Abstract
Anionic nanoclays are layered double hydroxide nanoparticles (LDH-NPs) that have been shown to exhibit toxicity by inducing reactive oxidative species and a proinflammatory mediator in human lung epithelial A549 cells. However, the molecular mechanism responsible for this LDH-NP-induced toxicity and the relationship between oxidative stress and inflammatory events remains unclear. In this study, we focused on intracellular signaling pathways and transcription factors induced in response to oxidative stress caused by exposure to LDH-NPs in A549 cells. Mitogen-activated protein kinase (MAPK) cascades, such as extracellular signal-regulated kinase, c-Jun-N-terminal kinase (JNK), and p38, were investigated as potential signaling mechanisms responsible for regulation of oxidative stress and cytokine release. Src family kinases (SFKs), which are known to mediate activation of MAPK, together with redox-sensitive transcription factors, including nuclear factor kappa B and nuclear factor-erythroid 2-related factor-2, were also investigated as downstream events of MAPK signaling. The results obtained suggest that LDH-NP exposure causes oxidative stress, leading to expression of antioxidant enzymes, such as catalase, glucose reductase, superoxide dismutase, and heme oxygenase-1, via a SFK-JNK and p38-nuclear factor kappa B signaling pathway. Further, activation of this signaling was also found to regulate release of inflammatory cytokines, including interleukin-6 and interleukin-8, demonstrating the inflammatory potential of LDH-NP.
Introduction
Nanotechnology and engineered nanoparticles have been extensively applied in diverse industrial fields, including manufacturing, processing, materials, and consumer products.Citation1,Citation2 Although nanomaterials provide many benefits related to their small size, large surface area, and high reactivity, there is growing concern about their possible adverse effects on human health.Citation3,Citation4 Further, the toxic effects of nanoparticles may be closely associated with their size-related properties.Citation5,Citation6 Of the various types of nanoparticles, inorganic layered double hydroxide nanoparticles (LDH-NPs), also known as anionic nanoclays, have attracted much attention as heat retention additives in plastic films, flame retardants, stabilizing agents for polymers, precursors of catalytic and magnetic materials, and drug delivery carriers in biology and medicine.Citation7,Citation8 LDH-NPs have the potential for widespread use due to their lamellar structures, which allow intercalation of anionic molecules between layers, enabling their controlled release and protecting them from surrounding environmental conditions.Citation9
The toxicity of LDH-NPs has been reported to differ according to the chemical compositions, cell lines, and concentrations used.Citation10 Although LDH-NPs are known to be less toxic than other inorganic nanoparticles, it has been reported that they might generate reactive oxygen species (ROS) and interleukin (IL)-8, a proinflammatory mediator, in cultured cells, suggesting that LDH-NPs are potentially toxic in terms of inducing oxidative stress and inflammation.Citation11 Nanoparticle-induced oxidative stress has been reported to mediate adverse physiological responses, such as inflammation, genotoxicity, fibrosis, and carcinogenesis.Citation12 It has also been demonstrated that generation of ROS and inflammation have an interdependent relationship in cells exposed to nanoparticles, as a reflection of protective attempts by the cell to destroy nanoparticles.Citation13 Indeed, nanoparticle-mediated oxidative stress can contribute to activation of signaling pathways involved in proinflammatory cascades.Citation14 However, little is known about LDH-NP-induced toxicity or its molecular mechanism, and information on this would be useful for prediction of potential toxicity after prolonged exposure.
To understand the mechanism responsible for the toxicity of LDH-NPs, we evaluated the involvement of oxidative stress-responding and inflammation-regulating signal transduction pathways and of associated transcription factors. Mitogen-activated protein kinase (MAPK) cascades, such as extracellular signal-regulated kinase (ERK), c-Jun-N-terminal kinase (JNK), and p38, have been investigated as upstream signaling mechanisms responsible for regulating oxidative stress and cytokine release.Citation15,Citation16 The MAPK cascades are multifunctional signaling pathways that are initiated in response to diverse extracelluar stimuli, including growth factors, oxidants, DNA damage, cytokines, toxins, and physical stress, and regulate a variety of cellular responses, such as apoptosis, induction of phase II detoxifying enzymes, immune activation, and inflammation.Citation17,Citation18 On the other hand, Src family kinases (SFKs), which are non-receptor protein tyrosine kinases, are known to mediate activation of MAPK in response to extracellular stimuli (eg, ROS or ultraviolet radiation).Citation19,Citation20 Hence, we considered that activation of SFKs could play a role in nanoparticle-induced oxidative stress and inflammation.
Redox-sensitive transcription factors implicated in LDH-NP-induced toxicity were also investigated, with a focus on nuclear factor kappa B (NF-κB) and nuclear factor-erythroid 2-related factor-2 (Nrf-2) as downstream events in the MAPK pathway. The NF-κB complex controls DNA transcription and is activated by harmful cellular stimuli, including oxidative stress, and consequently induces expression of a variety of proteins required for immunological and cellular defense.Citation21,Citation22 Nrf-2 is a basic transcription factor and regulates cellular antioxidant and anti-inflammatory responses.Citation23 Nrf-2 dissociates from Kelch-like ECH-associated protein 1 in cytoplasm and then translocates to the nucleus, where it binds antioxidant response elements required for the transcriptional activation of genes, including cytoprotective and antioxidant genes.Citation24,Citation25 Many researchers have suggested that cytosolic NF-κB and Nrf-2 are phosphorylated and translocated into the nucleus in response to the MAPK pathway under conditions of oxidative stress.Citation26,Citation27
Accordingly, the aim of this study was to investigate the phenomenon of LDH-NP-induced toxicity and to determine the molecular mechanism involved by focusing on oxidative stress and inflammation, in order to better understand and predict the potential adverse effects of LDH-NPs on human health. In our previous study, because LDH-NP generated the most ROS and induced the proinflammatory mediator IL-8 in A549 human alveolar epithelial cancer cells, we used the same cell line for the present study.Citation11 Oxidative stress induced by LDH-NPs was evaluated by estimating intracellular ROS generation and antioxidant enzyme activity, including those of superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR), in A549 lung epithelial cells. Expression of heme oxygenase-1 (HO-1), an antioxidant enzyme induced in response to oxidative stress, was also investigated. In addition, we evaluated the induction of proinflammatory cytokines, such as IL-1, IL-6, IL-8, and tumor necrosis factor α (TNF-α), in LDH-NP-treated cells. Finally, signaling pathways and transcription factors activated by LDH-NP exposure were investigated, with a focus on the relationship between oxidative stress and induction of inflammation.
Materials and methods
Preparation of nanoparticles
LDH-NPs (Mg0.68Al0.32(OH)2(CO3)0.16·0.1H2O; particle size ~100 nm) were prepared by coprecipitation as described previously.Citation11 Briefly, an aqueous solution of 0.5 M NaOH/0.5 M NaHCO3 was added dropwise to a mixed Mg/Al nitrate solution at room temperature to pH ~9.5. The resulting white precipitate was kept at 100°C for 24 hours under hydrothermal conditions to obtain uniformly sized homogeneous nanoparticles. The nanoparticles were analyzed by powder X-ray diffraction (PW3710 diffractometer [Phillips, Eindhoven, the Netherlands] with Ni-filtered CuKα radiation [λ=1.5418 Å]), Fourier transform infrared spectroscopy (Spectrum One B, PerkinElmer, Wellesley, MA, USA), and scanning electron microscopy (S-4300, Hitachi Tokyo, Japan). The mean particle size of the LDH-NPs was determined by randomly selecting 200 particles in scanning electron microscopy images.
Cell culture
Human lung alveolar carcinoma epithelial (A549) cells were purchased from the Korean Cell Line Bank and cultured in Roswell Park Memorial Institute 1640 medium under a humidified atmosphere (5% CO2/95% air) at 37°C. The medium was supplemented with 10% heat inactivated fetal bovine serum (Welgene Biopharmaceuticals, Daegu, Korea), 100 units/mL penicillin, and 100 μg/mL streptomycin.
Cell proliferation
The effect of LDH-NPs on cell proliferation was measured using the WST-1 assay (Roche, Basel, Switzerland). Briefly, cells (5×103/100 μL) were exposed to 1–1,000 μg/mL LDH-NPs for 24 hours or 500 μg/mL LDH-NPs for times ranging from 1 hour to 24 hours. Next, 10 μL of WST-1 solution (Roche) was added to each well, and cells were incubated for a further 4 hours. Absorbance was then measured using a plate reader at 440 nm (Dynex Technologies, Chantilly, VA, USA). Cells incubated in the absence of LDH-NPs were used as the control.
Intracellular ROS generation
Intracellular ROS levels were monitored using a peroxide-sensitive fluorescent probe, carboxy-2′,7′-dichlorofluorescein diacetate (H2DCFDA, Molecular Probes, Eugene, OR, USA), according to the manufacturer’s guidelines. Briefly, cells (5×103) were incubated with the nanoparticles under standard conditions as described above, washed with phosphate-buffered saline, collected by centrifugation, and incubated with 40 μM carboxy-H2DCFDA for 60 minutes at 37°C. After washing with phosphate-buffered saline, dichlorofluorescein fluorescence was immediately measured using a fluorescence microplate reader (SpectraMax® M3, Molecular Devices, Silicon Valley, CA, USA), and excitation and emission wavelengths were 490 nm and 530 nm, respectively. Cells not treated with nanoparticles were used as the control.
Antioxidant enzyme activity
The activity of CAT, GR, and SOD was estimated using a Chemical SOD assay kit (Cayman Chemical Company, Ann Arbor, MI, USA), the CAT assay kit (Enzo Life Science, Farmingdale, NY, USA), and the Chemical GR assay kit (Cayman), respectively, according to the manufacturer’s protocols. Enzyme activity is presented as a percentage of the enzyme activity of controls.
Western blot analysis
Cells were exposed to nanoparticles (500 μg/mL) for designated times (1, 3, 6, 10, and 24 hours), washed twice in ice-cold phosphate-buffered saline, and harvested using a scraper. Cell pellets were then collected by centrifugation. After washing the pellets twice with phosphate-buffered saline, cell lysis RIPA buffer (Sigma-Aldrich, St Louis, MO, USA) containing protease inhibitor cocktails (Thermo Fisher Scientific Inc, Pittsburgh, PA, USA) was added, and the pellets were incubated for 20 minutes at 4°C and centrifuged at 14,000× g for 30 minutes at 4°C to obtain total cell lysates. Nuclear and cytosol fractions were obtained using a Nuclear/Cytosol fractionation kit (BioVision Inc, Milpitas, CA, USA) according to the manufacturer’s protocol. Protein concentrations in cell lysates were determined using the Bradford assay (Bio-Rad Hercules, CA, USA).
Aliquots of lysates containing 30 μg of proteins were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and after transfer to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), the blots were preblocked with 5% skim milk in Tris buffered saline-Tween 20 (TBS-T) solution (20 mM Tris-HCl, 150 mM NaCl, pH 7.4, and 0.05% Tween 20) for 4 hours, and incubated overnight with rabbit polyclonal antibodies against HO-1 (1:100, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), p38 (1:1,000, Enzo Life Sciences), p-p38 (1:1,000), ERK (1:1,000), p-ERK (1:200), JNK (1:1,000), p-JNK (1:800), c-Src (1:1,000) (all from Santa Cruz Biotechnology), p-Src (1:800), NF-κB (1:1,000), Nrf-2 (1:1,000), β-actin (1:1,000), or lamin B (1:1,000) (all from Enzo Life Sciences). The blots were washed three times and incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (1:5,000) for 2 hours (Bethyl Laboratories, Montgomery, TX, USA). Blotting analysis was performed using the luminal Western blotting detection kit (Santa Cruz Biotechnology Inc) and a luminescent image analyzer (LAS-4000, Fujifilm, Tokyo, Japan).
Enzyme-linked immunosorbent assay
A549 cells were exposed to nanoparticles for designated times, and the supernatants were then collected, centrifuged to remove any remaining nanoparticles, and stored at −80°C. Concentrations of the proinflammatory cytokines, IL-1, IL-6, IL-8, and TNF-α, were determined by enzyme-linked immunosorbent assay, according to the instructions supplied by the manufacturer (BD Bioscience, Franklin Lakes, NJ, USA). Cells incubated without nanoparticles were used as controls. Absorbance was measured at 450 nm and quantified using a microplate reader (Dynex Technologies).
Inhibitors or antioxidant treatment
Cells were pretreated with a JNK inhibitor (SP600125, 10 μM), a p38 inhibitor (SB203580, 5 μM), or an SFK inhibitor (PP2, 5 μM) for 1 hour, and then incubated with the nanoparticles. Expression levels of MAPK and SFK and induction of proinflammatory mediators were evaluated by Western blot analysis and enzyme-linked immunosorbent assay, respectively. Alternatively, A549 cells were preincubated with the antioxidant ascorbic acid (5 mM) for 2 hours, and then incubated with the nanoparticles. ROS generation, expression levels of MAPK or SFK, and induction of proinflammatory mediators were investigated as described above.
Statistical analysis
The statistical analysis was performed using the Student’s t-test for unpaired data, and one-way analysis of variance (Tukey’s test, version 11.0) was conducted using SAS software (SAS Institute, Cary, NC, USA) to determine the significance of differences between experimental groups. All results are presented as the mean ± standard deviation and P<0.05 were considered to be statistically significant.
Results
Characterization of LDH-NPs
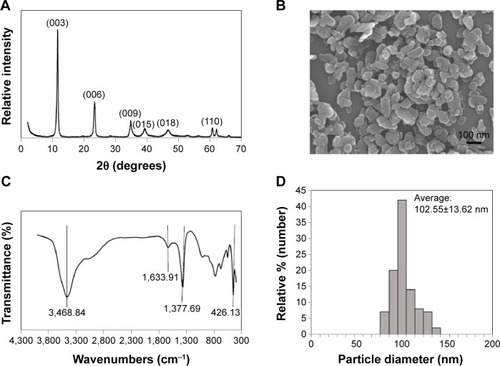
The X-ray diffraction pattern for the LDH-NPs revealed a typical MgAl layered structure, as indicated by (003), (006), (009), and (110) peaks (). Fourier transform infrared data confirmed that MgAl-CO3 LDH-NPs formed with peaks at 3,468.84, 1,633.91, 1,377.69, and 426.13 cm−1, which were attributed to OH− stretching, H2O binding, CO3−2 group, and O-M-O vibration, respectively (). SEM images of LDH-NP showed a typical hexagonal shape and an average particle size of 102.55±13.62 nm ().
Figure 1 Physicochemical characterization of LDH-NPs. X-ray diffraction pattern (A), scanning electron micrographs (B), Fourier transform infrared data (C), and size distribution of LDH-NPs determined by randomly selecting 200 particles in scanning electron micrographs (D).
Abbreviation: LDH-NPs, layered double hydroxide nanoparticles.
Cell proliferation and ROS generation
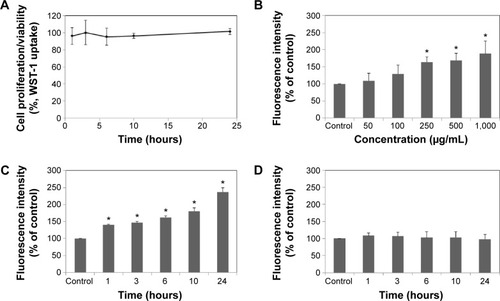
The cytotoxicity of LDH-NPs was evaluated by measuring cell proliferation/viability using the WST-1 assay. A549 cells were used because oxidative stress and IL-8 were highly induced in this cell line by LDH-NPs. Nanoparticles present at concentrations of up to 1,000 μg/mL did not affect the proliferation or viability of cells after 24 hours of incubation (data not shown), and at a concentration of 500 μg/mL, LDH-NPs did not affect proliferation or viability when incubated for 1–24 hours (). However, LDH-NP generated ROS in cells treated at 250–1,000 μg/mL for 24 hours (), and ROS levels were found to be elevated in cells exposed to 500 μg/mL LDH-NPs for 1–24 hours (). Interestingly, no significant increase in ROS levels was detected in LDH-NP-incubated cells in the presence of an antioxidant, ascorbic acid (). These results indicate that LDH-NPs generate ROS at high concentrations.
Figure 2 Cell proliferation of A549 cells treated with 500 μg/mL of LDH-NPs (A). Generation of reactive oxygen species by cells treated with different concentrations of LDH-NPs for 24 hours (B), 500 μg/mL LDH-NPs for different periods of time (C), and under the same conditions as those mentioned in (C) in the presence of an antioxidant, ascorbic acid (D).
Notes: The results are expressed as the mean ± standard deviation of three independent experiments. *Denotes a significant difference from the non-treated control (P<0.05).
Abbreviation: LDH-NPs, layered double hydroxide nanoparticles.
Antioxidant enzyme activity
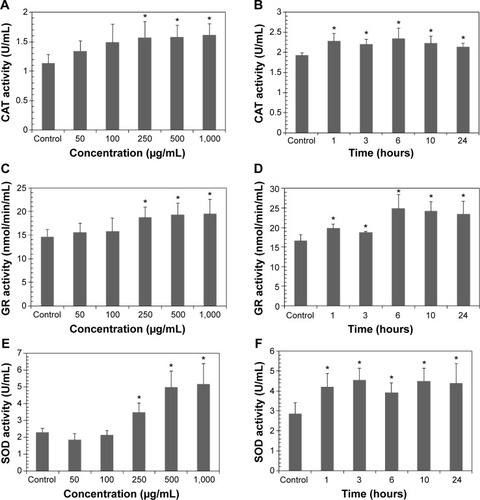
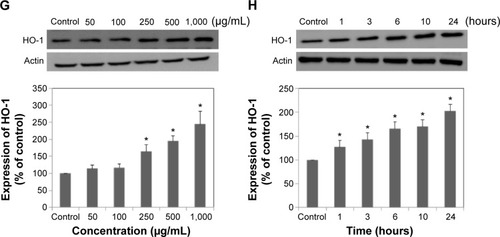
The activity of several antioxidant enzymes was evaluated to examine changes in the levels of protective enzymes in A549 cells exposed to LDH-NPs. The activity of all the antioxidant enzymes examined, ie, that of CAT, GR, and SOD, were elevated by LDH-NPs at 250–1,000 μg/mL (). Furthermore, enzyme activity was significantly increased by 500 μg/mL LDH-NPs after incubation for 1–24 hours (), which agreed well with the observed generation of ROS by LDH-NPs (). In addition, HO-1 (an antioxidant enzyme induced in response to oxidative stress) activity was also significantly increased by LDH-NPs in a concentration-dependent and time-dependent manner (). These results demonstrate that antioxidant enzyme activity, which plays a role in the scavenging of ROS, was increased by LDH-NPs, thus confirming induction of oxidative stress by LDH-NPs.
Figure 3 Activity of antioxidant enzymes in A549 cells exposed to LDH-NPs. For the concentration-dependent experiment, cells were incubated with LDH-NPs for 24 hours (A, C, E, G), and for the time-dependent experiment, cells were exposed to 500 μg/mL LDH-NPs (B, D, F, H).
Notes: The results are presented as the mean standard deviation of three independent experiments. *Denotes a significant difference from the non-treated control (P<0.05).
Abbreviations: LDH-NPs, layered double hydroxide nanoparticles; CAT, catalase; GR, glucose reductase; SOD, superoxide dismutase; HO-1, heme oxygenase-1.
Cytokine induction
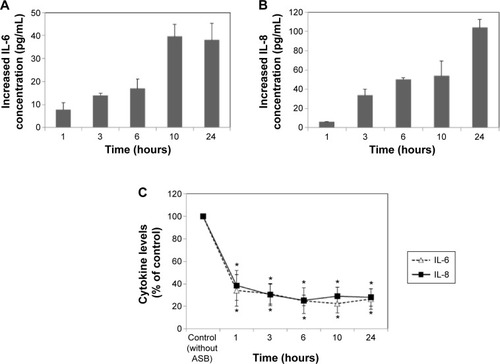
We also examined the effects of LDH-NPs on proinflammatory cytokines, ie, IL-1, IL-6, IL-8, and TNF-α. Levels of IL-1 and TNF-α were not significantly increased by LDH-NPs (data not shown), whereas IL-6 and IL-8 levels were considerably increased in a time-dependent manner (). On the other hand, elevation of IL-6 and IL-8 levels was significantly reduced in the presence of ascorbic acid, indicating the role of oxidative stress in cytokine induction.
Figure 4 Induction of the proinflammatory cytokines IL-6 and IL-8 in A549 cells exposed to 500 μg/mL LDH-NPs (A, B) and under the same conditions in the presence of ASB (C). Cytokine concentrations are presented as increases in IL-6 or IL-8 levels after subtracting basal IL-6 or IL-8 levels detected in untreated controls.
Notes: The results are presented as the mean ± standard deviation of three independent experiments. *Denotes a significant difference from the non-treated control (P<0.05).
Abbreviations: ASB, ascorbic acid; IL, interleukin; LDH-NPs, layered double hydroxide nanoparticles.
Signal transduction and transcription factors
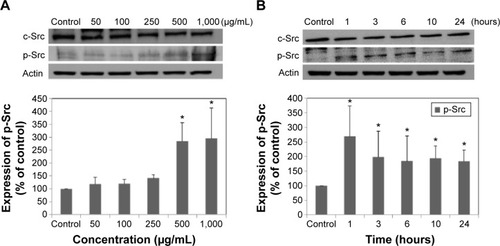
Molecular events were evaluated in A549 cells exposed to LDH-NPs in order to elucidate the mechanism responsible for their effects on oxidative stress and cytokine release. Expression of the phosphorylated forms of p38 and JNK was significantly elevated in cells exposed to LDH-NPs at 500–1,000 μg/mL (). In particular, JNK phosphorylation was remarkably increased after incubation with 500 μg/mL LDH-NP for only 1 hour (), whereas expression of ROS p-ERK did not change significantly (). On the other hand, when the expression level of Src, which lies upstream of the MAPK pathway, was examined, its phosphorylation was found to be significantly increased after treatment with 500 μg/mL LDH-NPs for 1–24 hours ().
Figure 5 Expression of MAPK in A549 cells exposed to different concentrations of LDH-NPs for 24 hours (A) or to 500 μg/mL LDH-NPs for different time periods (B). Relative density of expression of ERK, JNK, and p-38 were normalized versus β-actin and are presented relative to the non-treated control.
Notes: The results are presented as the mean ± standard deviation of three independent experiments. *Denotes a significant difference from the non-treated control (P<0.05). **Denotes a significant difference from the non-treated control (P<0.01).
Abbreviations: LDH-NPs, layered double hydroxide nanoparticles; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun-N-terminal kinase.
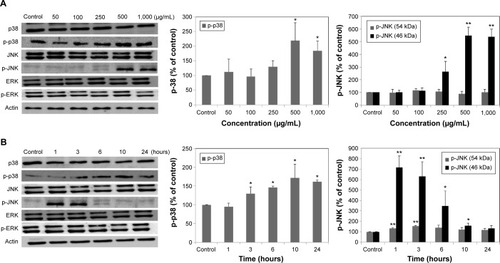
Figure 6 Expression of Src family kinases in A549 cells exposed to different concentrations of LDH-NPs for 24 hours (A) or to 500 μg/mL LDH-NPs for different time periods (B). The relative density of expression of c-Src (intact) and p-Src (phosphorylated) was normalized versus β-actin and is presented as relative to the non-treated control.
Notes: The results are presented as the mean ± standard deviation of three independent experiments. *Denotes a significant difference from the non-treated control (P<0.05).
Abbreviation: LDH-NPs, layered double hydroxide nanoparticles.
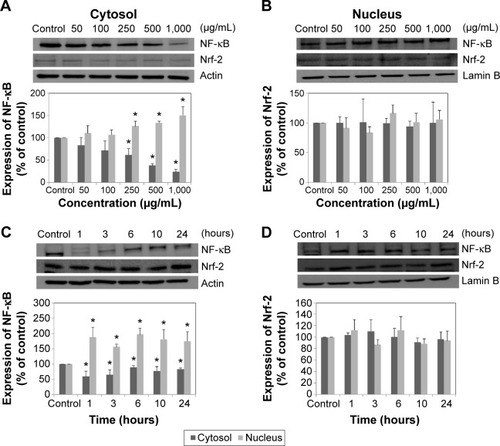
The molecular mechanism responsible for the toxicity of LDH-NPs was investigated further by examining the transcription factors, NF-κB and Nrf-2, which lie downstream of the MAPK signaling pathway (). Nuclear translocation of NF-κB was found to be promoted in a concentration-dependent manner (), whereas nuclear translocalization of Nrf-2 was not ().
Figure 7 Expressions of NF-κB and Nrf-2 in cytosolic and nuclear fractions of A549 cells exposed to different concentrations of LDH-NPs for 24 hours (A, B) or to 500 μg/mL LDH-NPs for different time periods (C, D). Relative density of expression of NF-κB and Nrf-2 was normalized versus β-actin in cytosolic fractions or versus lamin B in nuclear fractions, respectively, and are presented relative to the non-treated control.
Notes: The results are presented as the mean ± standard deviation of three independent experiments. *Denotes a significant difference from the non-treated control (P<0.05).
Abbreviations: LDH-NPs, layered double hydroxide nanoparticles; NF-κB, nuclear factor kappa B; Nrf-2, nuclear factor-erythroid 2-related factor-2.
Relationship between SFK-MAPK signaling pathway and cytokine induction
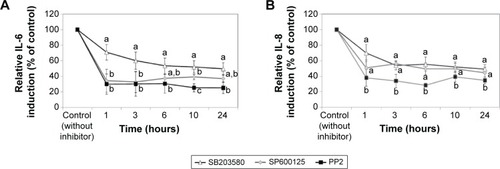
Induction of IL-6 and IL-8 in cells treated with LDH-NPs was evaluated in the presence of an SFK inhibitor (PP2), a p38 inhibitor (SB203580), and a JNK inhibitor (SP600125). As shown in , induction of IL-6 and IL-8 by LDH-NPs was considerably reduced by all three inhibitors. In particular, both cytokine inductions were markedly diminished by PP2.
Figure 8 Involvement of SFKs, JNK, and p38 MAPK in LDH-NP-induced IL-6 (A) and IL-8 (B) release from A549 cells. Cells were exposed to 500 μg/mL LDH-NPs only (controls) or pretreated with the p38 inhibitor SB203580, the JNK inhibitor SP600125, or the SFK inhibitor PP2, and then treated with 500 μg/mL LDH-NPs for different time periods. Cytokine release was measured by enzyme-linked immunosorbent assay, according to the instructions supplied by the manufacturer (BD Bioscience).
Notes: The results represent the mean ± standard deviation of three independent experiments and are presented as relative cytokine induction versus LDH-NP-treated controls (100% induction). Different letters in the figure indicate a statistically significant difference (P<0.05).
Abbreviations: IL, interleukin; JNK, c-Jun-N-terminal kinase; SFKs, Src family kinases; LDH-NPs, layered double hydroxide nanoparticles; MAPK, mitogen-activated protein kinase.
Discussion
This study was undertaken to evaluate the toxic effects of LDH-NP and to determine the molecular mechanism involved in relation to oxidative stress and inflammatory cytokine induction. Diverse types of nanoparticles have been reported to induce oxidative stress or inflammatory responses.Citation28–Citation30 However, few studies have addressed the mechanism responsible for nanoparticle-induced toxicity. In a previous study, we found that LDH-NPs generated ROS in human alveolar epithelial cancer A549 cells, human osteosarcoma (HOS) cells, and human cervical adenocarcinoma HeLa cells, but not in normal human lung epithelial L-132 cells, and because LDH-NP generated the most ROS and induced the proinflammatory mediator IL-8 in A549 cells, we used this cell line for the present study.Citation11 In terms of the effect of particle size on toxicity, LDH-NPs 50 nm in size were determined to be more toxic than 100–350 nm particles.Citation31 However, small-sized particles less than 100 nm tend to form aggregates in cell culture medium, so 100 nm particles were selected for the mechanistic study.
The LDH-NPs used in the present study had a homogeneous distribution and a particle size of ~100 nm. A549 cells were exposed to high concentrations of LDH-NPs in the mechanistic study because ROS was found to be generated when cells were treated with LDH-NPs at 250–1,000 μg/mL for 1–24 hours () and because cytokine release was marked at more than 500 μg/mL (data not shown). It is worthy of note that the actual concentration of LDH-NP as a delivery carrier is less than 100 μg/mL. Further, at 500 μg/mL, LDH-NPs produced ROS within only 1 hour (), but notably, cell proliferation was unaffected by treatment with LDH-NPs at this concentration for 24 hours (). On the other hand, no significant increase in ROS levels was detected in cells exposed to LDH-NPs in the presence of ascorbic acid (), suggesting that ROS generation by LDH-NPs is associated with oxidative stress.
Oxidative stress induction by LDH-NPs was confirmed by analyzing the activity and induction of anti-oxidant enzymes. As shown in , the activity of CAT (decomposes hydrogen peroxide to water and oxygen), GR (reduces glutathione disulfide to glutathione), and SOD (catalyzes the dismutation of superoxide radicals to oxygen and hydrogen peroxide) significantly increased in cells exposed to ≥250 μg/mL LDH-NPs, and their enzyme activity increased after exposure for only 1 hour when A549 cells were treated with 500 μg/mL LDH-NPs. On the other hand, HO-1 protein levels also increased in response to LDH-NP treatment (). Further, a strong correlation was observed between ROS generation () and increased activity of the antioxidant enzymes (). These results show that antioxidant activity increased in cells exposed to LDH-NP, probably to control and neutralize the ROS generated. These results may also explain why LDH-NPs did not affect cell proliferation ().
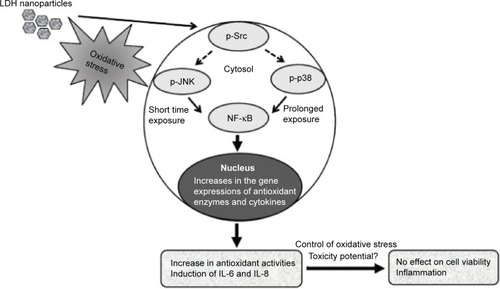
Western blot analysis revealed that phosphorylation of p38 was significantly induced in A549 cells exposed to LDH-NPs at concentrations ≥500 μg/mL for 3–24 hours (), whereas induction of phosphorylated JNK was observed after 1–10 hours at LDH-NP concentrations ≥250 μg/mL, that is, JNK was more strongly and rapidly activated than p38. This suggests that JNK was activated immediately after exposure to LDH-NPs, and that activation of p38 was associated with regulation of LDH-NP-induced oxidative stress at a later stage. ERK expression remained constant after incubation with LDH-NPs. On the other hand, Src, which lies upstream of MAPK, was also activated in cells treated with LDH-NPs at high concentrations (≥500 μg/mL) at 1–24 hours (), in a manner similar to that observed for JNK (). Further, nuclear translocation of NF-κB, which occurs downstream of MAPK, was increased at LDH-NP concentrations ≥250 μg/mL after 1–24 hours of exposure, whereas cytosolic and nuclear levels of Nrf-2 remained constant. These results suggest that LDH-NPs provoke oxidative stress, and that this subsequently induces activation of SFK, followed by activation of JNK and p38 MAPK, finally leading to activation of NF-κB (). Nuclear translocation of NF-κB is likely to be primarily controlled by JNK after 1–6 hours, and p38 mediates NF-κB translocation after prolonged exposure. Activation of NF-κB signaling by LDH-NP has been demonstrated by Li et al but seems to be involved in activation of dendritic cells.Citation32
Figure 9 Schematic illustration of the proposed mechanism of LDH-NP-induced toxicity in A549 cells. Oxidative stress caused by LDH-NPs activates SFK-JNK and p38-NF-κB signaling pathway, which increases expression of protective antioxidant enzymes, such as CAT, GR, SOD, and HO-1. However, this signaling activation concomitantly leads to induction of the proinflammatory mediators, IL-6 and IL-8.
Abbreviations: IL, interleukin; JNK, c-Jun-N-terminal kinase; SFK, Src family kinase; LDH-NPs, layered double hydroxide nanoparticles; NF-κB, nuclear factor kappa B; CAT, catalase; GR, glucose reductase; SOD, superoxide dismutase; HO-1, heme oxygenase-1.
CeO2 nanoparticles have been reported to cause oxidative stress and subsequent induction of HO-1 via a p38-Nrf-2 signaling pathway.Citation26 However, oxidative stress and expression of molecular entities associated with oxidative stress-related events were induced after 24 hours of exposure to a low concentration (1 μg/mL) of CeO2 nanoparticles.Citation26 This contrasts with what was observed for LDH-NPs because they generated ROS and activated signaling pathways at concentrations exceeding 250 μg/mL after incubation for 1–24 hours. Further, it has been reported that oxidative stress caused by both fumed and porous silica nanoparticles (1 μg/mL) activated Nrf-2 via the ERK MAPK signaling pathway after incubation for 24 hours.Citation33 In another study, single-walled carbon nanotubes (10 μg/mL) were found to induce oxidative stress and augment activation of NF-κB in human keratinocytes, but unfortunately the upstream signaling mechanism involved was not investigated.Citation34
The toxic effects of LDH-NPs have also been reported to be due to induction of the proinflammatory mediator, IL-8.Citation11,Citation31 In the present study, LDH-NPs were found to induce IL-6 and IL-8 (), but not IL-1 or TNF-α in A549 cells (data not shown). Interestingly, induction of IL-6 and IL-8 by LDH-NPs was highly suppressed in the presence of ascorbic acid (), suggesting that oxidative stress caused by LDH-NPs plays an important role in induction of cytokines. It is worth noting that induction of IL-6 and IL-8 by LDH-NPs was highly suppressed by pretreating cells with SFK, JNK, or p38 inhibitors (). Further, inhibition of SFK, which acts upstream of the JNK and p38 signaling pathways, most effectively suppressed cytokine induction, indicating that cytokine release is governed by the SFK-JNK and SFK-p38 MAPK pathways. Based on the observation that IL-6 release was considerably reduced by pretreating cells with the JNK inhibitor SP600125, JNK activation appears to be closely related to IL-6 induction. A small number of studies have shown that nanoparticle-induced oxidative stress contributes to the inflammatory response. For example, it has been reported that single-wall carbon nanotubes induce ROS and activate the MAPK and NF-κB signaling pathways, which are associated with the inflammatory response,Citation14 and that multiwalled carbon nanotubes induce a fibrogenic response via ROS-mediated activation of NF-κB signaling.Citation35 Titanium dioxide nanoparticles have been reported to induce oxidative stress and IL-8 release through the p38 and/or ERK MAPK pathways in BEAS-2B cells.Citation36 p38 and SFK-ERK1/2 pathways were found to regulate crystalline silica-induced IL-8 release in A549 cells, although ROS generation was not examined.Citation28 It was interesting that cell proliferation was unaffected by LDH-NPs in the present study, and that LDH-NPs, unlike other nanoparticles, induced oxidative stress at high concentrations. We believe that this is probably associated with the increased activity of antioxidant enzymes via the SFK-JNK and p38-NF-κB signaling pathway. Coincidentally, activation of the SFK-JNK and p38-NF-κB pathway via the oxidative stress induced by LDH-NPs led to induction of IL-6 and IL-8, suggesting that LDH-NPs have inflammatory potential ().
Conclusion
Our results suggest that the toxic effects of LDH-NPs are due to oxidative stress, which can also provoke induction of proinflammatory cytokines. The study shows that exposure to LDH-NPs induces ROS and expression of antioxidant enzymes, such as CAT, GR, SOD, and HO-1, via a SFK-JNK and p38-NF-κB signaling pathway, and that these protect against LDH-NP-induced oxidative stress. Further, activation of this signaling pathway concomitantly induces expression of proinflammatory cytokines, including IL-6 and IL-8. Therefore, it is likely that oxidative stress caused by LDH-NP mediates the inflammatory response in human lung epithelial cells.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (2013R1A1A3009283), and by Nano Material Technology Development Program (2014M3A7B6020163) of MSIP/NRF.
Disclosure
The authors report no conflicts of interest in this work.
References
- BarryRCLinYWangJLiuGTimchalkCANanotechnology-based electrochemical sensors for biomonitoring chemical exposuresJ Expo Sci Environ Epidemiol20091911819018275
- CormodeDPSkajaaTFayadZAMulderWJNanotechnology in medical imaging: probe design and applicationsArterioscler Thromb Vasc Biol200929992100019057023
- ChoiSJLeeJKJeongJToxicity evaluation of inorganic nanoparticles: considerations and challengesMol Cell Toxicol20139205210
- KrpeticZAnguissolaSGarryDKellyPMDawsonKANanomaterials: impact on cells and cell organellesAdv Exp Med Biol201481113515624683031
- ShangLNienhausKNienhausGUEngineered nanoparticles interacting with cells: size mattersJ Nanobiotechnology201412524491160
- ChoiSJChoyJHEffect of physico-chemical parameters on the toxicity of inorganic nanoparticlesJ Mater Chem20112155475554
- ChoiSJChoyJHLayered double hydroxide nanoparticles as target-specific delivery carriers: uptake mechanism and toxicityNanomedicine (Lond)2011680381421793673
- EvansDGDuanXPreparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicineChem Commun (Camb)2006548549616432560
- ChoyJHChoiSJOhJMClay minerals and layered double hydroxides for novel biological applicationsAppl Clay Sci200736122132
- KuraAUHusseinMZFakuraziSArulselvanPLayered double hydroxide nanocomposite for drug delivery systems; bio-distribution, toxicity, and drug activity enhancementChem Cent J201484725177361
- ChoiSJOhJMChoyJHToxicological effects of inorganic nanoparticles on human lung cancer A549 cellsJ Inorg Biochem200910346347119181388
- MankeAWangLRojanasakulYMechanism of nanoparticle-induced oxidative stress and toxicityBiomed Res Int2013201392916
- DonaldsonKPolandCAInhaled nanoparticles and lung cancer – what we can learn from conventional particle toxicologySwiss Med Wkly2012142w1354722714122
- PacurariMYinXJZhaoJRaw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cellsEnviron Health Perspect20081161211121718795165
- TakedaKMatsuzawaANishitohHRoles of MAPKKK ASK1 in stress-induced cell deathCell Struct Funct200328232912655147
- JayakumarARPanickarKSMurthyChRNorenbergMDOxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytesJ Neurosci2006264774448416672650
- PuddicombeSMDaviesDEThe role of MAP kinases in intracellular signal transduction in bronchial epitheliumClin Exp Allergy20003071110606925
- LiDQLuoLChenZJNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cellsExp Eye Res20068258859616202406
- HsuHYChiuSLWenMHChenKYHuaKFLigands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathwaysJ Biol Chem2001276287192873011390374
- KitagawaDTanemuraSOhataSActivation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic functionJ Biol Chem200227736637111694531
- PinkusRWeinerLMDanielVRole of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expressionJ Biol Chem199627113422134298662787
- JinWWangHYanWDisruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injuryMediat Inflamm20082008725174
- OwuorEDKongANAntioxidants and oxidants regulated signal transduction pathwaysBiochem Pharm20026476577012213568
- ItohKChibaTTakahashiSAn Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elementsBiochem Biophy Res Commun1997236313322
- ZhangXChenXSongHChenHZRovinBHActivation of the Nrf2/antioxidant response pathway increases IL-8 expressionEur J Immunol2005353258326716220540
- EomHJChoiJOxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2BToxicol Lett2009187778319429248
- WangJLiNZhengLP38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2Biol Trace Elem Res201114018619720422311
- OvrevikJLagMSchwarzePRefsnesMp38 and Src-ERK1/2 pathways regulate crystalline silica-induced chemokine release in pulmonary epithelial cellsToxicol Sci20048148049015240896
- ParkEJParkKOxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitroToxicol Lett2009184182519022359
- FoucaudLGoulaouicSBennasrouneAOxidative stress induction by nanoparticles in THP-1 cells with 4-HNE production: stress biomarker or oxidative stress signalling molecule?Toxicol In Vitro2010241512152020638469
- ChoiSJOhJMChoyJHSafety aspect of inorganic layered nanoparticles: size-dependency in vitro and in vivoJ Nanosci Nanotechnol200885297530119198442
- LiAQinLZhuDZhuRSunJWangSSignalling pathways involved in the activation of dendritic cells by layered double hydroxide nanoparticlesBiomaterials20103174875619853910
- EomHJChoiJOxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2BToxicol In Vitro2009231326133219602432
- MannaSKSarkarSBarrJSingle-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytesNano Lett200551676168416159204
- HeXYoungSHSchwegler-BerryDChisholmWPFernbackJEMaQMultiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-kappaB signaling, and promoting fibroblast-to-myofibroblast transformationChem Res Toxicol2011242237224822081859
- ParkEJYiJChungKHRyuDYChoiJParkKOxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cellsToxicol Lett200818022222918662754
