?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Microbial colonization on implanted devices and biofilm formation is a recurrent complication in implant surgery and may result in loss of implants. The aim of this study was to deposit silver nanoparticles on a titanium surface to obtain antibacterial properties. In the present study, we prepared a silver nanoparticle-modified titanium (Ti-nAg) surface using silanization method. The morphology and chemical components of the Ti-nAg surface were characterized by scanning electron microscopy (SEM) equipped with energy-dispersive spectroscopy (EDS). Two species of bacteria, Staphylococcus aureus and Escherichia coli, were utilized to test the antibacterial effect of the Ti-nAg treated surface. The SEM examination revealed that a small quantity of silver nanoparticles was sparsely deposited on the titanium surface. The diameter of these nanoparticles ranged from ten to several hundred nm. EDS analyses revealed that there was 4.26% of Ag present on the surface. After a 24-hour incubation, 94% of Staphylococcus aureus and over 95% of Escherichia coli had been killed on the Ti-nAg surface, and the SEM examination of anti-adhesive efficacy test showed that there were less bacteria attached to Ti-nAg surface than to a control surface of untreated Titanium. These data suggest that silver nanoparticle-modified titanium is a promising material with an antibacterial property that may be used as an implantable biomaterial.
Introduction
Implanted devices such as artificial hip or knee joints, and dental implants are suitable substrates for microbial colonization and biofilm formation, which is a recurrent complication in implant surgery.Citation1 Because of excellent resistance to corrosion and great biocompatibility, titanium has been widely used as an implantable biomaterial for devices of dental implants, fracture fixation, joint replacement, vertebral column orthopedic and percutaneous-transmucosal surgery. Multiple factors affect the outcome of orthopedic and dental implants, including osseointegration of bone-implant and the degree of aggregation of bacteria surrounding the implants. Previous studies have revealed that inflammatory diseases around dental implants may cause peri-implantitis and loss of supporting bone, which is associated with the reduction of long-term “survival” of implants.Citation2 It is known that microorganisms and host cells compete for the substrate in a process called “race for the surface”.Citation3 The avoidance of attached microorganisms on the surfaces of implanted devices is best practice for the survivability of any implant. However, infected implants and their consequent loosening is most common in titanium devices and this is still a challenge in clinical practice. In the past decades, many strategies have been developed to prevent or reduce bacterial colonization of the surface of titanium devices. Such strategies include: surface modifications to reduce concavity, such as polishing; and modification of the physical properties of the device such as surface-free energy.Citation4,Citation5
Silver is well known for its antimicrobial properties and has been used for many years in the medical field for antimicrobial purposes.Citation6 Silver impregnated hydroxyapatite film on an alumina substrate has shown excellent antibacterial properties against both Gram-positive and Gram-negative bacteria.Citation7 It has also been reported that silver-zeolite inhibits the growth of bacteria under anaerobic conditions.Citation8,Citation9 In addition to this, it has been shown that silver is capable of preventing human immunodeficiency virus (HIV) from binding to host cells.Citation10,Citation11 Consequently it has been suggested that silver inhibits bacterial invasion by several mechanisms. Because silver nanoparticles possess a large total surface area available for bacterial interaction they exhibit a stronger antibacterial effect than regular metallic silver.Citation12 As a consequence the incorporation of silver nanoparticles with other materials, to increase an antibacterial capability is currently the subject of intense study and several methods have been developed such as: silver-doped hydroxyapatite;Citation13 polymer-silver nanoparticles;Citation14 and silver nanoparticles on TiO2.Citation15 In light of the above findings, we hypothesized that a titanium surface upon which silver nanoparticles adhere could exhibit excellent antibacterial properties. To test this hypothesis, we prepared a silver nanoparticle-modified titanium (Ti-nAg) surface using silanization method,Citation16 and determined the size, shape and distribution of the silver nanoparticles with scanning electron microscopy (SEM) equipped with energy-dispersive spectroscopy (EDS). Furthermore, we examined the antibacterial and anti-adhesive properties using three well established microbiological assays.
Materials and methods
Materials
Silver nitrate (AgNO3, purity, 99.9%), concentrated sulfuric acid (purity, 98%), trisodium citrate (purity, 99%), hydrogen peroxide, sodium borohydride (NaBH4, purity, 98%), acetone 3-(aminopropyl) triethoxysilane (APS, purity, 99.5%), and sodium hydroxide (purity, 99.99 %) were used in the present study. All of above reagents were obtained from Sigma-Aldrich (St Louis, MO, USA). Titanium sheets (mass ratio 99%, 12 mm diameter) were purchased from BaoTi (Xian, China).
Methods
The preparation of titanium plate
Titanium coupons were ground into the shape of a plate using 1200 grit, then polished using 0.3-μm aluminum before being ultrasonically cleaned with acetone and distilled water. The polished titanium surface was first etched with “piranha solution” for 1.5 hours at 75°C (piranha solution: 3:7 30% hydrogen peroxide/concentrated sulfuric acid). The titanium coupons were then rinsed extensively with deionized (DI) water and dried in a stream of dry nitrogen (N2). The cleaned titanium coupons was silanized using a 2% APS solution for 1.5 hours,Citation17 followed by extensive rinsing with ethanol and water. Following this the coupons were sonicated in ethanol for 30 seconds. Finally, the APS-treated titanium coupons were again rinsed with water and dried in a stream of N2.
Deposition of silver nanoparticles onto titanium surface
Firstly 20 mL of an aqueous solution of silver nitrate; with a final concentration of 0.25 mM, and 0.25 mM trisodium citrate was prepared. While stirring vigorously, 0.6 mL of 10 mM NaBH4 was added to the solution.Citation18 Following this the silver nanoparticles were deposited onto the APS-treated titanium coupons by a 3-hour incubation in the silver nanoparticle solution. The resultant coupons was then rinsed with DI water and dried in a stream of N2.Citation19
Characterization of the silver nanoparticles on titanium surface
The morphology and composition of the Ti-nAg surface of the titanium coupons was characterized using an SEM (HITACHI S-4800, Tokyo, Japan) equipped with EDS (alpha ray spectrometer, Oxford, UK).
Antibacterial tests
The antimicrobial activity of the Ti-nAg surface was tested against the Gram-negative bacteria Escherichia coli (ATCC 8099, Rockville MD, USA) and Gram-positive Staphylococcus aureus (ATCC 6538, Rockville MD, USA). Zones of inhibition (ZoI) tests were carried out to determine the extent of silver ion release from the Ti-nAg surface. A film applicator coating (FAC) assay was used to test the antimicrobial effect by directly incubating microbial cells on Ti-nAg or polished titanium (Ti-polished, control titanium) surfaces. In the ZoI test, the concentration of Staphylococcus aureus was adjusted to 1 × 106 colony-forming units (CFUs)/mL and spread evenly on Luria-Bertani medium agar plates. Ti-nAg and the non-modified control titanium coupons were placed on the above prepared agar plates separately. The plates were incubated at 37°C in an aerobic petri dish for 24 hours and photographed to record the results. In the FAC assay the concentration of each bacterial strain was adjusted to 1 × 104 cells/mL in phosphate buffer solution (PBS: NaCl 137 mmol/L, KCl 2.7 mmol/L, Na2HPO44.3 mmol/L, KH2PO4 1.4 mmol/L) and Luria Bertani Medium (tryptone, yeast extract, NaCl). The titanium coupons were then put on these culture plates and kept in wet boxes. Then 10 μL bacterial suspension was applied to the Ti-nAg and the titanium control coupons, respectively. Following incubation at 37°C in an aerobic petri dish for 24 hours, the bacterial suspensions on the coupons were then transferred separately into tubes containing 10 mL of sterilized PBS, followed by vigorous vortex mixing for 5 minutes. Following this 10 μl of bacterial solutions from the mixtures were then spread on Luria Bertani medium broth-agar plates. The plates were incubated aerobically for 24 hours. The viable cells on each of the plates were counted by quantifying the CFUs. Each of the groups were respectively compared, using an independent Student’s t-test. For all statistical analyses, the probability of type I errors less than or equal to 0.05 was considered as statistically significant. Each test was run in triplicate and repeated on three separate occasions. The antibacterial effect in each group was calculated as a bactericidal ratio which was calculated as follows:
CFU represents colony-forming unit; CG is the Ti-polished coupons (control group); and EG is Ti-nAg coupons (experimental group).
Anti-adhesive test
The anti-adhesive activity of the Ti-nAg coupons towards Staphylococcus aureus and Escherichia coli was qualitatively evaluated by SEM. Ti-nAg and the control titanium coupons were immersed in 10 mL of the bacterial suspensions, which contained 1 × 108 cells/mL, cultured in Luria Bertani medium for 24 hours. The loosened and unattached bacteria cells were removed by sinking the plates into sterile PBS. The coupons were subsequently fixed with 2.5% glutaraldehyde solution for 2 hours at room temperature. After dehydration in an ethanol series (30%, 50%, 70%, 80%, 90% and 100%), the ethanol in the plates was removed by soaking in an amyl acetate solution for 1 hour at room temperature. Following this the plates were dried and coated with 8 nm Au using a sputter coater before being visualized with SEM.
Results and discussion
Characterization of silver nanoparticle-modified titanium surface
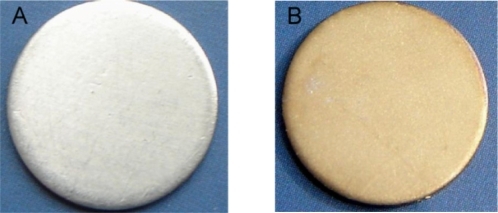
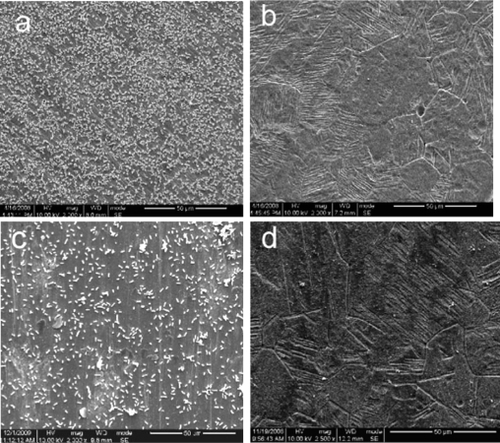
During the process of preparing the surfaces one noticeable change was the color of the titanium sheet which became gold after it had been etched using the piranha solution and treated with silver nanoparticle solution (). The SEM micrographs showed silver nanoparticles sparsely deposited on the titanium surface, with a diameter of approximately 100 nm. Some silver nanoparticles formed aggregates (–d). In contrast, the surface of the titanium sheet that has been etched by piranha solution, although not treated with the silver nanoparticle solution, showed many nanopores with a diameter of approximately 50 nm, (). After the Ti coupons had been polished () no nanopores were observed. To verify the chemical elements present in the nanoparticles EDS analyses were performed. As shown in , whenever the probe detected a silver nanoparticle (), the peak value of titanium reduced and the peak value of Ag increased ().The EDS analyses revealed that the nanoparticles on Ti-nAg surface were Ag and they were 4.26% Ag (, ).
Figure 2 Morphological characterization of Ti-Ag surface. SEM micrographs show Ti-nAg surface at different magnifications: (a) ×500; (b) ×10000; (c) ×40000; and (d) ×120000. Ti-polished surface (e) magnification ×60000, and titanium surface after etched by piranha solution but before treated with silver solution (f) magnification: ×60000.
Notes: (working distance: 15000 μm; accelerating voltages: 25 KV).
Abbreviation: SEM, scanning electron microscopy.
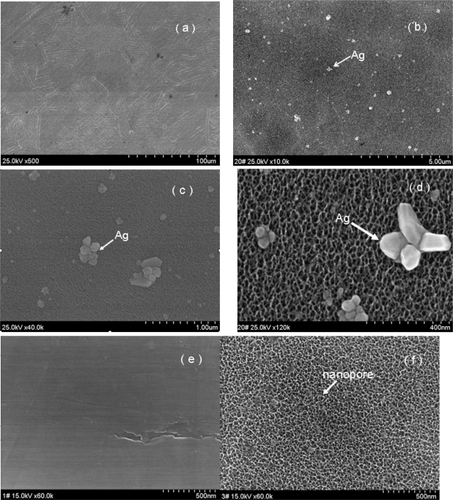
Figure 3 Line scanning of EDS image of the particle on the Ti-nAg surface.
Notes: The green line stands for Ag and the red line stands for titanium.
Abbreviations: EDS, energy-dispersive spectroscopy; Ti-nAg, silver nanoparticle-modified titanium.
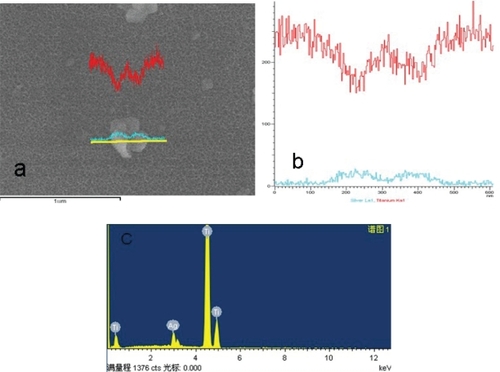
Figure 4 Photographs showing the ZoI tests for Ti-nAg (a) and Ti-polished (b).
Abbreviation: ZoI, zone of inhibition.
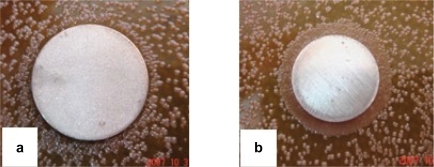
Table 1 Results of EDS analysis for chemical elements of the Ti-nAg surface
Antimicrobial and anti-adhesive properties
The antibacterial mechanism of Ti-nAg surface is attributable to the presence of the silver nanoparticles and released silver ions. The silver nanoparticles show efficient antimicrobial property due to their extremely large surface area, which provides an effective contact with microorganisms. The nanoparticles release silver ions in aqueous solution, which enhances their bactericidal activity.Citation20 It has been reported that Ag ions bind strongly to electron donor groups present in biological molecules containing sulfur, oxygen or nitrogen.Citation21 Since Staphylococcus aureus and the Escherichia coli have been reported to be the most common pathogens in biomaterial-associated infections,Citation22 these two bacteria were deemed appropriate for testing the antibacterial properties of the Ti-nAg surface. There are at least two mechanisms of inhibiting the formation of microbial plaque. The first is to inhibit the initial adhesion of bacteria, and the second is to inhibit the colonization of bacteria. In this study, we adopted two methods (ZoI and FAC tests) to determine antibacterial activities and one test for anti-adhesive activities of the Ti-nAg surface.
As shown in , there was no zone of inhibition observed around either the Ti-nAg plate () or the Ti-polished plate (). Since the ZoI test was based on the leaching of silver ions from the surface, the inhibition of the bacterial growth depends on a sufficient concentration of silver ions in the surrounding aqueous environment. However, our SEM examination revealed that only a small percentage of the modified titanium surface was covered by silver nanoparticles, which accounted for just 4.26% of the surface chemical elements. In addition, because the elemental silver has a very low rate of dissolution in an aqueous environment,Citation23 it is possible that the silver dissociated from the titanium surface did not reach a concentration sufficient to inhibit bacterial growth. This observation is consistent with the study of Ahearn and colleagues, in which they found negligible zones of inhibition when testing the release of ions from ion beam with a silver coating.Citation24
Figure 5 Counts of CFU (Mean ± SD) and bactericidal ratio in FAC test.
Abbreviations: CFU, colony-forming unit; CG, control group; EG, experimental group; SD, standard deviation; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli.
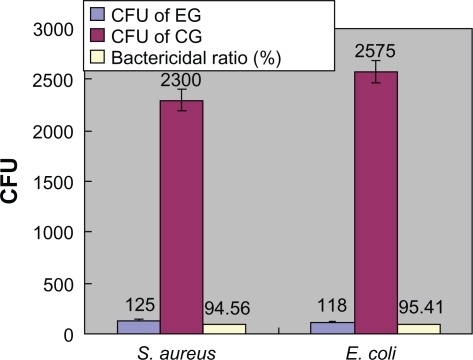
In contrast, in FAC assay, the Ti-nAg surface exhibited a strong antibacterial property. Following 24-hour incubation of bacteria on Ti-nAg surface, 94% Staphylococcus aureus and more than 95% Escherichia coli in the bacteria suspension were killed (). Ti-nAg specimens significantly inhibited the growth of both Staphylococcus aureus and Escherichia coli (P < 0.05) than Ti-polished specimen. The discrepancy between ZoI and FAC tests may be due to the difference of the antibacterial mechanism. The former relied on leaching of the silver ions from the surface, whereas the latter mediated an inhibitory effect by direct contact.
The SEM examination of anti-adhesive test showed that there were much less bacteria adherent to Ti-nAg surface than to the control titanium surface (). This was due to the direct contact inhibition and the anti-adhesive properties of the Ti-nAg surface. Therefore it is suggested that the Ti-nAg surface reduces the risk of bacterial colonization.
Conclusion
In the present study, we prepared a silver nanoparticle-modified titanium surface using a silanization method. The SEM images show that a small amount of silver nanoparticles were successfully coated on the titanium surface. The diameter of silver nanoparticles ranged from ten to several hundred nanometers. EDS analysis of the surface showed that atomic percentage of silver was only 4.26%. FAC assays and anti-adhesive testing showed that the Ti-nAg surface had remarkable antibacterial and anti-adhesive activities towards Staphylococcus aureus and Escherichia coli. These data suggest that silver nanoparticle-modified titanium is a promising material with the antibacterial properties that may be used as an implantable biomaterial.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchierholzJMMorsczeckNBrennerDPSpecial aspects of implant-associated infection in orthopaedic surgeryDer Orthopade20043339740415141663
- EspositoMHirschJLekholmUThomsenPBiological factors contributing to failures of osseointegrated oral implants.(II). KtiopathogenesisEur I Oral Sci1998106721764
- GristinaAGBiomaterial-centered infection: Microbial adhesion versus tissue integrationScience1987237158815953629258
- MeredithDOEschbachLRiehleMOCurtisASGRichardsRGHuman fibroblast reactions to standard and electropolished titanium and Ti-6Al-7Nb, and electropolished stainless steelJ Biomed Mater Res200575A541555
- Bacte-KoernerRJButterworthLAMayerIVBacterial adhesion to titanium-oxy-nitride (TiNOX) coatings with different resistivities: a novel approach for the development of biomaterialsBiomaterials200223142835284012069322
- CredeKSFDie Verhutung der Augenentzundung der Neugeborenen, der haufigsten und wuchtigsten Ursache der BlindheitBerlinA. Hirschwald1894
- FengQing LingKimTaik NamWuJingParkEui SeoKimJong OckAntibacterial effects of Ag-HAp thin films on alumina substratesThin Solid Films1998335214219
- FengQLKimTNWuJAntibacterial effects of Ag-HAp thin films on alumina substratesThin Solid Films1998335214219
- KawaharaKTsurudaKMorishitaMUchidaMAntibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions200016452455
- AltVBechertTSteinrückePAn in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cementBiomaterials2004254383439115046929
- LeeHYParkHKLeeYMKimKParkSBA practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applicationsChem Commun20072829592961
- KvitekLPanacekASoukupovaJKolarMVecerovaRPrucekREffect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs)J Phys Chem C200811258255834
- ArumugamSKSastryTPSreedharSBMandalASOne step synthesis of silver nanorods by autoreduction of aqueous silver ions with hydroxyapatite: an inorganic-inorganic hybrid nanocompositeJ Biomed Mater Res200780A391398
- MinYAkulutMKristairsenKGolanYIsraelachviliJThe role of interparticle and external forces in nanoparticle assemblyNat Mater2008752753818574482
- ZhengJHuaYXinjunLShanqingZEnhanced photocatalytic activity of TiO2 nano-structured thin film with a silver hierarchical configurationAppl Surf Sci2008254616301635
- AslanKLakowiczJRRapid deposition of triangular silver nanoplates on planar surfaces: application to metal-enhanced fluorescenceJ Phys Chem B2005109136247625116851692
- GeddesCDCaoHGryczynskiIGryczynskiZFangJLakowiczJRMetal-enhanced fluorescence (MEF) due to silver colloids on a planar surface: potential applications of indocyanine green to in vivo imagingJ Phys Chem A20031071834433449
- HengleinAGiersigMFormation of colloidal silver nanoparticles: capping action of citrateJ Phys Chem B19991034495339539
- AslanKLakowiczJRGeddesCDRapid deposition of triangular silver nanoplates on planar surfaces: application to metal-enhancedJ Phys Chem20051091362476251
- MoronesJRElechiguerraJLCamachoARamirezJTThe bactericidal effect of silver nanoparticlesNanotechnology20051623462353
- SongHYKoKKOhLHLeeBTFabrication of silver nanoparticles and their antimicrobial mechanismsEur Cells Mater200611Suppl 158
- GristinaAGCostertonJWBacterial adherence to bio-materials and tissue. The significance of its role in clinical sepsisJ Bone Joint Surg19856722642733881449
- SambhyVMacBrideMMPetersonBRSenASilver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materialsJ Am Chem Soc2006128309798980816866536
- AhearnDMayLGabrielMAdherence of organisms to silver-coated surfacesJ Ind Microbiol19951543723768605074