?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Despite significant advances in highly active antiretroviral therapy (HAART), the prevalence of neuroAIDS remains high. This is mainly attributed to inability of antiretroviral therapy (ART) to cross the blood–brain barrier (BBB), thus resulting in insufficient drug concentration within the brain. Therefore, development of an active drug targeting system is an attractive strategy to increase the efficacy and delivery of ART to the brain. We report herein development of magnetic azidothymidine 5′-triphosphate (AZTTP) liposomal nanoformulation and its ability to transmigrate across an in vitro BBB model by application of an external magnetic field. We hypothesize that this magnetically guided nanoformulation can transverse the BBB by direct transport or via monocyte-mediated transport. Magnetic AZTTP liposomes were prepared using a mixture of phosphatidyl choline and cholesterol. The average size of prepared liposomes was about 150 nm with maximum drug and magnetite loading efficiency of 54.5% and 45.3%, respectively. Further, magnetic AZTTP liposomes were checked for transmigration across an in vitro BBB model using direct or monocyte-mediated transport by application of an external magnetic field. The results show that apparent permeability of magnetic AZTTP liposomes was 3-fold higher than free AZTTP. Also, the magnetic AZTTP liposomes were efficiently taken up by monocytes and these magnetic monocytes showed enhanced transendothelial migration compared to normal/non-magnetic monocytes in presence of an external magnetic field. Thus, we anticipate that the developed magnetic nanoformulation can be used for targeting active nucleotide analog reverse transcriptase inhibitors to the brain by application of an external magnetic force and thereby eliminate the brain HIV reservoir and help to treat neuroAIDS.
Introduction
Human immunodeficiency virus (HIV-1) appears to be harbored in the brain, as indicated by the presence of large quantities of unintegrated viral DNA in the brain of HIV-infected individuals.Citation1 The exact mechanism of virus entry into the brain is not clearly elucidated; however the resulting infection leads to a number of central nervous system (CNS) disorders collectively known as neuroAIDS.Citation2,Citation3 Currently, no specific treatment exists for neuroAIDS, which is mainly attributed to the poor penetrability of antiretroviral therapy (ART) across the blood–brain barrier (BBB). The selective permeability of the BBB is due to the distinct morphology and enzymatic properties of endothelial cells that enable them to form complex tight junctions with minimal endocytic activity. This provides a physiological barrier that limits the transport of many blood-borne elements such as macromolecules and circulating leukocytes to the brain.Citation4 Previous studies report that delivery of ART to the brain is limited especially due to the physical structure of the BBB, presence of efflux pumps and higher expression of metabolizing enzymes, which makes BBB an effective barrier against many antiretroviral drugs.Citation5 Therefore, in order to increase the efficacy of ART, novel approaches to deliver antiretroviral drugs to the brain are warranted.
In recent years, advent of nanotechnology has stimulated the development of innovative systems for the delivery of drugs and diagnostic agents.Citation6 It is now possible to synthesize, characterize and specifically tailor the functional properties of nanoparticles for various clinical as well as diagnostic applications. Additionally, nanoformulations of small drugs delivered systemically are more efficacious and less toxic than the same drug delivered in free form. This effectiveness of nanoparticle based drug delivery systems is attributed to their small size, controlled time release of the drug and modification of drug pharmacokinetics and biodistribution profile.Citation6,Citation7 In this regard, one type of nanoparticle that has gained increasing interest is magnetic nanoparticles, which mainly consist of nano-sized iron oxide particles (magnetite; Fe3O4 or maghemite; γ-Fe2O3). The use of magnetic nanoparticles contributes to a precise delivery of drugs to the exact site (eg, inflammation, cancer) by application of an external magnetic field.Citation8 In cancer chemotherapy magnetically guided drug targeting has been attempted in order to increase the efficacy and reduce the deleterious side effects.Citation9,Citation10 Previous reports have shown successful delivery of anticancer drugs bound to magnetic nanoparticles to treat brain carcinomas.Citation11 Further, magnetic nanoparticles have been used as an imaging agent in the brain for diagnostic purposes.Citation12
Previous studies have also attempted to use monocytes/macrophage based drug carrier for targeted delivery to the brain.Citation13,Citation14 Such an approach utilizes the ability of phagocytes to cross the BBB and migrate towards inflammatory sites via the process known as diapedesis and chemotaxis. Also, studies report that monocytes/macrophages can cross an intact BBB under normal physiological condition.Citation15 Likewise, Jain et al has reported monocytes/neutrophils mediated delivery of Arg-Gly-Asp (RGD) anchored magnetic liposomes to the brain.Citation16 As a first step towards specific drug targeting to brain to treat neuroAIDS, we have shown direct binding of 3’-azido-3’-deoxythymidine-5’-triphosphate (AZTTP, active form of AZT) to magnetic nanoparticles and inhibition of HIV-1 replication in peripheral blood mononuclear cells (PBMCs).Citation17 In the current study, we report encapsulation of magnetic nanoparticles bound AZTTP (MP-AZTTP) within the liposomes followed by transmigration of MP-AZTTP liposomes across the in vitro BBB model system in presence of an external magnetic field. We hypothesize that MP-AZTTP liposomes can be transported across the BBB via direct transport or via monocyte-mediated transport, and that by application of an external magnetic field results in more effective transmigration of magnetic liposomes and magnetic monocyte across the BBB in neuroAIDS.
Material and methods
Preparation of liposomes loaded with MP-AZTTP liposomes
Magnetic nanoparticle synthesis and AZTTP binding to nanoparticles were performed according to the procedure we previously described.Citation17 In our previous study we determined the most optimal reaction conditions for production of MP-AZTTP. A reaction time of 2 hour with a ratio of 0.2 mg AZTTP to 3 mg magnetic nanoparticles (Fe3O4, magnetite) yields magnetic nanoparticles with 35 μg of AZTTP per mg of magnetic nanoparticles. Magnetic liposomes were prepared by reverse-phase evaporation method according to the procedure described earlier.Citation16 L-α-Soya phosphatidyl choline (PC) and cholesterol (CHOL) were obtained from Sigma (St. Louis, MO). In a typical optimized procedure, PC and CHOL (1.2:1 mole ratio) were dissolved in chloroform, followed by evaporation of a solvent in a rotary evaporator resulting in formation of dry lipid film. After completely removing all solvent traces, the lipid film was rehydrated under constant shaking with 1 mL of 0.9% saline containing 3 mg of MP-AZTTP nanoparticles. This resulted in encapsulation of MP-AZTTP into the liposomes. The unencapsulated magnetic nanoparticles were removed by magnetic decantation using a weak magnet. Fluorescent rhodamine labeled magnetic liposomes (Rho magnetic liposomes) were prepared by incorporation of 1 mol% of rhodamine-DHPE (Invitrogen Molecular Probes, Oregon, USA) during the formation of the lipid film.
Transmission electron microscopic (TEM) examination of MP-AZTTP liposomes
The size of the MP-AZTTP liposome was determined using negative staining technique of transmission electron microscopy. A drop of magnetoliposome sample was placedon a Formvar-coated grid and was allowed to dry. The sample was then stained with uranyl acetate dye (0.5% uranyl acetate). The grid samples were examined using a JEOL (Tokyo, Japan) transmission electron microscope operating at 80 kV.
MP-AZTTP encapsulation efficiency of the liposomes
MP-AZTTP encapsulation efficiency in liposomes was determined by a modification of a method reported previously.Citation18 Briefly, unentrapped MP-AZTTP was separated from MP-AZTTP liposomes using Sephadex G-50 mini-columns. 150 μL of MP-AZTTP liposomal formulation was added in 2.0 mL methanol and incubated for 1 min, followed by addition of 1.0 mL of water. The particles were separated magnetically followed by measuring the absorbance of the supernatant at 267 nm using Spectronic Genesys Bio10 spectrophotometer. Standard curve of AZTTP in the absence and presence of liposomes were used to determine the amount of liposome encapsulated AZTTP. The amount of encapsulated magnetite was estimated based on ferrous ion by using O-phenanthroline method.Citation16 The calibration curve was generated with several different amounts of magnetite. Another sample of the MP-AZTTP liposomes was assayed for phospholipid content by the method of Steward-Marshall.Citation19 The calibration curve was generated with several different amounts of a phospholipid solution.
Anti-HIV efficacy of MP-AZTTP liposomes
Magnetic AZTTP liposomes was assessed for anti-HIV activity in an in vitro model system using HIV-infected PBMCs. Briefly, PBMCs (1 × 106 cells/mL) were infected with HIV-1 IIIB [×4] (NIH AIDS Research and Reference Reagent Program Cat# 398) at a concentration of 103.0 TCID50/mL cells for 3 hours as described by the HIV-1 IIIB supplier. Cells were washed and cultured in the presence of free AZTTP or MP-AZTTP liposomes (1–100 nM AZTTP equivalent) once for 7 and 14 days, the culture supernatants were quantitated for p24 antigen by RETRO-TEK HIV-1 p24 antigen ELISA kit (Cat#: 0801200, Zeptometrix, Buffalo, NY). Untreated HIV infected PBMCs were included as a control. The minimum assay detection limit for p24 antigen by ELISA is 7.8 pg/mL as provided by the manufacturer (Zeptometrix, Buffalo, NY).
In vitro BBB model
The BBB model was established according to the procedure described earlier.Citation20 Primary human brain microvascular endothelial cells (BMVEC) and astrocytes were procured from Sciencell Research Laboratories, Carlsbad, CA. BMVECs were characterized by immunofluorescent method with antibodies to von Willebrand factor (VWF/Factor VIII) and CD31 (P-CAM). Human astrocytes were characterized by immunofluorescent method with antibody to glial fibrillary acidic protein. The cultures were also tested for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. Both the cultures were used for experiments between passages 2 and 8.
The BBB model consisted of 2-compartment wells in a culture plate with the upper compartment separated from the lower by a cyclopore polyethylene terephthalate membrane (Collaborative Biochemical Products, Becton Dickinson, San Jose, CA) with a pore diameter of 3 μm. In a 24-well cell culture insert (surface area 0.3 cm2), 1 × 105 BMVEC were grown to confluency on the upper side while a confluent layer of human astrocytes were grown on the underside. Intactness of the BBB was judged by measuring the transendothelial electrical resistance (TEER) using Millicell ERS microelectrodes (Millipore). A mean TEER value of 150 to 200 ohms/cm2 cell culture insert is consistent with the formation of the BBB. The BBB model was used for experiments atleast 5 days after cell seeding.
Transmigration of MP-AZTTP liposomes across the in vitro BBB
All transmigration experiments were conducted on day 6 of the BBB culture after membrane integrity was established by TEER measurement. Prior to transmigration experiment culture media was replaced with buffered Ringer’s solution and allowed to equilibrate for 15 min. The basolateral chamber contained 0.9 mL of buffer, whereas the apical chamber contained 0.35 mL to ensure that no pressure gradient existed. The transmigration experiment was performed in apical to basal direction. The apical chamber contained MP-AZTTP liposomes (50 μmol AZTTP equivalent) and the culture inserts were incubated for 4 hours at 37°C in the presence or absence of a magnet placed below the trans-well chamber. After incubation, the buffer from the lower chamber was collected and the magnetic liposomes were concentrated by centrifugation at 25,000 g for 15 min. AZTTP was extracted from liposome by treatment with a mixture of methanol and water (1:1) followed by HPLC analysis to estimate AZTTP concentration. The permeability coefficient was calculated according to the following formula as previously descried.Citation21
where
Papp: apparent permeability (cm/min)
dQ/dt: amount of MP-AZTTP liposomes accumulation in lower (basal) chamber as a function of time (nmol/min)
A: area of transwell (cm2)
C0: initial concentration of MP-AZTTP liposomes added in upper (apical) chamber (nmol/mL)
HPLC analysis of AZTTP
HPLC analysis of AZTTP was performed by a modification of a method reported earlier.Citation22 HPLC analysis was carried out with a Thermo-Finnigan chromatograph (Thermo Electron Corporation, San Jose, California) consisting of a SpectraSystem SMC1000 solvent delivery system, vacuum membrane degasser, P4000 gradient pumps, AS3000 auto sampler, SpectraSystem UV6000LP detector set at 254 nm and ChromQuest 4.1 software. A C18 RP Hypersil GOLD column (RP5, 250 × 4.6 mm, pore size 5 μm, Thermo Electron Corporation) was used and the eluting system consisted of a 0.2 M ammonium acetate supplemented with 8 mM TBAS (pH 7.5) and acetonitrile in volume ratio ammonium acetate: acetonitrile of 95:5. The flow-rate was 1.0 mL/min at room temperature.
Monocyte uptake studies
Peripheral blood monocytes were isolated from donor leucopacks using density gradient centrifugation procedure described by us.Citation23 The purity of isolated monocytes was assessed by flow cytometry using a fluorochrome-conjugated CD14 antibody and the purity was found to be >90%. The isolated monocytes were cultured in RPMI medium supplemented with 10% fetal bovine serum, penicillin 100 U/mL and streptomycin 100 μg/mL, and 2 mM L-glutamine (Gibco-BRL, Gaithersburg, MD) in a 6-well plate at cell density of 0.8 × 106 cells/mL for 24 hours. The monocyte uptake experiment was performed with fluorescent magnetic liposomes. The rhodamine labeled magnetic liposomes (Rho ML) were added to each test well after dilution in RPMI buffer, about 100 nmol total lipids per 106 monocytes was used followed by incubation of culture plate for 2 and 4 hours. After incubation, monocytes were washed 3 times with phosphate buffer saline (pH 7.4) to remove non-associated magnetic liposomes. The monocytes were then visualized and images acquired with an Olympus DP70 digital camera mounted on a fluorescence microscope with excitation at 546 nm and emission collection with a long pass filter at 590 nm (Zeiss, Germany). All images were processed using Adobe Photoshop software.
Transendothelial migration of magnetic liposome loaded monocytes across the in vitro BBB
As mentioned above, all transmigration assays were performed on day 6 of the BBB culture. 1 × 105 monocytes (with or without magnetic liposomes loaded) in 100 μl transendothelial migration (RPMI 1640 without phenol red + 1% BSA) medium was added to the upper chamber of the in vitro BBB system, the chambers were then incubated at 37°C, 5% CO2. A block magnet of strength 0.3 Tesla was placed below the artificial BBB chambers. Migrated monocytes were counted in the lower chamber after 2 and 4 hours incubation. Percent transendothelial migration was calculated with respect to the initial total number of cells added to the upper chamber. The number of cells transmigrated was counted using a hemocytometer. Cell viability was assessed by trypan blue staining.
Statistical analysis
All experiments were performed at least three times in duplicates. Data are presented as mean ± SE. The anti-HIV efficacy of AZTTP (free and liposome) was analyzed using a one way ANOVA with the Bonferroni adjustment (significance level P < 0.005). The transmigration experiment data was analyzed using unpaired Student’s t-test. Results were considered significant at P < 0.05, with a two-tailed test.
Results
Characteristics of MP-AZTTP liposomes
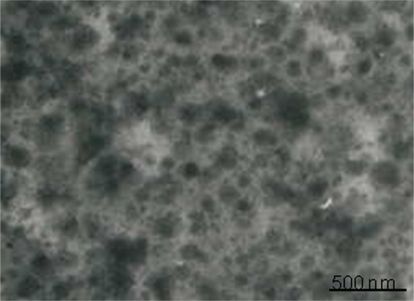
In the past decade, magnetic liposomes have attracted significant attention for various biological and medical applications.Citation24,Citation25 In this study, magnetic liposomes containing AZTTP were prepared by reverse-phase evaporation method by encapsulation of AZTTP modified magnetic nanoparticles within the core of liposomes. The size and morphology of the magnetoliposomes was determined by TEM with the negative staining technique using uranyl acetate dye. A typical TEM image of a magnetoliposome sample is shown in . The micrograph clearly shows the occurrence of relatively uniform, spherical shaped nanosized particles. The particles are well separated and their average size is ∼25 nm. The average size of magnetic liposomes is about ∼150 nm.
Also, the MP-AZTTP liposomes formed a pellet when kept near an external magnet (field strength ∼0.3 Tesla) indicating their susceptibility to an external magnetic field. The encapsulation efficiency of MP-AZTTP in liposomes was 54.5 ± 6 % (n = 6). Magnetite encapsulation ratio per mol of phospholipid was calculated and found to be 11.3 g magnetite per mol of phospholipid with percentage encapsulation to be about 45.3%.
Inhibition of p24 antigen production by MP-AZTTP liposomes
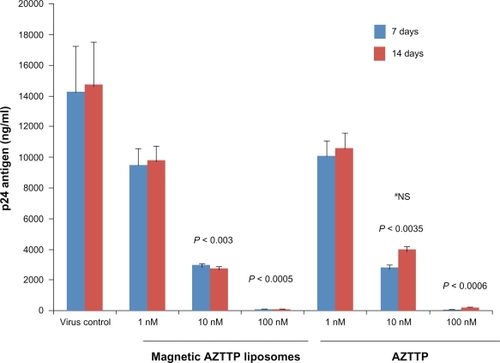
The magnetic AZTTP liposomes were examined for their ability to inhibit HIV-1 replication in an in vitro model system using HIV-infected PBMCs. After HIV-1 infection PBMCs were cultured with either free AZTTP or magnetic AZTTP liposomes at concentration from 1 to 100 nM. Culture supernatants were monitored for viral replication by measuring p24 antigen levels on day 7 and 14 post infection using an ELISA kit. On day 7 a dose-dependent decrease in p24 antigen production was observed with treatment of MP-AZTTP liposomes by HIV-1 infected PBMCs at 1 nM (9750 ± 875 ng/mL), 10 nM (3000 ± 115 ng/mL, P < 0.003) and 100 nM (110 ± 20ng/mL, P < 0.0005) compared to the untreated HIV-1 infected control cultures (14290 ± 2945) (). Further, the anti-HIV activity of free AZTTP (positive control) and MP-AZTTP liposomes were found to be comparable at various doses tested on day 7. However, at day 14 the p24 antigen production was found to be slightly lower (although not significant, P < 0.06) in magnetic AZTTP liposomes treated samples compared to free AZTTP (10 nM).
Figure 2 Magnetic AZTTP liposomes inhibit HIV-1 p24 production. PBMCs (1 × 106 cells/mL) obtained from normal subjects were infected with native HIV-1 IIIB (NIH AIDS Research and Reference Reagent Program Cat# 398) at a concentration of 103.0 TCID50/mL cells for 3 hours and washed 3 times with Hank’s balanced salt solution (GIBCO-BRL, Grand Island, NY) before being returned to culture with and without free AZTTP or magnetic AZTTP liposomes (1–100 nM) for 7 and 14 days. The culture supernatants were quantitated for HIV-1 p24 antigen using a p24 ELISA kit (ZeptoMetrix Corporation, Buffalo, NY). The data represent the average of 3 independent experiments and are expressed as ng/mL. Statistical analysis was done using one way ANOVA with Bonferroni adjustment.
#Comparison between MP-AZTTP liposome and free AZTTP group.
Abbreviations: AZTTP, azidothymidine 5’-triphosphate; MP-AZTTP, magnetic nanoparticles bound AZTTP.
Further, we also examined the non-specific cytotoxicity of MP-AZTTP liposomes to PBMCs. The results show that MP-AZTTP liposomes (100 nM) were not cytotoxic to PBMCs as evaluated by XTT viability assay ().
Table 1 Cytotoxicity of AZTTP and MP-AZTTP liposomes
Transmigration of MP-AZTTP liposomes across the in vitro BBB in the presence of a magnet
We then examined the ability of magnetic AZTTP liposomes to migrate across the artificial BBB model system under the influence of an external magnetic field. The intactness of the in vitro BBB model was established by TEER measurement. A mean TEER value of 150 to 200 ohms/cm2 cell culture insert is consistent with the formation of the BBB and was also reported earlier.Citation26 represents the apparent permeability of AZTTP transported across the in vitro BBB as free AZTTP and as magnetic liposomes. The results showed that permeability of MP-AZTTP liposomes in the presence of a magnet is significantly higher than that of free AZTTP, 3.64 × 10−3 cm/min as compared to 1.28 × 10−3 cm/min respectively (P < 0.0001). Further, results show no significant effects on the TEER values of the BBB model before and after treatment with the MP-AZTTP liposomes ().
Figure 3 Transmigration of MP-AZTTP liposomes across the blood–brain barrier (BBB) model. Apparent permeability coefficients (Papp) of MP-AZTTP transport across the BBB model as free and in magnetic liposomes. The data represents the mean ± SE of 3 independent experiments and is expressed as cm/min. Statistical analysis was performed using unpaired Student’s t-test.
Abbreviations: AZTTP, azidothymidine 5’-triphosphate; MP-AZTTP, magnetic nanoparticles bound AZTTP.
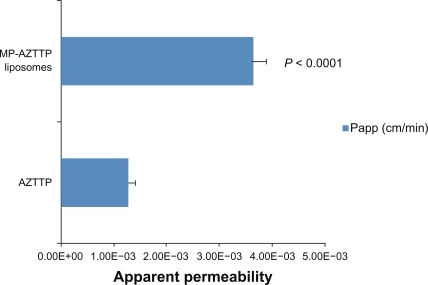
Table 2 Transendothelial electrical resistance (TEER) values of the in vitro blood–brain barrier model before and after treatment with magnetic nanoformulation
Uptake of magnetic liposomes by peripheral blood monocytes
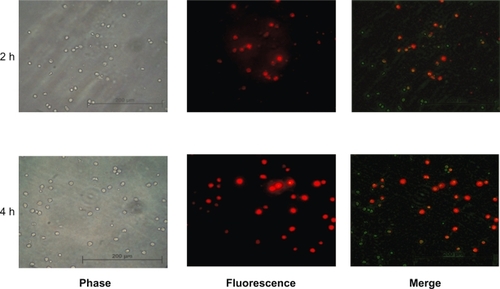
Previous studies have attempted to use monocytes/macrophages as cell based drug delivery carriers.Citation13,Citation14,Citation27–Citation29 In this experiment, we evaluated the ability of human monocytes to phagocytose magnetic liposomes. We co-cultured human monocytes in the presence of rhodamine labeled magnetic liposomes (Rho-ML) for 2- and 4-hour time intervals. As shown in the rhodamine fluorescence increased with incubation time. At 4 hours of incubation, most of the fluorescence was associated with monocytes indicating that >90% of monocytes had taken up Rho ML.
Figure 4 Uptake of magnetic liposomes by human monocytes. Monocytes were co-cultured with rDHPE-magnetic liposomes for 2 and 4 hours and their intracellular localization was assessed by fluorescence microscopy (Zeiss, Germany). Engulfed magnetic liposomes can be visualized as red fluorescence located within the cell cytoplasm.
Transendothelial migration of magnetic liposomes loaded monocytes
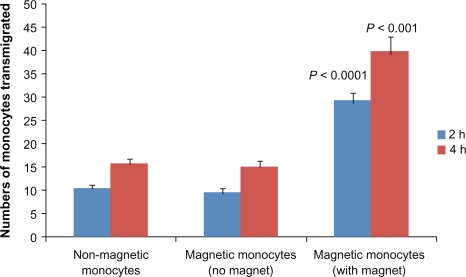
Previous studies have reported that engulfment of magnetic liposomes or magnetic nanoparticles by monocytes provide a magnetic property to these cells, which then responds to an external magnetic field.Citation16,Citation29 Therefore, we tested the ability of magnetic liposome loaded monocytes to transmigrate across the artificial BBB system with or without the magnet place underneath the transwell for the duration of the experiment. represents the number of monocytes transmigrated across the in vitro BBB. There were statistically significant differences, and on post hoc analysis we found that a significantly greater number of magnetic monocytes in the magnet group had transmigrated across the BBB at both 2 hours and 4 hours compared to the non-magnetic monocyte group (control) 28 ± 2 versus 10 ± 1 (P < 0.0001) and 40 ± 3 versus 15 ± 1 (P < 0.001). Further, in the within group post-hoc comparisons we found that in the magnetic monocytes with magnet group there were a significantly greater number of magnetic monocytes that transmigrated at 4 hours than at 2 hours (40 ± 3 versus 28 ± 2, P < 0.04). Additionally, TEER values were not affected before and after the treatment of BBB with magnetic monocytes ().
Figure 5 Transmigration of monocytes across the in vitro blood–brain barrier (BBB). Magnetic liposome loaded monocytes were added in the upper chamber of the BBB model with or without a magnet placed underneath for the duration of experiment. At 2 and 4 hours after plating, migrated monocytes were counted in the lower chamber. Results are expressed as mean ± SE of three independent experiments. Statistical significance was determined using unpaired Student’s t-test.
Discussion
Although highly active antiretroviral therapy (HAART) has greatly reduced the disease severity, thereby improving survival and quality of life, still individual patient responses are quite variable, and the prevalence of neurological complications remains high.Citation30,Citation31 Additionally, nucleoside and nucleotide analog reverse transcriptase inhibitors (NRTIs) that are important component of HAART have low intracellular ability to convert to active nucleoside 5’-triphosphate (NTP) form due to inefficiency of enzyme thymidylate kinase in human cells.Citation32 This leads to the development of drug resistance, toxicity and insufficient effective drug levels in virus target tissue.Citation33,Citation34
Recently, nanotechnology-based drug delivery systems have shown tremendous potential for delivery of drugs across the BBB.Citation35 In this report; we describe a magnetically guided delivery system for targeting AZTTP, an active form of AZT across the BBB. Delivery of active phosphorylated form of NRTIs offers an advantage by bypassing the first step of intracellular phosphorylation; however it poses a challenge in terms of protecting the NTPs from cellular phosphatases and neutralizing the electronegative charge of NTP. Thus, developing a magnetic nanocarrier by encapsulation of AZTTP within the core of liposome may provide an efficient approach for delivery of active NRTIs to the brain. In the current study, magnetic AZTTP liposomes were prepared successfully using a reverse phase evaporation method. TEM examination of sample stained with 0.5% uranyl acetate shows the occurrence of the layer surrounding each particle, which is attributed to the phospholipid coating (). The prepared magnetoliposomes were characterized for in vitro magnetic susceptibility, percent drug and magnetite encapsulation efficiency. The prepared magnetoliposomes were found to be responsive to an external magnetic field and form a pellet in less than 2 min when kept in front of a magnet of strength 0.3 Tesla. The maximum drug and magnetite loading achieved was 54.5% and 45.3%, respectively, which is comparable to that reported previously where diclofenac was encapsulated in magnetic liposomes.Citation16
The anti-HIV activity of magnetic AZTTP liposomes were compared with free AZTTP in HIV-1 infected human PBMCs cultures (). Both free and magnetic AZTTP formulations were effective inhibitors of HIV-1 in nanomolar concentration range. However, on day 14 post infection p24 antigen levels were found to be slightly lower (although not significant) in magnetic AZTTP liposome treated cultures compared to free AZTTP cultures. This observation indicates a possible sustained release effect of AZTTP due to higher retention time in liposomes. This is in agreement to previous report where 2’, 3’-dideoxycytidine-5’-triphosphate was encapsulated in liposomes and it remained stable for days.Citation36 Thus, the encapsulation of magnetic AZTTP in liposomes would increase their efficacy, biocompatibility, and also protect the active NRTIs from degradation by cellular phosphatases. Similarly, previous few studies have shown increased drug activity and reduced cytotoxicity of 5’-triphosphates of NRTI encapsulated within the nanogel carriers or erythrocytes.Citation37,Citation38
HAART has considerably reduced HIV disease morbidity and mortality; however the associated neuroAIDS continues to increase, which is mainly attributed to the inability of HAART to cross the BBB in sufficient amounts.Citation39 In addition, it is reported that under normal circumstance AZTTP is unable to cross the BBB.Citation40 We used an established in vitro BBB model to evaluate the ability of magnetic AZTTP liposomes to cross the BBB under the influence of an external magnetic field. The results obtained show that the apparent permeability of magnetic AZTTP liposomes was 3-fold higher than free AZTTP (). This result indicates that transmigration ability of AZTTP across the BBB increased significantly on binding to magnetic nanoparticles followed by transport under the influence of an external magnetic field. Previous studies using magnetoliposomes for cancer treatment have shown that administration of magnetic adriamycin (ADR) liposomes under an external magnetic force produced approximately 4-fold higher maximum ADR concentration in the tumor than did administration of ADR solution alone. Citation41 More recently, magnetic drug targeting has shown success in delivery of anticancer drugs to treat brain carcinoma.Citation11 Thus, brain-specific delivery of active NRTIs through an effective carrier would provide significant therapeutic benefits for treatment of neuroAIDS.
In recent years, several studies have described monocytes/macrophage-based nanocarrier drug delivery system for targeting ART to the brain.Citation13,Citation14 Therefore, we evaluated the uptake of rhodamine labeled magnetic liposomes by monocytes. Our results show that the rhodamine fluorescence increased with incubation time and at 4 h it was evenly distributed throughout the whole cytoplasm, indicating that magnetic liposomes were efficiently taken up by monocytes. The phagocytosis of magnetic nanoparticles/liposomes by monocytes makes them magnetic cells that respond to an external magnetic field.Citation16,Citation29 We then checked the ability of monocytes to transmigrate across the in vitro BBB model with a magnet placed underneath the trans-well plate. Our results indicate that magnetic liposomes loaded monocytes showed enhanced migration across the BBB model in response to an external magnetic force at 2 and 4 h of incubation. Similarly, Muthana and co-workers have demonstrated increased migration of magnetic monocytes across a human endothelial cell layer into a tumor spheroid indicating the potential of magnetic approach for gene therapies.Citation29 Also, RGD-anchored magnetic liposomes have been successfully targeted to brain via monocyte/neutrophil-mediated transport.Citation16 Further, in our studies no change was observed in the TEER values before and after treatment with magnetic nanoformulations, which indicates no effect on the integrity of BBB due to transport of the formulation across the BBB.
Thus, we envisaged that this magnetic liposomal drug delivery could provide a viable approach to overcome the problem of poor BBB penetration of several antiretroviral agents. Also, the transport of magnetic nanoformulation across the BBB occur by two routes, 1) direct delivery of magnetic nanoformulation under the influence of an external magnetic field and 2) uptake of these nanoformulations by circulating monocytes/macrophages which can then traverse the BBB.
In our studies on specific drug targeting to the brain to eliminate the remaining HIV-1 reservoirs, we have developed for the first time magnetic AZTTP liposomes and shown that they effectively inhibit HIV-1 replication in in vitro infection model. In addition, our result indicates that magnetic AZTTP nanoformulation migrates across an established BBB model via direct and monocyte mediated transport by application of an external magnetic field. Further studies to evaluate the drug release kinetics and stability of the developed nanoformulation are currently pursued in our lab. Therefore, the delivery of AZTTP using magnetic liposomes is expected to be more therapeutic and may reduce the risk of developing drug resistant viral strains and further reduces the clinical toxicities associated with the use of high doses of NRTIs.
Acknowledgments/disclosures
This work was supported in part by National Institute on Drug Abuse grant R37DA025576 and RO1DA027049. Also, we are sincerely grateful to Horacio Priestap, Department of Biological Sciences, Florida International University, for his assistance in HPLC analysis. The authors report no conflicts of interest.
References
- PangSKoyangiYMilesSWileyCHarryVChenIHigh levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patientsNature19903485892296295
- SpencerDCPriceRWHuman immunodeficiency virus and the central nervous systemAnnu Rev Microbiol1992466556931444270
- LiptonSAGendelmanHEDementia associated with the acquired immunodeficiency syndromeN Engl J Med19953329349407877652
- LesniakMSBremHTargeted therapy for brain tumorsNat Rev Drug Discov20043649950815173839
- NowacekAGendelmanHENanoART, neuroAIDS and CNS drug deliveryNanomed20094557574
- SuriSSFenniriHSinghBNanotechnology-based drug delivery systemsJ Occup Med Toxicol200721618053152
- SarinHRecent progress towards the development of effective systemic chemotherapy for the treatment of malignant brain tumorsJ Transl Med200977719723323
- HofmannHPetriAChastellainMHofmannMSuperparamagnetic nano-particle preparation for medical applicationEur Cell Mater200122930
- ItoAShinkaiMHondaHKobayashiTMedical application of functionalized magnetic nanoparticlesJ Biosci Bioeng200510011116233845
- SafarikISafarikovaMMagnetic nanoparticles and biosciencesMon Chem2002133737759
- ChertokBMoffatBADavidAEIron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumorsBiomaterials200829448749617964647
- RiviereCMartinaMSRiviereCMagneting targeting of nanometric magnetic fluid loaded liposomes to specific brain intravascular areas: A dynamic imaging study in miceRadiology200724443944817562813
- DouHGrotepasCBMcMillanJMMacrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of NeuroAIDSJ Immunol200918366166919535632
- DouHDestacheCJMoreheadJRDevelopment of a macrophage-based nanoparticle platform for antiretroviral drug deliveryBlood20061082827283516809617
- PerryVHAnthonyDCBoltonSJBrownHCThe blood-brain barrier and inflammatory responseMol Med19973335341
- JainSMishraVSinghPDubeyPKSarafDKVyasSPRGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targetingInt J Pharm2003261435512878394
- SaiyedZMGandhiNHNairMPNAZT 5’-triphosphate nanoformulation suppresses HIV-1 replication in peripheral blood mononuclear cellsJ Neurovirol200915343347
- PhillipsNCTsoukasCLiposomal encapsulation of azidothymidine results in decreased hematopoietic toxicity and enhanced activity against murine acquired immunodeficiency syndromeBlood1992795113711431371411
- Steward-MarshallJCColorimetric determination of phospholipids with ammonium ferrothyocianateAnal Biochem198010410146892980
- PersidskyYStinsMWayDA model for monocytes migration through the blood brain barrier during HIV-1 encephalitisJ Immunol1997158349935109120312
- Martínez-EstradaOMRodríguez-MillánEGonzález-De VicenteEReinaMVilaróSFabreMErythropoietin protects the in vitro blood-brain barrier against VEGF-induced permeabilityEur J Neurosci20031892538254414622154
- MolemaGJansenRWVisserJMeijerDKSimultaneous analysis of azidothymidine and its mono-, di-and triphosphate derivatives in biological fluids, tissue and cultured cells by a rapid high-performance liquid chromatographic methodJ Chromatogr1992579104114
- GandhiNHSaiyedZMThangavelSRodriguezJRaoKVKNairMPNDifferential effect of HIV-1 clade B and clade C Tat protein on expression of pro- and anti-inflammatory cytokines by primary monocytesAIDS Res Human Retrovir20092569169919621989
- SaiyedZMTelangSDRamchandCNApplication of magnetic techniques in the field of drug discovery and biomedicineBioMagn Res Technol200311812646069
- PankhurstQAConnollyJJonesSKDobsonJApplications of magnetic nanoparticles in biomedicineJ Phys D Appl Phys20033613167181
- HaorahJKnipeBLeibhartJGhorpadeAPersidskyYAlcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunctionJ Leukoc Biol2005781223123216204625
- AferganEEpsteinHDahanRDelivery of serotonin to the brain by monocytes following phagocytosis of liposomesJ Control Release2008132849018805446
- BatrakovaEVLiSReynoldsADA macrophage-nanozyme delivery system for parkinson’s diseaseBioconjugate Chem20071814981506
- MuthanaMScottSDFarrowNA novel magnetic approach to enhance the efficacy of cell-based gene therapiesGene Therapy20081590291018418420
- RobertsonKRHallCDAssessment of NeuroAIDS in the International settingJ Neuroimmune Pharmacol2007210511118040833
- MurriRLepriACPaolaCIs moderate HIV viremia associated with a higher risk of clinical progression in HIV-infected people treated with highly active antiretroviral therapy: evidence from the Italian Cohort of Antiretroviral-Naïve Patients StudyJ Acquir Immune Defic Syndr200641233016340469
- KohliEHanHYZemanADVinogradovSVFormulations of biodegradable nanogel carriers with 5’-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activityJ Control Release2007121192717509713
- Doualla-BellFTurnerDLoembaHPetrellaMBrennerBWainbergMAHIV drug resistance and optimization of antiviral treatment in resource-poor countriesMed Sci200420882886
- AntonelliGTurrizianiOVerriALong-term exposure to zidovudine affects in vitro and in vivo the efficiency of phosphorylation of thymidine kinaseAIDS Res Hum Retrovir1996122232288835200
- SilvaGANanotechnology approaches for drug and small molecule delivery across the blood brain barrierSurg Neurol20076711311617254859
- SzebeniJWahlSMBetageriGVInhibition of HIV-1 in monocyte/macrophage cultures by 2’,3’-dideoxycytidine-5’-triphosphate, free and in liposomesAIDS Res Hum Retroviruses199066917022163269
- VinogradovSVKohliEZemanADCrosslinked polymeric nanogel formulation of 5’-triphosphates of nucleoside analogs: role of the cellular membrane in drug releaseMol Pharm2005244946116323952
- MagnaniMRossiLFraternaleAFeline immunodeficiency virus infection of macrophages: in vitro and in vivo inhibition by dideoxycytidine-5’-triphosphate-loaded erythrocytesAIDS Res Hum Retrovir199410117911867826702
- PotulaRRamirezSKnipeBPeroxisome proliferator-activated receptor-γ activation suppresses HIV-1 replication in an animal model of encephalitisAids2008221539154918670212
- VinogradovSVPolymeric nanogel formulations of nucleoside analogsExpert Opin Drug Deliv20074151717184158
- KuboTSugitaTShimoseSNittaYIkutaYMurakamiTTargeted delivery of anticancer drugs with intravenously administered magnetic liposomes in osteosarcoma-bearing hamstersInt J Oncol200017230931510891540