Can nanocarriers conquer gene therapy?
Evaluation of: Magalhães M, Farinha D, Pedroso de Lima MC, Faneca H. Increased gene delivery efficiency and specificity of a lipid-based nanosystem incorporating a glycolipid. Int J Nanomedicine. 2014;9:4979–4989.
Hepatocellular carcinoma (HCC) is a devastating disease that makes up 70%–85% of all cases of liver cancer, affecting millions of people worldwide.Citation1 Unfortunately, HCC is aggressive and deadly; patients typically live for only 6 to 20 months after diagnosis, highlighting the dire need for new effective therapies. Although gene therapy has the potential to offer new biologically based medicines, the efficient, selective, and safe delivery of DNA- or RNA-based drugs to target cells including hepatocytes is still a major limiting factor to the broad applications of gene therapy in cancer diseases.Citation2–Citation4
However, to address the problem of gene delivery, recent advances use clinically relevant materials such as block copolymers, cyclodextrins, copolypeptides, charged lipids, and cholesterol-modified small interfering RNA (siRNA) via lipoprotein-based advanced nanocarriers with an ability to successfully deliver siRNA into target cells.Citation5 A new study by Magalhães et alCitation5 describes the development of a new lipid-based combination therapeutic approach using EPOPC:Chol:lactosyl-PE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-lactosyl [ammonium salt]), an advanced design of drug delivery nanocarrier platform that is expected to lead to more effective and selective nonviral gene delivery into target cells. For example, the preliminary results have attracted extensive interest by incorporating 15% of the designed molecule lactosyl-PE with liposome particles, which showed the most potent delivery to hepatocytes, not only in terms of therapeutic effect, but also in terms of the percentage of transfected HCC target cell types (). The potential advantage of these increasing efforts will be the opportunity to treat specific cancer cell types that have thus far been resistant to available therapies and keeping toxic drugs out of healthy tissues, as one of the biggest uses of future nanomedicine.Citation6
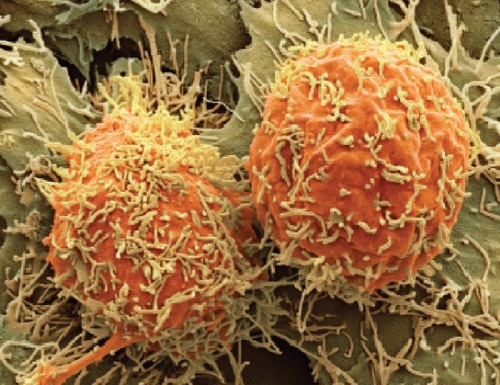
Figure 1 A colored scanning electron micrograph of two hepatocellular carcinoma cells (Steve Gschmeissner/SPL).
Notes: Patients with the disease typically live for only 6 to 20 months after diagnosis. Reproduced with permission from Scudellari M. Drug development: try and try again. Nature. 2014;516(7529):S4–S6.
Taken together, the promise of a revolution in human health in terms of targeted medicine remains quite real. With profound emerging knowledge of new molecular-based medicines founded on pharmacogenomic and genetic understanding of cancer coupled with the current advances of nanomedicine, humankind is on the verge of gaining immense new power to heal. Significantly, advances of new drug delivery nanocarriers have the potential to conquer gene therapy to combat tumor cells in a targeted manner; however, many questions remain.
Is oral insulin therapy still an uphill task?
Evaluation of: Song L, Zhi ZL, Pickup JC. Nanolayer encapsulation of insulin-chitosan complexes improves efficiency of oral insulin delivery. Int J Nanomedicine. 2014;9: 2127–2136.
Diabetes is a major health problem and is a leading cause of vision loss, kidney failure, amputations among adults, and a host of other conditionsCitation7 and is increasingly getting worse. Presently, most existing drugs for diabetes typically only treat symptoms in order to achieve the most stable glycemic levels, but they are noticeably every bit as fraught with the major concerns of drug resistance and side effects.Citation8 Hormonal therapy still remains a dream, yet it must deal with powerful proteolytic enzymes and the gastrointestinal (GI) tract.
However, the long anticipated development of drug delivery is a hallmark of advancing nanomedicine, as a variety of sophisticated strategies are being investigated for engineering advanced novel delivery platformsCitation9–Citation11 in order to address many confounding drug delivery limitations that remain to be resolved by other conventional methods. In a recent issue of International Journal of Nanomedicine, Song et alCitation12 investigated the encapsulation of a protein ‘insulin’ in greater detail using a size-tunable nanolayer combination of polyethylene glycol (PEG) with chitosan, a cationic naturally occurring polysaccharide, without creating a new chemical entity that often interferes with drug activity. Encapsulating drugs in nanomaterials enhances their stability, solubility, and pharmacokinetics, and increases the permeability and efficiency of therapeutics. Most importantly, the design of such a novel delivery platform has the ability to safely and effectively deliver insulin through a difficult-to-cross GI tract, which is a real obstacle for many therapeutics. However, further continued study of this important concept will help to establish new toxicogenomic approaches as scientifically accepted practices in the process of drug risk assessment and its therapeutic translational potential.Citation13
In summary, this is an excellent starting point for both endocrinologists as well as academic researchers who have been trying to devise a successful, painless oral insulin therapy, which would benefit a significant number of patients, especially given that drugs for this chronic disease must be taken for years. Oral insulin nanotherapeutics is a real promise; the generation of a nanolayered insulin-loadable molecule onto a drug-loadable delivery nanoparticle is a bioinspired approach to biomaterial design and may prove to be a useful strategy to overcome the tough barriers.
Next generation imaging tools
Evaluation of: Rizvi SB, Rouhi S, Taniguchi S, et al. Near-infrared quantum dots for HER2 localization and imaging of cancer cells. Int J Nanomedicine. 2014;9:1323–1337.
Quantum dots (QDs) are luminescent size- and shape-tunable nanocrystals with potential applications in labeling cells and molecular biomarkers.Citation14 In contrast to organic fluorophores, QDs demonstrate the outstanding features of bioconjugation and photostability, enabling constant molecular image acquisition, which is critical for accurate quantitative analysis and to control their form at the nanoscale with great precision. This offers new capabilities for multicolor optical coding in gene expression studies, high-throughput molecular screening, and in vivo imaging.Citation15
Recently, Rizvi et al demonstrated new ‘near-infrared-emitting quantum dot antiHER2-antibody bioconjugates’ to detect the expression and location of HER2 receptor gene status for the first time in living cancerous cells under in vivo conditions. This is remarkable because such an approach has the potential to lead a successful plethora of antibody–QD conjugates into clinical development and could provide a powerful tool for early diagnosis of important cancer diseases such as HCC,Citation1 where early detection is still a clinical challenge. Further, the development of these next generation imaging tools should offer the possibility of early diagnosis and treatment, and may prove to be an important consideration in controlling the pharmacokinetic exposure levels of a given antibody–QD combination,Citation16 with any potential toxicity possibly being mitigated by faster clearance. Importantly, other modifications must also be investigated in an effort to increase the specificity of cancer cell binding by using bispecific antibodies that could recognize two antigens at once and thus can substantially minimize toxicity in healthy tissues.
However, despite this great promise, QD-based fluorescence molecular imaging technology has yet to show major improvements over organic molecular probes, as current multiplexing capability is far below the expectation of creating a true molecular portrait of the individual cell with state of the art imaging of molecular targets with custom-designed QD molecular probes.
Acknowledgments
The authors would like to acknowledge Dr Aijaz Rashid, Indian Institute of Technology, Mumbai, India for his valuable comments.
Disclosure
The authors report no conflicts of interest in this work.
References
- ScudellariMDrug development: try and try againNature20145167529S4S625470198
- DavisMEZuckermanJEChoiCHEvidence of RNAi in humans from systemically administered siRNA via targeted nanoparticlesNature201246472911067107020305636
- SukhanovaAEven-DesrumeauxKKisserliAOriented conjugates of single-domain antibodies and quantum dots: towards a new generation of ultrasmall diagnostic nanoprobesNanomedicine20128451652521839049
- JainRKStylianopoulosTDelivering nanomedicine to solid tumorsNat Rev Clin Oncol201071165366420838415
- DongYLoveKTDorkinJRLipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primatesProc Natl Acad Sci U S A2014111113955396024516150
- BourzacKNanotechnology: carrying drugsNature20124917425S58S6023320289
- HoltRIMitchellAJDiabetes mellitus and severe mental illness: mechanisms and clinical implicationsNat Rev Endocrinol2014112798925445848
- ScheenAJPharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitusDrugs2015751335925488697
- ShiekhFAHighlights from recent advances in nanomedicineNanomedicine (Lond)2014991287128925204819
- ShiekhFASynthetic nanocarriers as a new paradigmNanomedicine (Lond)20149152259226225413853
- ShiekhFATargeted nanotherapeutics in cancerInt J Nanomedicine201491627162824741302
- SongLZhiZLPickupJCNanolayer encapsulation of insulin-chitosan complexes improves effi- ciency of oral insulin deliveryInt J Nanomedicine201492127213624833901
- GershonDToxicogenomics gains impetusNature200241568694511796961
- TangRXueJXuBShenDSudlowGPAchilefuSTunable ultrasmall visible-to-extended near-infrared emitting silver sulfide quantum dots for integrin-targeted cancer imagingACS Nano20159122023025560768
- RizviSBRouhiSTaniguchiSNear- infrared quantum dots for HER2 localization and imaging of cancer cellsInt J Nanomedicine201491323133724648731
- LiuXJiXLiuMHigh-performance ge quantum dot decorated graphene/zinc-oxide heterostructure infrared photodetectorACS Appl Mater Interfaces2015742452245825561422
- LinGOuyangQHuRIn vivo toxicity assessment of non-cadmium quantum dots in BALB/c miceNanomedicine201511234135025461291
