modified hydroxycamptothecin loaded nanoparticles with enhanced transport across Caco-2 cell monolayerJ Nanosci Nanotechnol200662912292017048498
- MaYDZhengYZengXWNovel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatmentInt J Nanomedicine201162679268822114498
- MaWXChenMSKaushalSPLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responsesInt J Nanomedicine201271475148722619507
- GullottiEParkJYeoYPolydopamine-based surface modification for the development of peritumorally activatable nanoparticlesPharm Res2013301956196723609560