Abstract
Graphene has attracted the attention of the entire scientific community due to its unique mechanical and electrochemical, electronic, biomaterial, and chemical properties. The water-soluble derivative of graphene, graphene oxide, is highly prized and continues to be intensely investigated by scientists around the world. This review seeks to provide an overview of the currents applications of graphene oxide in nanomedicine, focusing on delivery systems, tissue engineering, cancer therapies, imaging, and cytotoxicity, together with a short discussion on the difficulties and the trends for future research regarding this amazing material.
Introduction
Graphene consists of a monoatomic layer of carbon atoms in a honeycomb latticeCitation1 and is one of the strongest materials ever tested with tensile strengths greater than 100 GPa and a tensile modulus of 1 TPa.Citation2 Biologists have shown a keen interest in this newly discovered material because of its unique chemical structure, material, and biomedical properties.Citation3,Citation4 Graphene and graphene oxide (GO) sheets are easily synthesized via the Hummers method or variants thereof.Citation5–Citation8 GO is hydrophilic and its surface easily modified with a host of biocompatible polymers such as chitosan,Citation9 polyethylene glycol (PEG),Citation10 poly(ε-caproplactone),Citation11 poly-L-lysine (PLL),Citation12 and polyvinylalcohol.Citation13 GO contains a large amount of hydrophilic groups on its edge or basal planes; thus, sheets of small size and lower concentrations should be much more biocompatible. These properties make GO extremely attractive to a large swath of scientists with new applications in the fields of drug delivery,Citation14–Citation25 parasitology,Citation26,Citation27 tissue engineering (TE),Citation28–Citation35 antibacterials,Citation36–Citation44 cancer therapy,Citation45–Citation49 sensorsCitation50–Citation65 imaging, and diagnosticsCitation66–Citation75 reported monthly. To use GO in a clinical setting, it is essential to confirm its toxicity and bio-compatibility through extensive in vitro and in vivo studies using specific cell lines, theoretical and animal models.Citation76–Citation78 However, the safety and toxicity issue regarding GO and its potential health benefits to society are far from resolved.Citation79 Many previous investigations have shown GO and its hybrid structures to induce low cell toxicity, but reports remain conflicting.Citation80 The source of this conflict may be due to subtle epigenetic processes associated with aberrant gene expression.Citation81 Epigenetic mechanisms include DNA methylation at specific sites in regulatory regions such as phosphorylation, ubiquitination, and ATP-ribosylation that lead to chromatin remodeling.Citation81 The role of deregulated epigenetic mechanisms caused by GO and graphene-based exposure in disease pathogenesis is yet to begin.Citation82–Citation84 In addition to its application in toxicity assays, functionalized GO sheets and nanoparticles (NPs) are frequently used as tissue scaffolds, fillers, and composite meshes in many areas of regenerative medicine. Studies on the relationship between stem cell differentiation and the properties of graphene derivatives are generating a tremendous impetus in the fields of cardio and neuroregeneration. GO easily combines with a host of other nanoscale materials leading to new applications in the fields of drug delivery, TE, cancer therapeutics, bioimaging, and diagnostics. This review will selectively examine the benefits and limitations of these applications and highlight the approaches that have been currently developed to clarify biocompatible and toxicity issues surrounding graphene derivatives and graphene-based hybrid biomaterials.Citation85–Citation90
Delivery systems
Drug delivery
GO and its functional derivatives exhibit an exceptional set of material properties that are frequently used to carry different therapeutics such as DNA, antibodies, proteins, genes, and small drug molecules.Citation91 The properties of GO relevant to drug delivery include surface area, layer number, lateral dimensions, and surface chemistry.Citation92 The high surface area (2,600 m2 g−1) of the single layer permits high drug loading capacity compared with other nanomaterials, but its lack of rigidity means that cell penetration is poor.Citation93 Lateral dimensions of GO nano-sheets do not affect drug-loading capacity but could have limitations regarding blood–brain transport, renal clearance, and biodegradation.Citation94 The success of a GO-based drug delivery vehicle is dependent on three factors.Citation78 The first is constructing a carrier with an optimal loading capacity. The second is to confirm the degree of toxicity and biocompatibility, a prerequisite prior to preclinical and clinical testing. The third is to design a system able to release drugs in a controlled manner at a designated site (tumor) for successful therapy. A common strategy to achieve efficient tumor targeting is to conjugate drug carriers with specific ligands such as polyclonal antibodies,Citation95 folic acid,Citation96 and transferrinCitation97 that recognize molecular signatures on the target surface.
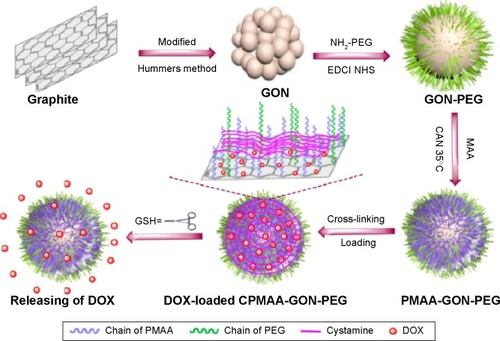
A simpler approach to targeted drug delivery is to directly immobilize the drug onto the unmodified graphene surface. Good examples of this approach can be found in the studies by Yang et al,Citation98 Depan et al and Mendes et alCitation99,Citation100 who showed that the anticancer molecule doxorubicin (DOX) forms a strong bond with the GO surface and that the release of DOX is more extensive in acidic or tumor environments than normal tissues. Several groups have sought to exploit the acidic environment of cancer cells by developing graphene-based vehicles containing pH-sensitive polymers. Of particular note is the work by Bai et alCitation102 in which a pH-sensitive GO/polyvinylalcohol hydrogel for loading and unloading the trial drug VB12 at physiological pH was developed. It was found that the percentage of drug released was dependent on the pH and salt concentration of the buffered solution. Compared with normal cells, cancer cells contain a higher level of reductive cysteine or glutathione (GSH) in their cytoplasm and endolysosomes.Citation102 In a recent article by Zhao et alCitation103 a cross-linked GO-PEG (cysteine polymethacrylic acid cross-linked nano graphene oxide polyethylene glycol) carrier possessing a novel reductive-triggering switch suited to the intracellular environment of tumor tissues was developed. The carrier released DOX six times faster at pH 5.0 in the presence of 10 mM GSH than at pH 7.4 with 10 µM GSH (stimulated normal tissues). A schematic of the fabrication process is illustrated in . There are many other chemical strategies (esterification and biodegradation) that can be incorporated into the carrier to control the release of a drug. For example, Lu et al prepared a single layer of polyacrylic acid (PAA)-GO (1.9 nm); then it reacted with 1,3-bis(2-chloroethyl)-1-nitrosourea,Citation104 a commercial cancer drug. The multifunctional vehicle enhanced the thermal stability of the drug and significantly extended the half-life of bound 1,3-bis(2-chloroethyl)-1-nitrosourea from 19 to 43 hours compared with the free drug and showed efficient intracellular uptake by GL261 cancer cells. More recently Xiong et alCitation105 used biodegradable PEGylated NGO conjugates (nano graphene oxide disulfide linked PEG) with cleavable disulfide bonds for the photothermal therapy of A549 cells. Nano graphene oxide disulfide linked PEG showed a higher efficacy for A549 cells than the control. The drug and gene delivery applications of GO-based vehicles are summarized in .
Table 1 Drug and gene delivery applications of GO-based vehicles
Figure 1 Schematic illustration of the preparation, ideal structural transformation, drug loading, and reduction-triggered release of the cysteine polymethacrylic acid cross-linked nano graphene oxide polyethylene glycol carriers. Reproduced with permission from Zhao X, Yang L, Li X, et al. Functionalized graphene oxide nanoparticles for cancer cell specific delivery of antitumor drug. Bioconjug Chem. 2015;26(1):128–136.Citation103 Copyright © 2015 American Chemical Society.
Abbreviations: PEG, polyethylene glycol; GSH, glutathione; DOX, doxorubicin; CPMAA, cysteine polymethacrylic acid; PMAA, polymethacrylic acid.
In addition to internal cellular changes in pH, ion concentration, and temperature, there are other external methods such as ultrasound, magnetic, and electric fields than can be used to trigger the release of a drug from the carrier. A good example of external triggered release is the study by Zhou et alCitation106 in which a magnetic field was used to release a drug from a graphene/Fe3O4 nanocomposite. It was found that the weight ratio of the loaded drug to the GO carrier could reach 200%. Other examples of this concept can be found in studies by Liu et alCitation107 and more recently by Servant et al.Citation108 The latter study showed that the release of a drug from pristine graphene/methacrylic acid scaffolds could be controlled in a pulsatile fashion upon the ON/OFF application of low electrical voltages, at low graphene concentrations (0.2 mg mL−1) while maintaining their structural integrity. The incorporation of highly conductive pristine graphene sheets into the methacrylic-acid based hydrogel significantly decreased the resistive heat generated from the hydrogel matrix, thus minimizing necrosis to surrounding skin and tissue.
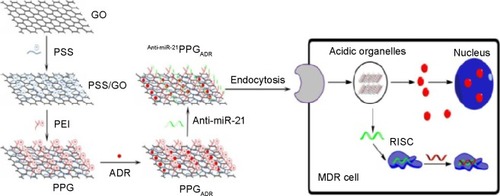
Combination therapy can be defined as the simultaneous administration of two or more active or preactive pharmacological agents that are known to disrupt multiple targets, resulting in a more efficient solution to cancer treatments. The concept of multidrug delivery was utilized by Zhang et alCitation27 by loading two anticancer drugs DOX and camptothecin (CPT) onto a folic acid GO carrier. The codelivery of both drugs had a better target efficacy and higher cytotoxicity than GO loaded with either DOX or CPT alone. Current chemotherapy for glioma is rarely satisfactory due to low therapeutic and efficiency and systemic side effects. A glioma-targeted drug delivery systems based on GO was recently reportedCitation109 in which targeted peptide chlorotoxin-conjugated graphene oxide sheets were loaded with DOX. Cytotoxicity experiments showed that chlorotoxin-conjugated GO/DOX mediated the highest rate of death of glioma cells compared with free DOX or GO loaded with DOX only. It is well known that code-livery is an effective treatment of cancer and other disease states.Citation110–Citation112 However, multidrug resistance frequently occurs in aggressive cancers and in patients with a terminal prognosis. Recently the codelivery of novel multidrug resistance (MDR)-reversing agents and anticancer drugs to cancer cells has shown great promise as a cancer treatment. MicroRNA-21 (miR-21) overexpression is associated with the development and progression of MDR in breast cancer, and it is emerging as a novel and promising MDR-reversing target. In a recent study by Zhi et alCitation113 a multifunctional nanocomplex composed of polyethylenimine (PEI)/poly(sodium 4-styrenesulfonate) (PSS)/GO termed PPG was used to evaluate the reversal effects of PPG as a carrier for adriamycin (ADR) along with miR-21 targeted small-interfering RNA (siRNA) (anti-miR-21) in cancer drug resistance. Cell experiments showed that PPG significantly enhanced the accumulation of ADR in MCF-7/ADR cells (an ADR-resistant breast cancer cell line) and exhibited much higher cytotoxicity than free ADR, suggesting that PPG could effectively reverse ADR resistance of MCF-7/ADR. A schematic of the PPG fabrication process and MDR reversion is shown in .
Figure 2 Fabrication of polyethylenimine poly(sodium 4-styrenesulfonate) graphene oxide delivery vehicle and MDR reversion. Reproduced from Zhi F, Dong H, Jia X, et al. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro. Plos One. 2013;8(3):e60034.Citation113
Abbreviations: GO, graphene oxide; PLL, poly-l-lysine; PSS, poly(sodium 4-styrenesulfonate); PEI, polyethylenimine; PPG, poly(sodium 4-styrenesulfonate) (PSS)/GO; ADR, Adriamycin.
Gene delivery
Nonviral gene therapy is a promising approach to treat various diseases caused by genetic disorders. These carriers can transfect cells with new genes from the liquid phase in a conventionally bulky approach or from the surface of the predeposited solid phase in a substrate-mediated manner. The gene vehicle or vector must protect the loaded DNA from degradation from cellular nucleases facilitating its uptake with high transaction efficiency. The major challenge preventing the achievement of these goals is the lack of efficient and nonmutagenic vectors or gene vehicles.Citation114–Citation117 Given the unpredictability of viral vectors, many researchers have turned to synthetic vectors composed of liposomes or more recently graphene derivatives. It has been shown that GO derivatives can improve the penetration of siRNA or plasmid DNA (pDNA) into cells protecting DNA from enzyme cleavage.Citation118 Moreover, the cytotoxicity of cationic PEI is significantly reduced after complexation or conjugation with GO.Citation21 In addition, Li et alCitation119 managed to pattern preconcentrated PEI/pDNA on absorbent GO mediating highly localized and efficient gene delivery. The patterned substrates exhibited excellent biocompatibility and enabled effective gene transfection for various cell lines including stem cells. The distinguishing property of PEI-GO compared to other vehicles is its ability to condense DNA at a low mass ratio (+49 mV)Citation116 and effectively transport pDNA through the cytoplasm to the nucleus. In addition, other carbon vectors such as GO/chitosan,Citation120 GO-PEG,Citation121 and GO/polyamidoamine (PAMAM)Citation122,Citation123 can also be used to deliver pDNA and siRNA. Liu et alCitation123 showed that graphene oleate PAMAM exhibited good compatibility and greatly improved green fluorescent protein gene transfection efficiency (18.3%) in contrast to ultrasonicated graphene (1.4%) and GO PAMAM without oleic modification (7.0%).
Besides its ability to protect DNA, graphene possesses the unique optical property of absorbing near infrared (NIR) light. Tian et alCitation124 showed that localized NIR heating of GO-PEG-Ce6 increased its uptake and efficacy against cancer cells. They attributed the enhanced uptake of GO-PEG-Ce6 to an increase in membrane fluidity upon NIR heating. Moreover, Kim et alCitation125,Citation126 demonstrated that NIR irradiation of functionalized reduced GO can change the membrane integrity of endosomes, thus improving the intracellular lifetime of the drug or gene and their delivery efficacy.
Tissue engineering
As well as DNA, GO is also used to deliver specific proteins such as bone morphogenetic proteins (BMPs) and substance P (SP) factors.Citation127–Citation129 Among BMPs, BMP-2 is a well-known growth factor used for bone regeneration. A large dose of BMP-2 leads to several side effects such as over bone growth, inflammation, and uncontrolled bone formation. In a recent study, La et alCitation35 demonstrated that the surface of a Ti-GO implant can be preloaded with several BMPs and SP. BMP-2 delivery using GO-Ti or GO-coated Ti exhibited a higher alkaline phosphatase activity in bone-forming cells in vitro compared with bare Ti. The dual delivery of BMP-2 and SP (a selective agent for mesenchymal cell differentiation) showed the greatest formation of bone growth in mouse calvaria compared with the other groups.
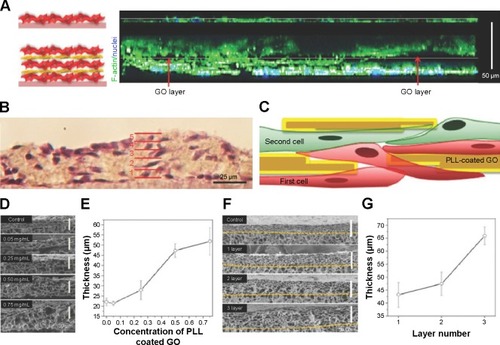
The development of highly organized and functional 3D complex scaffolds in vitro is of great importance in TE, since native tissues and organs exhibit highly organized and multifunctional architectures composed of extracellular matrix, different cell types, and chemical and physical signaling clues. Cardiomyocytes are particularly interesting forming dense quasi-lamellar and high vascularized tissue in heart muscle.Citation130,Citation131 Mimicking the vascularized structures of the myocardium with various types of cell still remains one of the major challenges in TE. Some of the most commonly used methods are bottom up assemblyCitation132 or the layer-by-layerCitation133,Citation134 (LBL) approach. In a recent article by Shin et alCitation135 high interlayer conductivity and strong cellular adhesion was achieved in a multilayer cell construct using functional PLL-GO NPs (GONs) and the LBL approach. The 3L construct made with PLL-GO promoted thicker tissue growth (65 µm) compared with the construct without PLL-GO as a control (23 µm). The thickness and size of the layers PLL-GO layers ranged from a few microns to 10 µm, which is much thicker than tissue grown on using fibronectin, gelatin (G), and nanofilms (6.2 nm). The advantages of using PLL-GO layers can be seen in the confocal cross-sectional images of the 3L tissue constructs and the control group after 2 days of culture ().
Figure 3 (A) Confocal cross-sectional images of the control group (top) and the 3L tissue constructs (bottom) after 2 days of culture. F-actin and cell nuclei were labeled with green and blue fluorescent dyes, respectively. The 3T3 fibroblasts were found to connect the cells on the first layer to the cells on the second layer through noncontinuous PLL-coated GO layer (red arrow, empty black area). (B) Hematoxylin and eosin (H&E) stain images of 3L 3T3 fibroblasts. The solid red lines indicate the interfaces between each layer. (C) Schematic illustration of the cross-section of the 2L construct showing the cells residing above and below the PLL-coated GO nanofilms. (D) SEM images showing the cross-section and (E) the thickness of 2L constructs fabricated with various concentrations of PLL-coated GOs as interlayer GO films. (F) SEM images showing the cross-section, and (G) the thickness of 1L, 2L, and 3L constructs. The thickness of the constructs was estimated from the corresponding SEM images. Reproduced from Shin SR, Aghaei-Ghareh-Bolagh B, Gao X, et al. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv Mater. 2014;22(39):6136–6144.Citation135 Copyright © 2015 Wiley ACH.
Abbreviations: GO, graphene oxide; PLL, poly-L-lysine; SEM, scanning electron microscope.
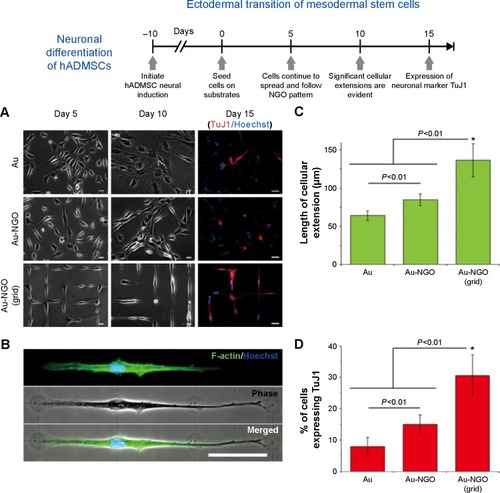
Silk fibrin (F) proteins are routinely employed in tissue generation as substitutes for bone and skin tissues and blood vessels. Utilizing the material advantages of Fibrin and GO, Wang et al fabricated a nanocomposite film by simply casting the two components together.Citation138 Fibrin functionalized graphene oxide and GO can also be used as nucleation sites for the growth of hydroxyapatite (HA). Deepachitra et alCitation139 showed that fibrin-graphene hydroxyapatite (FGHA) was an excellent platform for osteoblast cell growth and maturation, showing very high viability rates compared with GO, GOHA, and functionalized graphene oxide. Chaudhuri et al have sought to overcome the problems of toxicity and biocompatibility by blending the insulating polymer polycaprolactone with GO nanoplatelets resulting in a highly conductive biocompatible scaffold.Citation138 The resulting scaffold was used to differentiate human cord blood-derived mesenchymal stem cells into skeletal muscle cells. It was concluded that the addition of GO nanoplatelets enhanced both conductivity and the dielectric constant of the GO-polycaprolactone scaffold stimulating highly oriented multinucleated myotube formation. Studies have proved the ability of GO to promote stem cell differentiation into osteogenic, cardio, neuronal, and adipogenic lineages.Citation140–Citation143 Of particular note is the recent work by Kim et alCitation33 in which a novel strategy to guide stem cell differentiation into specific cell lineages by employing combinatorial GO hybrid-patterns of specific geometries was reported. NGO combinatorial pattern-arrays, with different sizes and geometries, were successfully transferred to various substrates such as Au-coated glass, molded polystyrene, flexible polydimethylsiloxane, and even biodegradable poly(lactic-co-glycolic acid) film. The NGO line patterns generated on both rigid gold substrates and flexible polymers were effective for guiding osteogenic differentiation of human adipose-derived mesenchymal stem cells (hADMSCs) with conversion efficiencies as high as 54.5% and 41%, respectively. In addition, patterned GO resulted in a conversion ratio of MSCs to neurons of up to 30%. The enhanced neuronal differentiation of hADM-SCs via patterned NGO could result in improved treatments of serious neurological disorders such as Parkinson’s disease. A schematic showing neuronal differentiation of hADMSCs using different NGO grid-patterned substrates is shown in .
Figure 4 Neuronal differentiation of hADMSCs using NGO grid-patterned substrate.
Notes: (A) Images of neural-induced hADMSCs grown on poly-L-lysine-coated Au (Au), NGO-coated Au (Au-NGO), and NGO grid-patterned substrates (Au-NGO (Grid)). All substrates were coated with laminin to facilitate cell attachment. Cellular growth and morphology were monitored over 15 days, followed by staining for the neuronal marker TuJ1 (red) and nucleus (blue). Scale bars =20 µm. (B) Phase-contrast and fluorescence images of cells stained for F-actin (green) and nucleus (blue) after 15 days of cultivation show extensive cellular extension on NGO-grid patterns. Scale bar =50 µm. (C) Quantitative comparison of the length of cellular extension on various substrates (n=3; *P<0.01, Student’s unpaired t-test). (D) Quantitative comparison of the percentage of cell expressing the neuronal marker TuJ1 on various substrates (n=3; *P<0.01, Student’s unpaired t-test). Reproduced with permission from Kim TK, Shah S, Yang L. Controlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arrays. ACS Nano. 2015:9(4):3780–3790.Citation33 Copyright ©2015 American Chemical Society.
Abbreviations: NGO, nano graphene oxide; TuJ1, class III beta-tubulin.
GO is also known to play a significant role both in endothelial and hepatocyte cell proliferation and morphology differentiaton. Zhou et alCitation136 also showed that a LBL 3D composite layer composed of PSS (polyanion) and polyacrylamide (poly-cation) grafted to GO exhibited excellent anticoagulant bioactivities indicating heparin-mimicking activity. In another LBL study,Citation137 assembled GO nanocomposite films were constructed aimed at improving the mechanical properties of polyelectrolyte multilayer (PEM) films containing PSS and poly(allylamine hydrochloride). It was found that a single layer of GO improved the elastic modulus of a PEM film by up to 181%.Citation137 When compared with native PEM films, fibroblast cells grew quicker and over a larger area, forming numerous and better organized adhesion points on the GO composite films.
Imaging techniques
In the last 10 years, a lot of effort has been dedicated in exploiting graphene derivatives as contrast agents (CAs) for intracellular imaging in vitro and in vivo. There are many examples of functionalized GO being employed as fluorescence and photoluminescent vehicles in cellular imaging. Of particular note is the recent work by Sreejith et alCitation144 in which a hybrid material composed of organic dyes, mesoporous silica nanoparticles (MSNPs), and GO was synthesized. Squarine dyes were loaded inside MSNPs, and the MSNP surfaces were then wrapped with ultrathin GO sheets. The hybrid was biocompatible, noncytotoxic exhibiting significant potential for in vitro fluorescence imaging as confirmed by the imaging studies with HeLa cells.
Magnetic resonance imaging (MRI) is a central whole-body imaging techniques used to visualize anatomical structures in biomedical research and clinical medicine. Researchers have been developing MRI CAs since the 1980s.Citation145 CAs are complexes of gadonlinium (Gd3+), manganese (Mn2+), or iron (Fe2+). Gd3+ chelate-based T1 MRI CAs currently dominate the market (have >95% market share) with nearly half of all MRI procedures in the United States using MRI CAs. However, the Food and Drug Administration recently restricted the clinical use of Gd3+ chelates for patients affected by renal failure. CAs using Mn2+ ions have been proposed as possible alternatives. Kanakia et alCitation146 showed that GO/Mn2+/Dextran (GNP-Dex) agents performed particularly well. The results indicated that at high concentrations between 0.1 and 100.0 mg/mL, the GNP-Dex formulations were hydrophilic, stable in deioinzed water, as well as iso-osmolar (upon addition of mannitol) isoviscous to blood. At potential steady state equilibrium concentrations of blood (0.1–10.0 mg/mL), protein binding, and histamine release studies indicated that GNP-Dex formulations are thermally stable and elicit negligible allergic response. The r1 relaxivity of GNP-Dex was 92 mM−1 s−1 (per-Mn2+ ion, 22 MHz proton Larmor frequency); approximately 20- to 30-fold greater than that of clinical Gd3+- and Mn2+-based CAs.
Other examplesCitation147 utilizing magnetize GO hybrids for imaging can be found in the study by Gollavelli et al in which reduced graphene was covalently modified with a PAA bridge then linked to fluorescein o-methacrylate. The PAA bridge was found to inhibit both vehicle aggregation and graphene-induced fluorescence quenching of conjugated fluorescein o-methacrylate. Toxicological studies showed the resultant hybrid to be nontoxic with insignificant amounts of reactive oxygen species (ROS) and apoptosis in HeLa cells. Confocal laser scanning microscopy images further revealed images that the hybrid was localized in the cytoplasm at the cellular level and exhibited a broad distribution from the head to the tail in zebrafish (animal model). Considering their large surface GO sheets can also be integrated with various types of NPs to form multifunctional nanomaterials for different application purposes.
MR and X-ray computed tomography (CT) imaging modalities are widely used for various experimental and clinical applications. MR offers high sensitivity and good discrimination particularly in soft tissue but shows no signal for high-density calculus and gland calcification. CT affords better spatial and density resolution than other modalities but is limited by the poor performance of iodine-based CAs in soft tissue. Thus, combining MR imaging with CT modality could achieve more useful information of soft tissues or tumors with enhanced accuracy. Recently, a GO-BaGdF5 nanocompositesCitation148 for multimodal imaging was fabricated using a solvo-thermal method in the presence of PEG; BaGdF5 NPs were firmly attached on the surface of GO nanosheets to form the GO/BaGdF5/PEG. The composite showed low cytotoxicity, positive MR contrast effect, and better X-ray attenuation property than lohexol, which enabled effective dual-modality MR and X-ray CT imaging of a tumor model in vivo. Moreover, histological examination and serum biochemistry assay revealed no apparent toxicity of the CA to mice after treatment. GO/BaGdF5/PEG may be further conjugated with different targeting ligands to construct multifunctional systems for targeted theranosis of cancers. A schematic summarizing the dual-imaging capabilities of GO/BaGdF5/PEG is shown in .
Figure 5 A schematic diagram of magnetic resonance (MR)/computed tomography (CT) imaging and near infrared photothermal therapy (PTT) using the graphene oxide/BaGdF5/polyethylene glycol (PEG) nanocomposites. Reproduced with permission from Zhang H, Wu H, Wang J, et al. Graphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials. 2015;42:66–77.Citation148 Copyright © 2015 Elsevier.
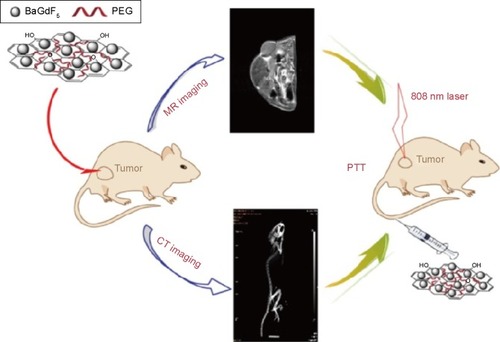
Hong et alCitation149 fabricated a targeted multifunctional GO hybrid via the covalent linkage of PEG, fluorescein isothiocyanate, 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), and TRC105 (a monoclonal antibody that binds to CD105) on GO. The pharmacokinetics and tumor-targeting efficacy of the NOTA/TRC105/GO hybrid were investigated with serial noninvasive positron emission tomography imaging and biodistribution studies, indicating excellent stability and target specificity. New preparationCitation150 methods were also employed in the construction and detection of graphene-based hybrids for magnetic resonance/fluorescence imaging. A magnetic Fe3O4-doped carbogenic nanocomposite (IOCNC) was synthesized by thermal decomposition of organic precursors in the presence of F3O4 NPs with a mean diameter of 6 nm. Magnetic studies confirmed the superparamagnetic behavior nature of IOCNC at room temperature. IOCNC showed MR contrast behavior by affecting the proton relaxation phenomena. The measured longitudinal (r1=T1) and transverse (r2=T2/T2*) relaxivity values are 4.52 and 34.75 mM−1 s−1, respectively. The hybrid showed a biocompatible nature with no apparent cytotoxicity. In vivo MR studies indicated both T1 and T2 contrast behaviors of the hybrid. Fluorescence imaging indicated selective uptake of IOCNC by macrophages in spleen. Pharmacokinetics and tumor targeting efficacy of the hybrid was evaluated via positron emission tomography imaging using Ga as the radiolabel. GO linked covalently with PEG was conjugated to NOTA and TRC105, making the hybrid specific toward CD105 in cell culture. In 4T1 tumor-bearing mice, the Ga/NOTA/GO/TRC105 and Ga/NOTA/GO composites were primarily cleared through the hepatobiliary pathway. Ga/NOTA/GO/TRC105 was accumulated quickly in 4T1 tumors with uptake remaining stable up to 24 hours postinjection. In active targeting, GONs with antibody, peptide, or protein coatings can bind specifically to the surface of tumor cells or to neovascular endothelial cells. However, certain challenges remain, as shown by the limited performance of the first clinically approved PEGlyated liposome (Doxil™). In immunodeficient animal models, the liposome exhibited marked antitumor effects, but in clinical applications, lipo-somes exhibited efficacy against only a limited number of tumors, such as Kaposi’s sarcoma. This can be attributed to the complexity of the tumor morphology during the successive stages of inflammation, fibrillization, hemorrhage, and repair that occur repeatedly in the process of tumor formation and growth in humans.Citation151
Photodynamic therapies
The strong optical absorbance of graphene-based nanomaterials in the NIR region makes them generally applicable as prognostic, diagnostic, and therapeutic agents in the treatment of cancer and other disease states. Photodynamic therapy (PDT)Citation152 is a popular cancer therapy method that involves the delivery of photosensitizers (PS)Citation153 into the cancer cells generating cytotoxic ROS (photodynamic) or generating heat (photo-thermal) that are capable of killing cells through photoablation. In addition, PDT has been shown to damage tumor vasculature through direct effects on vascular endothelial cells. Ideally, PDT agents should exhibit strong absorbance. However, clinical application of PDT is limited by the hydrophobic nature and poor tumor selectivity of existing PSs.Citation154 2-(1-Hexyloethyl)-2-devinyl pyropheophorbide-alpha (HPPH, Photochlor))Citation155–Citation157 is a second-generation PS currently progressing through phase I/II clinical trials and has shown excellent safety and efficacy for the treatment of lung, Barett’s esophageal, and head and neck cancers. GO and reduced graphene oxide (rGO) are reported to induce lung toxicity in mice when delivered orally or intravenously, but when coated with PEG and chitosan are nontoxic to cells in vitro and can be cleared via renal and hepatic routes. Recent work using HPPH loaded onto GO by Rong et alCitation158 showed a dramatic improvement in photodynamic cancer cell kill efficacy due to the increased tumor delivery of HPPH within the tumor compared with free HPPH upon 671-nm laser irradiation. The study highlighted the advantages of GO as a carrier for PDT resulting improvements in PDT efficacy and long-term survival rates of tumor mice following treatment.
Ultrafast laser is an effective tool for nanofabrication due to the high-pulse energy; the temperature of GO can increase by more than 1,000°C in microseconds, simultaneously reducing GO to rGO. Moreover, the ultrafast reduction of GONs with a femtosecond laser beam creates extensive microbubbling.Citation159 The instant collapse produces a microcavitation effect that brings about localized mechanical damage. A study by Li et alCitation160 showed that when microbubbles are produced the effective laser power was reduced to less than half of what is needed when microbubbling is absent. Gastric cancer cells labeled with PEG-transferrin required only a few scans of a 4 mW laser source for cell therapy, while 15 scans of a 9 mW source resulted in the death of only a few cells labeled with rGONs. This technique may be particularly useful in dealing with fibrotic intractable tumors often accounted in pancreatic cancers. A detailed review regarding the theranostic applications of graphene in cancer can be found in a recent article by Chen et al.Citation161
Cytotoxicity
PEGlyated GO and GO exhibit certain advantages in vitro and in vivo drug delivery, such as high drug-loading efficiency, passive and active targeting capabilities, and reversal effects against cancer drug resistance.Citation93 PEGylation is known to improve the solubility of hydrophobic nanomaterials and is widely used in many areas of nanomedicine.Citation25 Reports have shown that incubation of several cell cultures, such as glioblastoma cell line (U87MG), breast cancer cells (MCF-7), human ovarian carcinoma cell line (OVCAR-3), colon cancer cell lines (HCT-116), and lymphoblastoid cells (RAJI) with GO-capped PEGCitation162–Citation164 exhibit no cytotoxicity up to 100 µg/mL. The ability of macrophages to internalize and remove graphene materials from the site of deposition serves to enhance their cellular biocompatibility. For example, two phagocytic cell lines were able to internalize and micronized GO with different lateral sizes showing a selective internalization. After internalization, GO accumulated in the cytoplasm, perinuclear space, and nucleus of the cell.Citation165 Mu et al revealed that C2C12 progenitor cells used clathrin-mediated endocytosis to internalize medium-sized GO (500 nm) and phagocytosis for larger micron (1–2 µm)-sized sheets. Shortly after, both types of GO entered lysosomes for excretion. Almost no inhibition of cell proliferation was found at doses up to 100 µg/mL.Citation166
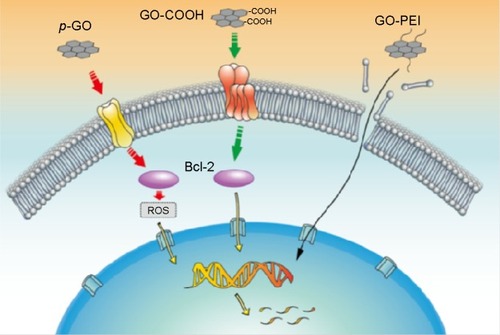
The in vitro hemocompatibility and genotoxicity of GO with human primary blood components remains a hotly contested issue. Initial studies examining the hemocompatibility of graphene and GO showed that graphene exerted a slightly higher cytotoxic effect than GO due to its strong hydrophobic interaction with cell membranes with both materials exerting insignificant hemolytic effect (up to 75 µg/mL).Citation167 In contrast, Liao et alCitation168 demonstrated that submicron-sized GO sheets induced the greatest hemolytic activity, whereas aggregated graphene sheets exhibited the lowest hemolytic activity. Coating the oxidized sheets with chitosan almost eliminated hemolytic activity. It was concluded that the toxicity of graphene and GO was dependent on the exposure environment (ie, whether or not aggregation occurs) and mode of interaction with cells (ie, suspension versus adherent cell types). In a recent investigation, Ding et alCitation169 examined the hemocompatibility of GO on human peripheral blood T lymphocytes and human serum albumin (HSA). In that work, the underlying toxic mechanisms of pristine GO (p-GO) and functionalized GO (GO-COOH and GO-PEI) to primary human peripheral blood T-lymphocytes and HSA were investigated. p-GO was found to interact directly with the protein receptors to inhibit their ligand-binding ability, leading to ROS-dependent apoptosis through the B-cell lymphoma-2 (Bcl-2) pathway; GO-COOH exhibited a similar degree of toxicity on T lymphocytes except keeping a normal ROS level. Ding et al proposed that GO-COOH inhibits protein-ligand binding and passes the passive apoptosis signal to nucleus DNA through a ROS-independent mechanism. GO-PEI showed severe hematotoxcity to T lymphocytes by inducing membrane damage. For HSA, the binding of GO-COOH resulted in minimal conformational change and HSA’s binding capacity to bilirubin remained unaffected, while the binding of p-GO and GO-PEI exhibited strong toxicity on HSA. A schematic of the toxic mechanism of GO on T lymphocytes is depicted in . These apparent contradictions in the literature are most probably due to poor-quality GO being used (broad lateral distributions >500 nm and the presence of contaminants, Mn2+, Fe3+, Cu2+) and inconsistencies in assay design (MTT false positives and GO’s strong autofluorescence signal). At concentrations approximate to 50 µg/mL or higher, freshly prepared GO begins to show toxicity against erythrocytes, fibroblasts, and, in some reports, PC12 cells as well. PEGylation significantly improves biocompatibility, but the chemical bonds linking GO with the surfactant can be broken releasing PEG and its derivatives into the surrounding environment. The influence of PEG to suppress heme destruction and improve peroxidase function was recently reported by Mao et al.Citation170 It was found that horseradish peroxidase (HRP) inactivation is significantly mitigated in the presence of PEG. In addition, recent reports show that the concentration of HRP oligomers produced from the biocatalysis of GO was undetectable.Citation171 It is well reported that carbon nanotubes are rapidly degraded by HRP, myeloperoxidase, eosinophil peroxidase with HRP-catalyzed oxidation of single walled carbon nanotubes and GO (single-walled carbon nanotubes) reported to induce DNA damage.Citation172 Whether the localized release of PEG from modified GO impacts other HRP inactivation pathways and hemotoxicity remains unknown. However, small lateral (l)-sized GO (200 nm) fragments are known to interact with DNA.Citation173 These interactions include DNA intercalation and the scission of DNA by GO/Cu2+ complexes. Furthermore, it has been shown that GO/Mn2+ and GO/Fe2+ complexes also cleave DNA. In addition, several investigations have shown that treatments of various cell lines with carbon nanomaterials such as rGO, graphene, and graphite can elevate the expression of p53, Rad 51, and MOGG1-1 reflecting chromosomal damage. Until recently, it was unclear whether DNA damage induced by graphene-based materials caused mutagenesis. In a recent study by Liu et alCitation174 GO treatments at concentrations of 10 and 100 µg/mL were found to alter gene expression in 101 genes involved in DNA-damage control, cell apoptosis, cell cycle, and metabolism. Intravenous injection of conventionally prepared GO at 4 mg/kg for 5 consecutive days induced formation of micronucleated polychromic erythrocytes in mice, and its mutagenesis potential appeared to be comparable with cyclophosphamide, a classic mutagen. However, traditionally prepared GO often contains high concentrations of Mn2+ (97 ppm) and Fe2+ WC. As stated previously, both metals are highly mutagenic in the presence of GO, nonspecific release of these ions from traditionally prepared GO might result in unusually high levels of toxicity and random scission of DNA. Consequently, researchers have started to use nontoxic oxidizing agents with greener exfoliating methods.Citation175 Of particular note is the recent work by Peng et alCitation176 in which an Fe2+-based green strategy produced a single layer of GO in just 1 hour. Their approach resulted in the production of high purity GO containing 0.025 ppm of Mn2+ and 0.13 ppm Fe2+, respectively. Results regarding the cytotoxicity of graphene-based nanomaterials remain conflicting (particular for GO). These discrepancies may be due to differences in the quality of the nanomaterials tested.Citation177,Citation178
Figure 6 Schematic diagram showing proposed toxic mechanisms of GO on T lymphocytes based on the current data. From left to right are p-GO, GO-COOH, and GO-PEI, respectively. Dotted line indicates signal pathway, and full line indicates the way of GO-PEI transport. Reproduced with permission from Ding Z, Zhang Z, Ma H, Chen Y. In vitro hemocompatibility and toxic mechanism of graphene oxide on human peripheral blood T lymphocytes and serum albumin. ACS Appl Mater Interfaces. 2014;6(22):19797–19807.Citation169 Copyright ©2015 American Chemical Society.
Abbreviations: Bcl-2, B-cell lymphoma-2; PEI, polyethylenimine; p-GO, pristine graphene oxide; ROS, reactive oxygen species.
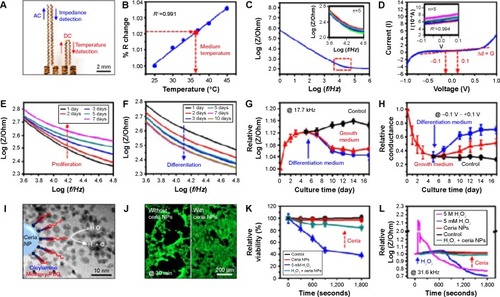
Finally, there are many cautionary warnings in the literatureCitation178 regarding the PEGlyated forms of GO and its ability to generate ROS in mammalian cells. However, ceria NPs are known to scavenge ROS and are therefore attractive candidates for inclusion into a graphene-based vehicles or TE platforms.Citation179 With this in mind, Kim et alCitation180 recently reported a graphene-based multifunctional platform that can suppress ROS generation. The multifunctional platform was capable of aligning plated cells and in situ monitoring of cellular physiological characteristics during proliferation and differentiation. Cell viability was represented by changes in impedance and monitored by an instrumented cell-culture platform (). Treatment with 5 M H2O2 in differentiated C2C12 cells induced instant death in the majority of cells, resulting in a dramatic increase in impedance, whereas treatment with 5 mM H2O2 in the presence of ceria NPs yielded only minimal changes in impedance.
Figure 7 Physiological monitoring of C2C12 myoblasts by impedance/temperature sensors and in vitro tests of the efficacy of ROS scavenging nanoparticles.
Notes: (A) Impedance and temperature sensors integrated on a PDMS substrate. (B) Calibration curve of temperature sensor: normalized resistance (% R change) as a function of temperature. The red arrow indicates the temperature of the growth medium during the culture. (C) Electrical characterization of the impedance sensor in the growth medium at 37°C. Impedance curve measured from 1 Hz to 1 MHz with a bias voltage of 0.01 V. The inset shows the magnified view of the red-dotted region. Repeated measurements show minor deviations. (D) Current-voltage (I–V) curve, whose slope indicates the conductance. The inset shows the magnified view. Repeated measurements confirm the stability of the sensor. (E and F) The impedance curve changed as (E) the proliferation and (F) the differentiation proceeded. (G) Impedance value measured at 17.7 kHz as the culture proceeded. Red and blue curves show the cells in growth and differentiation media, respectively. The control (black) used human dermal fibroblasts. (H) Conductance values calculated from IV curves with a range from −0.1 to +0.1 V. (I) Schematic illustration and TEM image (background image) of ROS-scavenging ceria nanoparticles. The ceria NPs are functionalized by oleylamine and methoxy-polyethylene glycol. (J and K) Fluorescence image of C2C12 myoblasts (stained with calcein AM) after 30 minutes of H2O2/Ceria NP treatment (J) and relative viability plot from fluorescence images (K). (L) Plots of impedance as a function of time in different treatment groups. Reproduced with permission from Kim SJ, Cho HR, Cho KW, et al. Multifunctional cell-culture platform for aligned cell sheet monitoring, transfer printing, and therapy. ACS Nano. 2015;9(3):2677–2688.Citation180 Copyright ©2015 American Chemical Society.
Abbreviations: ROS, reactive oxygen species; NP, nanoparticle; TEM, transmission electron microscope; PDMS, polydimethylsiloxane.
Conclusion
This review represents a snapshot of the current state of GO research and the opinions that govern its development. There is no doubt that GO has led to rapid improvements in many areas of biomedical science, including drug delivery, TE, sensors, imaging, and diagnosis within the last decade. Moreover, the recent advances in green fabrication methodsCitation174 further extend the application potential of GO to a larger field of scientists. If this potential can then be coupled with the digitization and real-time monitoring of the cell sample (microdroplets), GO could be clinically exploited in the very near future. In our humble opinion, the great expectations fueling graphene-based research warrant a “digital shotgun” approach, thus clarifying any doubts regarding its efficacy and applicability not just in nanomedicine, but its future impact on environmentalCitation181 and public health.
Acknowledgments
This research was supported by the National Research Foundation Korea project number 2012014335.
Disclosure
The authors report no conflicts of interest in this work.
References
- NovoselovKSGeimAKMorozovSVTwo-dimensional gas massless Dirac fermions in grapheneNature2005438706519720016281030
- LeeCWeiXKysarJWHoneJMeasurement of the elastic properties and intrinsic strength of monolayer graphemeScience2008321588738538818635798
- WangYLiZWangJLiJLinYGraphene and graphene oxide biofunctionlization and applications in biotechnologyTrends Biotechnol201129520521221397350
- LiuZRobinsonJTTabakmanSMYangKDaiHCarbon materials for drug delivery and cancer therapyMater Today2011147–8316323
- RaoCNRSoodAKSubrahmanyamKSGovindarajAGraphene the new two dimensional nanomaterialAngew Chem Int Ed Engl200948427752777719784976
- HummersWSOffemanREPreparation of graphitic oxideJ Am Chem Soc19588961339
- DreyerDRParkSBielawskiCWRuoffRThe chemistry of graphene oxideChem Soc Rev201039122824020023850
- MarcanoDCKosynkinDVBerlinJMImproved synthesis of graphene oxideACS Nano2010484806481420731455
- FanHWangJWuHFabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan compositesBiomacromolecules20101192345235120687549
- MaJLiuCLiRWangJProperties and structural characterization of oxide starch/chitosan/graphene oxide biodegradable nano-compositesJ Appl Polym Sci2012123529332944
- WojtoniszakMChenXKalenczukRJWajdaASynthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxideColloids Surf B Biointerfaces2012891798521962852
- YangKWanJZhangSZhangYLeeS-TLiuZIn vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in miceACS Nano20105151652221162527
- WangHQiuZCrystallization behaviors of biodegradable poly (l-lactic acid)/graphene oxide nano-composites from the amorphous statesThermochim Acta20115261–2229236
- WanCChenBPoly(ε-caprolactone)/graphene oxide biocomposites: mechanical properties and bioactivityBiomed Mater20116505501021921319
- ZhangJQiuZMorphology, crystallization behavior, and dynamic mechanical properties of biodegradable poly(ε-caprolactone)/thermally reduced graphene nanocompositesInd Eng Chem Res201150241388513891
- LiuJQCuiLDusanLGraphene and graphene oxide as new nanocarriers for drug delivery applicationsActa Biomater20139129243925723958782
- FengLWuLQuXNew horizons for diagnostics and therapeutic applications of graphene and graphene oxideAdv Mater201325216818623161646
- PanYSahooNGLiLThe application of graphene oxide in drug deliveryExpert Opin Drug Deliv20121291365137623005029
- ShenHZhangLLiuMZhangZBiomedical applications of grapheneTheranostics20122328329422448195
- ChenYTanCZhangHWangLTwo dimensional graphene analogues for biomedical applicationsChem Soc Rev20154492681270125519856
- ChenBALiuMZhangLMHuangJYaoJLZhangZJPolyethylenimine-functionalized graphene oxide as an efficient gene delivery vectorJ Mater Chem20112177367741
- RanaVKChoiMCKongJYSynthesis and drug-delivery behavior of chitosan functionalized graphene oxide hybrid nanosheetsMacromol Mater Eng20112962131140
- KakranNGSMBaoHPanYLiLFunctionalized graphene oxide as nanocarrier for loading and delivery of ellagic acidCurr Med Chem201118194503451221864287
- LiuKZhangJJChengFFZhengTTWangCZhuJJGreen and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug deliveryJ Mater Chem201121321203412040
- LiuZRobinsonJTSunXDaiHPEGylated nanographene oxide for delivery of water-insoluble cancer drugsJ Am Chem Soc200813033108761087718661992
- KimMGParkJYMiaoWLeeJOhYKPolyaptamer DNA nanothread-anchored, reduced graphene oxide nanosheets for targeted deliveryBiomaterials20154812913625701038
- ZhangLXiaJZhaoQLiuLZhangZFunctional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugsSmall20106453754420033930
- WenHDongCDongHEngineered redox-responsive peg detachment mechanism in pegylated nano-graphene oxide for intracellular drug deliverySmall20128576076922228696
- MudavathSLTalatMRaiMSrivastavaONSundarSCharacterisation and evaluation of amine modified graphene amphotericin B for the treatment of visceral leishmaniasis: in vivo and in vitro studiesDrug Des Devel Ther2014812351247
- PrajapatiVKAwasthiKYadavTPRaiMSrivastavaONSundarSAn oral formulation of amphotericin B attached to functionalized carbon nanotube is an effective treatement for experimental visceral leishmaniasisJ Infect Dis2012205233333622158723
- WangYWangHLiuDSongSWangXZhangHGraphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapyBiomaterials201334317715772423859660
- LeeWCLimCHYXShiHOrigin of enhanced stem cell growth and differentiation on graphene and graphene oxideACS Nano2011597334734121793541
- KimTKShahSYangLControlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arraysACS Nano2015943780379025840606
- TangLALeeWCShiHHighly wrinkled cross-linked graphene oxide membranes for biological and charge-storage applicationsSmall20128342343122162356
- LaWGJinMParkSYoonHHJeongGJDelivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regenerationInt J Nanomedicine20149110711624872706
- LimHNHuangNMLimSSHarrisonIChiaCHFabrication and characterization of graphene hydrogel via hydrothermal approach as a scaffold for preliminary study of cell growthInt J Nanomedicine201161817182321931479
- ShinSRAghaei-Ghareh-BolaghBGaoXNikkhahMJungSMLayer-by-layer assembly of 3d tissue constructs with functionalized grapheneAdv Funct Mater201424396136614425419209
- ChaudhuriBBhadraDMoroniLPramanikKMyoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide–polymer composite fibrous meshes: importance of graphene oxide conductivity and dielectric constant on their biocompatibilityBiofabrication20157101500925691492
- GurunathanSHanJWDayemAAEppakaylaVKimJHOxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosaInt J Nanomedicine201275901591423226696
- NandaSSAnSSAYiOxidative stress and antibacterial properties of a graphene oxide-cystamine nanohybridInt J Nanomedicine20151054955625609960
- KurantowiczNSawoszEJaworskSInteraction of graphene family materials with Listeria monocytogenes and Salmonella entericaNanoscale Res Lett2015102310.1186/s11671-015-0749-y.
- ChenHGaoDWangBGraphene oxide as an anaerobic membrane scaffold and antagonistic effects against pathogenic E. coli and S. aureusNanotechnology2014251616510124670485
- ZhanSZhuDMaSHighly efficient removal of pathogenic bacteria with magnetic graphene compositeACS Appl Mater Interfaces2015774290429825634911
- MangadlaoJDSantosCMFelipeMJLLeonACCRodriguesDFAdvinculaRCOn the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett filmsChem Commun2015511428862889
- LiuSZengTHHofmannMAntibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stressACS Nano2011596971698021851105
- HuWPengCLuoWGraphene-based antibacterial paperACS Nano2010474317432320593851
- GurunathanSHanJWDayemAAAntibacterial activity of dithiothreitol reduced graphene oxideJ Ind Eng Chem201319412801288
- MogharabiMAbdollahiMFaramarziMASafety concerns to application of graphene compounds in pharmacy and medicineJ Pharm Sci201422110.1186/2008-2231-22-23.
- NguyenPBerryVGraphene interfaced with biological cells: opportunities and challengesJ Phys Chem Lett2012381024102926286566
- Mejias CarpioIESantosCMWeiXRodriguesDFToxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cellsNanoscale20124154746475622751735
- SreeprasadTSMaliyekkalMSDeeptiKChaudhariKXavierPLPradeepTTransparent, luminescent, antibacterial and patternable film forming composites of graphene oxide/reduced graphene oxideACS Appl Mater Interfaces2011372643265421688808
- HuangNLiuMLiMZhangYYaoSSynergetic signal amplification based on electrochemical reduced graphene oxide-ferrocene derivative hybrid and gold nanoparticles as an ultrasensitive detection platform for bisphenol AAnal Chim Acta201585324925725467466
- GaoLLianCZhouYGraphene oxide-DNA based sensorsBiosens Bioelectron201460222924768760
- ZhangBLiQCuiTUltrasensitive suspended nanocomposite cancer sensors with strong suppression of electrical noiseBiosens Bioelectron201231110510922051545
- GuYJuCLiYDetection of circulating tumor cell in prostate cancer based on carboylated graphene oxide modified light addressable potentiometric sensorBiosens Bioelectron201566243125460877
- YaoYXueYImpedance analysis of quartz crystal microbalance humidity sensors based on nanodiamond/graphene oxide nanocomposite filmSens Actuators B Chem20152115258
- GeSSunMLiuWDisposable electrochemical immune-sensors based on peroxidase-like magnetic silica graphene composites for detection of cancer antigen 153Sens Actuators B Chem2014192317326
- JaqueDMartinez MaestroLdel RosalBNanoparticles for photothermal therapiesNanoscale20146169494953025030381
- JohannsenMGneveckowUEckeltLClinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial techniqueInt J Hyperthermia200521763764716304715
- LimDKBarhoumiAWylieRGEnhanced photothermal effect of plasmonic nanoparticles coated with reduced graphene oxideNano Lett20131394075407923899267
- HuschkaRZuloagaJKnightMWBrownLVNordlanderPHalasNJLight-induced release of DNA from gold nanoparticles: nanoshells and nanorodsJ Am Chem Soc201113331122471225521736347
- ShiXGongHLiYWangCChengLLiuZGraphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapyBiomaterials201334204786479323557860
- HuangJZhangLMChenBANanocomposites of size-controlled gold nanoparticles and graphene oxide: Formation and applications in SERS and catalysisNanoscale20102122733273820936236
- LiuQWeiLWangJCell imaging by graphene oxide based on surface enhanced Raman scatteringNanoscale20124227084708923070130
- HuangJZongCShenHMechanism of cellular uptake of graphene oxide studied by surface-enhanced Raman spectroscopySmall20128162577258422641430
- LiuZMGuoZYZhongHQQinXCWanMMYangBWGraphene oxide based surface-enhanced Raman scattering probes for cancer cell imagingPhys Chem Chem Phys20131582961296623340832
- WeitmanSDLarkRHConeyLRDistribution of the folate receptor GP38 in normal and malignant cell lines and tissuesCancer Res19925212339634011596899
- ZwickeGLMansooriGAJefferyCJUtilizing the folate receptor for active targeting of cancer nanotherapeuticsNano Rev2012310.3402/nano.v3i0.18496
- GurunathanSHanJWEppakaylaVKimJHGreen synthesis of graphene and its cytotoxic effects in human breast cancer cellsInt J Nanomedicine201381015102723687445
- HinzmannMJaworskiSKutwinMNanoparticles containing allotropes of carbon have genotoxic effects on glioblastoma multiforme cellsInt J Nanomedicine201392409241724876774
- WangHGuWXiaoNYeLXuQChlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drugInt J Nanomedicine201491433144224672236
- ChowdhurySMSurhlandCSanchezZGraphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiformeJ Nanomed Nanotechnol2015111109118
- ShenHZhangLLiuMBiomedical applications of grapheneTheranostics20122328329422448195
- ShiSYangKHongHTumor vasculature targeting and imaging in living mice with reduced graphene oxideBiomaterials201334123002300923374706
- QinXCGuoZYLiuZMFolic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapyJ Photochem Photobiol B201312015616223357205
- BussyCBoucettaAKostarelosKSafety considerations for graphene: Lessons learnt from carbon nanotubesAcc Chem Res201346369270123163827
- DallavelleMCalvaresiMBottoniAFrancoMMZerbettoFGraphene can wreak havoc with cell membranesACS Appl Mater Interfaces2015774406441425648559
- LiuJCuiLLosicDGraphene and graphene oxide as new nanocarriers for drug delivery applicationsActa Biomater20139129243925723958782
- SmolkovaBYamaniNECollinsARGutlebACDusinskaMNanoparticles in food. Epigenetic changes induced by nanomaterials and possible impact on healthFood Chem Toxicol201577647325554528
- GeorgantzopoulouAEffects of Silver Nanoparticles and Ions and Interaction with the First Line of Defense. [PhD]WageningenWageningen University2015188
- NilssonEESkinnerMKEnvironmentally induced epigenetic transgenerational inheritance of disease susceptibilityTransl Res20141651121724657180
- DubeyPMataiIKumarSUSachdevABhushanBGopinathPPerturbation of cellular mechanistic system by silver nanoparticles toxicity: cytotoxic, genotoxic and epigenetic potentialAdv Colloid Interface Sci201522142125935324
- YaoYCostaMGenetic and epigenetic effects of nanomaterialsJ Mol Genet Med20137410.4172/1747-0862.1000086.
- AhluwaliaABoraschiDByrneHJThe bio-nano interface as a basis for predicting nanoparticle fate and behavior in living organisms: towards grouping and catergorizing of nanomaterials and nanosafety by designBio Nano Materials201314195216
- SeabraABPaulaAJLimaRAlvesOLDuranNNanotoxicity of graphene and graphene oxideChem Res Toxicol201427215916824422439
- ServantABiancoAPratoMKostarelosKGraphene for multifunctional synthetic biology: the last ‘zeitgeist’ in nanomedicineBioorg Med Chem Lett20142471638164924594351
- ShiSChenFEhlerdingEBCaiWSurface engineering of graphene based nanomaterials for biomedical applicationsBioconjug Chem20142591609161925117569
- GoenkaSSantVSantSGraphene-based nanomaterials for drug delivery and tissue engineeringJ Control Release2014173758824161530
- ZhangHGrunerGZhaoYRecent advancements of graphene in bio-medicineJ Mater Chem B Mater Biol Med201312025422567
- SoenenSJParakWJReijmanJManshianB(Intra) Cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applicationChem Rev201511552109213525757742
- ParveenSMisraRSahooSKNanoparticles: a boon to drug delivery, therapeutics, diagnostics and imagingJ Nanomed Nanotechnol20128214766
- YangKZhangSAZhangGXSunXMLeeSTLiuZAGraphene in mice:ultrahigh in vivo tumor uptake and efficient photothermal therapyNano Lett20101093318332320684528
- SunXMLiuZWelsherKNanographene oxide for cellular imaging and drug deliveryNano Res20081320321220216934
- Ali BoucettaHBitounisDRaveendran-NairRServantAVan den BosscheJKostarelosKPurified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicityAdv Healthc Mater20132343344123184580
- DinauerNBalthasarSWeberCKreuterJLangerKvon BriesenHSelective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytesBiomaterials20052629589890615949555
- NasongklaNShuaiXAiHCRGD functionalized polymer micelles for targeted doxorubicin deliveryAngew Chem Int Ed Engl20041164664836487
- DanielsTRDelgadoTHelgueraGPenichetMLThe transferrin receptor part II: targeted delivery of therapeutic agents into cancer cellsClin Immunol2006121215917616920030
- YangXYZhangXYLiuZFMaYFHuangYChenYHigh-efficiency loading and controlled release of doxorubicin hydrochloride on gra-phene oxideJ Phys Chem C Nanomater Interfaces2008112451755417558
- DepanDShahJMisraRDKControlled release of drug from folate-decorated and graphene mediated drug delivery system: synthesis, loading efficiency, and drug release responseMater Sci Eng C Mater Biol Appl201131713051312
- MendesRGBachmatiukABüchnerBCunibertiGRümmeliMHCarbon nanostructures as multi-functional drug delivery platformsJ Mater Chem B Mater Biol Med201314401428
- ZhangYBAliSFDervishiECytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cellsACS Nano2010463181318620481456
- BaiHLiCWangXLShiGQA pH-sensitive graphene oxide composite hydrogelChem Commun2010461423762378
- ZhaoXYangLLiXFunctionalized graphen oxide nanoparticles for cancer cell specific delivery of antitumor drugBioconjug Chem201526112813625525819
- LuYJYangHWHungSCImproving thermal stability and efficacy of BCNU in treating glioma cells using PAA functionalized graphene oxideInt J Nanomedicine201271737174722619524
- XiongHGuoZZhangWZhongHLiuSJiYRedox-responsive biodegradable PEGylated nanographene oxide for efficiently chemo-photothermal therapy: a comparative study with non-biodegradable PEGylated nanographene oxideJ Photochem Photobiol B201413819120124976623
- ZhouKZhuYYangXLiCOne-pot preparation of graphene/Fe3O4 composites by a solvothermal reactionNew J Chem2010341229502955
- LiuHWHuSHChenYWChenSYCharacterization and drug release behavior of highly responsive chip-like electrically modulated reduced graphene oxide poly(vinyl alcohol) membranesJ Mater Chem201222331731117320
- ServantALeonVJasimDGraphene-based electroresponsive scaffolds as polymeric implants for on-demand drug deliveryAdv Healthc Mater2014381334134324799416
- WangHGuWXiaoNYeLXuQChlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drugInt J Nanomedicine201491433144224672236
- YangKFengLLiuZThe advancing uses of nanographene in drug deliveryExpert Opin Drug Deliv201512460161225466364
- KimTHLeeGJKangJHKimHJKimTOhJMAnticancer drug-incorporated layered double hydroxide nanohybrids and their enhanced anticancer therapeutic efficacy in combination cancer treatmentBiomed Res Int2014201419340110.1155/2014/19340124860812
- BalciogluMBuyukbekarBZYavuzMSYigitMVSmart-polymer-functionalized graphene nanodevices for thermo-switch-controlled biodetectionACS Biomater Sci Eng2015112736
- ZhiFDongHJiaXFunctionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitroPlos One201383e6003423527297
- FengLZhangSLiuZGraphene based gene transfectionNanoscale2011331252125721270989
- El-AneedAAn overview of current delivery systems in cancer gene therapyJ Control Release200494111414684267
- LiYRenTLiLCaiXDongHLiuSEngineered polyethylenimine/graphene oxide nanocomposite for nuclear localized gene deliveryPolym Chem20123925612569
- WhiteheadKALangerRAndersonDGKnocking down barriers: advances in siRNA deliveryNat Rev Drug Discov20098212913819180106
- ZhangLMLuZXZhaoQHHuangJShenHZhangZJEnhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxideSmall20117446046421360803
- LiKFengLShenJPatterned substrates of nano-graphene oxide mediating highly localized and efficient gene deliveryACS Appl Mater Interfaces2014585900590724673573
- TripathiSKGoyalRGuptaKCKumarPFunctionalized graphene oxide mediated nucleic acid deliveryCarbon201351224235
- BaoHQPanYZPingYChitosan-functionalized graphene oxide as nanocarriers for drug and gene deliverySmall20117111569157821538871
- ZhangLMWangZLLuZXPEGylated reduced graphene oxide as a superior ssRNA delivery systemJ Mater Chem B Mater Biol Med201316749755
- LiuXMaDTangHPolyamidoamine dendrimer and oleic acid-functionalized graphene as biocompatible and efficient gene delivery vectorsACS Appl Mater Interfaces20146118173818324836601
- TianBWangCZhangSFengLLiuZPhoto-thermally enhanced photodynamic therapy delivered by nanographene oxideACS Nano2011597000700921815655
- KimHLeeDKimJKimTKimWJPhoto-thermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxideACS Nano2013786735674623829596
- KimHKimWJPhotothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocompositeSmall201410111712623696272
- GautschiOPFreySPZellwegerRBone morphogentic proteins in clinical applicationsANZ J Surg200777862663117635273
- TermaatMFDen BoerFCBakkerFCPatkaPHaarmanHJBone morphogenetic genetic proteins. Development and clinical efficacy in the treatment of fractures and bone defectsJ Bone Joint Surg Am20058761367137815930551
- GovenderSCsimmaCGenantHKBMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study GroupRecombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospeactive, controlled, randomized study of four hundred and fifty patientsJ Bone Joint Surg Am200284A122123213412473698
- MadeiraCSanthagunamASalgueiroJBCabralJMSAdvanced cell therapies for articular cartilage regenerationTrends Biotechnol2015331354225466849
- CampbellSMaitlandDHoareTEnhanced pulsatile drug release from injectable magnetic hydrogels with embedded thermosensitive microgelsACS Macro Lett201543312316
- MatsusakiMAjiroHKidaTSerizawaTAkashiMLayer-by-layer assembly through weak interactions and their biomedical applicationsAdv Mater201224445447422213201
- NishiguchiAYoshidaHMatsusakiMAkashiMRapid. Construction of three-dimensional multilayered tissues with endothelial tube networks by the cell-accumulation techniqueAdv Mater201123313506351021728193
- JustinRChenBBody temperature reduction of graphene oxide through chitosan functionalisation and its application in drug deliveryMater Sci Eng C Mater Biol Appl201434505324268232
- ShinSRAghaei-Ghareh-BolaghBGaoXLayer-by-layer assembly of 3D tissue constructs with functionalized grapheneAdv Mater2014223961366144
- ZhouHChengCQinHSelf-assembled 3D compatible and bioactive layer at the macro-interface via graphene based supermoleculesPolym Chem201451135633575
- QiWXueZYuanWWangHLayer-bylayer assembled graphene oxide composite films for enhanced mechanical properties and fibroblast cell affinityJ Mater Chem B Mater Biol Med201423325331
- WangLChunxiangLZhangBZhaoBWuFGuanSFabrication and characterization of flexible silk fibroin films reinforced with graphene oxide for biomedical applicationsRSC Adv20144764031240320
- DeepachitraRNigamRProhitSDIn vitro study of hydroxyapatite coatings on fibrin functionalized/pristine graphene oxide for bone graftingMater Manuf Process2014306804811
- RyuSKimBCulture of neural cells and stem cells on grapheneJ Tissue Eng Regen Med20131023946
- HongSWLeeJHKangSHEnhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetric substratesBiomed Res Int201410.1155/2014/21249
- KimTShahSYangLControlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arraysACS Nano2015943780379025840606
- BressanEFerroniLGardinCGraphene based scaffolds effects on stem cells commitmentJ Transl Med20141229631025344443
- SreejithSMaXZhaoYGraphene oxide wrapping on squaraine-loaded mesoporous silica nanoparticles for bioimagingJ Am Chem Soc201213442173461734922799451
- AnantaJSGodinBSethiRGeometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrastNat Nanotechnol201051181582120972435
- KanakiaSToussaintJDChowdurySMPhysicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agentInt J Nanomedicine201382821283323946653
- GollavelliGLingYCMultifunctional graphene as an in-vitro and in-vivo imaging probeBiomaterials20123382532254522206596
- ZhangHWuHWangJGraphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapyBiomaterials201542667725542794
- HongHZhangYEngleJWIn vivo targeting and positron emission tomography imaging of tumor vasculature with 66Ga-labeled nano-grapheneBiomaterials201233164147415622386918
- SrivastavaSAwasthiRTripathiDMagnetic-nanoparticle-doped carbogenic nanocomposite: an effective magnetic resonance/fluorescence multimodal imaging probeSmall2012871099110922328128
- CianfroccaMScDSLRoennJVRandomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcomaCancer2010116163969397720564162
- JosefsenLBBoyleRWUnique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranosticsTheranostics20122991696623082103
- AgostinisPBergKCengelKAPhotodynamic therapy of cancer: an updateCA Cancer J Clin20116125028121617154
- YangKWanJZhangSTianBZhangYLiuZThe influence of surface chemistry and particle size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser powerBiomaterials20123372206221422169821
- ZhengXMorganJPandeySKConjugation of 2′-(1′-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivoJ Med Chem200952144306431819507863
- EthirajanMChenYHJoshiPPandeyRKThe role of porphyrin chemistry in tumor imaging and photodynamic therapyChem Soc Rev201140134036220694259
- SrivatsanAEthirajanMPandeySKConjugation of cRGD peptide to chlorophyll a based photosensitizer (HPPH) alters its pharmacokinetics with enhanced tumor-imaging and photosensitizing (PDT) efficacyMol Pharm2011841186119721702452
- RongPYangKSrivastanAPhotosensitizer loaded nano-graphene for multimodal imaging guided tumor photodynamic therapyTheranostics20144322923924505232
- ChenHHwangJHUltrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumorsJ Ther Ultrasound201311010.1186/2050-5736-1-1025512858
- LiJLHouXLBaoHCGraphene oxide nanoparticles for enhanced photothermal cancer cell therapy under the irradiation of a femtosecond laser beamJ Biomed Mater Res A201410272181218823852749
- ChenDDoughertyCAZhuKHongHTheranostic applications of carbon nanomaterials in cancer: focus on imaging and cargo deliveryJ Control Release201521023024525910580
- FengLLiuZGraphene in biomedicine: opportunities and challengesNanomedicine20116231732421385134
- RobinsonJTTabakmanSMLiangYUltrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapyJ Am Chem Soc2011133176825683121476500
- ChangYYangSTLiuJHIn vitro toxicity evaluation of graphene oxide on A549 cellsToxicol Lett2011200320121021130147
- YueHWeiWYueZThe role of the lateral dimension of graphene oxide in the regulation of cellular responsesBiomaterials201233164013402122381473
- MuQSuGLiLSize-dependent cell uptake of protein-coated graphene oxide nanosheetsACS Appl Mater Interfaces2012442259226622409495
- SasidharanAPanchakarlaLSSadanandanARHemocompatibility and macrophage response of pristine and functionalized grapheneSmall2012881251126322334378
- LiaoKHLinYSMacoskoCWHaynesCLCytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblastsACS Appl Mater Interfaces2011372607261521650218
- DingZZhangZMaHChenYIn vitro hemocompatibility and toxic mechanism of graphene oxide on human peripheral blood T lymphocytes and serum albuminACS Appl Mater Interfaces2014622197971980725371999
- MaoLLuoSHuangQLuJHorseradish peroxidase inactivation heme destruction and influence of polyethylene glycolSci Rep20133312610.1038/srep0312624185130
- BaiHJiangWKotcheyGPInsight into the mechanism of graphene oxide degradation via the photo-fenton reactionJ Phys Chem C Nanomater Interfaces201411819105191052924860637
- ZhuLChangDWDaiLHongYDNA damage induced by multi-walled carbon nanotubes in mouse embryonic stem cellNano Lett20077123592259718044946
- RenHWangCZhangJDNA cleavage system of nanosized graphene oxide sheets and copper ionsACS Nano20104127169717421082807
- LiuYLuoYWuJGraphene oxide can induce in vitro and in vivo mutagenesisSci Rep2013310.1038/srep03469.
- GurunathanSHanJWKimJHGreen chemistry approach for the synthesis of biocompatible grapheneInt J Nanomedicine201382719273223940417
- PengLXuZLiuZAn iron-based green approach to 1-h production of single layer graphene oxideNat Commun20156571610.1038/ncomms671625607686
- NezakatiTCousinsBGSeifalianAMToxicology of chemically modified graphene-based materials for medical applicationArch Toxicol201488111987201225234085
- YinPTShahSChhowallaMLeeKBDesign, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplicationsChem Rev201511572483253125692385
- KarakotiASinghSDowdingJMSealSSelfWTRedox-active radical scavenging nanomaterialsChem Soc Rev201039114422443220717560
- KimSJChoHRChoKWMultifunctional cell-culture platform for aligned cell sheet monitoring, transfer printing, and therapyACS Nano2015932677268825687418
- HouWCChowdhuryIGoodwinDGPhotochemical transformation of graphene oxide in sunlightEnviron Sci Technol20154963435344325671674
