?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Medicinal plants serve as rich sources of diverse bioactive phytochemicals that might even take part in bioreduction and stabilization of phytogenic nanoparticles with immense therapeutic properties. Herein, we report for the first time the rapid efficient synthesis of novel platinum–palladium bimetallic nanoparticles (Pt–PdNPs) along with individual platinum (PtNPs) and palladium (PdNPs) nanoparticles using a medicinal plant, Dioscorea bulbifera tuber extract (DBTE). High-resolution transmission electron microscopy revealed monodispersed PtNPs of size 2–5 nm, while PdNPs and Pt–PdNPs between 10 and 25 nm. Energy dispersive spectroscopy analysis confirmed 30.88%±1.73% elemental Pt and 68.96%±1.48% elemental Pd in the bimetallic nanoparticles. Fourier transform infrared spectra indicated strong peaks at 3,373 cm−1, attributed to hydroxyl group of polyphenolic compounds in DBTE that might play a key role in bioreduction in addition to the sharp peaks at 2,937, 1,647, 1,518, and 1,024 cm−1, associated with C–H stretching, N–H bending in primary amines, N–O stretching in nitro group, and C–C stretch, respectively. Anticancer activity against HeLa cells showed that Pt–PdNPs exhibited more pronounced cell death of 74.25% compared to individual PtNPs (12.6%) or PdNPs (33.15%). Further, Pt–PdNPs showed an enhanced scavenging activity against 2,2-diphenyl-1-picrylhydrazyl, superoxide, nitric oxide, and hydroxyl radicals.
Introduction
Nanoparticles of noble metals are gaining immense importance recently, owing to their noteworthy physicochemical properties unlike the bulk metal.Citation1 At the same time search for safe and environmentally benign route toward synthesis of nanoparticles useful to human health has got prime attention. Nanoparticles are synthesized using diverse approaches like chemical, physical, and biological methods.Citation2–Citation9 However, use of toxic chemicals for nanoparticles synthesis and stabilization lead to environmental hazards and limits their medicinal importance. Thus, biological synthesis of nanoparticles by plants, bacteria, and fungi are being investigated widely. However, there are only preliminary reports on biological synthesis of platinum nanoparticles (PtNPs) or palladium nanoparticles (PdNPs). PtNPs are used as catalysts for power generation in fuel cell. Further, they can reduce the toxic components of pollutants and play a key role in most of the important reactions for synthesis such as hydrogenation, oxidation, and hydration.Citation10 Most recently, synthesis of monodispersed PtNPs is considered to be one of the important aspects due to its shape and size dependent activity. Pt nanocubes and nano-octahedra are recently reported to exhibit efficient catalytic activity for methanol and ethanol electro-oxidation, and also in hydrogen adsorption, desorption, ethylene hydrogenation, benzene hydrogenation, and p-nitrophenol hydrogenation, as well as for electrocatalytic applications.Citation11–Citation14 Pt compound, cisplatin (cis-diaminedichloroplatinum) is a well known anticancer agent although it has high possibilities of treatment associated side effects.Citation15 Similarly, another such noble metal, Pd is also well known for its catalytic properties in carbon–carbon and carbon–heteroatom coupling reactions. It is also used for synthesizing complex organic molecules.Citation16 PdNPs are synthesized by various approaches like electrochemical deposition, sonochemical decomposition, and reduction of Pd salts by a variety of reducing agents that mostly involve toxic and hazardous chemicals.Citation17 There are only preliminary reports on PdNPs to induce disturbances in cell cycle arrest.Citation18 Till date the anticancer activity of bimetallic platinum–palladium nanoparticles (Pt–PdNPs) has never been tested.
In view of this background, there is a need to develop environmental friendly route to synthesize Pt–PdNPs with enhanced medicinal properties without involvement of toxic chemicals. Unfortunately, there are no reports on synthesis of bimetallic Pt–PdNPs by medicinal plants like Dioscorea bulbifera. Medicinal plants have always been preferred over other biological methods owing to their biocompatibility, rapid and efficient synthesis mechanism.Citation19–Citation23 D. bulbifera, has immense therapeutic significance in traditional complementary and alternative medicine for treatment of diabetes, sore throat, ulcers, tumor, cancer, and oxidative stress related degenerative disease.Citation24–Citation27 Similarly, it has been extensively used for synthesis of metal nanoparticles like gold, silver, and gold core silver shell (AucoreAgshellNPs) bimetallic nanoparticles.Citation28–Citation30
Hereby, as a part of our growing interest in nanobiotechnology, herein we report for the first time, the synthesis of Pt–PdNPs by a medicinal plant, D. bulbifera. The bioreduced nanoparticles were further investigated for anticancer and antioxidant activities.
Materials and methods
Plant material and preparation of extract
D. bulbifera tuber extract (DBTE) was prepared as per our earlier report.Citation30 In short, the tubers were collected from Western Ghats of Maharashtra, India, which were rinsed thoroughly under running tap water followed by chopping into thin slices and shade drying for 2 days at room temperature. After complete drying, the slices were pounded in an electric blender. Five gram of finely ground tuber powder was taken in a 300 mL Erlenmeyer flask with 100 mL of sterile distilled water and boiled for 5 minutes before final decantation and filtration through Whatman No 1 filter paper. The filtrate was collected and stored at 4°C for further use.
Synthesis of PtNPs, PdNPs, and Pt–PdNPs and UV–visble spectroscopy
PtCl62− ions were reduced by addition of 5 mL of DBTE to 95 mL of 10−3 M aqueous H2PtCl6·6H2O solution. Similarly, PdNPs were synthesized by addition of 5 mL of DBTE to 95 mL of 10−3 M aqueous PdCl2. Synthesis of PtNPs and PdNPs were achieved by carrying out the reaction at 100°C for 5 hours, which was monitored by UV–visble spectroscopy on a spectrophotometer (SpectraMax M5, Molecular Devices Corporation, Sunnyvale, CA, USA) operated at a resolution of 1 nm. In case of synthesis of Pt–PdNPs, 5 mL of DBTE was added to 95 mL of aqueous solution containing 10−3 M of both H2PtCl6·6H2O and PdCl2. The conditions for the synthesis were kept similar as that of the individual synthesis of PtNPs and PdNPs. In order to set up control experiments with variable elemental ratio in bimetallic particles, synthesis was tried by varying the Pt and Pd salt concentration in ratio 1:2, as well as 2:1. Chemical synthesis of PtNPs, PdNPs, and Pt–PdNPs was carried out as per an earlier report by Shafii et al,Citation31 with some modifications.Citation31 In short, 10−3 M PdCl2 were converted to H2PdCl4·nH2O by addition of 100 µL of concentrated HCl, then 10−3 M H2PtCl6·6H2O followed by addition of 1.11 g polyvinylpyrrolidone (PVP) to the mixture of methanol (130 mL) and distilled water (150 mL) acting as solvent. Mixing of the solution was carried out in 500 mL three-neck round bottomed flask with drop wise addition of 20 mL of 0.1 M NaOH in methanol under vigorous stirring. Bimetallic Pt–PdNPs were synthesized by conventional refluxing of the above mixture for 3 hours. Similar process was followed for individual synthesis of PtNPs and PdNPs. The resulting final colloidal nanoparticles solution with a dark brown color was stored in dark bottles at 4°C for further use. Control experiments with DBTE, chemically synthesized nanoparticles only as well as with DBTE and Pt–PdNPs with varied elemental ratio were run for all biological experiments.
HRTEM and EDS
Surface morphology and particle size of bioreduced PtNPs, PdNPs, and Pt–PdNPs were determined using transmission electron microscope (Tecnai 12 cryo TEM, FEI, Eindhoven, the Netherlands). The morphology and size of bioreduced nanoparticles were characterized by JEOL-JEM-2100 (JEOL, Akishima, Tokyo, Japan) high-resolution transmission electron microscope (HRTEM). Energy dispersive spectra recorded in the energy dispersive spectroscopy (EDS) equipped in JEOL JSM 6360A analytical scanning electron microscope at an energy range 0–20 keV confirmed the synthesis of PtNPs, PdNPs, and Pt–PdNPs using DBTE. The diffraction data for the dry powder were recorded on a Bruker X-ray diffractometer using a Cu Kα (1.54 Å) source. Phase formation was confirmed from characteristic peaks such as (111), (200), and (220).
FTIR spectroscopy
PtNPs, PdNPs, and Pt–PdNPs synthesized after 5 hours of reaction were centrifuged at 10,000 rpm for 15 minutes at room temperature, following which the pellet was redispersed in sterile distilled water to remove any plant materials. The process of centrifugation and redispersion in sterile distilled water was repeated thrice to ensure better separation of free entities from the nanoparticles. The purified pellet was then dried and subjected to Fourier transform infrared (FTIR; IRAffinity-1, Shimadzu Corp, Tokyo, Japan) spectroscopy measurement using the potassium bromide pellet technique in the diffused reflection mode at a resolution of 4 cm−1. Nanoparticle powders were mixed with potassium bromide and subjected to IR source 500–4,000 cm−1. Similar process was used for the FTIR study of DBTE before and after bioreduction.
Cell toxicity assay
Antiproliferative activities of PtNPs, PdNPs, and Pt–PdNPs were evaluated against HeLa cells employing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as per our earlier report.Citation19 In short, HeLa cell line (human epithelial cervical cancer) was obtained from National Centre for Cell Science, Pune, India and cultured in Dulbecco’s Modified Eagle’s Medium, supplemented with 10% fetal bovine serum, streptomycin (100 g mL−1) and penicillin (100 U mL−1) and were maintained in a humidified atmosphere at 37°C and 5% CO2. Cells (4×104)were seeded per well in a 96-well plate. The nanoparticles were added to the cells at a concentration of 10 µg mL−1 after 24 hours of seeding. After 48 hours, media was removed and the cells were washed with phosphate-buffered saline. MTT (0.5 mg mL−1) was added to the wells and incubated for 2 hours. The formazan crystals were dissolved using acidified isopropanol and the absorbance was measured at 570 nm on a microplate reader (SpectraMax M5, Molecular Devices Corporation). Control experiments were also run with DBTE, chemically synthesized nanoparticles only as well as with DBTE and Pt–PdNPs with varied elemental ratio. The statistical analysis was done by using one-way analysis of variance.
Flow cytometric analysis
Confirmation of apoptosis as most predominant mechanism behind the antiproliferative activity of the bioreduced nanoparticles was carried out by a flow cytometric analysis of cells treated with respective nanoparticles. Cells (5×105) were seeded in a T-25 flask and incubated for 24 hours followed by treatment with PtNPs, PdNPs, and Pt–PdNPs at a concentration of 10 µg mL−1. After 48 hours of incubation the cells were harvested and stained with Annexin-V (AV)-fluorescein isothiocyanate (FITC) and propidium iodide (PI) at a dilution of 1:20, for 10–15 minutes at 4°C. These were then acquired in BD FACSCalibur and analyzed using Cell Quest Pro software.
Confocal microscopy
HeLa cells were seeded on to glass cover slips at a density of 5×104 cells. After 24 hours of incubation the cells were treated as per the above protocol. Treated cells were stained with AV-FITC and PI, both at a dilution of 1:20 for 15 minutes at 4°C and were fixed in 4% paraformaldehyde followed by observation under LSM 780 confocal laser scanning microscope, Carl Zeiss (magnification ×40).
DPPH radical scavenging assay
Twenty microliters of each of PtNPs, PdNPs, and Pt–PdNPs were mixed with 80 µL of methanolic solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH; 100 µM) and incubated in darkness for 30 minutes in a 96-well plate.Citation32,Citation33 Absorbance was recorded at 517 nm in a 96-well plate reader (SpectraMax M5, Molecular Devices Corporation) and scavenging activity was found out by the following formula:
Superoxide anion scavenging assay
Nitroblue tetrazolium reduction in a non-enzymatic phenazine methosulfate-nicotinamide adenine dinucleotide (PMS-NADH) system through reaction of PMS, NADH, and oxygen was used to evaluate superoxide anion scavenging assay. Three hundred microliters of each of PtNPs, PdNPs, and Pt–PdNPs was added in 3 mL of Tris-HCl buffer (100 mM, pH 7.4) containing 750 µL of nitroblue tetrazolium (300 µM) solution and 750 µL of NADH (936 µM) solution. Seven hundred and fifty microliters of PMS (120 µM) was added to the mixture and incubated for 5 minutes at room temperature, followed by recording of absorbance at 560 nm. The following formula was used to calculate superoxide anion scavenging activity:
Nitric oxide scavenging assay
Five hundred microliters of each PtNPs, PdNPs, and Pt–PdNPs was added to a mixture of 2 mL of 10 mM sodium nitroprus-side in 500 µL phosphate buffer saline (pH 7.4) followed by incubation at 25°C for 2.5 hours. Next, 500 µL from above reaction mixture was added into 1 mL sulphanilic acid reagent (33% in 20% glacial acetic acid) and incubated at room temperature for 5 minutes. Eventually, 1 mL naphthylethylenediamine dihydrochloride (0.1% w/v) was added and incubated for 0.5 hour at room temperature. Absorbance was recorded at 540 nm and the scavenging activity was calculated using the following equation:
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity was estimated according to our earlier report.Citation24 In short, 100 µL of PtNPs, PdNPs, and Pt–PdNPs were added separately to 400 µL of phosphate buffer, followed by addition of 100 µL of ethylenediaminetetraacetic acid (1.04 mM), 100 µL of FeCl3 (1.0 mM), and 100 µL of 2-deoxyribose (60 mM). Initiation of reaction was achieved by addition of 100 µL of ascorbic acid (2 mM) and 100 µL of H2O2 (10 mM) into the mixture keeping in water bath at 37°C. One microliter of cold thiobarbituric acid (10 g L−1) was added after 1 hour followed by 1 mL of HCl (25 %) and boiled for 15 minutes at 100°C. Absorbance was recorded at 532 nm. The following formula was used to evaluate hydroxyl radical scavenging capacity:
Results
UV–visible spectroscopy
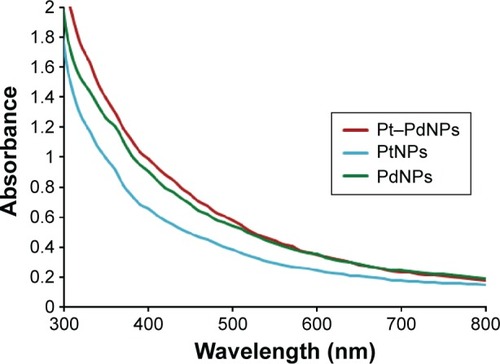
Bioreduction of Pt ions to PtNPs on exposure to DBTE was monitored by tracking the color change and with UV–visble spectroscopy. Facilitation of the bioconversion at an elevated temperature of 100°C was indicated by gradual color change of the light yellow Pt (IV) solution initially to brown representing Pt (II) and finally from brown to black marking the formation of Pt (0). Entire reaction was found to be completed in 5 hours. The UV–visible spectra indicates a lower rate of PtNPs synthesis, which might be probably due to difficulty in initially forming Pt nuclei, indicating the requirement of longer duration and higher temperature for complete synthesis of PtNPs. Similarly, addition of DBTE into Pd ion solution showed a gradual color change from transparent orange to dark brown over the time span of 5 hours. The observed emergence of brown color, attributed due to the excitations of surface plasmon vibrations, indicates the synthesis of PdNPs, which was further confirmed by UV–visible analysis (). The observed absence of peak at 420 nm specific to Pd2+ ions in reaction mixture indicated the complete reduction of the Pd2+ ions to PdNPs after 5 hours at 100°C. Bimetallic Pt–PdNPs showed an intense dark brown color on completion of the bioreduction after 5 hours. The absorbance was found to be higher as compared to both PtNPs and PdNPs alone. The as synthesized nanoparticles were used for all further characterization, while the obtained nanoparticles were collected by centrifugation and washed by repetition of alternate redispersion in distilled water and centrifugation for further biological studies.
HRTEM, EDS, and XRD analysis
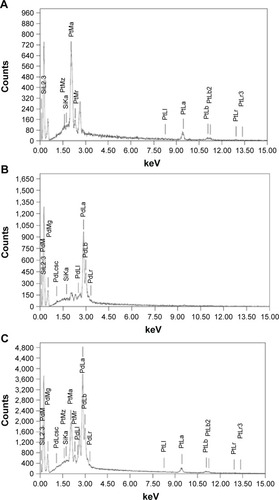
Morphologies of the nanoparticles synthesized during bioreduction were confirmed by employing HRTEM analysis. PtNPs exhibited uniformly distributed spherical nanostructures between 2 and 5 nm (). The particles were not seen to be agglomerated and were well stabilized in the matrix of biomaterial. In case of the PdNPs, a mixture of spherical and blunt ended cubes was observed ranging from 10 to 20 nm (). It is important to note that the PdNPs were also found to be discrete and well in isolation. Pt–PdNPs were found in irregular shape ranging between 20 and 25 nm (). TEM images of the chemically synthesized nanoparticles are given in . EDS analysis confirmed the presence of elemental Pt up to 99.65%±3.14% in PtNPs indicating the purity of the bioreduced nanoparticles. Similarly, elemental Pd content up to 99.18%±2.84% in PdNPs supported its purity and establishes the efficiency of the DBTE to form pure metallic nanoparticles. Further, EDS analysis clearly indicated that bimetallic nanoparticles were comprised of 30.88%±1.73% elemental Pt and 68.96%±1.48% elemental Pd (). EDS analysis data for chemically synthesized and other control nanoparticles are included in . shows X-ray diffraction (XRD) pattern of the PtNPs, PdNPs, and Pt–PdNPs synthesized by DBTE. The (111), (200), and (220) planes in PtNPs at the respective 2θ positions of 39.7°, 46.2°, and 67.4° confirm the face centered cubic phase as per the JCPDS card no 04-0802. Pt–PdNPs also showed the presence of (111), (200), and (220) peaks, with their respective diffraction positions shifted slightly toward higher 2θ values. The noise in the XRD data of the PdNPs suppressed their characteristic peaks, but the EDS data supports the presence of the PdNPs.
Figure 2 High-resolution transmission electron micrographs of nanoparticles synthesized by DBTE.
Notes: (A) PtNPs with inset showing color change on bioreduction of Pt salt to PtNPs; (B) PdNPs with inset showing color change on bioreduction of Pd salt to PdNPs; (C) Pt–PdNPs with inset showing color change on bioreduction of Pt and Pd salt solution to Pt–PdNPs.
Abbreviations: DBTE, Dioscorea bulbifera tuber extract; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles.

Figure 3 Representative spot EDS profile confirming the presence of respective metals in the nanoparticles synthesized by DBTE.
Notes: (A) PtNPs showing presence of elemental platinum; (B) PdNPs showing presence of elemental palladium; (C) Pt–PdNPs showing presence of elemental both platinum and palladium.
Abbreviations: DBTE, Dioscorea bulbifera tuber extract; EDS, energy dispersive spectroscopy; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles.
FTIR analysis
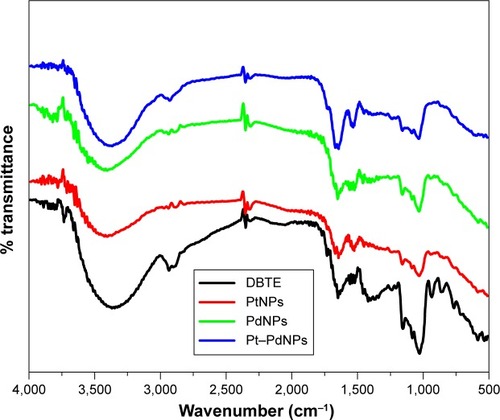
FTIR spectrum of DBTE showed strong peaks at 3,373 cm−1, which is attributed to hydroxyl group in polyphenolic compounds (). Additionally, the other peaks in the spectrum of DBTE corresponding to the characteristic groups were found to be in agreement to our previous report.Citation29 Sharp peaks at 2,937, 1,647, 1,518, and 1,024 cm−1, associated with C–H stretching, N–H bending in primary amines, N–O stretching in nitro group, and C–C stretch, respectively, were also observed in the DBTE. The presences of almost all the characteristic peaks of DBTE in the bioreduced nanoparticles indicated the stabilization of nanoparticles by DBTE may be due to coating. As seen in the other spectra, the peak intensity is reduced in the nanoparticles indicating relatively lesser concentration of DBTE taking part in the stabilization process. The peak at 1,415 cm−1, attributed to carboxylate group was found to diminish in the nanoparticles, indicating the possible conjugation of the nanoparticles with the extract through carboxylate group. Overall, it is supported herewith that DBTE plays a key role in reducing the metal ions to their corresponding metallic nanoparticles and further, through conjugation, making them stable in the medium.
Figure 4 Fourier transform infrared absorption spectra of dried Dioscorea bulbifera tuber extract (DBTE) before bioreduction and after complete bioreduction of PtNPs, PdNPs, and Pt–PdNPs.
Abbreviations: PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles.
Cell viability
MTT assay demonstrated cytotoxic effect of PtNPs (12.6%), PdNPs (33.15%), and Pt–PdNPs (74.25%), with Pt–PdNPs to be the most toxic out of all the three nanoparticles, at a concentration of 10 µg mL−1 (). An enhanced cytotoxic effect of Pt–PdNPs could be attributed to a synergistic effect of both the components. Chemically synthesized nanoparticles were found to be comparatively less potent, the data for which along with other control nanoparticles are included in .
Figure 5 Antiproliferative activity of PtNPs, PdNPs, and Pt–PdNPs against HeLa cells. The data are expressed as a percentage of the control MTT reduction (always taken as 100%) and represent the average ± SEM (n=3); *indicates P=0.05.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum palladium bimetallic nanoparticles; SEM, standard error of the mean.

Flow cytometric analysis
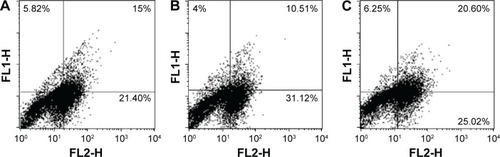
Immunofluorescence analysis of the cells treated with nanoparticles indicated apoptosis of treated cells. Percentage of dual positive cells (AV-FITC+PI+) was observed to be higher in the groups treated with Pt–PdNPs (20.60%) represented in the upper right quadrant of as compared to treatment groups of PtNPs and PdNPs alone as evident in similar quadrants of , respectively. The dual positivity indicated the cells in late apoptosis. Moreover, the total number of cells undergoing cell death was much higher in Pt–PdNPs as compared to PtNPs or PdNPs alone, indicating a higher anticancer activity of Pt–PdNPs (). The total number of cells in all of the three quadrants (indicating cells in early as well as late apoptosis) was much higher in representing treatment with Pt–PdNPs as compared to individual PtNPs or PdNPs.
Figure 6 Flow cytometric analysis of phosphatidylserine externalization (annexin V binding) and cell membrane integrity (PI staining) for HeLa cells treated with PtNPs, PdNPs, and Pt–PdNPs for 48 hours.
Notes: The dual parametric dot plots combining annexin V-FITC and PI fluorescence show the viable cell population (lower left quadrant, annexin V− PI−), the early apoptotic cells (lower right quadrant, annexin V+PI−), and the late apoptotic cells (upper right quadrant, annexin V+PI+). (A) Treatment with PtNPs; (B) treatment with PdNPs; (C) treatment with Pt–PdNPs.
Abbreviations: PdNPs, palladium nanoparticles; PI, propidium iodide; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles; FITC, fluorescein isothiocyanate.
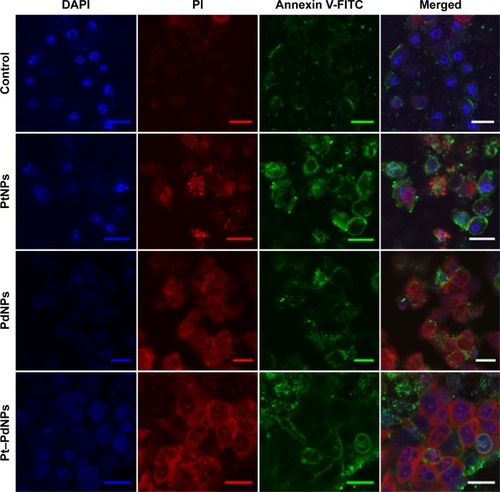
Immunofluorescence imaging of the cells treated with nanoparticles supported the quantitative analysis done by flow cytometry. It was observed that the number of apoptotic cells after PtNPs treatment () was less, whereas the cells treated with PdNPs were necrotic. Treatment with Pt–PdNPs caused cell death due to apoptosis, shown by large number of dual positive cells.
Figure 7 Apoptosis: HeLa cells seeded on coverslips were treated with 10 µg mL−1 of PtNPs, PdNPs, and Pt-PdNPs for 48 hours, and then stained for Annexin V-FITC and PI.
Note: Inset represents scale bar equivalent to 20 µm.
Abbreviations: PdNPs, palladium nanoparticles; PI, propidium iodide; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles; DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate.
Antioxidant activity
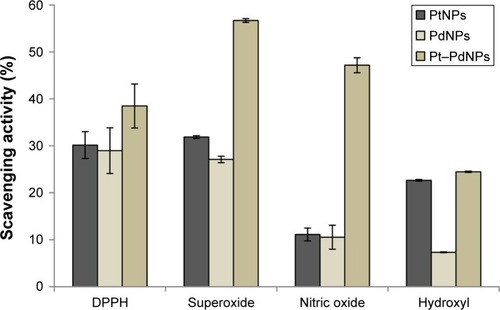
Antioxidant studies showed that PtNPs could scavenge DPPH radical up to 30.16%±2.86%, while PdNPs exhibited an activity up to 28.97%±4.87% (). However, Pt–PdNPs showed a superior activity of 38.49%±4.67% as compared to individual PtNPs and PdNPs. Similarly, a synergistic enhancement of scavenging activity against superoxide radical was also observed for Pt–PdNPs (56.71%±0.4%) as compared with only PtNPs (31.87%±0.29%) or PdNPs (27.1%±0.69%). In case of nitric oxide scavenging activity Pt–PdNPs showed a significantly superior activity of 47.17%±1.59%, while PtNPs and PdNPs individually showed a reduced activity of 11.11%±1.35% and 10.53%±2.54%, respectively. A similar trend was observed in case of hydroxyl radical scavenging where activity of Pt–PdNPs was found to be 24.48%±0.13%, which was higher compared to either of PtNPs (22.65%±0.17%) or of PdNPs (7.32%±0.08%). Chemically synthesized nanoparticles showed comparatively reduced free radical scavenging activity, the data for which along with other control nanoparticles are included in .
Discussion
Complex and unique phytochemistry of D. bulbifera is comprised of a wide family of bioactive natural components with potent pharmacological significance. These phytochemicals include saponins, reducing sugars, ascorbic acid, citric acid, phenolics, and flavonoids, which play a critical role in rapid synthesis and shape evolution of exotic metal nanoparticles. Hence, herein we achieved a rapid and efficient synthesis of PtNPs, PdNPs, and Pt–PdNPs within 5 hours at 100°C. Conversion of yellow color of the solution to black marked the completion of the synthesis of PtNPs. Our results are in close agreement with the recent report on synthesis of PtNPs by Ocimum sanctum leaf extract, where it was found that the rate of synthesis of PtNPs increased from 20% at 60°C to 100% at 95°C.Citation34 Rate of synthesis of PtNPs was observed to be slower as compared to the gold nanoparticles by D. bulbifera as reported earlier. This relatively low rate of bioreduction could be attributed due to the probable difficulty in initial formation of Pt nuclei. This could be reflected in the fact that 100% conversion of PtNPs by DBTE required longer time and higher temperature than gold nanoparticles.Citation35 PtNPs synthesized by DBTE were found to be very small (<5 nm) uniform and monodispersed unlike the bigger mixed aggregation (2–20 nm) comprised of mixture of spheres and plates as obtained during synthesis by Diospyros kaki leaf extract.Citation35
A similar high temperature and time as that of PtNPs was required for complete synthesis of PdNPs, as well. However, synthesis of PdNPs by DBTE was faster as compared to the recent report on synthesis of PdNPs by Gardenia jasminoides Ellis, which took 24 hours for complete reduction at 60°C.Citation36 Blunt ended Pd nanocubes or nanospheres in a range between 10 and 20 nm were found to be uniformly dispersed without showing any agglomeration. Similarly, results in case of G. jasminoides Ellis was concluded to be attributed due to natural stabilizers present in the crude aqueous extract and elevated temperature. Thus, the phytochemical mixtures of reducing and stabilizing agents in DBTE might be playing a predominant role in the particle assembly. Under excitation at higher temperature, availability of abundant Pd crystal nuclei facilitated the formation of tiny spherical particles, which further grew rapidly by consumption of Pd ions without any external addition of either reducing or stabilizing agents.Citation36 However, to the best of our knowledge there are no reports of bimetallic Pt–PdNPs from medicinal plant D. bulbifera. Thus, the reported synthesis ensured a unique irregular flower like architecture with synergistic enhancement of bioactivity. Chemically synthesized similar heterogeneous bimetallic nanostructures, reported recently, were expected to integrate diverse functionalities in a single crystal structure.Citation37
Bioreduced PtNPs showed anticancer activity against HeLa cells. Pt complexes are well known anticancer agents and drugs like cisplatin, carboplatin, and oxaliplatin, in addition to nedaplatin, lobaplatin, and heptaplatin.Citation38 In case of individual PtNPs, the activity was found to be low. However, the anticancer efficacy was considerably enhanced synergistically in Pt–PdNPs. It is well documented that Pt complexes shows a pronounced variability in triggering the death in cancer cells. The underlying mechanism of action plays a key role in this selectivity, which is reflected in case of the drugs like cisplatin that kills certain cancer cells but not others. Similarly, other Pt complexes fail to exhibit same levels of cytotoxicity to the cancer cells that are highly sensitive to cisplatin. Such variability was best explained by two possible reasons: 1) the physicochemical properties of nanoparticles that derive from their structure and 2) the array of metabolic events like gene expression, protein synthesis, post-translational modification, and synthesis of small molecules, in the cell in response to the exposure to PtNPs, PdNPs, and Pt–PdNPs.Citation38 Enhancement of activity in Pt–PdNPs as compared to individual PtNPs reflects that combination with transition metal like Pd can synergistically increase anticancer activity. Our results are in close agreement to earlier reports, where it is documented that although drugs like satraplatin, miriplatin, prolindac, aroplatin, and picoplatin are in developmental stages, the side effects ranging from nephrotoxicity to drug resistance of the tumor cells have posed real challenges to researchers, which have prompted to consider the combination of transition metals with biologically active molecules as a successful strategy toward designing promising anticancer agents.Citation39 Flow cytometric analysis confirming maximum dual positive cells (AV-FITC+PI+) in Pd–PtNPs treated HeLa cells indicated the efficient and synergistic induction of apoptosis by both Pt and Pd components in Pt–PdNPs. FITC-AV binding is highly specific to early stages of apoptosis, which is marked by transposition of phosphatidylserine from the internal to the outer surface of cell membrane.Citation19,Citation40 Pt–PdNPs exhibited superior triggered translocation of phosphatidylserine as compared to individual PtNPs and PdNPs indicating extensive damage to cell membrane leading to apoptotic effects rather than necrosis. Antioxidants are considered to be one of the most promising cancer chemopreventive agent that can reverse, suppress, or prevent carcinogenic progression. Reactive oxygen species production is found to be extensive in tumor cells, which upregulates certain growth factors and matrix metalloproteinases, promoting angiogenesis, metastasis, and secondary tumor developments.Citation41 In our study we found that the nanoparticles were not only cytotoxic against the cancer cells, but also possess antioxidant properties. It was evident that the free radical scavenging potential increased in the bimetallic nanoparticles (Pt–PdNPs) as compared to individual NPs. Our results are strongly in agreement with a recent report, which has demonstrated that a mixture of Pd and Pt nanoparticles, used in Japan since last 60 years or more for treating chronic diseases, possess remarkable superoxide dismutase and catalase activity in in vivo mice model and thereby, considered as a powerful tool to control age related skin disease caused by oxidative damage.Citation42 Another such study provides a strong scientific rationale to the fact that PtNPs are superoxide dismutase/catalase mimetic, thus have significant antiaging properties by increasing the lifespan in Caenorhabditis elegans.Citation43 In view of the background, consideration of Pt as a component can serve as a pivotal strategy to design and explore combinatorial effect of bimetallic nanomaterials.
Conclusion
This is the first report on rapid and efficient synthesis of novel PtNPs, PdNPs, and Pt–PdNPs using traditional medicinal plant D. bulbifera within 5 hours. The particles were found to be almost monodispersed in the size range of 2–25 nm and stable. A synergistic activity enhancement was evident in the bimetallic Pt–PdNPs as compared to individual PtNPs or PdNPs. The particles exhibited potent anticancer and antioxidant activity. Hereby, it is of utmost scientific rationale to design and explore the combined effects of Pt–PdNPs as novel biomedicine. It can serve dual purpose of preventing cancer progression by its antioxidant properties as well as induction of apoptosis and cell death by its cytotoxic potential against cancer cells.
Acknowledgments
The authors acknowledge the help extended for the use of TEM and HRTEM facilities in Chemical Engineering and CRNTS funded by the DST through Nanomission and IRPHA schemes and Board of College and University Development (BCUD), Savitribai Phule Pune University for research grant (11SCI001810). S Ghosh thanks Council of Scientific and Industrial Research (CSIR, Government of India) for Senior Research Fellowship (09/137(0516)/2012-EMR-I). Dr Geetanjali Tomar thanks DST INSPIRE for the faculty position and the research grant (IFA13 LSBM73 and GOI-E-161(2), respectively).
Supplementary materials
Figure S1 Characterization by TEM.
Notes: (A) Chemically synthesized PtNPs; (B) chemically synthesized PdNPs; (C) chemically synthesized Pt–PdNPs; (D) DBTE synthesized Pt–PdNPs using 1:2 molar ratio of platinum and palladium salt; (E) DBTE synthesized Pt–PdNPs using 2:1 molar ratio of platinum and palladium salt.
Abbreviations: DBTE, Dioscorea bulbifera tuber extract; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles; TEM, transmission electron microscope.
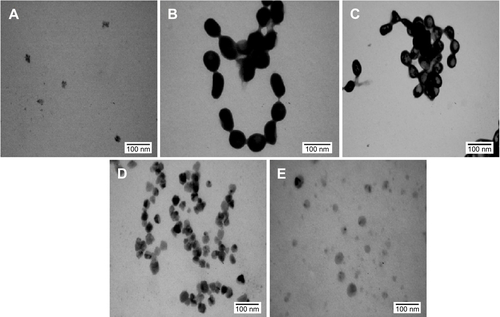
Figure S2 Energy dispersive spectroscopy analysis.
Notes: (A) Representative spot EDS profile of chemically synthesized PtNPs showing 87.32% elemental platinum. (B) Representative spot EDS profile of chemically synthesized PdNPs showing 7.25% elemental palladium. (C) Representative spot EDS profile of chemically synthesized Pt–PdNPs showing 31.59% elemental platinum and 21.49% elemental palladium. (D) Representative spot EDS profile of DBTE synthesized Pt–PdNPs using 1:2 molar ratio of platinum and palladium salt, respectively showing 36.29% elemental platinum and 41.20% elemental palladium. (E) Representative spot EDS profile of DBTE synthesized Pt–PdNPs using 2:1 molar ratio of platinum and palladium salt, respectively showing 14.5% elemental platinum and 4.16% elemental palladium.
Abbreviations: DBTE, Dioscorea bulbifera tuber extract; EDS, energy dispersive spectroscopy; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles.
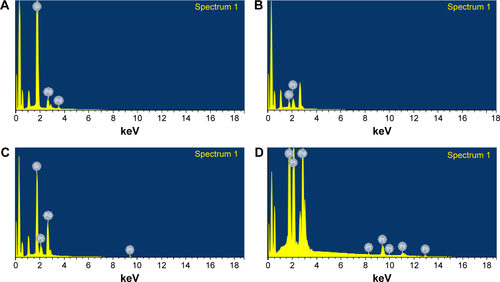
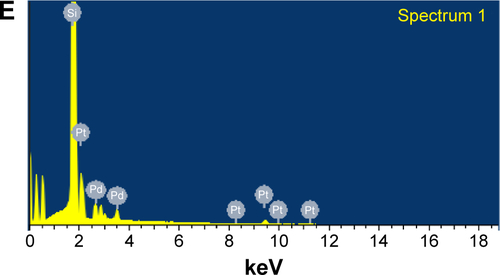
Figure S3 Representative X-ray diffraction profile of thin film PtNPs, PdNPs, and Pt–PdNPs synthesized by DBTE.
Abbreviations: au, arbitrary unit; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles; DBTE, Dioscorea bulbifera tuber extract.
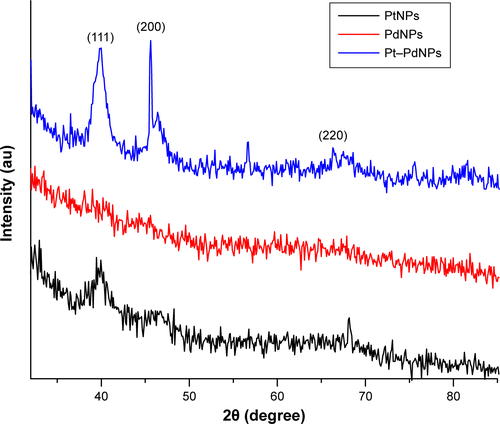
Figure S4 Antiproliferative activity against HeLa cells.
Notes: Comparative activities of chemically synthesized PtNPs (NPs1), chemically synthesized PdNPs (NPs2), chemically synthesized Pt–PdNPs (NPs3), DBTE + chemically synthesized PtNPs (NPs4), DBTE + chemically synthesized PdNPs (NPs5), DBTE + chemically synthesized Pt–PdNPs (NPs6), DBTE synthesized Pt–PdNPs using 1:2 molar ratio of platinum and palladium salt (NPs7), DBTE synthesized Pt–PdNPs using 2:1 molar ratio of platinum and palladium salt (NPs8), physical mixture of PtNPs and PdNPs (NPs9), and only DBTE.
Abbreviations: DBTE, Dioscorea bulbifera tuber extract; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles.
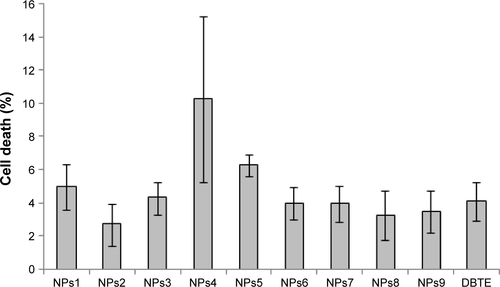
Figure S5 Comparative antioxidant activities of chemically synthesized PtNPs (NPs1), chemically synthesized PdNPs (NPs2), chemically synthesized Pt–PdNPs (NPs3), DBTE + chemically synthesized PtNPs (NPs4), DBTE + chemically synthesized PdNPs (NPs5), DBTE + chemically synthesized Pt–PdNPs (NPs6), DBTE synthesized Pt–PdNPs using 1:2 molar ratio of platinum and palladium salt (NPs7), DBTE synthesized Pt–PdNPs using 2:1 molar ratio of platinum and palladium salt (NPs8), physical mixture of PtNPs and PdNPs (NPs9), and only DBTE.
Abbreviations: DBTE, Dioscorea bulbifera tuber extract; PdNPs, palladium nanoparticles; PtNPs, platinum nanoparticles; Pt–PdNPs, platinum–palladium bimetallic nanoparticles; DPPH, 2,2-diphenyl-1-picrylhydrazyl.
Disclosure
The authors report no conflicts of interest in this work.
References
- SohnEKJohariSAKimTGAquatic toxicity comparison of silver nanoparticles and silver nanowiresBioMed Res Int2015201589304926125025
- SalunkeGRGhoshSSantosh KumarRJRapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm controlInt J Nanomed2014926352653
- GaidhaniSVYeshvekarRKShedbalkarUUBellareJHChopadeBABio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticusProcess Biochem2014491223132319
- ShedbalkarUSinghRWadhwaniSGaidhaniSChopadeBAMicrobial synthesis of gold nanoparticles: current status and future prospectsAdv Colloid Interface Sci2014209404824456802
- SinghRNawaleLUArkileMChemical and biological metal nanoparticles as antimycobacterial agents: a comparative studyInt J Antimicrob Agents201546218318826009020
- WadhwaniSAShedbalkarUUSinghRKarveMSChopadeBANovel polyhedral gold nanoparticles: green synthesis, optimization and characterization by environmental isolate of Acinetobacter sp. SW30World J Microb Biotechnol2014301027232731
- SantDGGujarathiTRHarneSRAdiantum philippense L. frond assisted rapid green synthesis of gold and silver nanoparticlesJ Nanopart2013201319
- GaidhaniSSinghRSinghDBiofilm disruption activity of silver nanoparticles synthesized by Acinetobacter calcoaceticus PUCM 1005Mater Lett2013108324327
- SinghRWaghPWadhwaniSSynthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibioticsInt J Nanomed2013842774290
- LongNVChienNDHayakawaTHirataHLakshminarayanaGNogamiMThe synthesis and characterization of platinum nanoparticles: a method of controlling the size and morphologyNanotechnology201021303560519966396
- SusutCNguyenTDChapmanGBTongYShape and size stability of Pt nanoparticles for MeOH electro-oxidationElectrochim Acta2008532161356142
- HanSBSongYJLeeJMKimJYParkKWPlatinum nanocube catalysts for methanol and ethanol electrooxidationElectrochem Commun200810710441047
- KimCLeeHShape effect of Pt nanocrystals on electrocatalytic hydrogenationCatal Commun2009111710
- ChenJLimBLeeEPXiaYShape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applicationsNano Today2009418195
- CutillasNYellolGSDe HaroCVicenteCRodríguezVRuizJAnticancer cyclometalated complexes of platinum group metals and goldCoord Chem Rev201325719–2027842797
- SawooSSrimaniDDuttaPLahiriRSarkarASize controlled synthesis of Pd nanoparticles in water and their catalytic application in C-C coupling reactionsTetrahedron2009652243674374
- HoPFChiKMSize-controlled synthesis of Pd nanoparticles from β-diketonato complexes of palladiumNanotechnology200415810591064
- PetrarcaCClementeEGiampaoloLDPalladium nanoparticles induce disturbances in cell cycle entry and progression of peripheral blood mononuclear cells: paramount role of ionsJ Immunol Res2014 Article ID 295092, 8 pages
- GhoshSMorePDerleADiosgenin functionalized iron oxide nanoparticles as novel nanomaterial against breast cancerJ Nanosci Nanotechnol2015159464947226682367
- GhoshSPatilSAhireMGnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potentialJ Nanobiotechnol20121017
- KittureRGhoshSMorePACurcumin-loaded, self-assembled Aloe vera template for superior antioxidant activity and trans-membrane drug releaseJ Nanosci Nanotechnol20151564039404526369010
- KittureRChordiyaKGawareSZnO nanoparticles-red sandalwood conjugate: a promising anti-diabetic agentJ Nanosci Nanotechnol20151564046405126369011
- KittureRGhoshSKulkarniPFe3O4-citrate-curcumin: promising conjugates for superoxide scavenging, tumor suppression and cancer hyperthermiaJ Appl Phys2012111064702064707
- GhoshSDerleAAhireMPhytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbiferaPLoS One2013812e8252924367520
- GhoshSMorePDerleADiosgenin from Dioscorea bulbifera: novel hit for treatment of Type II diabetes mellitus with inhibitory activity against α-amylase and α-glucosidasePLoS One201499e10603925216353
- GhoshSAhireMPatilSAntidiabetic activity of Gnidia glauca and Dioscorea bulbifera: potent amylase and glucosidase inhibitorsEvid Based Complement Alternat Med2012201292905121785651
- GhoshSPariharVSMorePDhavaleDDChopadeBAPhytochemistry and therapeutic potential of medicinal plant: Dioscorea bulbiferaMed Chem201554154159
- GhoshSJagtapSMorePDioscorea bulbifera mediated synthesis of novel AucoreAgshell nanoparticles with potent antibiofilm and antileishmanial activityJ Nanomater20152015562938
- GhoshSPatilSAhireMSynthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agentsInt J Nanomed20127483496
- GhoshSPatilSAhireMSynthesis of gold nanoanisotrops using Dioscorea bulbifera tuber extractJ Nanomater20112011354793
- ShafiiSLihuaWNordinMRYongLKSynthesis of palladium-platinum bimetallic nanoparticles and their catalytic activity towards the hydrogenation reaction of palm oleinJ Chem Eng Process Technol201231
- AdershAGhoshSMorePChopadeBAGandhiMNKulkarniARSurface defect rich ZnO quantum dots as antioxidant inhibiting α-amylase and α-glucosidase: a potential anti-diabetic nanomedicineJ Mater Chem B201532245974606
- PatilABGhoshSPhadatareSDEvaluation of malonic acid diamide analogues as radical scavenging agentsN J Chem20153912671273
- SoundarrajanCSankariADhandapaniPRapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applicationsBioprocess Biosyst Eng201235582783322167464
- SongJYKwonEYKimBSBiological synthesis of platinum nanoparticles using Diopyros kaki leaf extractBioprocess Biosyst Eng201033115916419701776
- JiaLZhangQLiQSongHThe biosynthesis of palladium nano-particles by antioxidants in Gardenia jasminoides Ellis: long lifetime nanocatalysts for p-nitrotoluene hydrogenationNanotechnology2009203838560119713585
- PengZYangHSynthesis and oxygen reduction electrocatalytic property of Pt-on-Pd bimetallic heteronanostructuresJ Am Chem Soc2009131227542754319438286
- WexselblattEYavinEGibsonDCellular interactions of platinum drugsInorganica Chim Acta20123937583
- KapdiRFairlambIJSAnti-cancer palladium complexes: a focus on PdX2L2, palladacycles and related complexesChem Soc Rev201443134751477724723061
- MallickAMorePGhoshSDual drug conjugated nanoparticle for simultaneous targeting of mitochondria and nucleus in cancer cellsACS Appl Mater Interfaces20157147584759825811662
- RajkumarVGuhaGKumarRAAntioxidant and anti-cancer potentials of Rheum emodi rhizome extractsEvid Based Complement Alternat Med2011201169798621792364
- ShibuyaSOzawaYWatanabeKPalladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity in micePLoS One2014910e10928825333617
- KimJTakahashiMShimizuTEffects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegansMech Ageing Dev2008129632233118400258