 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The cross-administration of nanocarriers modified by poly(ethylene glycol) (PEG), named PEGylated nanocarriers, a type of combination therapy, is becoming an increasingly important method of long-term drug delivery, to decrease side effects, avoid multidrug resistance, and increase therapeutic efficacy. However, repeated injections of PEGylated nanocarriers induces the accelerated blood clearance (ABC) phenomenon, prevents long circulation, and can cause adverse effects owing to alterations in the biodistribution of the drug. Although the nature of the ABC phenomenon that is induced by repeated injections of PEGylated nanocarriers has already been studied in detail, there are few reports on the immune response elicited by the cross-administration of PEGylated nanocarriers. In this study, we investigated the ABC phenomenon induced by the intravenous cross-administration of various PEGylated nanocarriers, including PEGylated liposomes (PL), PEG micelles (PM), PEGylated solid lipid nanoparticles (PSLN), and PEGylated emulsions (PE), in beagle dogs. The results indicated that the magnitude of the immune response elicited by the cross-administration was in the following order (from the strongest to the weakest): PL, PE, PSLN, PM. It is specifically PEG in the brush structure that elicits a significant immune response, in both the induction phase and the effectuation phase. Furthermore, the present study suggests that there is a considerable difference between the effect of repeated injections and cross-administration, depending on the colloidal structure. This work is a preliminary investigation into the cross-administration of PEGylated nanocarriers, and our observations can have serious implications for the design of combination therapies that use PEGylated vectors.
Introduction
The term combination therapy refers to the simultaneous or successive use of either multiple pharmacologically active agents or different types of therapies such as chemotherapy and radiotherapy.Citation1,Citation2 Owing to the complex pathology of many diseases, combination therapy is increasingly used as a long-term therapy to reduce side effects and avoid multidrug resistance,Citation1 and it is expected to become even more widespread in the future as an understanding of its underlying safety develops.Citation3 Recent years have seen the development of therapies involving the cross-administration of two or more therapeutic agents using different nanocarriers.Citation4,Citation5 One such regimen developed to decrease the risk of complications and achieve maximum therapeutic efficacy is currently undergoing clinical trials.Citation4 Although the use of multiple drugs can lead to new therapeutic possibilities, it can also make carrier design more complex and affect the safety of the carriers used.
Nanoscale drug delivery systems, or nanocarriers, are widely used in both the clinic and the laboratory to enable passive drug targeting and decrease the harmful side effects of drugs.Citation6–Citation8 The surface of nanocarriers is frequently modified by attaching the polymer poly(ethylene glycol) (PEG) in a process called PEGylation. Such modified nanocarriers are able to avoid uptake by the mononuclear phagocyte system and have a correspondingly higher biological half-life; therefore, they are more likely to spontaneously accumulate in solid tumors via the enhanced permeability and retention effect.Citation9,Citation10 PEGylation was regarded as a major breakthrough in the development of nanocarriers,Citation11 and offers new opportunities for cancer imaging, molecular diagnosis, and targeted therapy – especially combination therapy.Citation12
However, Moghimi and GrayCitation13 reported that particles that were coated with the amphiphilic polymer poloxamine lost their long-circulating characteristics after repeated injections at certain intervals. This phenomenon was named accelerated blood clearance (ABC) by Dams et al.Citation14 This abnormal immune response has serious implications for the use of repeated injections of PEGylated nanocarriers and, as a result, has attracted a great deal of attention in the context of PEGylated liposomes (PL),Citation14 PEGylated emulsions (PE),Citation15 polymeric micelles,Citation16 and PEGylated solid lipid nanoparticles (PSLN).Citation17 Furthermore, it is worth noting that the intravenous administration of PEG micelles (PM) accelerated the plasma clearance of a subsequent dose of PEG liposomes, but did not influence the pharmacokinetics of a subsequent PM dose, suggesting that the ABC phenomenon may depend on the exact combination of nanocarriers used.Citation18
Although the induction of the ABC phenomenon by repeated injections of PEGylated nanocarriers has already been studied in detail,Citation19 the immune response induced by the cross-administration of different PEGylated nanocarriers is not fully understood and a relevant systematic study is yet to be carried out. In this work, we investigated the effect of cross-administration on the ABC phenomenon by intravenously co-administering various PEGylated nanocarriers, including PL, PM, PSLN, and PE, in beagle dogs, and by comparing the magnitude of the ABC effect that was elicited by cross-administration and repeated injection. It is well known that the occurrence and the magnitude of the ABC phenomenon are influenced by physicochemical properties of PEGylated nanocarriers.Citation19 To investigate the effect of cross-administration on the ABC phenomenon, the influencing factors should be fixed, for example, the PEG length and the encapsulated drug. Based on nanoparticles modified by 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (mPEG2000-DSPE), which exhibited prolonged blood circulation time and were widely applied in the clinical setup,Citation20 mPEG2000-DSPE was used to modify nanocarriers. Tocopheryl nicotinate (TN) was employed as an indicator by virtue of its lack of effect on the immune organs. Furthermore, the mechanism of the immune response induced by the cross-administration was further investigated, based on the anti-PEG IgM levels and the ability of anti-PEG IgM to bind to the PEGylated nanocarriers. Our work is an important preliminary investigation into the cross-administration of PEGylated nanocarriers, and our observations can have serious implications for the design of combination therapies that use PEGylated vectors.
Materials and methods
Materials
TN was a gift from the Northeast Pharmaceutical Group Co., Ltd. (Shenyang, People’s Republic of China). Injectable soybean lecithin (S75) was purchased from Lipoid GmbH (Ludwigshafen, Germany). Glycerin monostearate (GMS) and glycerol distearate (GDS) were supplied by Sigma-Aldrich (St Louis, MO, USA). Medium-chain triglycerides (MCT) were obtained from the Beiya Medicated Oil Co., Ltd. (Tieling, People’s Republic of China). Cholesterol was provided by Nanjing Xinbai Pharmaceutical Co., Ltd. (Nanjing, People’s Republic of China). mPEG2000-DSPE was supplied by Genzyme Corporation (Cambridge, MA, USA). All other reagents were of chromatographic grade.
Animals
Male beagle dogs weighing 10–12 kg were purchased from the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, People’s Republic of China). Animal care and experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.Citation21
Preparation of various PEGylated nanocarriers
Preparation of PEGylated emulsions
PE consisted of TN, MCT, and S75 (1:5:1.165, weight ratio). A 9:1 molar ratio of S75 to mPEG2000-DSPE was used.
Emulsions were prepared as previously described.Citation15 Briefly, the oil phase (TN, MCT, S75, and mPEG2000-DSPE) was constantly stirred in a water bath at 55°C. The aqueous phase (sterile water) was heated to 55°C and added to the oil phase with rapid stirring. The emulsions were formed through agitation and incubation at 55°C for 10 min. The mixture was sonicated in an ice bath using an ultrasonic cell pulverizer (JY92-II, Ningbo Scientz Biotechnology Co., Ltd., Zhejiang, People’s Republic of China) at 200 W for 2 min and at 400 W for 6 min. The resultant emulsions were sized by extruding through polycarbonate membrane filters with pore sizes of 0.22 μm and were adjusted to an isotonic level by injecting 50% glucose.
Preparation of PEGylated liposomes
PL (TN:S75:cholesterol:mPEG2000-DSPE in a 3.9:5.4:3.6:1 molar ratio) was prepared using the thin film hydration method. Briefly, the lipid mixture was dissolved in ethanol, and the solvent was subsequently removed at 50°C. The resultant dry lipid film was hydrated at 50°C in sterile water under mechanical agitation to obtain an injectable solution with a final lipid concentration of 41.6 mg/mL. The resultant multilamellar vesicles were sonicated using a Vibra-cell probe sonicator (JY92-2D, Ningbo Xinzhi Biological Technology Co. Ltd., Ningbo, People’s Republic of China) equipped with a tapered tip for at least 2 min at 200 W, followed by sonication for 6 min at 400 W. In order to remove large particles, the suspensions were passed through polycarbonate membrane filters with pore sizes of 0.8, 0.45, and 0.22 μm after sonication, respectively.
Preparation of PEGylated solid lipid nanoparticles
Two solid lipid nanoparticle (SLN) preparations were used: PSLN-GMS and PSLN-GDS. They contained TN, GMS or GDS, S75, and mPEG2000-DSPE in a molar ratio of 7.5:56:9:1.
PSLN was prepared using the melt emulsification process, as previously described.Citation22 Briefly, TN, GMS (or GDS), S75, and mPEG2000-DSPE (7.5:56:9:1, molar ratio) were mixed and dispersed in absolute ethanol at 65°C, and then the solvent was removed. Sterile water was heated to 65°C and added to the mixture under mechanical agitation to give injectable solutions. Subsequently, the resultant mixture was treated using an ultrasonic cell pulverizer (JY92-II, Ningbo Scientz Biotechnology Co., Ltd.), for at least 2 min at 200 W, followed by 3 min at 400 W. Following sonication, the suspensions were sized through polycarbonate membranes with a pore size of 0.22 μm. The preparation was cooled to room temperature after the extrusion.
Preparation of PEG micelles
The TN-loaded mPEG2000-DSPE micelles were prepared using a self-assembly method.Citation23 TN and mPEG2000-DSPE (3:4, molar ratio) were dispersed in 0.5 mL of absolute ethanol, and sterile water was added to the homogeneous phase to achieve a clear, injectable micelle solution of volume 8 mL. The final solution was filtered through a polycarbonate membrane with a pore size of 0.22 μm.
A submicron particle sizer (Nicomp 380™, Particle Sizing Systems, Inc., Santa Barbara, CA, USA) was used to determine the mean diameters and zeta potentials of the emulsions, liposomes, SLNs, and micelles. Data are presented in .
Table 1 Characterization data of nanocarriers
Determination of fixed aqueous layer thickness
The fixed aqueous layer thicknesses (FALTs) of PE, PL, PSLN, and PM were determined using a common method based on the Gouy-Chapman theory.Citation24–Citation27 Zeta potentials were used to calculate the FALT using the following equation:
Pharmacokinetics of various PEGylated nanocarriers
Male beagle dogs were randomly divided into 11 groups (n=3). The injection protocols for the different PEGylated nanocarriers are presented in . The beagle dogs were treated with intravenous injections of various PEGylated nanocarriers at doses of 2.5 μmol phospholipid/kg. The interval between the two injections was 7 days. At 1 min, 3 min, 5 min, 10 min, 15 min, 0.5 h, 1 h, 2 h, 4 h, 6 h, 10 h, and 24 h after the second injection, blood samples (2 mL) were taken and centrifuged at 4,000 rpm for 10 min to isolate the plasma. Plasma samples were stored at −20°C until used.
Table 2 Injection protocols used to administer nanocarriers to normal beagle dogs
Measurement of TN concentration in plasma
High-performance liquid chromatography (HPLC) was applied to determine the concentration of TN in the plasma by using a previously reported method.Citation15 The HPLC system consisted of a P230 pump, a UV230 UV/Vis detector (Da Lian Elite Analytical Instruments Co., Ltd., Liaoning, People’s Republic of China), and a Hypersil BDS C18 column (5 μm, 200×4.6 mm). Chromatographic conditions were as follows: mobile phase (methanol:isopropanol =80:20, v/v), flow rate (1 mL/min), column temperature (30°C), UV detector wavelength (264 nm). Before analysis, methanol (100 μL), the internal standard (100 μg/mL tocopheryl acetate; 100 μL), and n-hexane (600 μL) were added to the plasma samples (100 μL), and the mixture was vortexed for 5 min. The supernatant (500 μL) was obtained by centrifuging the mixture at 10,000 rpm for 10 min and dried using a CentriVap centrifugal vacuum concentrator (Labconco Corporation, Kansas City, MO, USA). Mobile phase (methanol:isopropanol =80:20, v/v, 100 μL) was used to dissolve the dried mixture. Then, the mixture was vortexed for 1 min and centrifuged at 10,000 rpm for 10 min. The supernatant (20 μL) was collected and used for the HPLC analysis.
Detection of anti-PEG IgM antibodies in serum
Anti-PEG IgM levels in serum were determined by the enzyme-linked immunosorbent assay (ELISA) method.Citation28 A solution of mPEG2000-DSPE in absolute ethanol (10 nmol; 50 μL) was added to a 96-well plate (Corning Incorporated, New York, NY, USA). The plate was then air dried and blocked for 1 h with 50 mM Tris-buffered saline containing 0.14 mM NaCl and 1% bovine serum albumin (BSA; Biosharp, Seoul, South Korea). The plate was washed three times with washing solution (pH 8.0 Tris-buffered saline with 0.05% Tween® 20 [Sigma-Aldrich]). Diluted serum samples (1:1,000; 100 μL) were added to the 96-well plate, which was incubated for 1 h and washed five times as described earlier. Goat anti-dog IgM was purchased from Immunology Consultants Laboratory, Inc (Portland, OR, USA), and conjugated to horseradish peroxidase (HRP) (obtained from Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, People’s Republic of China). The diluted HRP-conjugated goat anti-dog IgM antibody (1 μg/mL) was added to the 96-well plate and incubated for 1 h. Then, the 96-well plate was washed five times. Staining was initiated by adding o-phenylene diamine (100 μL; 1 mg/mL, Sigma-Aldrich). The samples were then incubated for 15 min, and the reaction was terminated by the addition of H2SO4 (100 μL; 2 mol/L). The absorbance was measured at 490 nm using a microplate reader (Bio-Rad Laboratories Ltd., Hertfordshire, UK). The experiment was performed at room temperature.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis was performed using Student’s t-test with the SPSS 16.0 (SPSS Inc., Chicago, IL, USA) software. P<0.05 was considered statistically significant.
Results
The characteristics of various PEGylated nanocarriers
It has been reported that the ABC phenomenon may be influenced by the physicochemical properties, for example, size, surface charge, degree of PEGylation, and PEG chain length of the nanocarrier that is injected first.Citation29,Citation30 Therefore, nanocarriers need to be fully characterized. Apart from PM, which measured 14.3±1.4 nm, the mean particle size of the PEGylated nanocarriers was in the range of 120–140 nm. The FALTs of PEGylated nanocarriers were estimated based on the zeta potential data. In , the FALTs of PE, PL, PSLN-GMS, and PSLN-GDS, which contained 10 mol% mPEG2000-DSPE, were determined to be 3.5±0.2, 3.3±0.1, 2.1±0.2, and 2.8±0.4 nm, respectively. The FALT of PM was not calculated, because the zeta potential of PM was almost not changed in the various concentrations of NaCl. The particle size, polydispersity index, zeta potential, and FALT measurements of the PEGylated nanocarriers are summarized in .
Pharmacokinetics of PEGylated nanocarriers following a single intravenous injection
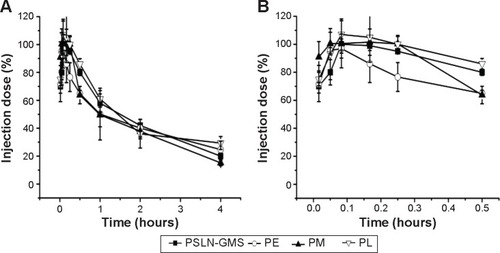
The beagle dogs were treated with PEGylated nanocarriers by an intravenous injection at a phospholipid dose of 2.5 μmol/kg, and the pharmacokinetics was determined using HPLC. As shown in , PEGylated nanocarriers exhibited a prolonged circulation time in the bloodstream and the plasma concentrations of PL, PE, PSLN-GMS, and PM at 4 h after the intravenous injection were 29.4%±4.7%, 26.7%±6.1%, 20.1%±2.1%, and 15.2%±2.1% of the injected dose, respectively. No statistical difference was found between the pharmacokinetic profiles of the first doses among the various PEGylated nanocarriers (P<0.05).
Figure 1 The blood clearance profile of a single intravenous injection of various PEGylated nanocarriers at a phospholipid dose of 2.5 μmol/kg in beagle dogs.
Notes: Data shown over 0–4 hours (A). A magnified view of the 0–0.5 hour period (B). Data show mean ± standard deviation of three repeats.
Abbreviations: GMS, glycerin monostearate; PE, PEGylated emulsions; PEG, poly(ethylene glycol); PL, PEGylated liposomes; PM, PEG micelles; PSLN, PEGylated solid lipid nanoparticles.
The immune response induced by the cross-administration of various PEGylated nanocarriers
Effect of the cross-administration of PEGylated nanocarriers on the ABC phenomenon in the induction phase
In order to investigate the inducement of the ABC phenomenon by cross-administration of PEGylated nanocarriers in the induction phase, the beagle dogs were administered the first injection of PEGylated nanocarriers (PE, PL, PSLN-GMS, or PM at a dose of 2.5 μmol phospholipid/kg), and 7 days later the second dose of PE was administered. The results shown in indicate that the extent of the ABC effect depended on the type of PEGylated nanocarrier. PL and PE induced the fastest clearance of the second dose of PE. The percentage of injected TN remaining was less than 10% at 1 min after the second injection. The clearance of PE was moderately affected by the first injection of PSLN (ABC index(0–30 min) =0.42±0.08). In contrast, PM only slightly affected the subsequent dose of PE, especially at 0–30 min after the injection.
Figure 2 Effect of various PEGylated nanocarriers on the induction of the ABC phenomenon in beagle dogs.
Notes: The beagle dogs were first given PE, PL, PSLN-GMS, or PM, at a dose of 2.5 μmol phospholipid/kg. Then, 7 days after the first injection, the beagle dogs were given PE. Data show mean ± standard deviation of three repeats.
Abbreviations: GMS, glycerin monostearate; PE, PEGylated emulsions; PEG, poly(ethylene glycol); PL, PEGylated liposomes; PM, PEG micelles; PSLN, PEGylated solid lipid nanoparticles.
Effect of the cross-administration of PEGylated nanocarriers on the ABC phenomenon in the effectuation phase
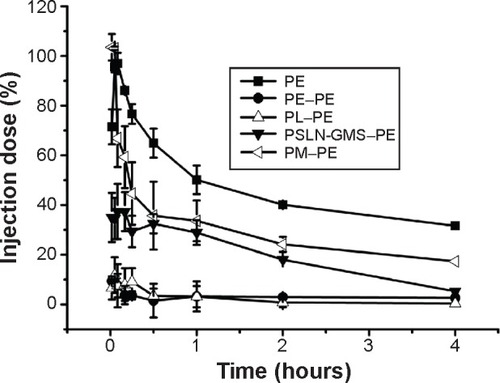
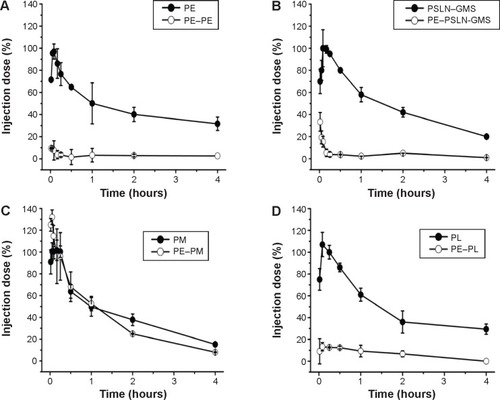
The beagle dogs were administered the first injection of PE (2.5 μmol phospholipid/kg), and 7 days later, a second injection of PE, PL, PSLN-GMS, or PM was administered. As shown in , the clearance rates of PE and PSLN were significantly faster when injected following a first injection of PE and the ABC indices(0–30 min) were 0.05±0.00 and 0.08±0.01, respectively. In comparison with the second dose of PSLN-GMS, the blood clearance rate of the second dose of PE was faster 0–10 min after the injection. The ABC phenomenon was less prominent in the case of PL (ABC index(0–30 min) =0.18±0.04), and the pharmacokinetics of the second dose of PM was not affected by an initial administration of PE (ABC index(0–30 min) =1.04±0.09).
Figure 3 Effect of PE on the pharmacokinetics of various PEGylated nanocarriers in beagle dogs.
Notes: The beagle dogs were first given PE at a dose of 2.5 μmol phospholipid/kg. Then, 7 days after the first injection, the beagle dogs were given PE (A), PSLN-GMS (B), PM (C), or PL (D). Data show mean ± standard deviation of three repeats.
Abbreviations: GMS, glycerin monostearate; PE, PEGylated emulsions; PEG, poly(ethylene glycol); PL, PEGylated liposomes; PM, PEG micelles; PSLN, PEGylated solid lipid nanoparticles.
Comparison of the ABC phenomenon induced by the repeated injection and the cross-administration of various PEGylated nanocarriers
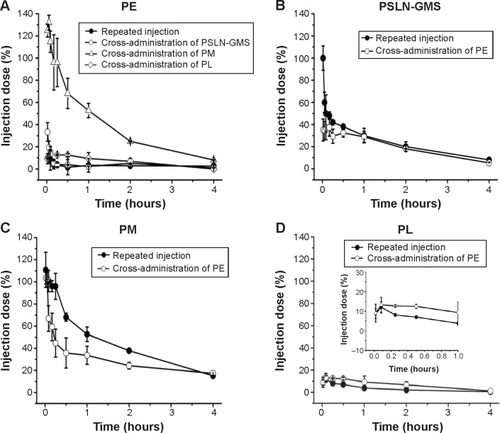
In recent years, treatment regimens involving cross- administration have been widely used. Compared to the conventional repeated injections, this regimen results in significantly lower toxic side effects; furthermore, it reduces the risk of complications. As shown in , the ABC phenomenon induced by repeated injection of various PEGylated nanocarriers was consistent with the results of previous literatures.Citation14–Citation17 Nevertheless, we found that the extent of the ABC phenomenon was different for repeated injections and cross-administration. It is difficult to accurately evaluate the extent of the immune response induced by repeated injections or cross-administration. Ishihara et alCitation31 calculated the ratio of the area under the curve (AUC)(0–24 h) for the second injection to the AUC(0–24 h) for the first injection and referred to this as the ABC index; a value of 1 indicates no evidence of the ABC phenomenon. Moreover, our previous study proved that the ratio of AUC(0–30 min), regarded as the ABC index(0–30 min), was suitable for evaluating the ABC phenomenon,Citation17 based on the fact that the ABC phenomenon is a rapid immune response. The results shown in indicated that, for PE, the most marked ABC phenomenon was induced by repeated injections, followed by that induced by the cross-administration with PSLN-GMS; however, the blood clearance rate of PM was not affected by the first injection of PE. The ABC indices(0–30 min) for the repeated injections and cross-administration of PSLN-GMS and PM were 0.05±0.00, 0.08±0.01, and 1.04±0.09, respectively. For PSLN-GMS, cross-administration with PE induced a more pronounced ABC effect compared with repeated injections of PSLN-GMS (ABC index(0–30 min) PSLN-PSLN =0.51±0.03 and ABC index(0–30 min) PSLN-PE =0.42±0.08). It is worth noting the lack of ABC phenomenon that was observed when using PM. Repeated injections of PM failed to induce an ABC effect, but the ABC phenomenon was markedly observed in beagle dogs following cross-administration of PM and PE (ABC index(0–30 min) PM-PE =0.68±0.05). In contrast, there was no statistical difference in the extent of the ABC phenomenon between repeated injections of PL and cross-administration of PE after PL, as evidenced by their ABC indices(0–30 min) (0.13±0.02 and 0.09±0.00 respectively).
Table 3 The ABC index(0–30 min) of repeated injections and cross-administration of various PEGylated nanocarriers
Figure 4 Comparison of the effects of repeated injections and cross-administration of various PEGylated nanocarriers on immune response.
Notes: PE (A); PSLN-GMS (B); PM (C); and PL (D). Data show mean ± standard deviation of three repeats.
Abbreviations: GMS, glycerin monostearate; PE, PEGylated emulsions; PEG, poly(ethylene glycol); PL, PEGylated liposomes; PM, PEG micelles; PSLN, PEGylated solid lipid nanoparticles.
Level of anti-PEG IgM after treatment with various PEGylated nanocarriers
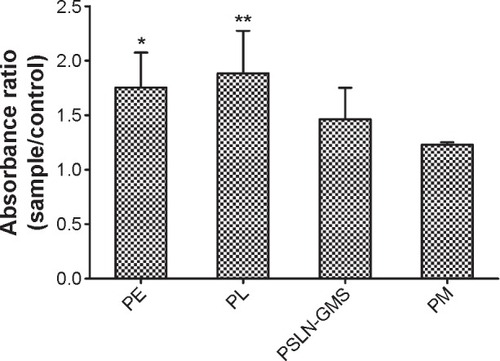
Wang et alCitation32 reported that the anti-PEG IgM production in the ABC phenomenon is involved in the accelerated clearance of the second dose. Thus, we determined the anti-PEG IgM levels produced by various PEGylated nanocarriers. In order to deduct the background value of different beagle dogs, the ratio of absorbance of anti-PEG IgM on day 7 (before the second injection) to that of anti-PEG IgM on day 0 (before the first injection) was used to evaluate the anti-PEG IgM level. As shown in , for the cross-administration of PEGylated nanocarriers, the absorbance ratio of the anti-PEG IgM ranked in the following order (from the highest to the lowest): PL, PE, PSLN-GMS, and PM.
Figure 5 The production of anti-PEG IgM induced by the initial priming dose of various PEGylated nanocarriers (PE, PL, PSLN-GMS, and PM) at doses of 2.5 μmol phospholipid/kg.
Notes: The anti-PEG IgM in the serum was determined using ELISA. Data show mean ± standard deviation of three repeats. P-values represent a significant difference compared with 1. *P<0.05; **P<0.01.
Abbreviations: GMS, glycerin monostearate; ELISA, enzyme-linked immunosorbent assay; PE, PEGylated emulsions; PEG, poly(ethylene glycol); PL, PEGylated liposomes; PM, PEG micelles; PSLN, PEGylated solid lipid nanoparticles.
Discussion
Generally, the first blood sample taken represents the maximum blood plasma concentration (Cmax), because no absorption is required when injecting drugs directly into the bloodstream. Nevertheless, we found that the first blood sample taken 1 min after an intravenous injection of PEGylated nanocarriers in beagle dogs was not the maximum plasma concentration. The Cmax values of various PEGylated nanocarriers were observed for the third blood sample, which was taken 5 min after injection (). The recognition and ultimate fate of foreign particles depends on the immune cells.Citation33 In this study, the determination of blood cells at different time points after the injection of PE was used to monitor the real-time changes in blood immune cells, provide a quantitative measurement of this change, and reflect the interaction between the immune system and nanocarriers (see Supplementary material). The size and shape of nanocarriers are very similar to those of pathogenic microorganisms, subcellular organelles, and cell debris.Citation34 The intravenous injection of PE was immediately recognized by the immune cells, and the immune cells migrated to the injected limb from the limb opposite to the injected limb. Subsequently, a large number of immune cells gathered around the injected PE and hindered its circulation from the injected limb to the opposite limb (from where the blood was taken). Therefore, the plasma concentration of PE was not at its maximum immediately after the intravenous injection. On the other hand, PEG coatings provide a steric barrier and prevent hydrophobic interactions between the surface of the nanocarriers and plasma opsonins, thus inhibiting their uptake by immune cells.Citation35 Therefore, the plasma concentration of PE peaked at 5 min after the intravenous injection; meanwhile, the values of immune cells at the injection side and the side opposite to the injection site returned to the baseline levels ().
The ABC phenomenon that was induced by repeated injections of PEGylated nanocarriers is divided into two phases: the induction phase, following the first injection in which the immune system is primed and the anti-PEG IgM is produced, and the effectuation phase, following the second injection in which PEGylated nanocarriers are recognized, bound by the anti-PEG IgM, and rapidly cleared from the blood circulation.Citation36 In this work, the immune response elicited by the cross-administration of PEGylated nanocarriers was investigated during the induction phase and the effectuation phase.
Laverman et alCitation36 proposed that the physicochemical properties of the second injection of nanoparticles influenced the magnitude of the ABC phenomenon. To investigate the effect of cross-administration of PEGylated nanocarriers on the immune response in the induction phase, beagle dogs were injected with PSLN, PL, PM, and PE, 7 days after the first injection; all groups were given PE. Although each type of PEGylated nanocarrier had a similar circulation time (), the blood clearance rate of the second dose of PE was completely different. As shown in , the immunogenicity of the PEGylated nanocarriers in decreasing order was PL, PE, PSLN, and PM. A possible explanation is that the PEGylated nanocarriers have different colloidal structures. Different materials caused different types of binding proteins to be bound to different degrees.Citation37 The lipids that make up the SLNs are solid at body temperature, which leads to the formation of tightly packed molecules in the emulsified layer.Citation38 The hydrophobic regions of PSLN are not easily exposed, and this limits the extent of protein binding. Nevertheless, it cannot be ignored that PL, PE, PM, and PSLN-GMS had similar circulation times after intravenous injection (), and that this means that they have similar protein binding characteristics. Another explanation is related to the surface arrangements of PEG on nanocarriers. Koide et alCitation16 reported that the ABC phenomenon was not observed in BALB/c SCID (T- and B-cell-deficient) mice, but that anti-PEG IgM expression was induced in BALB/c nu/nu (T-cell-deficient) mice following treatment with PEGylated liposomes, suggesting that PEGylated liposomes elicit an anti-PEG IgM response in a T-cell-independent manner. Furthermore, Ishida et alCitation39 proved that PEGylated liposomes promote an immune response against PEG in a manner similar to that induced by T-cell-independent type 2 (TI-2) antigens. In general, most TI-2 antigens have large multivalent molecules arranged on the surface and exhibit a prolonged circulation time in vivo.Citation40,Citation41 The ordered arrangement of polymer chains on the surface of PEGylated nanocarriers can interact with membrane receptors on B cells and induce a strong immune response. SLNs are defined as colloidal particles of lipid matrix that are solid at physiological temperature.Citation42 Compared to liposomes and emulsions, it is difficult for SLNs to form a highly ordered crystal structure,Citation43 leading to an irregular arrangement of PEG. Moreover, when the PEG on the surface of PSLN was arranged in a less orderly fashion, the ability of PSLN to activate B cells was diminished, resulting in the secretion of a small amount of anti-PEG IgM. Correspondingly, the ABC phenomenon induced by PSLN-GMS was weaker than that by PL and PE.
In addition, the repeating –(O–CH2–CH2)n- subunit of PEG is the immunogenic epitope of PEGylated nanocarriers and a binding site for the derived anti-PEG IgM.Citation44,Citation45 We speculated that the observed differences in the extent of the ABC phenomenon are related to the PEG conformation on the surface of the different nanocarriers. Theoretically, in a nanoparticle prepared with less than 4 mol% mPEG2000-DSPE, the PEG on the nanoparticle surface is arranged in the mushroom conformation; with 4–8 mol%, it is in the transition conformation; and with more than 8 mol%, it is in the brush conformation.Citation46 When PEG is arranged in the brush conformation, the neighboring PEG chains push against each other. In this study, in addition to PM, other nanocarriers contained 10 mol% mPEG2000-DSPE at their surfaces, indicating that the PEG chains are arranged in the brush conformation. However, GMS, which formed the solid matrix of PSLN-GMS, contains two hydroxyl groups, which allows it to form hydrogen bonds with the oxygen atoms of PEG, and therefore, the PEG chains on the surface of PSLN-GMS tended to fold toward the surface and form the mushroom-like structures. In contrast, the grafted PEG on the surface of PL and PE was arranged in the brush configuration (). We speculated that the repeat units of PEG are more exposed in the brush structure and, as a result, are more accessible to the membrane receptors on B cells. In order to study the relationship between the conformation of PEG and immune response, the ABC phenomenon elicited by PSLN prepared with GMS, which contains two hydroxyl groups, was compared to that elicited by PSLN prepared with GDS, which contains one hydroxyl group. It was found that PSLN-GDS induced a faster blood clearance of the second-dose PE than did PSLN-GMS (). Based on the modeling of PEG lipids by Needham et alCitation47 the FALTs of nanocarriers were used to characterize the PEG chain length on the nanocarrier surface. We calculated the FALTs of PSLN-GMS and PSLN-GDS. The FALT data () indicated that on the surface of PSLN-GMS, the PEG chain structure was more mushroom-like than brush-like, as evidenced by the thinner FALT for PSLN-GMS; this suggests that the PEG chains were more likely to fold toward the surface of PSLN-GMS.Citation48 This result further indicated that nanocarrier surfaces featuring brush structure of PEG induce a significant ABC phenomenon.
Figure 6 Diagram depicting the different PEG conformations on the surface of various nanocarriers.
Notes: PEG chains adopt a brush conformation on the surface of PE and PL, but have a mushroom-like conformation on the surface of PSLN-GMS. PL (A); PE (B), and PSLN-GMS (C).
Abbreviations: GMS, glycerin monostearate; PE, PEGylated emulsions; PEG, poly(ethylene glycol); PL, PEGylated liposomes; PSLN, PEGylated solid lipid nanoparticles.
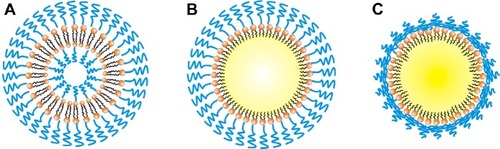
Figure 7 Effect of the types of lipid used to prepare PSLN on the induction of ABC phenomenon in beagle dogs. The beagle dogs were first given PSLN-GMS and PSLN-GDS at a dose of 2.5 μmol phospholipid/kg. Then, 7 days after the first injection, the beagle dogs were given PE. Data show mean ± standard deviation of three repeats.
Abbreviations: GDS, glycerol distearate; GMS, glycerin monostearate; PE, PEGy lated emulsions; PEG, poly(ethylene glycol); PSLN, PEGylated solid lipid nanoparticles.
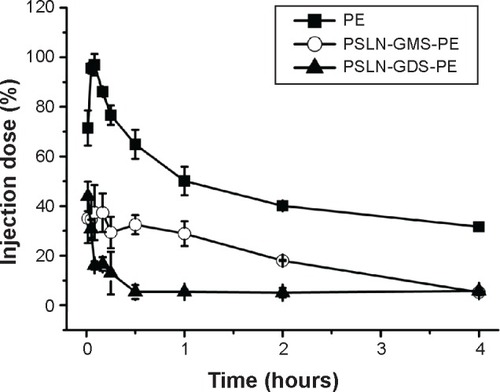
It is well known that the size of particles has a considerable impact on the extent of PEG conjugation. In other words, the flexibility, surface density, and the FALT of the PEG layer are related to the size of the particles. Therefore, for small nanocarriers, the number of PEG chains per unit area is small, allowing the chains to be more flexible and the FALT to be thin. The particle size of PM was 14.3±1.4 nm, which was much smaller than that of PL, PE, and PSLN. This means the PEG chains on the surface of PM were more flexible than those on PL, PE, and PSLN. Torchilin et alCitation49 reported that the more flexible the PEG is, the more independent the motion of any polymeric unit relative to the neighboring one is. This means that the chains have a larger number of possible conformations and a higher transition rate from one conformation to another. The flexible PEG chains on the PM surface did not interact effectively with the B cells, and no significant level of anti-PEG IgM was observed (). On the other hand, the molar percentage of mPEG2000-DSPE at the surface of PM was obviously greater than that at the surface of other PEGylated nanocarriers. Excessive cross-linking of membrane immunoglobulin was induced by the higher surface density of PEG,Citation50 leading to B cells being not able to produce anti-PEG IgM. Nevertheless, our results indicated that the administration of PM accelerated the plasma clearance of the subsequent dose of PE and the ABC index(0–30 min) was 0.68±0.05 (). We speculated that the first injection of PM stimulated the activation of the liver macrophages (Kupffer cells) in an IgM-independent manner, resulting in the enhanced intrinsic phagocytosis and high response ability of Kupffer cells to the second injection of PE; however, further investigation is required to obtain a profound understanding of the mechanism of the immune response triggered by the cross-administration of PM and PE.
According to the two-phase model, the key factors influencing the magnitude of the ABC phenomenon are the level of anti-PEG IgM production in the induction phase and the binding of anti-PEG IgM to the PEGylated nanocarriers in the effectuation phase. In order to investigate the effect that cross-administration of PEGylated nanocarriers has on the ABC phenomenon in the effectuation phase, the beagle dogs were administered the first injection of PE (the equivalent anti-PEG IgM level), and 7 days later, a second dose of PE, PSLN-GMS, PM, or PL was administered. Interestingly, the clearance rates of PSLN-GMS, PL, and PE were significantly accelerated by the first injection of PE, and the blood removal rates of PE and PL were faster than that of PSLN 0–10 min after the second injection (). One possible explanation is that the various PEGylated nanocarriers induce the production of antibodies with different antigens-recognition abilities – the antigens that induced the antibodies were more strongly recognized by said antibodies. Nevertheless, the results shown in show that the blood clearance rate of the second injection of PE was still faster than that of PSLN 0–10 min after the first injection of PSLN-GMS. Another explanation is related to the PEG conformation on the surface of nanocarriers. Hashimoto et alCitation51 reported that anti-PEG IgM that was generated in response to a single intravenous immunization of PEGylated liposomes had the ability to bind to the dense PEG that was mainly in a brush conformation. Compared with PSLN-GMS, the PEG chains in the PE formulation are more brush-like in structure; therefore, the anti-PEG IgM preferentially binds to the brush conformation of PEGylated nanocarriers. An exception is PM; the pharmacokinetics of the second-dose PM was not affected by an initial administration of PE (). The small particle size of PM was the reason it did not induce the ABC phenomenon. It is well known that IgM is the largest antibody and the molecular mass is 900 kDa, while the particle size of PM was only 14.3±1.4 nm. The smaller-sized PM could avoid the binding by the anti-PEG IgM.
A comparative study of the ABC phenomenon elicited by the repeated injection and the cross-administration was carried out. Surprisingly, there is a considerable difference between the immune response induced by repeated injections and the response induced by cross-administration of PEGylated nanocarriers (). The ABC index(0–30 min) was used to evaluate the extent of the ABC phenomenon. According to the results of the statistical analysis of the ABC index(0–30 min), we propose that the ABC phenomenon can be classified into four types: significant ABC phenomenon (ABC index(0–30 min) was 0.0–0.5), medium ABC phenomenon (ABC index(0–30 min) was 0.5–0.7), weak ABC phenomenon (ABC index(0–30 min) was 0.7–0.9), and no ABC phenomenon (ABC index(0–30 min) was 0.9–1.0). For PE, repeated injections elicited the strongest ABC phenomenon (ABC index(0–30 min) =0.05±0.00), while the cross-administration of PE and either PSLN or PL induced a relatively weak ABC effect (ABC indices(0–30 min) were 0.08±0.01 and 0.18±0.04, respectively). Moreover, the cross-administration of PM elicited no immune response (ABC index(0–30 min) =1.04±0.09). In contrast, for PSLN-GMS, the cross-administration of PE induced a stronger ABC phenomenon than repeated injections of PSLN-GMS (ABC indices(0–30 min) were 0.42±0.08 and 0.51±0.03, respectively). It is worth noting that with repeated injections of PM, the first injection had no effect on the pharmacokinetics of the second; however, cross-administration of PE resulted in a medium ABC phenomenon (ABC index(0–30 min) =0.68±0.05). These results strongly indicated that the immune response induced by cross-administration is completely different from that induced by repeated injections. Thus, when combinations of different types of nanocarriers are used, the immune response is completely different. Therefore, it is necessary for us to systematically investigate the immune response triggered by cross-administration.
Conclusion
In this report, we demonstrate that an immune response is induced by the cross-administration of PEGylated nanocarriers (PE, PL, PSLN, and PM). The study indicated that in addition to PM, nanocarriers with brush-like PEG structures could induce a significant ABC phenomenon. All else being equal, the anti-PEG IgM preferentially binds to PEGylated nanocarriers that have PEG chains in the brush conformation. For PM, the particle size played an important role in the attenuated ABC phenomenon by the cross-administration of PE and no immune response by repeated injections; however, further investigation is required to obtain a profound understanding of the mechanism. Importantly, there is a considerable difference between the ABC phenomenon induced by repeated injections and cross-administration. These findings provide novel insights into the methods that can be employed for reducing the immunogenicity of PEGylated nanocarriers. Furthermore, the results reported here have potential implications for the design, development, and clinical application of PEGylated products that require cross-administration.
Results and discussion
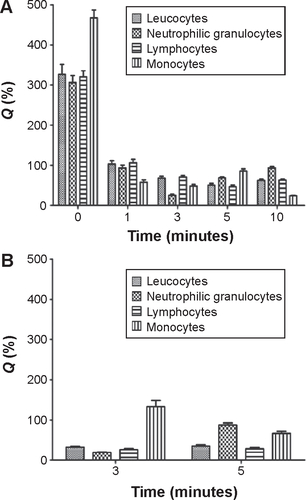
As shown in , immune cells immediately gathered in the injected limb, and the number of monocytes, leukocytes, lymphocytes, and neutrophilic granulocytes at the injection site increased by 467%, 326%, 320%, and 306%, respectively. The amount of immune cells returned to the baseline level within 3–5 min of administration. The number of immune cells on the side opposite to the injection site was reduced to below 50% of the control at 3 min after the injection, whereas the number of immune cells in the injected limb and in limb opposite to the injected limb returned to the baseline levels at 5 min after the injection ().
Figure S1 The percentage change in leucocytes, neutrophilic granulocytes, monocytes, and lymphocytes in left and right limbs after the intravenous injection.
Notes: The beagle dogs were treated with PE at a dose of 2.5 μmol phospholipid/kg. Right limb (A); left limb (B). Data show mean ± standard deviation of three repeats. Q% = (Qt/Q0)×100, where Qt is the value for the blood sample and Q0 is the control value.
Abbreviation: PE, PEGylated emulsions.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No 81072602) and the Shenyang Pharmaceutical University Students’ Innovation Undertaking Project of Liao Ning Province (Grant No 201410163006).
Supplementary materials
Hematological analysis
Method
Beagle dogs were injected with PE (PEGylated emulsions) via a vein in the right forelimb at a phospholipid dose of 2.5 μmol/kg. Before the injection, a blank blood sample was collected from the left forelimb. Blood samples were collected immediately in the right forelimb after the first injection, regarded as 0 min. At 1, 3, 5, and 10 min after the intravenous injections, blood samples were obtained from the right forelimb, except at 3 and 5 min when blood samples were collected from the left forelimb. All blood samples were collected in bottles containing ethylenediaminetetraacetic acid dipotassium salt (EDTA-2K) and analyzed using a hematology analyzer (LH-750, Beckman Coulter, Inc., Brea, CA, USA). Ratios between measured values and blank blood samples were calculated at each time point, designated Q, which is expressed by the following equation:
Disclosure
The authors report no conflicts of interest in this work.
References
- GrecoFVicentMJCombination therapy: opportunities and challenges for polymer–drug conjugates as anticancer nanomedicinesAdv Drug Deliv Rev200961131203121319699247
- BroxtermanHJGeorgopapadakouNHAnticancer therapeutics: “Addictive” targets, multi-targeted drugs, new drug combinationsDrug Resist Updat20058418319716154800
- GeeJHowellAGullickWConsensus statementEndocr Relat Cancer200512Suppl 1S1S716113086
- HoneckerFKollmannsbergerCQuietzschDPhase II study of weekly paclitaxel plus 24-h continuous infusion 5-fluorouracil, folinic acid and 3-weekly cisplatin for the treatment of patients with advanced gastric cancerAnticancer Drugs200213549750312045461
- PriottoGKasparianSMutomboWNifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trialLancet20093749683566419559476
- SuriSSFenniriHSinghBNanotechnology-based drug delivery systemsJ Occup Med Toxicol2007211618053152
- FarokhzadOCLangerRImpact of nanotechnology on drug deliveryACS Nano200931162019206243
- WagnerVDullaartABockA-KZweckAThe emerging nanomedicine landscapeNat Biotechnol200624101211121817033654
- YuanFDellianMFukumuraDVascular permeability in a human tumor xenograft: molecular size dependence and cutoff sizeCancer Res19955517375237567641188
- LaginhaKMumbengegwiDAllenTLiposomes targeted via two different antibodies: assay, B-cell binding and cytotoxicityBiochim Biophys Acta200517111253215904660
- StormGBelliotSODaemenTLasicDDSurface modification of nanoparticles to oppose uptake by the mononuclear phagocyte systemAdv Drug Deliv Rev19951713148
- NieSUnderstanding and overcoming major barriers in cancer nanomedicineNanomedicine20105452352820528447
- MoghimiSMGrayTA single dose of intravenously injected poloxamine-coated long-circulating particles triggers macrophage clearance of subsequent doses in ratsClin Sci19979343713799404230
- DamsETLavermanPOyenWJAccelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomesJ Pharmacol Exp Ther200029231071107910688625
- WangCChengXSuiYA noticeable phenomenon: thiol terminal PEG enhances the immunogenicity of PEGylated emulsions injected intravenously or subcutaneously into ratsEur J Pharm Biopharm201385374475124129310
- KoideHAsaiTHatanakaKParticle size-dependent triggering of accelerated blood clearance phenomenonInt J Pharm20083621–219720018586076
- ZhaoYXWangLYanMNRepeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beaglesInt J Nanomedicine201272891290022745552
- KaminskasLMMcleodVMPorterHChristopherJBoydBJDifferences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearanceJ Pharm Sci2011100115069507721721002
- Abu LilaASKiwadaHIshidaTThe accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manageJ Control Release20131721384723933235
- PozziDColapicchioniVCaraccioloGEffect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cellsNanoscale2014652782279224463404
- US Department of Health and Human ServicesPublic Health ServiceNational Institutes of HealthGuide for the Care and Use of Laboratory AnimalsWashington, DCNational Academy Press1985
- ZhaoYXWangCLWangLA frustrating problem: accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in ratsEur J Pharm Biopharm201281350651322580209
- SongHGengHRuanJDevelopment of Polysorbate 80/Phospholipid mixed micellar formation for docetaxel and assessment of its in vivo distribution in animal modelsNanoscale Res Lett201161112
- ShimadaKMiyagishimaASadzukaYDetermination of the thickness of the fixed aqueous layer around polyethyleneglycol-coated liposomesJ Drug Target1995342832898821002
- SadzukaYNakadeATsurudaTSonobeTStudy on the characterization of mixed polyethyleneglycol modified liposomes containing doxorubicinJ Control Release200391327128012932707
- SadzukaYNakadeAHiramaREffects of mixed polyethyleneglycol modification on fixed aqueous layer thickness and antitumor activity of doxorubicin containing liposomeInt J Pharm2002238117118011996821
- WilsonDRZhangNSilversALForstnerMBBaderRASynthesis and evaluation of cyclosporine A-loaded polysialic acid–polycaprolactone micelles for rheumatoid arthritisEur J Pharm Sci20145114615624075961
- IchiharaMShimizuTImotoAAnti-PEG IgM response against PEGylated liposomes in mice and ratsPharmaceutics20103111124310423
- IshidaTIchikawaTIchiharaMSadzukaYKiwadaHEffect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in miceJ Control Release200495340341215023452
- WangXYIshidaTIchiharaMKiwadaHInfluence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in ratsJ Control Release200510419110215866337
- IshiharaTTakedaMSakamotoHAccelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticlesPharm Res200926102270227919633820
- WangXIshidaTKiwadaHAnti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomesJ Control Release2007119223624417399838
- JanewayCATraversPWalportMShlomchikMJImmunobiologyNew York, NYGarland Science2001910
- SzebeniJMuggiaFGabizonABarenholzYActivation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and preventionAdv Drug Deliv Rev201163121020103021787819
- OwensDEIIIPeppasNAOpsonization, biodistribution, and pharmacokinetics of polymeric nanoparticlesInt J Pharm200630719310216303268
- LavermanPCarstensMGBoermanOCFactors affecting the accelerated blood clearance of polyethylene glycolliposomes upon repeated injectionJ Pharmacol Exp Ther2001298260761211454922
- JiskootWvan SchieRMCarstensMGSchellekensHImmunological risk of injectable drug delivery systemsPharm Res20092661303131419247815
- MüllerRRadtkeMWissingSSolid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparationsAdv Drug Deliv Rev200254S131S15512460720
- IshidaTWangXShimizuTNawataKKiwadaHPEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent mannerJ Control Release2007122334935517610982
- MondJJLeesASnapperCMT cell-independent antigens type 2Annu Rev Immunol19951316556927612238
- MondJJVosQLeesASnapperCMT cell independent antigensCurr Opin Immunol1995733493547546399
- JoshiMDMüllerRHLipid nanoparticles for parenteral delivery of activesEur J Pharm Biopharm200971216117218824097
- LiuKSunJHeZ-gDevelopment on novel nanostructured lipid carriersJ Shenyang Pharm Univ20083017
- ChengT-LChenB-MChernJ-WWuM-FRofflerSREfficient clearance of poly(ethylene glycol)-modified immunoenzyme with anti-PEG monoclonal antibody for prodrug cancer therapyBioconjug Chem200011225826610725103
- ChengT-LWuP-YWuM-FChernJ-WRofflerSRAccelerated clearance of polyethylene glycol-modified proteins by anti-polyethylene glycol IgMBioconjug Chem199910352052810346886
- LiS-DHuangLStealth nanoparticles: high density but sheddable PEG is a key for tumor targetingJ Control Release2010145317820338200
- NeedhamDStoichevaNZhelevDVExchange of monooleoylphosphatidylcholine as monomer and micelle with membranes containing poly(ethylene glycol)-lipidBiophys J1997735261526299370456
- GarbuzenkoOBarenholzYPrievAEffect of grafted PEG on liposome size and on compressibility and packing of lipid bilayerChem Phys Lipids2005135211712915921973
- TorchilinVPOmelyanenkoVGPapisovMIPoly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevityBiochim Biophys Acta19941195111207918551
- BastenASilveiraPAB-cell tolerance: mechanisms and implicationsCurr Opin Immunol201022556657420829011
- HashimotoYShimizuTMimaYAbu LilaASIshidaTKiwadaHGeneration, characterization and in vivo biological activity of two distinct monoclonal anti-PEG IgMsToxicol Appl Pharmacol20142771303824632081
