Abstract
Osteoporosis is a serious public health problem affecting hundreds of millions of aged people worldwide, with severe consequences including vertebral fractures that are associated with significant morbidity and mortality. To augment or treat osteoporotic vertebral fractures, a number of surgical approaches including minimally invasive vertebroplasty and kyphoplasty have been developed. However, these approaches face problems and difficulties with efficacy and long-term stability. Recent advances and progress in nanotechnology are opening up new opportunities to improve the surgical procedures for treating osteoporotic vertebral fractures. This article reviews the improvements enabled by new nanomaterials and focuses on new injectable biomaterials like bone cements and surgical instruments for treating vertebral fractures. This article also provides an introduction to osteoporotic vertebral fractures and current clinical treatments, along with the rationale and efficacy of utilizing nanomaterials to modify and improve biomaterials or instruments. In addition, perspectives on future trends with injectable bone cements and surgical instruments enhanced by nanotechnology are provided.
Introduction
Osteoporosis is a progressive bone disease characterized by reduced bone density and microarchitectural deterioration of bone tissue.Citation1 According to the definition given by the World Health Organization, a patient is diagnosed as having osteoporosis when his/her bone mineral density is 2.5 standard deviations or more below the mean peak bone mass (defined by the average value for young healthy adults).Citation2 For measuring bone density and evaluating osteoporosis or fracture risk prediction, dual X-ray absorptiometry is considered the “gold standard” method,Citation3 while ultrasound methodology has been developed for population screening and diagnosis in primary care.Citation4,Citation5
In clinical practice, according to osteoporotic condition, osteoporosis is usually categorized as primary or secondary osteoporosis. With the increased life expectancy and rapidly aging population worldwide, osteoporosis has become a major public health problem in many countries. According to the International Osteoporosis Foundation, osteoporosis is estimated to affect one in three women and one in eight men over the age of 50 years.Citation6,Citation7 In the USA, it is estimated that at least 10 million people suffer from osteoporosis and over 1.5 million cases of osteoporotic vertebral fracture (OVF) occur every year, generating direct health care costs of US$12–18 billion each year.Citation8 In fact, the direct annual costs of treating osteoporotic fractures in the workplace in the USA, Canada, and Europe alone are estimated to be approximately $48 billion.Citation9 As of 2006, in the Chinese population aged 50 years or older, 65 million people were estimated to have osteoporosis, while an additional 200 million were estimated to have osteopenia. Demographic studies also indicate that osteoporosis may soon reach epidemic proportions in the developing world.Citation10
One of the serious consequences of osteoporosis is an increased risk of fractures.Citation11 Fractures can occur in many locations of the skeleton and are often associated with high morbidity and mortality.Citation12 According to Burger’s model, fractures of the spine have a much higher incidence than fractures at other sites, especially in the patient with earlier osteoporosis. Spinal or vertebral fractures are fractures in one or more vertebral bodies and are usually classified as wedge, biconcavity, or compression fractures depending on the type of deformity.Citation13 Vertebral fractures caused by osteoporosis are also known as OVFs, which are the focus of this article. Patients experiencing a spinal fracture have an increased mortality rate of approximately 10%, and female OVF patients have a 23% higher mortality rate when compared with those without OVF.Citation14 OVF is also one of the major risk factors for secondary fractures, the probability of which is increased fourfold after the first vertebral fracture occurs.Citation15 OVF can also cause other severe physical, functional, and psychological problems or disorders, such as chronic back pain, kyphosis, and shortening of the segmental vertebrae.Citation16 Because of the extraordinary public health challenges and economic burden imposed by OVF worldwide, prevention and treatment of OVF are of considerable importance for the health care community.
Current treatments for OVF and their challenges
Clinical treatment of OVF aims at achieving pain relief and restoring height and functional stability of the fractured vertebral body.Citation17,Citation18 Treatment of OVF includes non-surgical and surgical approaches, which are summarized in . Although conservative non-surgical approaches (bed rest, medication, nutritional improvements) can relieve pain and alleviate reduce complications, they cannot restore the height and functional stability of the fractured vertebral body. Traditional surgical approaches include anterior decompression and fusion and/or posterior instrumentation and fusion, but these approaches may not be appropriate for elderly patients and still have high postoperative failure rates.Citation19,Citation20 Therefore, minimally invasive procedures like vertebroplasty and kyphoplasty are becoming increasingly attractive options for the treatment of OVF.Citation21
Table 1 Current approaches for treating osteoporotic vertebral fractures
Vertebroplasty and kyphoplasty are minimally invasive percutaneous surgical techniques that internally stabilize the vertebral body by injection of self-hardening biomaterials such as bone cements (). Both procedures are performed using a fluorescence detector (eg, a C-arm X-ray machine) and thus require the injectable bone cement to be radiopaque. Vertebroplasty was first developed in France by Herve Deramond et al in 1986Citation22 and was introduced to the USA in 1995.Citation23 Vertebroplasty is effective for pain relief, and statistics show that it eases pain in 80% of patients. Vertebroplasty is also used to strengthen vertebral bodies that are weakened but not yet fractured, thereby preventing further fractures.Citation24 However, vertebroplasty is not particularly effective in restoring the height of fractured vertebra or correcting spinal deformity. Another disadvantage of vertebroplasty is the high probability of bone cement leakages into the spinal canal, which increases the risks of neurological damage and pulmonary embolism.Citation25
Figure 1 Schematic of vertebroplasty and kyphoplasty procedures, which are both minimally invasive, percutaneous surgical approaches that can internally stabilize a fractured vertebral body via injection of self-hardening biomaterials like bone cement.
Note: The difference between vertebroplasty and kyphoplasty procedures is the utilization of a balloon that is inflated to create a cavity in the compressed vertebral body prior to injection of the cement. The blue lines represent the catheter; the yellow ovals represent the balloon; and the white oval represents the cement in each instance.
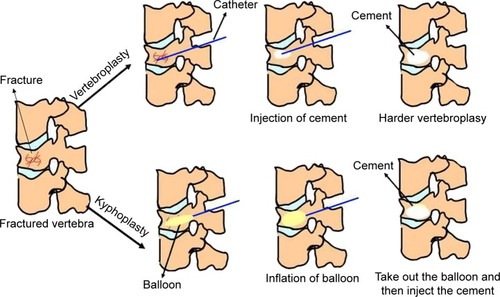
Kyphoplasty (also known as balloon-assisted vertebroplasty) was developed in the early 1990s as an improved approach to mitigate the complications of vertebroplasty and to restore the height of the fractured vertebra.Citation26 This procedure starts with inserting an inflatable balloon catheter into the center of the fractured vertebra, after which the balloon is inflated to recover the compressed vertebral height and correct the deformity, meanwhile creating a void for injecting bone cement ().Citation27 Kyphoplasty is now a well established procedure for the treatment of OVF and has been practiced in many countries.Citation28
Recently, an alternative procedure known as vertebral stenting, which is based on the principles of balloon kyphoplasty and vascular stenting, has also been developed. In vertebral stenting, after the balloon is removed, a metal stent remains within the created void to prevent the vertebral body from collapsing, so in an ideal scenario, a virtually physiological vertebral body height and shape can be restored and preserved.Citation29 However, vertebral stenting has not been widely accepted by surgeons due to the limited supply of appropriate stents.
Vertebroplasty and kyphoplasty or vertebral stenting face a number of problems in clinical practice, including those associated with bone cement, which will be further discussed. In addition, bacterial infection is a common complication associated with implants or devices for the treatment of OVF. Although minimally invasive surgery can significantly lower the risk of infection when compared with open surgery, bacterial colonization and formation of biofilm on the implanted device cannot be completely eradicated. The consequences of infection are severe, leading to considerable health care burden, prolonged patient suffering, and substantial morbidity and even mortality.Citation30 As a result, reducing infection in the surgical treatment of OVF has also become a persistent challenge.
Nanotechnology for orthopedic applications
Bone is a natural nanocomposite that consists of hierarchically arranged collagen fibrils, proteoglycans, and hydroxyapatite (HA) crystals, all at nanometer scale. Inspired by this nanostructure, it has been widely speculated that mimicking nanoscale features of natural bone in materials or creating topographies resembling nanoscale roughness of bone may enhance new bone growth or regeneration. This strategy provides innovative opportunities to design and fabricate novel material formulations, devices, and systems, or to modify existing ones for better outcomes. Based on a similar rationale, nanotechnology-based bone tissue engineering, which combines living cells and growth factors with appropriate biomaterial nanoscaffolds for restoration and regeneration of defective bone tissue, has also been developed.Citation31 Moreover, nanotechnology has demonstrated significant impacts on the development of novel bone substitutes, biological electronics such as biosensors, sensitive diagnostic systems, and controlled drug delivery systems.Citation32
Recent studies have shed light on the mechanism(s) behind the positive role of nanotechnology in orthopedic applications. On the one hand, this positive role is attributed to the factors such as vastly changed grain (or feature) size, surface roughness and surface area-to-volume ratio, surface wettability, and associated energetics, which have also been correlated with the superior physiochemical properties of nanomaterials, including their mechanical, electrical, optical, catalytic, and magnetic properties.Citation33 On the other hand, studies have arguably elucidated that favorable cell or tissue responses to nanomaterials are strongly correlated with greater adsorption or interactions of selected proteins compared with conventional micron-sized materials. This may be one of the underlying mechanisms accounting for why nanomaterials possess more intriguing biological properties than conventional micron-sized materials.Citation33
Increasing numbers of nanotechnologies and nanomaterials have been developed and are utilized in the field of orthopedics, and there is a growing interest in the research and development of nanotechnology for preventing and treating osteoporosis. For instance, nanotechnology has been utilized to enhance the bioavailability of calcium supplements, which can reduce the risk of developing osteoporosis. Nano calcium carbonate and calcium citrate were devised and prepared to increase bioavailability in the gastrointestinal tract.Citation34 The results suggested that nanosized calcium carbonate and calcium citrate are more bioavailable than orally administered microsized calcium carbonate and calcium citrate, respectively.
The present article addresses the frontiers of research on nanotechnology and nanomaterials tentatively used for treating OVF. New advances in injectable nanomaterials and surgical tools for vertebroplasty and kyphoplasty, such as improvements in the mechanical, biocompatible, antibacterial, and radiological properties of bone cements and tools, are also analyzed. However, since the nanotechnologies and nanomaterials for treatment of OVF are still in the early stages of development, most studies to date have been performed in vitro, and the efficacy and feasibility of these nanotechnologies and nanomaterials for clinical use have not been explicitly demonstrated. Therefore, this review focuses more on the potential and promise of nanotechnology and nanomaterials for treating OVF and future perspectives.
Injectable nanomaterials for treatment of OVF
Surgical procedures for treating OVF essentially aim at regaining sufficient strength and height of the fractured vertebral body with the assistance of implants and/or biomaterials.Citation35 Possible implants and biomaterials include metal fixtures (eg, screws, plates, rods) and bone fillers that can augment osteoporotic or weak vertebral bodies. In the aforementioned minimally invasive procedures like vertebroplasty and kyphoplasty, bone fillers need to be injectable and then able to solidify, so are also known as bone cements.Citation35 So far, the only clinically approved bone cement is polymethyl-methacrylate (PMMA) cement,Citation36 and others currently under development include calcium phosphate cement (CPC)Citation37 and calcium sulfate cement (CSC).Citation38 Meanwhile, new radiopacifiers for imaging purposes during minimally invasive surgery have also been developed. In addition, injectable hydrogel is a new candidate for bone healing and regeneration via minimally invasive procedures. In this section, current progress and advances with these three injectable materials are summarized.
Bone cements
A package of bone cement usually consists of a powder component and a liquid phase acting as a reaction medium. During surgery, bone cement is formed into a paste by mixing the powder and the liquid, and the paste can then either be injected through a channel or molded by the surgeon into the sites of bone defects or voids.Citation39 After setting and hardening, the paste conforms to the shape of the defect or void, and solidifies to achieve enough strength to support the vertebra. The characteristics and properties of PMMA, CPC, and CSC for vertebroplasty and kyphoplasty are shown in .
Table 2 Properties of PMMA, CPC, and CSC bone cements
An ideal bone cement for vertebroplasty and kyphoplasty is expected to have the following properties: high injectability and homogeneity during injection; appropriate setting properties allowing appropriate handling times; high biocompatibility and low risk of necrosis or infection; adequate mechanical strength and appropriate stiffness to match neighboring vertebral bodies; bioactivity and bioresorbability to stimulate new bone ingrowth or appropriate porous structures for osseointegration and angiogenesis; and high radiopacity for tracking during surgery. In order to achieve these goals, nanotechnology has recently been applied to improve the properties of various bone cements.
Polymethylmethacrylate cement
PMMA bone cement has excellent setting and injectability as well as adequate mechanical properties for augmentation of a fractured vertebral body ().Citation40 However, PMMA bone cements have clear disadvantages, including monomer toxicity, high polymerization temperature detrimental to tissue, a lack of biological potential to remodel or integrate into bone, and excessive stiffness that may cause fracture at the adjacent levels.Citation41 Among these problems, the lack of capability for osseointegration and excessive stiffness are intrinsic drawbacks that cannot be avoided by carefully adjusting the surgical procedure.Citation42
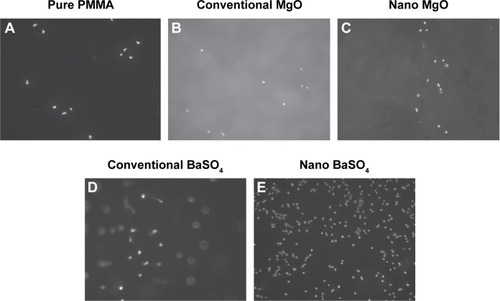
Nanotechnology has recently opened up new opportunities to resolve these problems. For improving osseointegration, nanomaterials with unique topography, surface roughness, surface hydrophilicity, and surface energy properties have shown a strong ability to mediate specific protein adsorption and subsequent cell behavior and tissue regeneration.Citation33 This rationale has been applied to modify PMMA bone cement by incorporating nanoparticles, nanofibers, and other nanostructures for introducing enhanced osseointegrative capability to bio-inert PMMA. For example, a number of studies have shown improved bone cell activity on PMMA which was modified with nanostructured additives even if the additives were not inherently bioactive. Ricker et alCitation43 reported PMMA bone cements with nanophase MgO and BaSO4 had higher osteoblast adhesion densities than pure PMMA cement samples after 4 hours of culture, as shown in . Also, the effect of incorporating 10 wt% MgO nanoparticles (12.8 nm in size) into PMMA on increasing surface roughness of the cement was investigated. The results showed that the bone cement with nanophase MgO significantly increased surface roughness when compared with PMMA modified with the same amount of microparticles.Citation43 Similarly, the increased nanometer surface roughness achieved by embedding TiO2 nanoparticles (32 nm in diameter) in polymer matrix was demonstrated to increase osteoblast activity.Citation39 Based on these in vitro studies, it appears that the improved osteoblast activity of PMMA bone cements are due to the nanoscale surface roughness resulted from addition of oxide nanoparticles.
Figure 2 Fluorescent images showing osteoblast adhesion (nuclei stained with DAPI) on PMMA modified with nano and conventional MgO and BaSO4 (magnification 100×). (A) Pure PMMA; (B) conventional MgO; (C) nano MgO; (D) conventional BaSO4; and (E) nano BaSO4.
Notes: Osteoblast adhesion was significantly increased on nanoparticle-modified PMMA compared with pure PMMA or PMMA modified with conventional particles. Copyright ©2008. Dove Medical Press. Reproduced from Ricker A, Liu-Snyder P, Webster TJ. The influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cement. Int J Nanomedicine. 2008;3:125–132.Citation43
Abbreviations: PMMA, polymethylacrylate; DAPI, 4,6-diamidino-2-phenylindole.
The ultrahigh stiffness of PMMA bone cement, which reaches 2–3 GPa (elastic modulus) and is 4–40 times higher than that of human cancellous bone (50–800 MPa depending on location), is another impediment to its application in treating OVF. This high stiffness has a stress-shielding effect, weakening neighboring vertebrae and ultimately causing them to fracture.Citation39 Creating porous structures in the hardened PMMA bone cement is becoming an effective strategy to overcome the ultrahigh stiffness of PMMA. For example, the elastic modulus and yield strength of PMMA could be decreased when mixed with a 2% aqueous solution of sodium hyaluronate gel.Citation44
In order to develop bone cement with anti-infective properties, silver nanoparticles has been investigated as a antimicrobial additive to bone cement. Alt et al showed that PMMA bone cement loaded with 1 wt% nanosilver (particle sizes of 5–50 nm) had satisfactory antibacterial activity against all bacterial strains tested, including S. epidermidis and methicillin-resistant strains of both S. epidermidis and S. aureus. Among three different concentrations of nanosilver (0.1, 0.5, and 1 wt%), PMMA with 1 wt% nanosilver exhibited the highest antibacterial activity, with no decrease in human osteoblast viability when compared with osteoblasts cultured without adding silver.Citation45 This suggests that PMMA incorporated with nanosilver may be a promising bone cement for clinical use due to it high antibacterial activity and lack of cytotoxicity.
Calcium phosphate cement
As an alternative to PMMA bone cement, CPCs have received much attention due to their chemical similarity to natural bone, high bioactivity and biodegradability, and the isothermal reaction during setting.Citation37 Usually, CPC consists of one or more calcium phosphate compounds (such as CaHPO4, Ca8H2(PO4)6·5H2O) and a liquid phase of water or phosphate-containing aqueous solution. Depending on the composition of the final product, CPCs are usually classified into brushite (dicalcium phosphate dihydrate) CPC or apatite CPC. Firstly developed by Brown and Chow in 1986, the attempt to use CPC for augmentation of osteoporotic bone started in 1992Citation46 and use of CPC for intravertebral reconstruction was proposed in 1995.Citation47 In the last two decades, CPC has also been investigated for its ability to reinforce osteoporotic vertebral bodies,Citation48 thoracolumbar burst fractures,Citation49 and pedicle screw fixation.Citation50 However, most of the commercially available CPC products are not suitable for treating OVF or replacing load-bearing bones due to insufficient mechanical properties, setting and hardening times that are too long, and uncontrolled degradation.
In order to tackle these problems, nanotechnology-enhanced CPCs have been developed by a number of companies and research groups ().Citation51–Citation53 Some of the CPCs are based on strategies of decreasing the particle size of the starting calcium phosphate compounds to a nanometer or submicron range or adding nanophase materials to existing CPC systems. Alpha- and beta-tricalcium phosphates with reduced particle sizes could substantially decrease setting time, facilitate nucleation of apatite crystals, and accelerate hardening of the cement without significantly affecting the final compressive strength (41±1.8 MPa).Citation54 In addition to the strategy of reducing particle size, ultrafine nanofibers prepared by electrospinning were also incorporated into CPC and the results showed clear increases in the fracture resistance of CPC. This CPC-nanofiber composite had an elastic modulus (15–50 GPa) comparable with that of human cortical bone, and degradation of the fibers could introduce pores and interconnective channels for bone ingrowth.Citation55
Table 3 Nanotechnology-enabled calcium phosphate cements
Although adding nanofibers has a positive effect by enhancing the mechanical properties of CPC, identification of suitable nanofibers that are bioresorbable and bioactive while being able to provide sufficient strength and fracture toughness remains challenging.Citation39 Recent studies have demonstrated that carbon nanotubes (CNTs) may be a reasonable choice. In the study by Wang et al, incorporation of 0.2 wt% and 0.5 wt% as-received CNTs into CPC resulted in an increase in compressive strength by 24%, and biomineralized CNTs led to a 120% increase in the compressive strength of CPC.Citation56 Similarly, Chew et alCitation57 reported a high-strength CPC achieved by reinforcement of multi-walled CNTs and bovine serum albumin. This CPC/multi-walled CNT/bovine serum albumin composite had substantially improved compressive strength (~16 MPa) compared with pure CPC cement (~1 MPa). Their study also suggested that hydroxyl functional groups on the surface of multi-walled CNTs improved their reactivity and wettability, leading to strong interfacial bonding with CPC. In addition, strong attractions of Ca2+ and PO34− ions with the functional groups of multi-walled CNTs-OH are expected to promote nucleation and growth of HA crystals in the bone cement, enhancing mechanical strength and osteoconductivity. Further, adding 5 wt% CaSiO3 nanofibers (with an aspect ratio of 9.6) achieved a significant 250% increase in the compressive strength of CPC (from 14.5 to 50.4 MPa) but no decrease in self-setting ability of the cement. Better than CNTs or other nanofibers, CaSiO3 can simultaneously release Ca and Si ions during hydrolysis, and the increases in Ca ion concentration and silica gel precipitation can facilitate precipitation of HA crystals in the cement setting reaction. This CaSiO3 nanofiber-based strategy of enhancing the mechanical properties of CPC is still under development but can potentially be used for the treatment of OVF.
Calcium sulfate cement
Calcium sulfate, also known as plaster of Paris or gypsum, has a long clinical history as a bone graft substitute.Citation58 Surgical-grade CSC is mainly calcium sulfate hemihydrate (2CaSO4·H2O, CSH), the physical properties of which are shown in . Compared with CPC, CSC has relatively higher mechanical strength but degrades much faster. Studies show that it may be fully absorbed within a few weeks after implantation in vivo.Citation59 For application in the treatment of OVF, this fast degradation rate generates problems with regard to insufficient mechanical stability and mismatch between bone remodeling and cement receding.
Incorporation of nanostructured materials has been shown to be an effective strategy to improve the mechanical properties of CSC. The influence of ceramic nanoparticles on the hydration reaction and consequent mechanical properties of the CSC cement was investigated.Citation60 The results showed that a compressive strength as high as 72 MPa was achieved after 42 days for the CSC doped with 10 wt% of electric arc furnace dust, which was mainly composed of ZnFe2O4 nanoparticles. Another study of a nanocomposite cement containing CSH and biomimetic nanocrystalline carbonated apatite revealed that initial setting time and injectability of the nanocomposite increased to 33 minutes and 95% compared with 7 minutes and 71% for pure CSH, respectively.Citation61 To control the degradation rate of CSC, Liu et al studied degradation of a nanocrystalline HA/CSH composite and showed that addition of 30 wt% HA nanocrystals to CSH had a significant effect on the degradation rate of the composite cement.Citation62
Other bone cements
Other types of injectable bone cements, including calcium silicate cement (CSiC), magnesium phosphate cement, and bioactive glass cement, have also received attention. CSiC is primarily used for replacement of dentine, but there is a highly basic calcium hydroxide byproduct that is potential toxic to osteogenic activity and bone regeneration. Some nanoparticles are used to reduce generation of this calcium hydroxide byproduct while also improving the mechanical properties of CSiC. For example, CSiC/Nano-Fe2O3 and CSiC/Nano-SiO2 composites have been developed,Citation63 and the compressive and flexural strengths of the CSiC cements containing nano-SiO2 and nano-Fe2O3 were both higher than those of the CSiC cement. Scanning electron microscopy (SEM) revealed that the nanoparticles acted not only as fillers but also as activators promoting hydration of the cement. Moreover, blended CSiC were fabricated by adding CNTs and nanoclay (kaolin clay) to the cement, and the nanoclay was found to improve CNT dispersion and interfacial interaction between cement phases. For example, replacement of CSiC by 6 wt% exfoliated nanoclay increased compressive strength by 18%, and combination of 6 wt% nanoclay and 0.02 wt% CNTs increased the compressive strength by 29%, all compared with unmodified CSiC cement.
In summary, combining nanomaterials with bone cement to create nanocomposite has showed great potential for improving the mechanical properties of bone cement in the treatment of OVF.Citation39 This strategy also has the ability to provide a bioactive environment for enhancing bone cell recruitment, adhesion, proliferation, and differentiation.Citation39 Therefore, nanotechnology-enhanced bone cements are expected to be better for the treatment of OVF and thus should be further studied and developed.
Injectable hydrogels
Because of their biomimetic structure similar to that of extracellular matrix, a number of hydrogels have been developed as injectable carriers of growth factors, drugs, or cells for bone repair or regeneration. Unlike the aforementioned injectable bone cements, injectable hydrogel has in situ cross-linking ability that can be initiated by temperature, pH, light, or ions. For example, a thermosensitive injectable hydrogel for long-term sustained and controlled drug delivery was created by adding biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanoparticles to chitosan.Citation64 Abdel-Bar et al developed an injectable and thermoreversible chitosan/β-glycerophosphate hydrogel system that enabled controlled release of vancomycin for the treatment of orthopedic infections.Citation65 Many hydrogels contain rich carboxylic groups that can chelate with calcium-containing phosphates, thus facilitating the formation of polymer/calcium phosphate composites resembling natural bone.
However, hydrogels alone are generally too soft to be applied for the repair or treatment of load-bearing bones like the spine. Incorporating nanoscale components into the hydrogel system is an effective approach to reinforce hydrogel and combine new functionalities. Campbell et al devised an injectable composite hydrogel of poly (N-isopropylacrylamide) reinforced by superparamagnetic iron oxide nanoparticles, which showed extremely high elasticity (G’ >60 kPa) and contrast for magnetic resonance imaging purposes.Citation66 Silk fibroin hydrogel containing HA nanoparticles (silk fibroin/HA composite hydrogel) is another promising injectable hydrogel developed for bone regeneration and repair.Citation67 Similarly, a variety of gelatin-based hydrogels modified by nanoparticles have been developed.Citation68,Citation69 For example, a composite hydrogel of photopolymerizable gelatin incorporating recombinant bone morphogenetic protein (BMP)-2 and sulfated chitosan nanoparticles was prepared and shown to have clear advantages for sustained and sequential delivery of growth factors (eg, BMP-2 and BMP-7).Citation68 Biodegradable gelatin hydrogel incorporating gold nanoparticles (average size 27±3 nm) was also studied.Citation70 The results showed that these gel-gold nanoparticles were degradable with collagenase and promoted significant alkaline phosphatase activity, proliferation, viability, and osteogenic differentiation of stem cells. Moreover, the gel-gold nanoparticles achieved significantly greater new bone formation in vivo, indicating its potential for treating fractured bones.
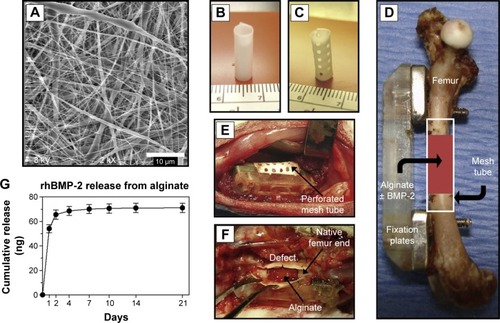
Although nanoparticle-modified hydrogels demonstrate great potential for delivering drugs to treat diseases of bone, use of hydrogels in the treatment of OVF remains challenging. A temporary fixation strategy was developed recently in order to expand the application of hydrogels to load-bearing bones like spine, as shown in . Specifically, a delivery system consisting of an electrospun polycaprolactone nanofibrous mesh tube was used to support alginate hydrogel until sufficient bone regeneration was achieved ().Citation71 The main advantages of the nanofibrous mesh tube were that it could support cell infiltration and bone formation while acting as a barrier to separate the osseous and nonosseous regions. Local delivery of BMP-2 via the hydrogel was tested, and the results showed that the BMP-2 delivery system could effectively repair large bone defects ().
Figure 3 An alginate-based hybrid system consisting of electrospun nanofibrous mesh for growth factor delivery and bone repair.
Notes: (A) Scanning electron micrograph of electrospun nanofibrous mesh illustrating the smooth and bead-free nanofibers. Tubular bone implants made from nanofibrous mesh (B) without and (C) with perforations. (D) Scheme of mesh tube implant in segmental bone defect, where modular fixation plates are used to stabilize the bone and a nanofibrous mesh tube is placed in a defect 8 mm long. Also, alginate hydrogel with or without rhBMP-2 may be injected into the hollow tube. (E) Photograph of the surgical site after placing a perforated mesh tube. (F) The mesh tube was retrieved 1 week after implantation and the mesh tube was cut open, where the alginate was still present inside the defect. (G) Curve showing release kinetics of rhBMP-2 from alginate over 21 days in vitro, and sustained release of the rhBMP-2 was observed during the 1st week. Reproduced from Biomaterials. Vol 32. Kolambkar YM, Dupont KM, Boerckel JD. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. 65–74. 2011, with permission from Elsevier.Citation71
Abbreviation: rhBMP, recombinant human bone morphogenetic protein-2.
Nanoparticles for localized drug delivery and treatment
Targeting of systemically administered drug conjugates and particles to bone is an attractive and minimally invasive option for treatment of osteoporosis and its complications. However, drug conjugates may suffer from instability of the active components during delivery to the site of action or at the site of action.Citation72 Therefore, a localized and controlled drug delivery system for OVF patients would be an attractive strategy for the enhancement of bone-implant integration during or after surgery. Nanotechnology also provides new ways to enable or enhance local treatment of OVF, and a number of examples are listed in .Citation72–Citation77 Specifically, a novel biodegradable thermosensitive drug carrier based on amphiphilic monomethoxypoly (ethylene glycol)-co-poly(lactic-co-glycolic acid) (mPEG-PLGA) diblock copolymers has been developed for treating osteomyelitis.Citation76 The prepared mPEG–PLGA hydrogels were useful for formulation of injectable drug depots, and an in vivo study confirmed sustained release of teicoplanin from mPEG-PLGA hydrogel, which could efficiently treat osteomyelitis in rabbits and avoid the disadvantages of PMMA cement beads.
Table 4 Nanotechnology-enabled local treatments of osteoporotic vertebral fracture (OVF) or osteoporosis
In addition, bisphosphonate-loaded calcium phosphate nanoparticles (nCaPs) have been prepared for treating problems associated with osteoporosis.Citation78 The peri-implant bone response to titanium implants coated with bisphosphonate-loaded nCaPs was evaluated in an established rat femoral condyle implantation model of osteoporotic and healthy bone under compromised medical conditions. The results demonstrated that combined use of nCaPs and bisphosphonates increased both bone formation and bone-to-implant contact, and also suggested that simultaneous targeting of bone formation (by nCaPs) and bone resorption (by bisphosphonates) represents an effective strategy for improving bone-implant integration, especially in the case of osteoporotic patients.
Nanostructured radiopacifiers
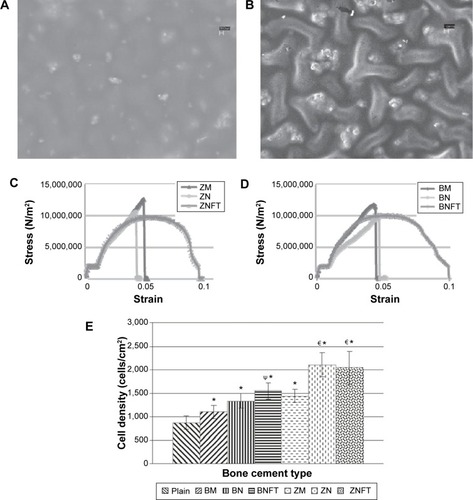
Since bone cement materials are not usually visible under X-ray radiation, adding radiopacifier becomes necessary to enable monitoring and tracking of bone cement during minimally invasive surgeries like vertebroplasty and kyphoplasty. Micron-sized BaSO4 and zirconium dioxide (ZrO2) particles have been used as radiopacifiers in bone cements for years.Citation79 However, the evidence shows that adding high contents of micron-sized BaSO4 or ZrO2 is detrimental to the mechanical and biological properties of bone cement. For example, addition of micron-sized BaSO4 particles decreased the tensile strength of PMMA bone cement from 45 MPa to 36 MPa, since agglomerates of BaSO4 could serve as initiation sites for fatigue cracks.Citation43 Based on the reinforcing strategy mentioned in the previous sections, there is a growing interest in developing a nanosized radiopacifier with high X-ray contrast which can promote the mechanical strengths and biological properties of the bone cement at the same time. For instance, when compared with conventional BaSO4 microparticles, addition of 10 wt% BaSO4 nanoparticles into commercial PMMA cement resulted in a 41% increase in tensile strain-to-failure, a 70% increase in tensile work-of-fracture, and a twofold increase in the fatigue life of the cement ().Citation80 Similar results has been found for bone cements containing ZrO2 nanoparticles (). Furthermore, a study by Ajeesh et alCitation81 showed improved osteoblast adhesion and proliferation on PMMA cement containing ZrO2 nanoparticles when compared with cement containing microparticles. In addition to the effect of decreased size, surface modification of a nanosized radiopacifier is also effective in improving both the biological and mechanical properties of bone cement.Citation36 For example, PMMA bone cements containing ZrO2 nanoparticles functionalized with 3-(trimethoxysilyl)propylmethacrylate showed greater in vitro osteoblast adhesion and improved mechanical strength when compared with conventional bone cements containing micron-sized particles. Mechanical tests also revealed that the failure modes of bone cement containing functionalized ZrO2 nanoparticles were less brittle and had a clear plastic deformation region. Moreover, bone cements containing functionalized nanoparticles showed greater radiopacity than their nonfunctionalized counterparts.
Figure 4 Morphology, mechanical properties and biocompatibility of bone cements containing functionalized nanoparticles.
Notes: Scanning electron micrographs of (A) ZNFT bone cement containing functionalized ZrO2 nanoparticles and (B) BNFT bone cement containing functionalized BaSO4 nanoparticles. Representative compressive stress-strain curves for (C) various bone cements containing ZrO2 particles, and (D) bone cements containing BaSO4 particles. (E) Twenty-four-hour osteoblast adhesion tests showing cell adhesion density as a function of bone cements type. ΨCompared to bone cements containing micron BaSO4 particles, adhesion was found to be greater on bone cements containing BaSO4 nano-particles functionalized with TMS (P<0.05). €WRT bone cements containing micron ZrO2 particles, adhesion was found to be greater on bone cements containing unfunctionalized ZrO2 nano-particles (P<0.05) and ZrO2 nano-particles functionalized with TMS (P<0.1). Copyright ©2010. Dove Medical Press. Reproduced from Gillani R, Ercan B, Qiao A, Webster TJ. Nanofunctionalized zirconia and barium sulfate particles as bone cement additives. Int J Nanomedicine. 2010;5:1–11.Citation79
Abbreviations: BM, BaSO4 micron particles; BN, BaSO4 nanoparticles; TMS, 3-(trimethoxysilyl) propyl methacrylate; BNFT, BaSO4 nanoparticles functionalized with TMS; ZM, ZrO2 micron particles; ZN, ZrO2 nanoparticles; ZNFT, ZrO2 nanoparticles functionalized with TMS.
Since CPC and CSC are biodegradable, radiopaque particles added to these cements can be released into the surrounding tissue as the cement degrades. Radiopacifiers such as BaSO4 and ZrO2 are barely soluble, and release of such particles may result in a biocompatibility hazard. Due to their large surface area-to-volume ratio, a lower concentration of radiopaque nanoparticles can have the same radiopacity as a large amount of micron-sized radiopacifier. This reduced dosage may have less of an adverse biological effect. In addition, biocompatible nanoparticles, such as iron oxide,Citation81 alumina,Citation82 strontium-modified titanium nanotubes,Citation83 and tantalum pentoxide,Citation84 have shown improved radiopacity when blended with bone cements. Since nanosized radiopacifiers may have greater radiopacity than their micron-sized counterparts, this advantage allows the nanoparticle modified-bone cement to have higher resolution and contrast when compared with the surrounding tissue, which is likely to allow patients to be subjected to lower X-ray doses.Citation39
Nanotechnology-enhanced instruments for treatment of OVF
Balloon catheter for kyphoplasty
A balloon catheter comprises a thin catheter tube and a distensible balloon located at the distal end of the catheter.Citation85 A balloon catheter is the key instrument used in kyphoplasty to create a cavity in the compressed or fractured vertebral body, thereby recovering the height of the fractured vertebra while creating room for cement reinforcement. However, a common complication of kyphoplasty is leakage of cement from the vertebral body, which may cause pulmonary embolism, nerve damage, paralysis, and even death.Citation86,Citation87 Special balloon catheters have been developed for restoring the height of a vertebral body or reducing/avoiding cement leakage.Citation88–Citation90 However, most of these balloon catheters are non-degradable and needed to be removed prior to the injection of bone cement.
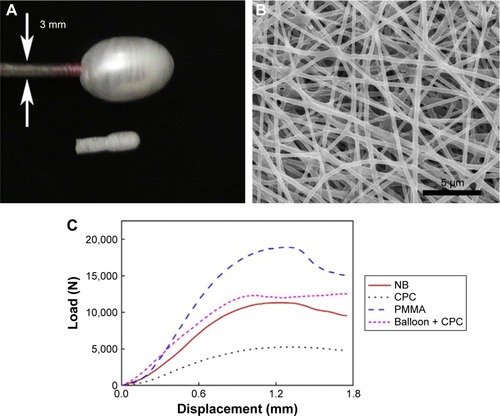
Recently, degradable nanofibrous poly(d,l-lactide-co-ε-caprolactone) balloons (ENPBs) were prepared by electrospinning ().Citation91 Preliminary studies showed that these nanotechnology-enhanced balloon catheters could separate the cement from the surrounding environment, indicating an ability to eliminate cement leakage and prevent the water-induced collapse of cement often seen with CPC. ENPBs filled with CPC also showed enough strength to restore the height of a fractured vertebral body. Further, ENPBs showed good biodegradability and cytocompatibility, and calcium-based bone cements can release calcium ions throughout the ENPB membrane. These advantages suggest that ENPBs could be effective balloon catheters for CPC cement, enabling simple, convenient, and safe delivery of bone cements in kyphoplasty.
Figure 5 Morphology and mechanical property of electrospun nanofibrous P(DLLA-CL) balloons (ENPBs).
Notes: (A) Photograph of inflated (top) and non-inflated (bottom) ENPBs. Arrows indicate the diameter of balloon catheter. (B) Scanning electron micrograph of electrospun nanofibers in an ENPB. (C) Typical force-displacement curves for natural bones, nature bones injected with CPC, natural bones injected with PMMA, and natural bones with balloon insertion and CPC injection, respectively. Reprinted from Nanomedicine. Vol 9. Sun G, Wei D, Liu X, et al. Novel biodegradable electrospun nanofibrous P(DLLA-CL) balloons for the treatment of vertebral compression fractures. 829–838;2013, with permission from Elsevier.Citation91
Abbreviations: CPC, calcium phosphate cement; ENPB, electrospun nanofibrous poly(d,l-lactide-co-ε-caprolactone) balloon; NB, nature bone; PMMA, polymethylacrylate.
Pedicle screws
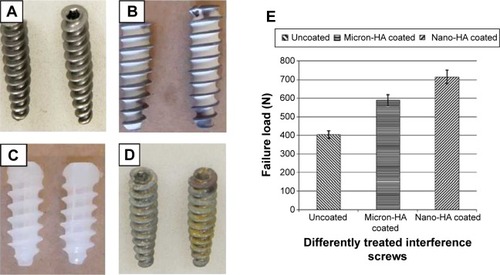
Pedicle screws usually serve as anchoring points for implantation of spinal instrumentation in the treatment of spinal fractures and deformities. The strength of the screw in contact with the surrounding bone diminishes as the bone degrades due to osteoporosis, which would cause screw loosening and subsequent instability in spinal instrumentation or even fixation failure.Citation92 Different strategies have been investigated for their ability to improve the fixation efficacy of pedicle screws in osteoporotic bone, including adjustments in thread design and screw shape as well as surface modification of the screws.Citation93 In the past few years, nanostructural modification of the surface of titanium pedicle screws has yielded promising results in research and clinical tests. It is now widely accepted that the surface topography of titanium and its alloys affects the attachment, proliferation, and differentiation of osteoblasts. This understanding is important for enhancing the osseointegrative capability of bone screws. Compared with screws made from uncoated Ti6Al4V and conventional HA-coated Ti6Al4V, a nanosized HA coating on Ti6Al4V screws significantly enhanced the fixation efficacy of the screws and led to better stability, bone ingrowth, and osseointegration.Citation94 shows images of Ti6Al4V and bioabsorbable screws before and after nano-HA coating. The variation in failure loads for coated Ti6Al4V interference screws after extraction are shown in . The nano HA-coated Ti6Al4V interference screws also showed good osteoblastic activity and minimal formation of vascular granulation tissue.
Figure 6 Images and failure loads of metallic interference screws before and after coating with HA.
Notes: Photographs of metallic (Ti6Al4V) interference screws (A) before and (B) after coating with nano-HA, (C) bioabsorbable interference screws, and (D) metallic screws retrieved after implantation. (E) Failure loads of metallic interference screws coated with micro-HA and nano-HA after extraction, compared with uncoated screws. Reproduced from Springer and Eur J Orthop Surg Traumatol. Vol 24, 2014:813–819. Influence of micro- and nano-hydroxyapatite coatings on the osteointegration of metallic (Ti6Al4V) and bioabsorbable interference screws: an in vivo study. Aksakal B, Kom M, Tosun HB, Demirel M. With kind permission from Springer Science and Business Media.Citation94
Abbreviation: HA, hydroxyapatite.
Nano-HA coatings have not been tested in humans, but Olerud et alCitation95 investigated the effects of HA coating on pedicle screws made from wrought stainless steel (SAF 2507). In their study, 23 consecutive patients undergoing lumbar fusion were randomly assigned to one of three treatment groups. The first group received uncoated stainless steel screws, the second group received screws partly coated with HA, and the third group received screws fully coated with HA. After 11–16 months, 21 screws had been extracted and the extraction torque was recorded. At removal, the extraction torques exceeded the upper limit of the torque wrench (600 N·cm) for many HA-coated screws. The calculated mean extraction torque was 29±36 N·cm for the uncoated group, 447±114 N·cm for the partly coated group, and 574±52 N·cm for the fully-coated group (P<0.001). Radiographs showed more radiolucent zones surrounding the uncoated screws than the HA-coated screws (P<0.001). The results suggested that HA coating of pedicle screws resulted in improved fixation with a reduced risk of loosening of the screws.
Anodization is another promising nanotechnology to improve osseointegrative ability on titanium.Citation96 A large number of studies have confirmed that adhesion, proliferation, and differentiation of osteoblasts are enhanced on anodized nanotubular titanium when compared with titanium surfaces containing anodized nanoparticles or without anodization.Citation97 These enhancements are arguably attributed to the increased initial adsorption of vitronectin and fibronectin (proteins known to promote cell adhesion) on the anodized nanotubular titanium compared with other titanium surfaces. For example, a cytocompatibility study of anodized nanotubular substrates showed that a 33% increase in osteoblast adhesion, which was correlated with a 18% increase in vitronectin adsorption and a 30% increase in fibronectin adsorption onto anodized titanium when compared with conventional titanium.Citation98 Studies also suggest a possibly higher clinical success rate for the anodized titanium implants in comparison with titanium surfaces of similar shape without anodization. In addition, when compared with conventional titanium screws, the anodized titanium screws resulted in enhanced skin growth and decreased infection.Citation99 Similarly, Oha et alCitation100 reported enhanced osteogenic differentiation of mesenchymal stem cells cultured on anodized nanotubular titanium compared with conventional titanium samples. They also reported that osteoblasts deposited more calcium and synthesized more osteocalcin, which are important for bone formation, on anodized titanium compared with conventional titanium.
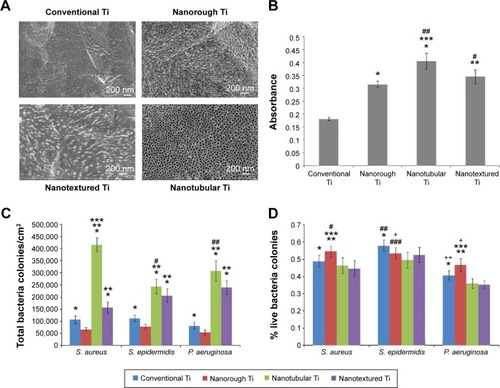
Infection of an orthopedic implant is another problem needing to be resolved. Some researchers have demonstrated that certain nanosized titanium topographies may be useful for reducing bacterial adhesion while promoting formation of bone tissue.Citation101–Citation103 Puckett et alCitation104 showed that the surface features of nanosized titanium are possibly a simple means for reducing bacterial adhesion and subsequent infection on titanium implants like pedicle screws. Specifically, nanorough titanium surfaces showed decreased adhesion of S. aureus, S. epidermidis, and Pseudomonas aeruginosa (three types of bacteria commonly associated with orthopedic implant infection), while nanotubular and nanorough titanium created by anodization resulted in increased bacterial attachment. shows the surface features of conventional titanium, anodized nanotubular titanium, fibronectin adsorption, and growth of bacteria cultured on conventional and anodized nanotubular titanium for 1 hour. shows that titanium with appropriate nanotopography may be useful for reducing bacterial adhesion while promoting formation of new bone, and so should be further studied for improving the efficacy of titanium-based instruments like pedicle screws.
Figure 7 Morphology and antibacterial capacity of conventional Ti, nanorough Ti, nanotubular Ti, and nanotextured Ti.
Notes: (A) Scanning electron micrographs of (clockwise) conventional Ti as purchased, nanorough Ti fabricated by electron beam evaporation, nanotextured Ti fabricated by anodization for 1 minute in 0.5% hydrofluoric acid at 20 V, and nanotubular Ti fabricated by anodization for 10 minutes in 1.5% hydrofluoric acid at 20 V. Scale bar 200 nm. (B) Increased fibronectin adsorption on nanorough, nanotubular, and nanotextured Ti surfaces compared with conventional Ti surface. (C) Decreased Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa colonies on nanorough and conventional Ti surfaces compared with nanotubular and nanotextured Ti surfaces after 1 hour. (D) The highest percentage of live bacteria colonies for S. aureus, S. epidermidis, and P. aeruginosa attached to the nanorough Ti surfaces after 1 hour compared with the conventional, nanotextured, and nanotubular Ti surfaces. *P<0.1 compared to nanotextured Ti; **P<0.01 compared to nanotextured Ti; ***P<0.05 compared to nanotubular Ti; #P<0.05 compared to conventional Ti; ##P<0.01 compared to nanotubular Ti; ###P<0.1 compared to nanotubular Ti; +P<0.1 compared to conventional Ti; ++P<0.1 compared to nanotubular Ti for respective bacteria lines. Reprinted from Biomaterials. Vol 31. Puckett S, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. 706–713;2010, with permission from Elsevier.Citation104
Abbreviation: Ti, titanium.
PEEK-based spinal implants
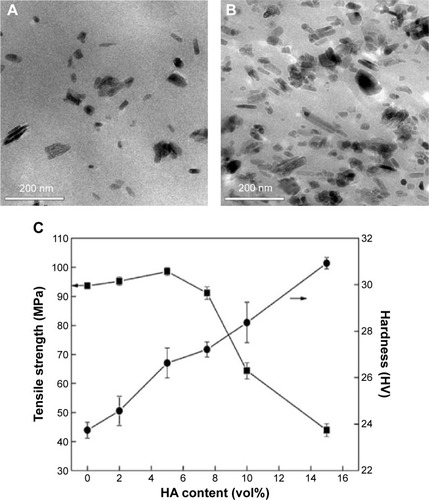
Polyether-ether-ketone (PEEK) has recently been attracting attention as a high-strength polymer with favorable imaging compatibility and stiffness that closely matches bone, rendering it suitable for orthopedic, trauma, and spinal implant applications.Citation105,Citation106 For spinal implants, PEEK has many advantages, including excellent mechanical properties, chemical inertness, ease of processing, biocompatibility, low toxicity, and radiolucency. PEEK and PEEK-based composites are commonly used for fabricating spinal fusion cages.Citation107 However, PEEK faces the problem of insufficient osseointegrative capability and the common solution of adding HA to PEEK suffers from dramatically decreased mechanical strength. To solve this problem, nanophase HA has been combined with PEEK and showed better mechanical properties due to the strong adhesion of HA fillers to the PEEK matrix.Citation108 show TEM images of PEEK-HA nanocomposites with 5.0 vol% and 15.0 vol% HA content. The nanocomposite showed enhanced mechanical properties, with tensile strength increased from 92 MPA to 98 MPa and hardness increased from 23 HV to 27 HV when the HA content increased from 0 vol% to 5 vol% ().Citation109 The study also revealed that well-dispersed HA nanoparticles bonded strongly to PEEK and no debonding was observed, suggesting that using nanoparticles is a possible solution to the debonding problem encountered with current PEEK-HA composites. However, the tensile strength decreased from 98 MPa to 24 MPa as the HA content increased from 5 vol% to 10 vol% due to widespread agglomeration of HA nanoparticles.
Figure 8 Transmission electron micrographs of PEEK-HA nanocomposites with (A) 5.0 vol% and (B) 15.0 vol% HA content. (C) Ultimate tensile strength and microhardness of PEEK-HA nanocomposites as a function of HA content.
Note: The arrow in C means the micro-hardness of the nanocomposites. Reprinted from Mater Sci Eng A. Vol 528 (10–11). Wang L, Weng LQ, Song SH, Zhang ZG, Tian SL, Ma R. Characterization of polyetheretherketone–hydroxyapatite nanocomposite materials. 3689–3696; 2011, with permission from Elsevier.Citation108
Abbreviations: HA, hydroxyapatite; PEEK, polyether-ether-ketone.
In a further attempt to avoid agglomeration of HA nanoparticles, a novel PEEK/HA nanocomposite was developed by in situ synthesis.Citation110 In this process, HA particles were first mixed into PEEK oligomers with short chains to achieve low viscosity, good wetting, and contact between HA nanoparticles and PEEK molecules. The PEEK oligomers then started to polymerize and wrap HA nanoparticles in the PEEK matrix. The tensile strength of the HA/PEEK composite reached as high as 108 MPa at an HA content of 6.1%. The composites with an HA content below 17.4% exhibited a plastic fracture mode, while a brittle fracture mode was observed in the composites with HA content above 17.4%.
To improve the mechanical and biological properties of PEEK, nano-TiO2 reinforced PEEK composites (n-TiO2/PEEK) have been studied.Citation111 In vitro tests showed that n-TiO2 promoted cell attachment and improved osteoblast spreading. In vivo tests showed that n-TiO2 improved bone regeneration around the implants compared with pure PEEK, as assessed by micro-computed tomography and histological analysis. Li et alCitation112 fabricated HA/PEEK nanocomposites containing 15.1, 21.6, 29.2, and 38.2 vol% nanosized HA and reported that the tensile strength and fracture strain of the nanocomposites filled with 21.6 vol% and 29.2 vol% nanosized HA closely matched those of human cortical bone. In vitro tests of immersion, cell adhesion, and proliferation in simulated body fluid also suggested that the 29.2 vol% nano HA/PEEK nanocomposite had better biocompatibility than the other specimens. Therefore, development of PEEK composites containing nanosized bioactive materials may be an effective way of obtaining both mechanical and biological benefits for PEEK-based spinal implants.
Safety concerns with nanotechnology
Nanotechnology offers vast potential for massive improvement in the field of orthopedic repair and regeneration, particularly with regard to improving the interaction between host bone and the implant.Citation113 Injectable nanomaterial and nanotechnology-enhanced instruments have demonstrated their superior properties in terms of bioactivity, radiopacity, mechanical strength, antibacterial effect, and osseointegration when compared with traditional materials and unmodified instruments.Citation114 However, the safety concern is still an important issue in implementing nanotechnology for the treatment of OVF and other orthopedic problems. For example, nanophase silver is of significant interest for preventing infections. However, silver nanoparticles themselves also have severe toxicity due to their size and shape and the silver ions released.Citation115 As a result, a lot of nanosilver-embedded composites developed to date have come with a risk of uncontrollable toxicity during degradation of the implanted composite in humans. The aforementioned BaSO4 nanoparticles that were added to bone cementsCitation116 also face the health risk of released barium ions, and the long-term influence of BaSO4 nanoparticles on human health is still unclear. Obviously, further studies on the extensive toxicity and safety risks of nanotechnology are necessary before nanotechnology can be translated into clinical treatments.
Summary and future directions
Emergence of nanotechnology has provided new strategies for improving the properties of biomedical materials for orthopedic applications. In this article we have summarized the recent progress and perspectives in nanotechnology for the surgical treatment of OVF. For developing better bone cements and surgical instruments for minimally invasive vertebroplasty and kyphoplasty, incorporating nanomaterials (eg, nanoparticles, nanofibers, nanocrystals) has been demonstrated to be an effective means of improving the biological properties, radiopacity, mechanical properties, and safety of bone cements, from PMMA to CPC. The positive and effective role of nanomaterials is probably a result of the appropriate surface roughness, nanotopography, hydrophobicity, and surface area generated by adding nanomaterials, which is favorable for cellular activity, protein absorption, enhanced radiopacity, and strengthening the interaction between nanoscale additives and bone cement components. Further research on these mechanisms may potentially lead to new bone cements or surgical tools with improved efficacy. Despite the fact that nanophase composites have shown great potential for improving bone cements or surgical instruments used for treating OVF, problems and unknown risks associated with these materials remain.
Future directions in this area are likely to encompass the following aspects. Firstly, extensive investigations on the agglomeration problem, toxicology, and safety concerns regarding nanoscale additives for bone cements or implantable materials will continue. In addition, optimized parameters for fabrication and modification of such nanocomposites will be further studied. Secondly, a number of new nanomaterials such as injectable hydrogels with advanced properties, including sensing, detecting, and environment-responsive capabilities are expected to play a more important role in the treatment of OVF. These new materials will be administered to defective sites in less invasive ways and allow more functionalities for drug delivery, cell therapy, and theranostic treatment of spinal fractures. Compared with the bone cements and metallic implants mentioned above, these materials are smart and responsive to temperature, pH, light, ionic, or biological change. Although many of these new smart biomaterials show promise, it is still challenging to use these materials for the treatment of OVF due to insufficient mechanical strength. Therefore, the third research direction will be to enhance the mechanical properties of bone cements for wider application in load-bearing bones including the spine. This review has shown some possible approaches to reinforce new smart materials like hydrogel by incorporating nanomaterials into its network, but further studies in this direction are still needed. Lastly, studies aiming at improving biocompatibility or osseointegration properties of spinal implants and decreasing the risk of infection associated with these implants are also of great interest. It is known that surface properties of metallic implants can directly affect bone and bacterial behaviors in both the short and long term. Therefore, surface modification through nanotechnology is expected to play an important role in design and fabrication of better spinal implants.
Acknowledgments
The authors would like to thank the Jiangsu Provincial Special Program of Medical Science (BL2012004), the project funded by the Priority Academic Program Development of Jiangsu High Education Institutions, National Basic Research Program of China (973 Program, 2014CB748600), the Jiangsu R&D Innovation Program (BY2014059-07), the National Natural Science Foundation of China (51472279), the Jiangsu Six Peak of Talents Program (2013-WSW-056), the Chinese Ministry of Education Star-up Fund for Oversea Scholars, and the Open Fund of State Key Laboratory of Nonlinear Mechanics for supporting this work.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- CummingsSRBlackDMRubinSMLifetime risks of hip, Colles, or vertebral fracture and coronary heart disease among white postmenopausal womenArch Intern Med1989149244524482818106
- World Health OrganizationAssessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study GroupWorld Health Organ Tech Rep Ser199484311297941614
- MessinaCBandiraliMSconfienzaLMPrevalence and type of errors in dual-energy x-ray absorptiometryEur Radiol2015251504151125428701
- PisaniPRennaMDConversanoFScreening and early diagnosis of osteoporosis through X-ray and ultrasound based techniquesWorld J Radiol2013539841024349644
- ConversanoFFranchiniRGrecoAA Novel ultrasound methodology for estimating spine mineral densityUltrasound Med Biol20154128130025438845
- SunyeczJAThe use of calcium and vitamin D in the management of osteoporosisTher Clin Risk Manag2008482783619209265
- HernlundESvedbomAIvergårdMOsteoporosis in the European Union: medical management, epidemiology and economic burdenArch Osteoporos2013813624113837
- BoughtonBOdleTOsteoporosisLongeJLThe Gale Encyclopedia of Medicine3rdFarmington Hills, MI, USAThompson Gale2006
- International Osteoporosis FoundationInvest in your bonesOsteoporosis in the workplace Available from: http://www.bbcbonehealth.org/documents/workplacereportEN.pdfAccessed June 6, 2015
- LiaoRXYuMJiangYXiaWBManagement of osteoporosis with calcitriol in elderly Chinese patients: a systematic reviewClin Interv Aging2014951552624729692
- KennethGSPietGProgress in osteoporosis and fracture prevention: focus on postmenopausal womenArthritis Res Ther20091125119849819
- KennethESJonathanRPElizabethAWFalls, fractures, and osteoporosis after stroke – time to think about protection?Stroke2002331432143611988628
- EastellRCedelSLWahnerHWClassification of vertebral fracturesJ Bone Miner Res199162072152035348
- HeZWZhaiQPHuMLBone cements for percutaneous verte-broplasty and balloon kyphoplasty: current status and future developmentsJ Orthop Translation20153111
- CosmanFBeurSJLeBoffMSClinician’s guide to prevention and treatment of osteoporosisOsteoporos Int2014252359238125182228
- UlmarBBrunnerAGühringMSchmälzleTWeiseKBadkeAInter- and intraobserver reliability of the vertebral, local and segmental kyphosis in 120 traumatic lumbar and thoracic burst fractures: evaluation in lateral X-rays and sagittal computed tomographiesEur Spine J20101955856619953277
- FreedmanBAPotterBKNestiLJGiulianiJRHamptonCKukloTROsteoporosis and vertebral compression fractures – continued missed opportunitiesSpine J2008875676218343730
- PratherHWatsonJOGilulaLANonoperative management of osteoporotic vertebral compression fracturesInjury200738S40S4817723791
- NakashimaHYukawaYItoKMachinoMIshiguroNKatoFCombined posterior-anterior surgery for osteoporotic delayed vertebral fracture with neurologic deficitNagoya J Med Sci20147630731425741039
- KoesBWTulderMWPeulWCDiagnosis and treatment of sciaticaBMJ2007231313131717585160
- MarcucciGBrandiMLKyphoplasty and vertebroplasty in the management of osteoporosis with subsequent vertebral compression fracturesClin Cases Miner Bone Metab20107516022461293
- GalibertPDeramondHRosatPLe GarsDNote préliminaire sur le traitement des angiomes vertébraux par vertébroplastie acrylique percutanée [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]Neurochirurgie198733166168 French3600949
- CloftHJJensenMEKyphoplasty: an assessment of a new technologyAm J Neuroradiol20072820020317296979
- SpivakJMJohnsonMGPercutaneous treatment of vertebral body pathologyJ Am Acad Orthop Surg2005131617
- HadjipavlouAGTzermiadianosMNKatonisPGSzpalskiMPercutaneous vertebroplasty and balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures and osteolytic tumoursJ Bone Joint Surg Br2005871595160416326869
- MathisJMOrtizAOZoarskiGHVertebroplasty versus kyphoplasty: a comparison and contrastAJNR Am J Neuroradiol20042584084515140732
- BouzaCLópez-CuadradoTCedielPSaz-ParkinsonZAmateJMBalloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysisBMC Palliat Care20098122119740423
- Van MeirhaegheJBastianLBoonenSRanstamJTillmanJBWardlawDA randomized trial of balloon kyphoplasty and nonsurgical management for treating acute vertebral compression fractures: vertebral body kyphosis correction and surgical parametersSpine (Phila Pa 1976)20133897198323446769
- RotterRMartinHFuerdererSVertebral body stenting: a new method for vertebral augmentation versus kyphoplastyEur Spine J20101991692320191393
- DavidBLaurieAKDisadvantages of infection surveillance by medical record chart reviewAm J Infect Control19819151710283811
- EngelEMichiardiANavarroMLacroixDPlanellJANanotechnology in regenerative medicine: the materials sideTrends Biotechnol200826394718036685
- LiuHWebsterTJNanomedicine for implants: a review of studies and necessary experimental toolsBiomaterials20072835436921898921
- ZhangLJWebsterTJNanotechnology and nanomaterials: promises for improved tissue regenerationNano Today200946680
- HassimARachmawatiHPreparation and characterization of calcium carbonate nanoparticles. The third nanoscience and nanotechnology symposium 2010AIP Publishing201012841195198
- LarssonSCement augmentation in fracture treatmentScand J Surg20069511111816821654
- AroraMChanEKGuptaSDiwanADPolymethylmethacrylate bone cements and additives: a review of the literatureWorld J Orthop20134677423610754
- BarinovSMKomlevVSCalcium phosphate bone cementsInorg Mater20114714701485
- NelsonCLMcLarenSGSkinnerRASmeltzerMSThomasJROlsenKMThe treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pelletsJ Orthop Res20022064364712168649
- NoYJRoohani-EsfahaniSIZreiqatHNanomaterials: the next step in injectable bone cementsNanomedicine (Lond)201491745176425321173
- RodriguezLCChariJAghyarianSGindriIMKosmopoulosVRodriguesDCPreparation and characterization of injectable brushite filled-poly (methyl methacrylate) bone cementMaterials2014767796795
- HarperEJBioactive bone cementsProc Inst Mech Eng H19982121131209612002
- GlimcherMJPMMAInstr Course Lect198736493325562
- RickerALiu-SnyderPWebsterTJThe influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cementInt J Nanomedicine2008312513218488423
- BogerABohnerMHeiniPProperties of an injectable low modulus PMMA bone cement for osteoporotic boneJ Biomed Mater Res B Appl Biomater20088647448218288697
- AltVBechertTSteinrückePAn in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cementBiomaterials2004254383439115046929
- BrownWEChowLCA new calcium phosphate setting cementJ Dent Res198362672679
- ChowLCEanesEDCalcium phosphate cementMonogr Oral Sci20011814816311758446
- NakanoMHiranoNMatsuuraKPercutaneous transpedicular vertebroplasty with calcium phosphate cement in the treatment of osteoporotic vertebral compression and burst fracturesJ Neurosurg2002973 Suppl28729312408381
- OnerFCVerlaanJJVerboutAJDhertWJCement augmentation techniques in traumatic thoracolumbar spine fractureSpine (Phila Pa 1976)20063111 SupplS89S9516685242
- VerlaanJJDhertWJVerboutAJOnerFCBalloon vertebroplasty in combination with pedicle screw instrumentation: a novel technique to treat thoracic and lumbar burst fracturesSpine (Phila Pa 1976)200530E73E7915682000
- LowKLTanSHZeinSHSMcPhailDSBoccacciniAROptimization of the mechanical properties of calcium phosphate/multi-walled carbon nanotubes/bovine serum albumin composites using response surface methodologyMater Des20113233123319
- PerezRAPatelKDKimHWNovel magnetic nanocomposite inject-ables: calcium phosphate cements impregnated with ultrafine magnetic nanoparticles for bone regenerationRSC Adv201551341113419
- El-FiqiAKimJ-HPerezRAKimH-WNovel bioactive nanocomposite cement formulations with potential properties: incorporation of the nanoparticle form of mesoporous bioactive glass into calcium phosphate cementsJ Mater Chem B2015313211334
- GinebraMPEspanolMMontufarEBPerezRAMestresGNew processing approaches in calcium phosphate cements and their applications in regenerative medicineActa Biomater201062863287320123046
- CanalCGinebraMPFibre-reinforced calcium phosphate cements: a reviewJ Mech Behav Biomed Mater201141658167122098867
- WangXYeJWangYChenLReinforcement of calcium phosphate cement by bio-mineralized carbon nanotubeJ Am Ceram Soc200790962964
- ChewKKLowKLSharif ZeinSHReinforcement of calcium phosphate cement with multi-walled carbon nanotubes and bovine serum albumin for injectable bone substitute applicationsJ Mech Behav Biomed Mater2011433133921316621
- PeltierLFThe use of plaster of Paris to fill defects in boneClin Orthop Res196121129
- ZhouWXueYDJiXBYinGYZhangNRenYXA novel injectable and degradable calcium phosphate/calcium sulfate bone cementAfr J Biotechnol2011101944919457
- RakiLLBeaudoinJLAlizadehRLMakarJSatoTJCement and concrete nanoscience and nanotechnologyMaterials20103918942
- HesarakiSMoztarzadehFNematiRNezafatiNPreparation and characterization of calcium sulfate–biomimetic apatite nanocomposites for controlled release of antibioticsJ Biomed Mater Res B Appl Biomater20099165166119582854
- LiuXWangXMChenZInjectable bone cement based on mineralized collagenJ Biomed Mater Res B Appl Biomater201094727920336741
- LiHXiaoHGYuanJOuJPMicrostructure of cement mortar with nanoparticlesCompos Part B Eng200435185189
- PengQSunXGongTInjectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulinActa Biomater201395063506923036950
- Abdel-BarHMAbdel-ReheemAYOsmanROptimized formulation of vancomycin loaded thermoreversible hydrogel for treatment of orthopedic infectionsJ Pharm Sci2014529362946
- CampbellSBPatenaudeMHoareTInjectable superparamagnets: highly elastic and degradable poly(N-isopropylacrylamide)-super-paramagnetic iron oxide nanoparticle (SPION) composite hydrogelsBiomacromolecules20131464465323410094
- CaoLWerkmeusterJAWangJGlattauerVMcLeanKMLiuCBone regeneration using photocrosslinked hydrogel incorporating rhB-MP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticlesBiomaterials2014352730274224438908
- KimHHParkJBKangMJParkYHSurface-modified silk hydrogel containing hydroxyapatite nanoparticle with hyaluronic acid-dopamine conjugateInt J Biol Macromol20147051652224999272
- ReddyNNVaraprasadKRavindraSEvaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocompositesColloids Surf A Physicochem Eng Asp201138512027
- HeoDNKoWKBaeMSEnhanced bone regeneration with a gold nanoparticle–hydrogel complexJ Mater Chem B2014215841593
- KolambkarYMDupontKMBoerckelJDAn alginate-based hybrid system for growth factor delivery in the functional repair of large bone defectsBiomaterials201132657420864165
- LuhmannTGermershausOGrollJBone targeting for the treatment of osteoporosisJ Control Release201216119821322016072
- ValenteJFAGasparVMAntunesBPCountinhoPCorreiaIJMicro-encapsulated chitosan-dextran sulfate nanoparticles for controlled delivery of bioactive molecules and cells in bone regenerationPolymer201354515
- IgnjatovićNLNinkovPSabetrasekhRUskokovićDPA novel nano drug delivery system based on tigecycline-loaded calcium phosphate coated with poly-DL-lactide-co-glycolideJ Mater Sci Mater Med20102123123919707858
- NguyenMKJeonOKrebsMDSchapiraDAlsbergESustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiationBiomaterials2014356278628624831973
- PengKTChenCFChuIMTreatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticlesBiomaterials2010315227523620381140
- HamdanSARuggeroBSanneKBSynergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic ratsBiomaterials2014355482549024731712
- AlghamdiHSBoscoRBothSKSynergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic ratsBiomaterials2014355482549024731712
- GillaniRErcanBQiaoAWebsterTJNanofunctionalized zirconia and barium sulfate particles as bone cement additivesInt J Nanomedicine2010511120161983
- GomollAHFitzWScottRDThornhillTSBellareANanoparticulate fillers improve the mechanical strength of bone cementActa Orthop20087942142718622848
- AjeeshMFrancisBFAnnieJHarikrishna VarmaPRNano iron oxide–hydroxyapatite composite ceramics with enhanced radiopacityJ Mater Sci Mater Med2010211427143420195889
- AbboudMCasaubieilhLMorvanFFontanilleMDuguetEPMMA-based composite materials with reactive ceramic fillers: IV. Radiopacifying particles embedded in PMMA beads for acrylic bone cementsJ Biomed Mater Res20005372873611074433
- KhaledSMCharpentierPARizkallaASSynthesis and characterization of poly (methyl methacrylate)-based experimental bone cements reinforced with TiO2-SrO nanotubesActa Biomater201063178318620170759
- HoekstraJWvan den BeuckenJJLeeuwenburghSCMeijerGJJansenJATantalumpentoxide as a radiopacifier in injectable calcium phosphate cements for bone substitutionTissue Eng C201117907913
- WardlawDCummingsSRMeirhaegheJVBastianLRabstamJEastellREfficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trialLancet20093731016102419246088
- PadovaniBKasrielOBrunnerPPeretti-VitonPPulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplastyAm J Neuroradiol19992037537710219399
- MathisJMPercutaneous vertebroplasty: complication avoidance and technique optimizationAm J Neuroradiol2003241697170613679295
- AntonioKLudwigOJensFHeight restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: a cadaveric studySpine J2015151092109824200410
- ZhengZLukKDKuangGVertebral augmentation with a novel vessel-X bone void filling container system and bioactive bone cementSpine2007322076208217762808
- HeiniPFOrlerRVertebroplasty in severe osteoporosis. Technique and experience with multi-segment injectionOrthopade2004331223014747907
- SunGWeiDLiuXNovel biodegradable electrospun nanofibrous P(DLLA-CL) balloons for the treatment of vertebral compression fracturesNanomedicine2013982983823318398
- GautschiOPSchatloBSchallerKSTessitoreEClinically relevant complications related to pedicle screw placement in thoracolumbar surgery and their management: a literature review of 35,630 pedicle screwsNeurosurg Focus201131E821961871
- BeckerSChavanneASpitalerRAssessment of different screw augmentation techniques and screw designs in osteoporotic spinesEur Spine J2008171462146918781342
- AksakalBKomMTosunHBDemirelMInfluence of micro- and nano-hydroxyapatite coatings on the osteointegration of metallic (Ti6Al4V) and bioabsorbable interference screws: an in vivo studyEur J Orthop Surg Traumatol20142481381923689912
- OlerudCPetrén-MallminMLarssonSHydroxyapatite coating improves fixation of pedicle screws: a clinical studyJ Bone Joint Surg Br20028438739112002498
- KimHSYangYKohJTFabrication and characterization of functionally graded nano-micro porous titanium surface by anodizingJ Biomed Mater Res B200988427435
- RodriguezRKimKOngJLIn vitro osteoblast response to anodized titanium and anodized titanium followed by hydrothermal treatmentJ Biomed Mater Res A200265A352358
- YaoCPerlaVMcKenzieJSlamovichEBWebsterTJAnodized Ti and Ti6Al4V possessing nanometer surface features enhances osteoblast adhesionJ Biomed Nano200516873
- ErcanaBWebsterTJThe effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfacesBiomaterials201031133684369320149926
- OhaSBrammeraKSLibYSStem cell fate dictated solely by altered nanotube dimensionProc Natl Acad Sci U S A20091062130213519179282
- WebsterTJEjioforJUIncreased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMoBiomaterials2004254731473915120519
- WebsterTJSiegelRWBiziosROsteoblast adhesion on nanophase ceramicsBiomaterials1999201221122710395391
- WebsterTJErgunCDoremusRHSiegelRWBiziosREnhanced functions of osteoblasts on nanophase ceramicsBiomaterials2000211803181010905463
- PuckettSTaylorERaimondoTWebsterTJThe relationship between the nanostructure of titanium surfaces and bacterial attachmentBiomaterials20103170671319879645
- KurtzSMDevineJNPEEK biomaterials in trauma, orthopedic, and spinal implantsBiomaterials2007284845486917686513
- TothJMWangMEstesBTScifertJLSeimHBIIITurnerASPolyetheretherketone as a biomaterial for spinal applicationsBiomaterials20062732433416115677
- ChoDYLiauWRLeeWYLiuJTChiuCLSheuPCPreliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc diseaseNeurosurgery2002511343134912445338
- WangLWengLQSongSHZhangZGTianSLMaRCharacerization of polyetheretherketone–hydroxyapatite nanocomposite materialsMater Sci Eng A201152810–1136893696
- WangLWengLSongSZhangZTianSMaRCharacterization of polyetheretherketone-hydroxyapatite nanocomposite materialsMater Sci Eng A2011528689696
- MaRWengLFangLLuoZSongSStructure and mechanical performance of in situ synthesized hydroxyapatite/polyetheretherketone nanocomposite materialsJ Solgel Sci Technol2012625256
- WuXLiuXWeiJMaJDengFWeiSNano-TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studiesInt J Nanomedicine201271215122522419869
- LiKYeungCYYeungKWKTjongSCSintered hydroxyapatite/polyetheretherketone nanocomposites: mechanical behavior and biocompatibilityAdv Eng Mater201214B155B165
- ShortkroffSTurellMBRiceKCellular response to nanoparticlesMRS ProceedingsCambridge, UKCambridge University Press2001
- DimitriouRJonesEMcGonagleDBone regeneration: current concepts and future directionsBMC Med201196621627784
- LubickNNanosilver toxicity: ions, nanoparticles or both?Environ Sci Technol200842861719192768
- RaeTTolerance of mouse macrophages in vitro to barium sulfate used in orthopedic bone cementJ Biomed Mater Res197711839846591525
