Abstract
Mimicking nature is a powerful approach for developing novel lipid-based devices for drug and vaccine delivery. In this review, biomimetic assemblies based on natural or synthetic lipids by themselves or associated to silica, latex or drug particles will be discussed. In water, self-assembly of lipid molecules into supramolecular structures is fairly well understood. However, their self-assembly on a solid surface or at an interface remains poorly understood. In certain cases, hydrophobic drug granules can be dispersed in aqueous solution via lipid adsorption surrounding the drug particles as nanocapsules. In other instances, hydrophobic drug molecules attach as monomers to borders of lipid bilayer fragments providing drug formulations that are effective in vivo at low drug-to-lipid-molar ratio. Cationic biomimetic particles offer suitable interfacial environment for adsorption, presentation and targeting of biomolecules in vivo. Thereby antigens can effectively be presented by tailored biomimetic particles for development of vaccines over a range of defined and controllable particle sizes. Biomolecular recognition between receptor and ligand can be reconstituted by means of receptor immobilization into supported lipidic bilayers allowing isolation and characterization of signal transduction steps.
Introduction
Particles are important in pharmaceutical and biomedical research since their size scale can be similar to that of proteins or DNA. They can mimic structural and functional aspects of viruses, bacteria and other biological assemblies and are currently being used in: imaging;Citation1 biosensing;Citation2 gene and drug delivery;Citation3 and vaccines.Citation4,Citation5 On the other hand, cationic lipids can be combined with negatively charged biomolecules or biological structures. Silica,Citation6 latex,Citation7 or hydrophobic drug particlesCitation8 have been coated with cationic lipids and characterized by means of adsorption isotherms, mean particle size from dynamic light scattering, surface potential analysis and colloidal stability. Properties of the intervening medium such as pH and ionic strength were systematically varied for achieving optimal lipid bilayer deposition and colloid stability.Citation6,Citation9 Polystyrene sulfate (PSS) nanoparticles covered with a dioctadecyldimethylammonium bromide (DODAB) bilayerCitation10 were used for adsorption of serum proteins,Citation11 cholera toxin,Citation11 Taenia crassiceps antigens,Citation12,Citation13 recombinant proteins,Citation13,Citation14 polysaccharidesCitation15–Citation17 and giant DNA.Citation18 Similarly, lipid assemblies such as liposomes, vesicles, solid lipid nanoparticles and lipidic bilayer fragments (BF) or disks are as versatile as particles. Alec Bangham produced the first liposomes in 1965 in Cambridge UK.Citation19 The liposome “membrane” model prepared from phospholipids has lead to much of our present knowledge on membrane properties. In addition, a variety of bilayer structures formed by dialkyldimethylammonium halidesCitation20 and other synthetic amphiphilesCitation21–Citation24 were introduced to mimic membrane properties furnishing unique opportunities to investigate structure-function relationships. Since the major requirement to form a supramolecular assembly of the bilayer type was an approximately cylindrical amphiphilic molecule with a geometric parameter between 0.5 and 1.0,Citation25 not only natural phospholipids were prone to form bilayers. Structural and functional aspects of biological membranes were also copied in a variety of biomimetic systems. Bilayers were the preferential supramolecular assembly for several synthetic amphiphiles as dialkyldimethylammonium bromide or chloride,Citation20 sodium dihexadecylphosphateCitation22,Citation26,Citation27 and many other molecules.Citation28,Citation29 However, lipid BF or disks remained as a much less explored bilayer assembly. Lately, our group has been developing novel formulations for hydrophobic drugs or vaccines based on these structures. Amphotericin B solubilized at the rim of DODAB BF provided a novel formulation with excellent activity against systemic candidiasis in mice at low drug doseCitation30,Citation31 with low nephrotoxicity.Citation32 BF have also been useful to cover particles such as silica, latex or insoluble drug particles with lipids.Citation33,Citation34 This mini-review aims at an overview on preparation, characterization and biomedical applications for lipid-based biomimetic particles.
Lipid BF or disks
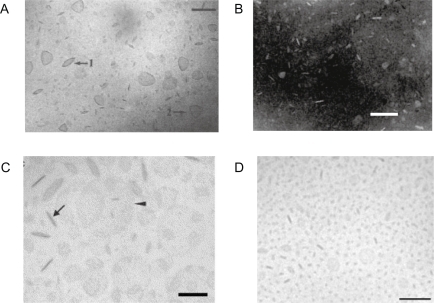
Upon probe sonication of aqueous egg yolk lecithin dispersions, the high kinetic energy given by the sonicator to the lipid particles, caused their breaking down on colliding with each other.Citation35 The collisions completely disrupted the multilamellar particles into short-lived bilayer fragments, which then reaggregated to form single-shelled vesicles of roughly uniform size.Citation35 From this early study up to now, discs have been reported for a variety of amphiphiles and lipids in aqueous dispersions over a broad range of experimental conditions.Citation10,Citation36 They were found from self-assembly of lipids and proteins,Citation37–Citation39 lipids and micelle-forming compoundsCitation40,Citation41 lipids and hydrophilic polymers such as polyethylene-glycol lipids (PEGylated lipids)Citation42,Citation43 or charged lipids dispersed by probe sonication.Citation27,Citation44–Citation46 The major repulsive interactions preventing fusion of lipid BF and discs in dispersion were electrostatic, steric and/or eletrosteric. In particular, probe sonication of the synthetic and cationic lipid dioctadecyldimethylammonium bromide (DODAB) can yield disrupted vesicles: the bilayer fragments, BF, or disks.Citation10 The existence of BF in aqueous dispersions of sodium dihexadecylphosphate, or dioctadecyldimethylammonium bromide or chloride obtained by probe sonication has been supported by the following evidences: (i) osmotic non-responsiveness of the dispersion indicative of absence of inner vesicle compartment;Citation47 (ii) transmission electron microscopy (TEM) micrographs with electronic staining;Citation27 (iii) cryo-TEM micrographs;Citation45 (iv) fluid and solid state coexistence and complex formation with oppositely charged surfactant;Citation48 (v) solubilization of hydrophobic drugs at the borders of DODAB bilayer fragments, which does not occur for DODAB closed bilayer vesicles.Citation49,Citation50 They differ from the closed vesicles by providing hydrophobic borders at their edges that are absent in closed bilayer systems such as vesicles or liposomes. Under conditions of low ionic strength, due to electrostatic repulsion, the charged BF remain colloidally stable in aqueous dispersions. These BF are not micelles; due to its cylindrical molecular shape DODAB or sodium dihexadecylphosphate (DHP) molecules self-assemble as bilayers.Citation51,Citation52 shows BF from different lipids.
Figure 1 A) Lipid BF of dioctadecyldimethylammonium bromide (DODAB)Citation45 or B) sodium dihexadecylphosphate (DHP)Citation27 or C) DSPC/cholesterol/PEG-DSPE(5000) mixtures at 12 mol% PEG-DSPE(5000)Citation42 or D) DSPC: cholesterol: ceramide-PEG5000 carrying bacteriorhodopsin.Citation43
Notes: With exception of micrograph in B) which was obtained by TEM after negatively staining the sample, all micrographs were obtained by cryo-TEM. In C), disks were observed edge-on (arrow) or face-on (arrow head). Bars denote 100 nm. Copyright © 1995 and 1991 American Chemical Society; © 2005 and 2007 Elsevier. Adapted with permission from Carmona-Ribeiro AM, Castuma CE, Sesso A, Schreier S. Bilayer structure and stability in dihexadecyl phosphate dispersions. J Phys Chem. 1991;95:5361–5366. Johansson E, Engvall C, Arfvidsson M, Lundahl P, Edwards K. Development and initial evaluation of PEG-stabilized bilayer disks as novel model membranes. Biophys Chem. 2005;113:183–192. Johansson E, Lundquist A, Zuo S, Edwards K. Nanosized bilayer disks: attractive model membranes for drug partition studies. Biochim Biophys Acta. 2007;1768:1518–1525. Andersson M, Hammarstrom L, Edwards K. Effect of bilayer phase transitions on vesicle structure, and its influence on the kinetics of viologen reduction. J Phys Chem. 1995;99(39):14531–14538.
Abbreviations: DHP, sodium dihexadecylphosphate; DODAB, dioctadecyldimethylammonium bromide; DSPE, distearoylphosphatidylethanolamine; PEG, polyethyleneglycol; TEM, transmission electron microscopy.
The interaction between particles and bilayers for vaccines
The interaction between one bilayer vesicle and two microspheres considering vesicles and particles of similar sizes can be electrostatic, electrodynamic (van der Waals) and/or hydrophobic.Citation6,Citation7,Citation53–Citation61 schematically illustrates possible interactions and assemblies.
Figure 2 The interaction between one bilayer vesicle and two particles. Citation6,Citation7, Citation53–Citation61 Copyright © 1999 Elsevier. Adapted with permission from Carmona-Ribeiro AM, Lessa MM. Interactions between bilayer vesicles and latex. Colloids Surf A. 1999;153:355–361.
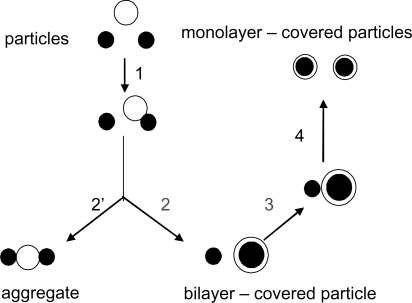
In the first step (step 1), electrostatic and/or van der Waals and/or hydrophobic attraction leads to aggregation of a vesicle and a particle. These same interaction forces may disrupt the vesicle bilayer and promote bilayer adsorption onto the microsphere (step 2) and/or further aggregation with the other microsphere (step 2′). The adsorbed bilayer may attract the second microsphere (step 3). The hydrophobic interaction between an eventually hydrophobic surface and the hydrocarbon chains in the bilayer may completely destroy the bilayer structure flip-flopping the hydrocarbon chains onto the particle surface and generating a monolayered coverage on each microsphere (step 4). Lipid deposition from lipidic vesicles onto a solid surface would be determined initially by the classical combination of a repulsive force arising from the interaction of the electrical double layers associated with the vesicle and the surface and the attractive dispersion force between the vesicle and the solid. Vesicles are not, however, permanent rigid structures, and depending on their size, chemical composition, physical state of the bilayer and aqueous medium composition they can distort, aggregate, disrupt and fuse with each other. Deposition of vesicles onto a solid surface could give rise to any particular one or a combination of these processes. Unilamellar phosphatidylcholine vesicles were reported to break open and adhere to a mica surface to form a bilayer coating.Citation62
Phospholipid monolayers with lipid haptens inserted were supported by hydrophobic glass and useful for specific adherence of macrophages and cell surface recognition studies, but did not serve as hosts for transmembrane proteins.Citation63 Dipalmitoylphosphatidylcholine (DPPC) and phosphatidylinositol (PI) from vesicles adsorbed onto negatively charged ballotini (hydrophobic) glass beads as a monolayer with their head groups uppermost.Citation64
The easiest method for preparing high quality phospholipid bilayers on a flat hydrophilic surface was the direct fusion of small unilamellar vesicles.Citation65 This method stemmed from making unilamellar membranes on glass coverslips for spectroscopic studies.Citation65 Phospholipid fusion at the hydrophilic surface such as freshly cleaved mica could be induced at elevated temperatures for those lipids of higher transition temperature with traces of divalent cations such as Ca2+. The other method for preparing supported membranes of biological interest was the controlled transfer of monolayers to the surface using the Langmuir. trough. Using this method the content in each leaflet was easily controlled.Citation66 The main advantages of the vesicle fusion method seemed to be simplicity and the most natural lateral pressure in the bilayer in comparison to the lateral pressures obtained with the Langmuir trough. However, the content in each leaflet could not be controlled using fusion. Palmitoyloleoylphosphatidylcholine (POPC) vesicles without major protruding molecular moieties spread on a glass surface and formed a supported planar bilayer.Citation67 In contrast, Escherichia coli (E. coli) lipid vesicles adsorbed as entire vesicles to the surface forming a supported vesicle layer on glass.Citation67 The difference in behavior upon deposition on glass was due to chemical structure of E. coli lipids. Lipopolysaccharides from E. coli are bulky and have strongly hydrated polar heads. Their vesicles simply adhered and formed a supported vesicle layer on glass.Citation67 Fusion in-between vesicles attached to the surface was prevented by steric repulsion.Citation67 For DPPC and DSPC bilayers on hydrophilic silicon/water interface, single and double bilayers have been prepared and characterized via neutron reflectivity.Citation68 This technique investigated the structure, hydration and roughness of the layers and allowed to determine the distance between two deposited bilayers.Citation68 The outermost bilayer was highly hydrated and floated at 2 to 3 nm above the first one.Citation68 Adhesion of a DODAB vesicle layer onto the rough and highly hydrated surface of cells was electrostatically driven. Cationic vesicles at low ionic strength surrounded the bacterial cell as a vesicle layer.Citation69 Absence of DODAB vesicle disruption upon interaction with the bacteria was depicted from absence of [Citation14C]-sucrose leakage from large vesicles in experiments where this marker was used to label the inner water compartment of the vesicles.Citation70 Given the quaternary ammonium moiety of the DODAB molecule, its antimicrobial effect was systematically evaluated and its differential cytotoxicity established as illustrated in .
Table 1 Differential cytotoxicity of DODAB against some eukaryotic* and prokaryotic cells**
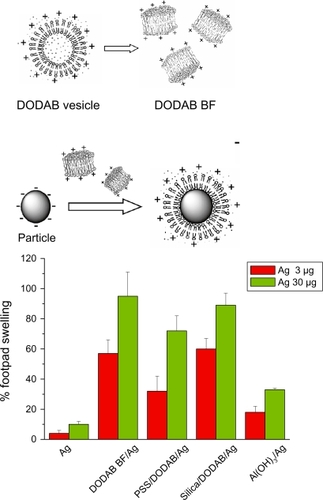
In spite of its dose dependent-toxicity, DODAB induced delayed-type hypersensibility (DTH), a marker for cell-mediated immune responses. This interesting property allowed DODAB to find many uses as an efficient immunoadjuvant mainly for veterinary uses but also in humans in a few instances.Citation13,Citation74–Citation79 Supramolecular assemblies of DODAB BF by themselves or after interaction with supporting particles were recently combined with three different model antigens in separate and tested as immunoadjuvants.Citation13 DODAB-based immunoadjuvants carrying antigens at reduced DODAB dose (0.01–0.1 mM) induced superior DTH responses in mice in comparison to alum. Thus, the cationic immunoadjuvant was either reduced to a single-component, nanosized system – DODAB BF – or was a dispersion of cationic particles with controllable nature and size as obtained after covering silica or polystyrene sulfate latex (PSS) with a cationic DODAB bilayer. DODAB BF interacted with proteins both via the hydrophobic effect and the electrostatic attraction at low ionic strength. DODAB based adjuvants exhibited good colloid stability while complexed with the antigens, complete absence of toxicity in mice (ie, local or general reactions) and a remarkable induction of Th1 immune response at reduced doses of cationic and toxic DODAB lipid. DODAB vesicle disruption by probe sonication at low ionic strength (0.1–5.0 mM monovalent salt) produced DODAB BF which remained electrostatically stabilized in dispersion by the electrostatic repulsion in between fragments. DODAB BF also interacted with oppositely charged particles such as silica or polystyrene sulfate (PSS) latex to produce the cationic particulates. schematically introduced the novel cationic immunoadjuvants based on reduced DODAB doses and their compared DTH response.Citation12–Citation14
Figure 3 Superior performance of novel DODAB-based adjuvants inducing DTH in mice as compared to alum. The same antigen (Ag) carried by each adjuvant was used for immunization. Ag was carried by DODAB BF at 0.1 mM DODAB (DODAB BF/Ag) or by PSS/DODAB or silica/DODAB particles at 0.01 or 0.05 mM DODAB (PSS/DODAB/Ag or silica/DODAB/Ag), respectively, or by alum (Al(OH)Citation3/Ag). After immunization, elicitation of the swelling response was done by injecting Ag alone in the footpad so that % footpad swelling was measured in comparison to alum. Copyright © 2007, 2009 Elsevier. Adapted with permission from Lincopan N, Espíndola NM, Vaz AJ, Carmona-Ribeiro AM. Cationic supported lipid bilayers for antigen presentation. In. J Pharm. 2007;340:216–222. Lincopan N, Espíndola NM, Vaz AJ, et al. Novel immunoadjuvants based on cationic lipid: preparation, characterization and activity in vivo. Vaccine. 2009;27:5760–5771. Lincopan N, Santana MRA, Faquim-Mauro E, da Costa MHB, Carmona-Ribeiro AM. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 2009;9:article 5.
Abbreviations: DODAB, dioctadecyldimethylammonium bromide; PSS, polystyrene sulfate; DTH, delayed-type hypersensibility; BF, bilayer fragments.
The final DODAB concentration required to cover all particles with a bilayer can be easily calculated from total surface area for particles and bilayers and from the mean molecular area for the lipid at the air-water interface.Citation6,Citation7,Citation11,Citation56 Sizing, zeta-potentials and polydispersity index for the novel cationic adjuvants as compared to the similarly cationic Al(OH)3 evidenced a superior colloid stability in contrast to the one exhibited by alum.Citation13,Citation14 illustrates the physical properties of DODAB BF, PSS/DODAB and silica/DODAB used in for antigen presentation.
Table 2 Physical properties of the novel cationic immunoadjuvants in a1 mM NaCl (pH 6.3) or b5 mM TrisHCl (pH 7.4) at 5 × 109 PSS particles/ml or 0.1 mg/ml silica or 0.1 mg/ml Al(OH)3. Copyright © 2009 Elsevier. Adapted with permission from Lincopan N, Espíndola NM, Vaz AJ, et al. Novel immunoadjuvants based on cationic lipid: preparation, characterization and activity in vivo. Vaccine. 2009;27:5760–5771.
Chemical, biological (eg, engineered viruses and bacteria), polymeric or liposomal adjuvants have been developed and tested for vaccines.Citation4,Citation5,Citation80–Citation83 However, most of them induced side effects and/or showed a lack of universality for different antigens or routes of administration. At present the only adjuvant licensed worldwide for humans is represented by aluminum salts, mainly aluminum hydroxide and aluminum phosphate.Citation84,Citation85 They elicit high and long lasting antibody titers and Th2 type responses but are poor inducers of cytotoxic T lymphocytes (CTLs), the most important cellular defense against infectious diseases caused by intracellular pathogens (eg, HIV and Mycobacterium tuberculosis) and tumors.Citation84 Furthermore, alum adjuvants can induce occasional local reactions.Citation86,Citation87 and production of immunoglobulin E (IgE) both associated with some allergic reactions in humans.Citation88,Citation89 In addition to Alum, MF59,Citation90–Citation95 virosomesCitation96–Citation98 and L-tyrosineCitation99 represented new and safe adjuvants and carrier systems recently licensed in some countries for human use.
Particles are taken up by antigen presenting cells (APC) more effectively than soluble antigen.Citation100,Citation101 Optimal APC uptake of positively charged particles with diameters of 500 nm and below was reported.Citation102 Furthermore, particles with a positive charge showed higher internalization into human breast cancer cells than those with negative charge, while the degree of internalization of the positively and negatively charged nanoparticles into human umbilical vein endothelial cells was almost the same.Citation103 Particles coated by cationic lipid were optimized regarding bilayer deposition.Citation6,Citation9,Citation12,Citation18 This was possible from systematic studies of effects of ionic strength and concentrations for particles and cationic lipid. Due to the microbicidal effect of cationic lipids and surfactants,Citation104 cationic particles turned out to be extremely toxic to bacteria, a major problem while trying to transform these microorganisms by means of cationic particle/DNA assemblies.Citation18 The bacteria cannot be genetically modified if they die. Silica,Citation6,Citation56,Citation57,Citation61 latexCitation7,Citation105 or hydrophobic drug particlesCitation4,Citation33,Citation49 have been coated with cationic lipids. Although DODAC or DHP electrostatically adsorbed to oppositely charged polystyrene microspheres, forming homodisperse bilayer-covered lattices, this took place only over a certain range of low lipid concentrations.Citation7,Citation12,Citation53,Citation54 Beyond bilayer deposition, there was vesicle adhesion to the bilayer-covered latex.Citation53 Using radiolabeled D-glucose inside the cationic vesicles at very low ionic strength, cationic liposome adsorption was accompanied of vesicle disruption evidencing formation of a bilayer on the solid particle surface.Citation53 A series of monodisperse PSS dispersions (76–412 nm mean diameter) were coated with DODAB bilayers so that the biomimetic particles are available over a range of controllable sizes.Citation18,Citation54 The zeta-potential of these bilayer-covered particles in water remained constant (and positive) over the entire range of sizes tested.
Whereas polystyrene microspheres have a hydrophobic surface, silica particles are good models for hydrophilic surfaces. Silica interacts with erythrocytes, lysosomes, macrophage plasma membranes and liposomesCitation106 but the mechanism of the interaction between silica and phospholipid membranes is still controversial. The main possibilities are: 1) silica particles binding dipalmitoylphosphatidylcholine (DPPC) through hydrogen bonds between Si-OH and O = P- groups; 2) tetraalkylammonium groups at the extracellular region of the erythrocyte membrane forming ion pairs with dissociated silanol on the silica particle and generating hemolytic effects observed for silica. Adsorption isotherms of 4 different bilayers on hydrophilic silica over a range of experimental conditions helped to clarify this issue.Citation56 The separate use of synthetic charged membranes with phosphate or tetraalkylammonium groups as polar heads such as are DODAB and DHP bilayer vesicles, to obtain adsorption isotherms on silica established the relative importance of phosphate or tetraalkylammonium on the mechanism of phospholipid deposition onto hydrophilic silica particles. Formation of ion pairs between the quaternary ammonium in the choline moiety of the phospholipid and the deprotonated silanol drove vesicle adhesion to the particle but vesicle rupture and bilayer deposition was determined by the cooperative occurrence of several hydrogen bridges between silanol and the phosphate moiety on the phospholipid.Citation56 There was a low affinity between neutral phospholipids and the silica surface and a high affinity for the cationic amphiphile over a range of pH values.Citation57 Tris-hydroxymethylaminomethane (Tris) used as a buffer increased the affinity between PC and silica at pH ≤ 7.4 due to Tris adsorption on silica with an increase in the surface density of hydroxyls on the surface available to hydrogen bridging with phosphate phospholipid groups. Bilayer deposition, however, was unambiguously confirmed by the three techniques only for the interaction DPPC vesicles/silica over 1 hours at 65°C and for the interaction DODAB vesicles/silica over the all range of experimental conditions tested.Citation57 A simple spectrophotometric method for identifying entire bilayer deposition onto solid particles was developed from incorporation of the optical probe merocyanine 540 onto the outer bilayer vesicle surface. Upon bilayer deposition on the particle, sandwiching the marker between bilayer and solid particle reduced light absorption. Thereby reduction of light absorption by merocyanine was quantitatively related to bilayer deposition.Citation57 For the interaction between cationic DODAB/DPPC and anionic PI/DPPC vesicles with zinc citrate dispersions the majority of the adsorption was in the form of intact liposomes.Citation107 When liposomes interacted with hydrophilic solid surfaces bearing ionizable groups such as citrate or silanol, the pH affected the extent of adsorption.Citation107 For anionic liposomes, adsorption decreased with pH. For cationic liposomes, adsorption increased with pH.Citation107 The fusion and spreading of phospholipid bilayers on negatively charged glass surfaces was dependent on pH and ionic strength.Citation108 Membrane fusion of negatively charged membranes was favored by low pH and high ionic strength whereas membrane fusion of positively charged membranes onto the surface occurred under all conditions tested.Citation108
The interaction between particles and bilayers for drug delivery
The particle concept encompasses a broad variety of particulates: lipid particles (eg, a bilayer fragment); polymeric; mineral or metallic particles; bacterial cells; viruses; mammalian cells with several organelles and particles of insoluble, hydrophobic drugs. The lipid covered-latexes were useful as hosts for receptors,Citation60,Citation109,Citation110 as coatings reducing protein adsorption on the particlesCitation110 and in chromatography.Citation111–Citation113 The potential of hybrid particle-lipid systems in diagnostics and therapeutics has also been realized.Citation8,Citation114–Citation116 In drug formulation, lipid nanoparticles of the anticancer drug chlorambucil were prepared by ultrasonication, using stearic acid as the core lipid and DODAB as surface modifier.Citation117 The presence of DODAB on the lipid nanoparticles resulted in greater accumulation of the drug in tumors.Citation117 For the encapsulation of cisplatin, bilayer-coating circumvented the limited solubility of cisplatin in water and produced cisplatin nanocapsules, bean-shaped nanoprecipitates of cisplatin coated by a lipid bilayer.Citation118 The nanocapsules represented a novel lipid formulation of cisplatin characterized by a very high cisplatin-to-lipid ratio and cytotoxicity against tumor cells in vitro as compared to the free drug. The formation of the nanocapsules critically depended on the presence of negatively charged phospholipids and positively charged aqua-species of cisplatin.Citation118,Citation119 The effect of PEG on the stability of the cisplatin nanocapsules was studied by incorporating PEG conjugated to phosphatidylethanolamine (DSPE-PEG2000).Citation120 Cisplatin release from the nanocapsules depended on the temperature, the surrounding medium, and the lipid composition of the bilayer coat. Sterically stabilized cisplatin nanocapsules containing 6 mol % DSPE-PEG served as the starting formulation for in vivo studies addressing the anti-tumor efficacy of cisplatin nanocapsules in tumor-bearing mice; there was a requirement of anionic phospholipid for successful nanoencapsulation of the cationic aqua-cisplatin.Citation120
Miconazole or amphotericin B were formulated in DODAB or DHP BF.Citation34,Citation49 Some of these formulations required low drug-to-lipid molar ratio due to limited drug loading capacity of the BF at their rims. For example, BF loading capacity for monomeric amphotericin B was 0.1 mM amphotericin B at 2 mM DODAB meaning that one drug molecule required 20 molecules of cationic lipid to become soluble in the BF nanostructure. At and above this 1:20 drug-to-lipid molar ratio, all solubilization sites at the rim of the BF were occupied. Therefore, further addition of drug resulted in appearance of aggregated amphotericin B in the dispersion as easily monitored by systematic determination of size distribution by means of photon correlation spectroscopy.
In order to formulate hydrophobic drugs with the DODAB lipid at high drug-to-lipid molar ratios, we took advantage of the “sticky” property of chaotropic dihydrogen phosphate anion which converted miconazole or amphotericin B drug particles into negatively charged particles. Thereafter, anionic drug particles could be coated by the DODAB cationic lipid.Citation34,Citation49 These formulations were tested against C. neoformans and Candida and were very effective due to DODAB activity against fungi. The cationic lipid alone exhibited minimal fungicidal concentrations (MFC) equal to 2 and 2 to >250 mg/L against C. neoformans and Candida, respectively. In combination, over the first hour, fungicidal activity was due to DODAB with lipid capsules retarding drug action. At 48 hours and 104 cfu/mL, MFC (mg/L) against Candida albicans was reduced from 4 to 1 amphotericin B (at 2 DODAB), and from 8 to 1 miconazole (at 1 DODAB). Calculations of synergism indexes showed synergistic action of both antimicrobial drugs: the cationic DODAB lipid and the microbicidal drugCitation34,Citation49 so that the DODAB/miconazole (MCZ) formulations should be further tested regarding therapeutic activity in vivo. illustrated the efficacy of MCZ in DODAB or DHP BF despite the low dose of drug.
Table 3 Minimal fungicidal concentration (MFC) for Zoltec® (fluconazol), miconazole (MCZ), MCZ/DODAB BF or MCZ/DHP BF against Candida albicans. Copyright © 2006 Elsevier. Adapted with permission from Vieira DB, Pacheco LF, Carmona-Ribeiro AM. Assembly of a model hydrophobic drug into cationic bilayer fragments. J Colloid Interface Sci. 2006;293:240–247.
In summary, two major strategies were developed to formulate hydrophobic drugs with bilayer fragments. These strategies can be better visualized in . In , coalescence of BF around the drug granule would encapsulate drug particles at high drug-to-lipid molar ratios.Citation33,Citation34,Citation49,Citation50 In , monomolecular drug solubilization would be achieved at low drug-to-lipid molar ratios.Citation30,Citation31,Citation33 Therefore, these discoidal, charged BF dispersed the hydrophobic drug particles in water both at low and high drug-to-lipid molar ratios.
Figure 4 A) Encapsulation of amphotericin B particle by a cationic bilayer at high drug to lipid molar ratio; B) Solubilization of amphotericin B at the rim of cationic BF at low drug to lipid molar ratio.

PEG decorated lipid bilayers are widely used in drug delivery.Citation121 In these hybrid polymer/lipid systems, there is a transition from a dispersed lamellar phase (liposomes) to a micellar phase mediated by the formation of small discoidal micelles. The onset of disk formation took place at low PEG-lipid concentrations (<5 mol%) and the size of the disks decreased as more PEG-lipid was added to the lipid mixture.Citation122 Stable dispersions dominated by flat bilayer disks could be prepared from a carefully optimized mixture of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-5000][PEG-DSPE(5000)]. By varying the content of PEG-DSPE (5000), the disks diameter varied from about 15 to 60 nm. Disks compared favorably to uni- and multilamellar liposomes for hydrophilic drug partitioning employing immobilized disks in glass capillaries.Citation42 The major repulsive interactions preventing fusion of these BF were steric. They provided larger areas than vesicles or liposomes for hydrophilic drug partitioning and from this point of view were considered as an attractive and sometimes superior alternative to liposomes.Citation43
Reconstitution of receptor-ligand recognition in artificial biomimetic particles is a very promising but hitherto unexplored area for research. The major advantage of these particles is the possibility of complete quantification of binding from simple analytical methods and techniques such as centrifugation for separation between free and bound receptors or free and bound ligands. Aiming at the production of bilayer covered silica particles, the interaction between silica particles and lipid vesicles or BF has been systematically studied by our group since 1997.Citation56–Citation61 As a result optimal coverage of silica particles with a PC bilayer was recently achieved.Citation59,Citation60 At pH 6.3, limiting PC adsorption indicative of one-bilayer deposition on each silica particle was obtained at and above 10 mM NaCl. Increasing ionic strength provided increasing attractive van der Waals attraction between vesicle and particle so that vesicles ruptured upon contact with particles and covered them with one bilayer.Citation59 Keeping ionic strength at 10 mM NaCl, the effect of increasing pH was decreasing affinity between PC and silica.Citation59 These experiments revealed an important role for hydrogen bonding between silanol on silica and phosphate on PC driving bilayer deposition. Bilayer deposition improved colloid stability of silica as shown from absence of particulate sedimentation.Citation59
Silica-based biomimetic particles successfully immobilized proteins or DNACitation13 or allowed isolation and reconstitution of receptor-ligand specific interaction.Citation60
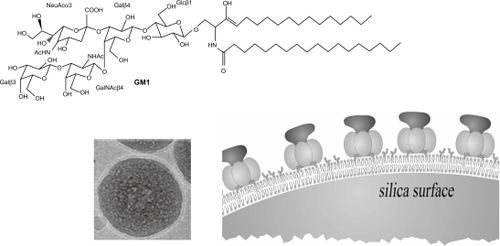
Cholera toxin (CT) and its receptor, the monosialoganglioside GM1, a cell membrane glycolipid, self-assembled on PC bilayer-covered silica at 1 CT/5 GM1, a molar ratio in perfect agreement with literature. illustrated this proof of concept.
Figure 5 Receptor-ligand recognition on biomimetic particles.Citation60 Cryo-TEM revealed the PC bilayer surrounding a silica particle. The GM1 receptor inserted in supported PC bilayers recognized its ligand, the cholera toxin. Copyright © 2005 American Chemical Society. Adapted with permission from Mornet S, Lambert O, Duguet E, Brisson A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 2005;5:281–285
Abbreviations: TEM, transmission electron microscopy; PC, phosphatidylcholine.
Conclusions
The intermolecular forces between lipids and particles have to be understood in depth before optimal biomimetic particles based on lipids can be obtained. Over the years our systematic studies on such interactions produced some examples of optimized bilayer deposition on silica or latex particles. Furthermore, charged BF solubilized hydrophobic drugs and complexed with antigens yielding novel supramolecular assemblies for drug and vaccine delivery either by providing hydrophobic edges for drug or antigen complexation or by covering silica, drug or polymeric particles with minute amounts of lipid. Low doses of cationic lipid in the formulations avoided their well described toxicity. Our contribution to the field may be summarized in a thorough description of the differential cytotoxicity of the DODAB cationic lipid, and the invention of novel charged carriers to formulate drugs or vaccines at reduced charged lipid dose. Encapsulation of drugs, reconstitution of biomolecular recognition, antigen presentation and antimicrobial therapy were some examples of biomedical applications for biomimetic particles that were provided by this mini-review.
Acknowledgements
Financial support from FAPESP and CNPq is gratefully acknowledged.
References
- El-SayedIHHuangXEl-SayedMASurface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancerNano Lett2005582983415884879
- MedintzARClappJSMelingerJRDeschampsHMattoussiAReagentless Biosensing assembly based on quantum dot-donor Förster resonance energy transferAdv Mater20051724502455
- Carmona-RibeiroAMBilayer-forming synthetic lipids: drugs or carriers?Curr Med Chem2003102425244614529483
- O’HaganDTSinghMUlmerJBMicroparticles for the delivery of DNA vaccinesImmunol Rev200419919120015233735
- CaputoASparnacciKEnsoliBTondelliLFunctional Polymeric nano/microparticles for surface adsorption and delivery of protein and DNA vaccinesCurr Drug Delivery20085230242
- MouraSPCarmona-RibeiroAMCationic bilayer fragments on silica at low ionic strength: competitive adsorption and colloid stabilityLangmuir20031966646667
- Carmona-RibeiroAMMidmoreBRSynthetic bilayer adsorption onto polystyrene microspheresLangmuir19928801806
- Carmona-RibeiroAMBiomimetic particles in drug and vaccine deliveryJ Liposome Res20071716517218027236
- PereiraEMAVieiraDBCarmona-RibeiroAMCationic bilayers on polymeric particles: effect of low NaCl concentration on surface coverageJ Phys Chem B20041081149011495
- Carmona-RibeiroAMLipid bilayer fragments and disks in drug deliveryCurr Med Chem2006131359137016719782
- LincopanNCarmona-RibeiroAMProtein assembly onto cationic supported bilayersJ Nanosci Nanotechnol200993578358619504885
- LincopanNEspíndolaNMVazAJCarmona-RibeiroAMCationic supported lipid bilayers for antigen presentationIn J Pharm2007340216222
- LincopanNEspíndolaNMVazAJNovel immunoadjuvants based on cationic lipid: preparation, characterization and activity in vivoVaccine2009275760577119664738
- LincopanNSantanaMRAFaquim-MauroEda CostaMHBCarmona-RibeiroAMSilica-based cationic bilayers as immunoadjuvantsBMC Biotechnol20099:article 5.
- VieiraDBLincopanNMamizukaEMPetriDFSCarmona-RibeiroAMCompetitive adsorption of cationic bilayers and chitosan on latex: optimal biocidal actionLangmuir200319924932
- CorreiaFMPetriDFSCarmona-RibeiroAMColloid stability of lipid/polyelectrolyte decorated latexLangmuir2004209535954015491183
- AraujoFPPetriDFSCarmona-RibeiroAMColloid stability of sodium dihexadecyl phosphate/poly(diallyldimethylammonium chloride) decorated latexLangmuir2005219495950116207027
- RosaHPetriDFSCarmona-RibeiroAMInteractions between bacteriophage DNA and cationic biomimetic particlesJ Phys Chem B2008112164221643019367939
- BanghamADLiposome LettersLondonAcademic Press19831405
- KunitakeTOkahataYTamakiKKumamaruFTakayanagiMFormation of the bilayer membrane from a series of quaternary ammonium saltsChem Lett197764387390
- HargreavesWRDeamerDWLiposomes from ionic, single-chain amphiphilesBiochemistry1978171837593768698196
- MortaraRAQuinaFHChaimovichHFormation of closed vesicles from a simple phosphate diester. Preparation and some properties of vesicles of dihexadecyl phosphateBiochem Biophys Res Commun19788110801086666808
- CzarnieckiMFBreslowRPhotochemical probes for model membrane structuresJ Am Chem Soc197910136753676
- SuedholterEJREngbertsJBFNHoekstraDJVesicle formation by two novel synthetic amphiphiles carrying micropolarity reporter head groupsJ Am Chem Soc198010224672469
- IsraelachviliJNMitchellDJNinhamBWTheory of self-assembly of lipid bilayers and vesiclesBiochim Biophys Acta1977470185201911827
- Carmona-RibeiroAMYoshidaLSSessoAChaimovichHPermeabilities and stabilities of large dihexadecylphosphate and dioctadecyldimethylammonium chloride vesiclesJ Colloid Interface Sci1984100433443
- Carmona-RibeiroAMCastumaCESessoASchreierSBilayer structure and stability in dihexadecyl phosphate dispersionsJ Phys Chem19919553615366
- FuhrhopJHFritschDBolaamphiphiles form ultrathin, porous and unsymmetric monolayer lipid membranesAcc Chem Res198619130137
- SegotaSTezakDSpontaneous formation of vesiclesAdv Colloid Interface Sci2006121517516769012
- VieiraDBCarmona-RibeiroAMSynthetic bilayer fragments for solubilization of amphotericin BJ Colloid Interface Sci2001244427431
- LincopanNMamizukaEMCarmona-RibeiroAMIn vivo activity of a novel amphotericin B formulation with synthetic cationic bilayer fragmentsJ Antimicrob Chemother20035241241812917237
- LincopanNMamizukaEMCarmona-RibeiroAMLow nephrotoxicity of an effective amphotericin B formulation with cationic bilayer fragmentsJ Antimicrob Chemother20055572773415761070
- PachecoLFCarmona-RibeiroAMEffects of synthetic lipids on solubilization and colloid stability of hydrophobic drugsJ Colloid Interface Sci200325814615412600782
- LincopanNCarmona-RibeiroAMLipid-covered drug particles: combined action of dioctadecyldimethylammonium bromide and amphotericin B or miconazoleJ Antimicrob Chemother200658667516636081
- FinerEGFlookAGHauserHMechanism of sonication of aqueous egg yolk lecithin dispersions and nature of the resultant particlesBiochim Biophys Acta197226049585062443
- NathAAtkinsWMSligarSGApplications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteinsBiochemistry2007462059206917263563
- BayburtTHSligarSGSingle-molecule height measurements on microsomal cytochrome P450 in nanometer-scale phospholipid bilayer disksProc Natl Acad Sci U S A2002996725673011997441
- LyukmanovaENShenkarevZOParamonovASLipid-protein nanoscale bilayers: a versatile medium for NMR investigations of membrane proteins and membrane-active peptidesJ Am Chem Soc20081302140214118229924
- HazelbauerGLFalkeJJParkinsonJSBacterial chemoreceptors: high-performance signaling in networked arrays200833919
- LawaczeckRKainoshoMChanSIThe formation and annealing of structural defects in lipid bilayer vesiclesBiochim Biophys Acta1976443313330963059
- AlmgrenMMixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactantsBiochim Biophys Acta2000150814616311090823
- JohanssonEEngvallCArfvidssonMLundahlPEdwardsKDevelopment and initial evaluation of PEG-stabilized bilayer disks as novel model membranesBiophys Chem200511318319215617826
- JohanssonELundquistAZuoSEdwardsKNanosized bilayer disks: attractive model membranes for drug partition studiesBiochim Biophys Acta200717681518152517451640
- PansuRBArrioBRoncinJFaureJVesicles versus membrane fragments in DODAC suspensionsJ Phys Chem199094796801
- AnderssonMHammarstromLEdwardsKEffect of bilayer phase transitions on vesicle structure, and its influence on the kinetics of viologen reductionJ Phys Chem199599391453114538
- MeyerHWRichterWRettigWStumpfMBilayer fragments and bilayered micelles (bicelles) of dimyristoylphosphatidylglycerol (DMPG) are induced by storage in distilled water at 4°CColloids Surf A: Physicochem Eng Aspects2001183185495504
- Carmona-RibeiroAMChaimovichHPreparation and characterization of large dioctadecyldimethylammonium chloride liposomes and comparison with small sonicated vesiclesBiochim Biophys Acta19837331721796882751
- CocquytJOlssonUOlofssonGvan der MeerenPTemperature quenched DODAB dispersions: fluid and solid state coexistence and complex formation with oppositely charged surfactantLangmuir2004203906391215969378
- VieiraDBPachecoLFCarmona-RibeiroAMAssembly of a model hydrophobic drug into cationic bilayer fragmentsJ Colloid Interface Sci200629324024716095603
- VieiraDBCarmona-RibeiroAMCationic nanoparticles for delivery of amphotericin B: preparation, characterization and activity in vitroJ Nanobiotechnol20086:article 6.
- Carmona-RibeiroAMSynthetic amphiphile vesiclesChem Soc Rev199221209214
- IsraelachviliJNIntermolecular and surface forces2nd edLondonAcademic Press Limited1992
- TsurutaLRLessaMMCarmona-RibeiroAMInteractions between dioctadecyldimethylammonium chloride or bromide bilayers in waterLangmuir19951129382943
- TsurutaLRLessaMMCarmona-RibeiroAMEffect of particle size on colloid stability of bilayer-covered polystyrene microspheresJ Colloid Interface Sci1995175470475
- TsurutaLRCarmona-RibeiroAMCounterion effects on colloid stability of cationic vesicles and bilayer-covered polystyrene microspheresJ Phys Chem199610071307134
- RapuanoRCarmona-RibeiroAMPhysical adsorption of bilayer membranes on silicaJ Colloid Interface Sci19971931041119299094
- RapuanoRCarmona-RibeiroAMSupported bilayers on silicaJ Colloid Interface Sci2000226299307
- Carmona-RibeiroAMLessaMMInteractions between bilayer vesicles and latexColloids Surf A1999153355361
- MouraSPCarmona-RibeiroAMBiomimetic particles: optimization of phospholipid bilayer coverage on silica and colloid stabilizationLangmuir200521101601016416229540
- MouraSPCarmona-RibeiroAMBiomimetic particles for isolation and reconstitution of receptor functionCell Biochem Biophys20064444645216679532
- MouraSPCarmona-RibeiroAMAdsorption behavior of DODAB/DPPC vesicles on silicaJ Colloid Interface Sci200731351952617532328
- HornRGDirect measurement of the force between two lipid bilayers and observation of their fusionBiochim Biophys Acta1984778224228
- LinLCWeisRMMcConnellHMInduction of helical liposomes by Ca2+-mediated intermembrane bindingNature19822961641657063019
- JacksonSReboirasMDLyleIGJonesMNAdsorption of phospholipid vesicles on solid surfacesFaraday Discuss Chem Soc198681291301
- BrianAAMcConnellHMAllogeneic stimulation of cytotoxic T cells by supported planar membranesProc Natl Acad Sci U S A198481615961636333027
- TammLKMcConnellHMSupported phospholipid bilayersBiophys J1985471051133978184
- NollertPKieferHJaehnigFLipid vesicle adsorption versus formation of planar bilayers on solid surfacesBiophys J199569144714558534815
- CharitatTBellet-AmalricEFragnetoGGranerFAdsorbed and free lipid bilayers at the solid-liquid interfaceEur Phys J B19998583593
- TápiasGNSicchierolliSMMamizukaEMCarmona-RibeiroAMInteractions between cationic vesicles and Escherichia coliLangmuir19941034613465
- MartinsLMSMamizukaEMCarmona-RibeiroAMCationic vesicles as bactericidesLangmuir19971355835587
- Carmona-RibeiroAMOrtisFSchumacherRIArmelinMCSInteractions between cationic vesicles and cultured mammalian cellsLangmuir19971322152218
- CampanhãMTNMamizukaEMCarmona-RibeiroAMInteractions between cationic vesicles and Candida albicansJ Phys Chem B200110582308236
- CampanhãMTNMamizukaEMCarmona-RibeiroAMInteractions between cationic liposomes and bacteria: the physical-chemistry of the bactericidal actionJ Lipid Res1999401495150010428986
- GallDThe adjuvant activity of aliphatic nitrogenous basesImmunology1966113693865924622
- DaileyMOHunterRLThe role of lipid in the induction of hapten-specific delayed hypersensitivity and contact sensitivityJ Immunol1974112152615344815095
- HilgersLASnippeHDDA as an immunological adjuvantRes Immunol19921434945031439129
- TsurutaLRQuintilioWCostaMHBCarmona-RibeiroAMInteractions between cationic liposomes and an antigenic protein: the physical chemistry of the immunoadjuvant action19973820032011
- Klinguer-HamourCLibonCPlotnicky-GilquinHDDA adjuvant induces a mixed Th1/Th2 immune response when associated with BBG2Na, a respiratory syncytial virus potential vaccineVaccine2002202743275112034101
- KorsholmKSAggerEMFogedCThe adjuvant mechanism of cationic dimethyldioctadecylammonium liposomesImmunology200712121622617302734
- GregoriadisGMcCormackBObrenovicMSaffieRZadiBPerrieYVaccine entrapment in liposomesMethods19991915616210525452
- PerrieYMohammedARKirbyDJMcNeilSEBramwellVWVaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigensInt J Pharm200836427228018555624
- O’HaganDTSinghMMicroparticles as vaccine adjuvants and delivery systemsExpert Rev Vaccines2003226928312899577
- XiangSDScholzenAMinigoGPathogen recognition and development of particulate vaccines: Does size matter?Methods2006401916997708
- GuptaRAluminum compounds as vaccine adjuvantsAdv Drug Delivery Rev199832155172
- JeffersonTRudinMDi PietrantonjCAdverse events after immunisation with aluminium-containing DTP vaccines: systematic review of the evidenceLancet Infectious Diseases20044849014871632
- ClementsCGriffithsEClementsCGriffithsEThe global impact of vaccines containing aluminium adjuvantsVaccine200220S24S3312184361
- TrollforsBBergforsEInerotAVaccine related itching nodules and hypersensitivity to aluminiumVaccine20052397597615620469
- LindbladEAluminium adjuvants-in retrospect and prospectVaccine2004223658366815315845
- GuptaRSiberGAdjuvants for human vaccines – current status, problems and future prospectsVaccine199513126312768585280
- SinghMUgozzoliMKazzazJA preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigensVaccine2006241680168616300864
- SinghMO’HaganDAdvances in vaccine adjuvantsNature Biotechnology19991710751081
- OttGBarchfeldGChernoffDRadhakrishnanRvan HoogevestPVan NestGMF59. Design and evaluation of a safe and potent adjuvant for human vaccinesPharm Biotechnol199562772967551221
- TraquinaPMorandiMContorniMVan NestGMF59 adjuvant enhances the antibody response to recombinant hepatitis B surface antigen vaccine in primatesJ Infect Dis1996174116811758940205
- GranoffDMcHughYRaffHMokatrinAVan NestGMF59 adjuvant enhances antibody responses of infant baboons immunized with Haemophilus influenzae type b and Neisseria meningitis group C oligosaccharide-CRM197 conjugate vaccineInfect Immun199765171017159125551
- PoddaADel GiudiceGMF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profileExpert Rev Vaccines2003219720312899571
- CusiMApplications of influenza virosomes as a delivery systemHum Vaccin200621717012895
- GlückRBurriKMetcalfeIAdjuvant and antigen delivery properties of virosomesCurr Drug Deliv2005239540016305442
- HuckriedeABungenerLStegmannTThe virosome concept for influenza vaccinesVaccine200523S26S3816026906
- BaldrickPRichardsonDWheelerAReview of L-tyrosine confirming its safe human use as an adjuvantJ Appl Toxicol20022233334412355563
- Kovacsovics-BankowskiMClarkKBenacerrafBRockKLEfficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophagesProc Natl Acad Sci U S A199390494249468506338
- VidardLKovacsovics-BankowskiMKraeftSKChenLBBenacerrafBRockKLAnalysis of MHC class II presentation of particulate antigens of B lymphocytesJ Immunol1996156280928188609400
- FogedCBrodinBFrokjaerSSundbladAParticle size and surface charge affect particle uptake by human dendritic cells in an in vitro modelInt J Pharm200529831532215961266
- OsakaTNakanishiTShanmugamSTakahamaSZhangHEffect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cellsColloids and Surf B: Biointerfaces2009712325330
- Carmona-RibeiroAMVieiraDBLincopanNCationic surfactants and lipids as anti-infective agentsAnti-Infective Agents in Medicinal Chemistry200653351
- Carmona-RibeiroAMBilayer vesicles and liposomes as interface agentsChem Soc Rev200130241247
- NashTAllisonACHaringtonJSPhysico-chemical properties of silica in relation to its toxicityNature19662102592614289018
- CatuognoCJonesMNThe interaction of cationic and anionic vesicles with zinc citrate dispersionsColloids Surf A2000163165176
- CremerPSBoxerSGFormation and spreading of lipid bilayers on planar glass supportsJ Phys Chem199910325542559
- SicchierolliSMCarmona-RibeiroAMIncorporation of the cholera toxin receptor in phospholipid-covered polystyrene microspheresColloids and Surf B: Biointerfaces199555764
- SicchierolliSMCarmona-RibeiroAMBiomolecular recognition at phospholipid-covered polystyrene microspheresJ Phys Chem19961001677116775
- HautalaJTLindenMVWiedmerSKSimple coating of capillaries with anionic liposomes in capillary electrophoresisJ Chromatogr A20031004819012929964
- HaratakeMHidakaSOnoMNakayamaMPreparation of an ion-exchangeable polymer bead wrapped with bilayer membrane structures for high performance liquid chromatographyAnal Chim Acta2007589768317397656
- GulcevMDLucyCAFactors affecting the behavior and effectiveness of phospholipid bilayer coatings for capillary electrophoretic separations of basic proteinsAnal Chem2008801806181218232711
- Al-JamalWTKostarelosKLiposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applicationsNanomedicine20072859817716195
- SinghRTianBKostarelosKArtificial envelopment of nonenveloped viruses: enhancing adenovirus tumor targeting in vivoFASEB J2008223389340218556649
- SooPLDunneMLiuJAllenCNano-sized advanced delivery systems as parenteral formulation strategies for hydrophobic anti-cancer drugsBiotechnology: Pharmaceutical Aspectsde VilliersMMAramwitPKwonGSHeidelbergSpringer2009349383
- SharmaPGantaSDennyWAGargSFormulation and pharmacokinetics of lipid nanoparticles of a chemically sensitive nitrogen mustard derivative: ChlorambucilInt J Pharm200936718719418930127
- BurgerKNStaffhorstRWde VijlderHCNanocapsules: lipid-coated aggregates of cisplatin with high cytotoxicityNature Medicine200288184
- ChupinVde KroonAIPde KruijffBMolecular architecture of nanocapsules, bilayer-enclosed solid particles of cisplatinJ Am Chem Soc2004126138161382115493941
- VelinovaMJStaffhorstRWMulderWJPreparation and stability of lipid-coated nanocapsules of cisplatin: anionic phospholipid specificityBiochim Biophys Acta2004166313514215157616
- LasicDDSterically stabilized vesiclesAngew Chem Int Ed Engl1994331716851683
- JohnssonMEdwardsKLiposomes, disks, and spherical micelles: aggregate structure in mixtures of gel phase phosphatidylcholines and poly(ethylene glycol)-phospholipidsBiophys J2003853839384714645073
- MornetSLambertODuguetEBrissonAThe formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopyNano Lett2005528128515794611