Abstract
Prostate cancer is one of the leading causes of cancer-related deaths among the Caucasian adult males in Europe and the USA. Currently available diagnostic strategies for patients with prostate cancer are invasive and unpleasant and have poor accuracy. Many patients have been overly or underly treated resulting in a controversy regarding the reliability of current conventional diagnostic approaches. This review discusses the state-of-the-art research in the development of novel noninvasive prostate cancer diagnostics using nanotechnology coupled with suggested diagnostic strategies for their clinical implication.
Introduction
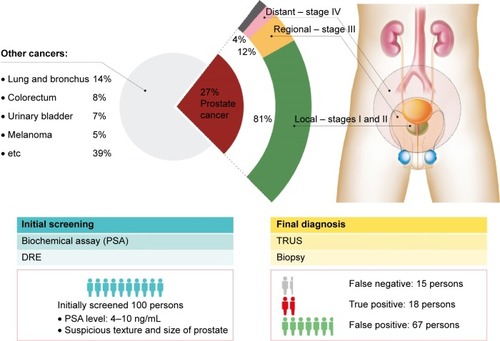
As the name implies, prostate cancer originates from a gland in the male reproductive system found near the bladder. It is one of the leading causes of cancer-related deaths among Caucasian males in the USA, and it is the most commonly diagnosed form of cancer in both Europe and the USA.Citation1,Citation2 A statistical report predicts new cases and deaths in the USA to be 220,800 and 27,540, respectively, for 2015. As shown in , the disease can advance to a more aggressive malignant form, which can be stratified into four discrete stages: I, II, III, and IV. Upon biopsy examination, the stages are determined and stratified according to Gleason’s score method.Citation3,Citation4 Stages I and II are localized in the prostate gland, whereas stages III and IV exhibit regional spread to the nearby bladder and distant spread to other organs, such as liver and bone, which are far away from the prostate gland.Citation5–Citation11 The prevalence of local, regional, and distant forms of the cancer is known to be 81%, 12%, and 4%, respectively. No apparent symptoms appear in stage I, but they start to show and become apparent as the disease progresses. Patients diagnosed with prostate cancer at stage I, II, or III have a high 5-year survival rate, but patients with stage IV cancer have a low 5-year survival rate of <27%, highlighting the importance of early detection.
Figure 1 Prostate cancer diagnosis statistics.
Notes: Prostate cancer accounts for 27% of all male cancer cases meaning every one out of four men is diagnosed with prostate cancer in the USA. The localized, regional, and metastasized tumor tissue accounts for 81%, 12%, and 4%, respectively, of all prostate cancer diagnosis with the unknown stage remaining. Typical subjects with prognoses to have prostate cancer are 85 persons per 100 persons after the initial screening, while the other 15 persons are not predicted to have prostate cancer. Only 18 persons of the 85 persons with positive initial screening result are diagnosed with prostate cancer. Surprisingly, the other 15 persons, who are believed to have no prostate cancer based on the initial screening result, are turned out to be diagnosed with prostate cancer. Data from Siegel et al.Citation1
Abbreviations: DRE, digital rectal examination; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography.
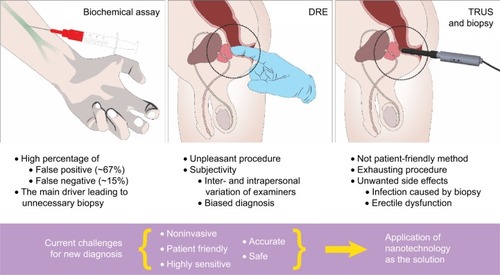
Currently available clinical diagnostic methods for prostate cancer include biochemical assay,Citation12–Citation20 biopsy,Citation21–Citation27 digital rectal examination (DRE),Citation28–Citation32 and transrectal ultrasonographyCitation33–Citation49 as described in . Among these methods, the biochemical assay is used for initial screening. The biochemical assay measures serum – a term meaning the processed medium from whole blood – prostate-specific antigen (PSA) level where a concentration >4 ng/mL is considered to indicate a risk of prostate cancer.Citation12–Citation20 Since approval by the US Food and Drug Administration (FDA) 25 years ago, it has been used as the gold standard for the initial screening of the disease.
Figure 2 Current conventional approach of prostate cancer diagnosis.
Notes: Current conventional diagnostic methodologies for the patients with prostate cancer hold several drawbacks. Patients with PSA levels 4–10 ng/mL are subjected to further examinations. The assay gives a significant number of false positives, which raises a question of its reliability. Concurrently, DRE is performed in an initial screening for prostate cancer but introduces subjectivity to the examination. The last method is TRUS, which takes a visual image of the tumor tissue in the gland, and, in most cases, biopsies are performed with a risk of potential infections from the rectum. Due to the drawbacks of current diagnosis, a novel, noninvasive, effective, initial screening prior to diagnosis is in high demand with excellent sensitivity and accuracy.
Abbreviations: DRE, digital rectal examination; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography.
The patients with >4 ng/mL PSA level undergo further DRE. DRE is performed in order to inspect the prostate gland condition by examining its texture and size. Combined with a PSA screening result, the decision is made whether to do a biopsy for further examination. However, DRE is not useful for the early detection of prostate tumor because of a possibility that a tumor could originate from the ventral or other untouchable sides of the gland.Citation28–Citation32 In addition to the poor sensitivity of the examination, it is an unpleasant procedure for a patient to undergo.
After DRE patients undergo transrectal ultrasonography. It can offer a visualization of the gland for examination and can be used to guide immediate, subsequent biopsies.Citation33–Citation49 Upon detecting a suspicious portion of the gland, specimens are collected. Typically, 12 specimens are collected and evaluated according to the Gleason scoring system, and the most reliable, accurate diagnosis is finally made.Citation21–Citation27,Citation50–Citation56 Patients may undergo radical prostatectomyCitation57 or hormone therapy depending on the extent of malignancy.Citation58–Citation65 The major drawback of a biopsy is the possibility of a potential infection caused by microbes that have migrated from the rectum which can cause inflammation in the diseased gland.
In addition to the traditional diagnostic methods, a bone scan is also carried out to scan the whole body for the presence of metastatic prostate cancer. ProstaScint- scan, positron emission tomography (PET) scanning, and computer-aided tomography (CT) with PET have all been used for prostate cancer detection. Prostate-specific membrane antigen (PSMA) has been selected as a target in the detection of prostate cancers with these techniques.Citation66–Citation72
A notable molecular imaging technology is 68Ga-PSMA PET/CT, which targets PSMA as the biomarker. The FDA has recently approved a clinical trial of this technology. It is the first time in the history of the development of prostate cancer diagnosis, prognosis, or monitoring, where a diagnostic tool is used to target PSMA. This technique uses an agent that is a monoclonal antibody to detect the intracellular domain of PSMA. It is possible that this agent may only capture the dead cells of the prostate cancer cells.
Despite the fact that PSA has been the gold standard for the initial screening, whether PSA screening has provided a major contribution in early prostate cancer detection remains uncertain.Citation73,Citation74 Its poor sensitivity and specificity has led to false-negative (15%) and false-positive (66%) diagnoses, which leads to a loss of confidence in the biomarker as an effective tool for initial screening.Citation75–Citation79 As a consequence, almost two-thirds of prostatic biopsies made annually in the USA and Europe are unnecessary.Citation1,Citation2
To circumvent the aforementioned procedures, an accurate biochemical assay is desperately needed. However, the accuracy and preciseness of the prostate cancer initial screening is currently being challenged. Inaccurate diagnoses lead to overdiagnosis or undertreatment, which exacerbates the physiological state of the tissue, makes treatment more difficult, and remains a persistent clinical problem.Citation78–Citation82 The inaccuracy of conventional, initial screening is largely due to the drawbacks of the biochemical assay and DRE described in . Improvement of the diagnosis accuracy will contribute to bringing the best clinical decision to match a patient to an appropriate therapy. Thus, it is inevitable to develop a novel diagnostic strategy that will eliminate the drawbacks of the methods and accurately diagnose prostate cancer. An accurate diagnosis even at the biochemical assay level will not only minimize the complications but also maximize the efficacy of subsequent therapies for the diagnosed patients. Moreover, an earlier diagnosis also demands a more accurate probe that requires highly sensitive and specific sensors to detect highly reliable biomarkers. Thus, just like for any other diseases, an accurate diagnosis is very important for delivering the appropriate treatment to the right patient. In this regard, nanotechnology with a new biomarker for prostate cancer has been chosen as a promising tool for future noninvasive diagnosis of prostate cancer.
Nanotechnology has been employed in the development of various biomedical applications such as drug development.Citation83–Citation104 On the other hand, nanotechnology is also being employed for diagnostic development.Citation101,Citation103 The rationale for the employment of diagnostics development is to achieve a higher accuracy of diagnosis. Improvements in the sensitivity and specificity of nanotechnology are warranted and promising.Citation105,Citation106 Recent studies have demonstrated such improvements and suggested it as a promising tool for next-generation diagnostics, because such improvements could contribute to making a more accurate diagnosis.Citation86,Citation107–Citation115
In addition to employing nanotechnology, an accurate initial screening also requires a highly reliable prostate cancer biomarker. Since biomarkers reflect the physiological state of tissue from which they are secreted, it is best to use one that is both tissue and cancer specific. PSA itself is only tissue specific rather than cancer specific which has resulted in controversy regarding its reliability for initial screening of prostate cancer. In order to achieve a more reliable biochemical assay other biomarkers that are specific to prostate cancer beyond PSA are highly favored. Detecting biomarkers that are specific to prostate cancer from a body fluid would make novel diagnosis less invasive and more accurate.
Herein, this review introduces the current state of nanotechnology applied toward the development of diagnostics for prostate cancer. From the standpoint of diagnostic development for prostate cancer, the relevant research trend is discussed along with clinical implications of nanotechnology-based prostate cancer detection in initial screening.
From bodily fluids to nanotechnology-based bioassays
Bodily fluids for noninvasive diagnosis
Semen has become a promising proximal fluid for prostate cancer diagnosis by detecting related biomarkers and monitoring the disease’s pathological process.Citation116 Due to the fact that seminal fluids are directly transported from prostate glands, they contain more biomarkers than other fluids such as serum and urine.Citation116
Serum is plasma after the removal of blood cells. Since all cells require oxygen that is delivered through the circulatory system, serum contains biomarkers of diseased tissues from any location of the body. Thus, serum is an outstanding source for diagnosis. However, the environment of serum is likely to hinder detection of biomarkers due in part to proteolytic degradation activity and a high concentration of albumin, which takes up to 50% of the weight of the whole serum proteome.Citation117–Citation121 Low-level protein biomarkers are subjected to degradation by proteases and peptidases, which divide into different classes. Typical protease classes are aspartic, cysteine-, serine-, and metallopeptidases. Peptidases belong to one of the three classes, including endo-, exo-, and carboxypeptidases. High-abundant proteins can create a masking effect by noncovalently attaching to the low-level protein biomarkers.
Urine is rich in proteome, including the biomarkers of various diseases. Since urine comes from blood through glomerular filtration, urine is in contact with the genitourinary tract that releases biomarkers of the disease. Urine has gained interest as a source or medium for diagnosis due to it being a noninvasive procedure. Many reports have demonstrated that urine contains a variety of biomarkers that could indicate diseases.Citation122–Citation126 Urine contains both urinary and systemic information since urine is in direct contact with urogenital organs and gets filtered from serum via glomerular filtration. Considering genitourinary organs are in direct contact with the body fluid, it is appealing to use urine for prostate cancer diagnoses in a noninvasive manner.Citation127 However, some challenges lie in using urine as the source because of the low pH level, high salt concentrations, and interference from other biomarkers of higher abundance.Citation128
Reliable biomarkers and emerging nanotechnology for noninvasive diagnostic strategies
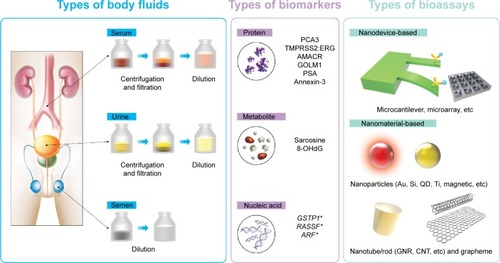
As a source for diagnosis, various bodily fluids have been exploited because they contain metabolized biomolecules that are biomarkers of diseases. illustrates the typical body fluids of semen,Citation116 serum,Citation129 and urine.Citation127,Citation129 The wide variety of biomarkers from the bodily fluids indicates the body’s physiological or pathophysiological state. For example, diseased tissues alter the physiological metabolisms and produce nonphysiological levels of biomolecules, enzymatic activity, or a new biomolecule species such as TMPRSS2:ERG that does not exist in the physiological state otherwise. The fusion gene is a result of a rearrangement on chromosome 21, and the TMPRSS2:ERG fusion protein is an oncogene that deregulates cytological metabolisms.Citation130–Citation144 The biomolecules are diverse in types, which are divided into carbohydrates, metabolites, nucleic acids, and proteins.Citation127
Figure 3 New diagnostic strategy based on nanotechnology (bioassay) with non-PSA biomarkers in various body fluids.
Notes: Different body fluids are subjected to appropriate sample preparations for subsequent analysis. The sample preparations are carried out to create the best environments for the nanoprobe’s function. These specimens may be centrifuged and/or diluted. Various biomarkers can be collected or detected by the nanoprobes from the body fluids. Nanotechnology-based bioassays detect various prostate cancer biomarkers. The *denotes the detection of epigenetic change.
Abbreviation: PSA, prostate-specific antigen.
The current FDA-approved biomarker PSA shows poor sensitivity and specificity and often leads to negative biopsies indicating its poor specificity in prostate cancer diagnosis. In order to compensate for the limitations of PSA as the effective biomarker for prostate cancer, novel biomarkers beyond PSA have been proposed and are under investigation. The main idea with novel biomarkers is exploiting them in conjunction with PSA. For instance, the currently FDA-approved prostate cancer gene 3 test requires urinary PSA level information so that it can generate a prostate cancer gene 3 score that would aid the diagnostic decision making.Citation17,Citation145 Thus, in addition to the biomarker discovery, a previous study has used panels of biomarkers for prostate cancer detection and evaluated their specificity toward prostate cancer detection.Citation127 The trend with use of a panel of biomarkers for prostate cancer detection indicates the realization that there is no such thing as a single perfect biomarker.
The more reliable biomarkers they are, the more effective prognosis or initial screening will be. Aspiration for noninvasive patient-friendly prostate cancer detection is one of the main drivers of novel biomarker discovery from bodily fluids. These features will add an enormous benefit by minimizing the number of unnecessary nonpatient-friendly DRE and invasive biopsies for prognosis and diagnosis. The conventional methods, PSA assay and DRE, for initial screening put a huge burden on the patients considering the high false-positive rate of initial screening leading to unnecessary repeated biopsies.
There is no nanotechnological approach made in clinical settings, yet. Attempts are currently underway to develop bioassays with high sensitivity and specificity that could analyze bodily fluids, and nanotechnology has emerged as a promising tactical tool. However, there are challenges in detecting the novel biomarkers in body fluids. Urine, especially, has a range of different pH levels, high concentrations of salts, and interferences by a variety of high abundance biomolecules, which all contribute to the obstruction of detecting the desired biomarkers with low sensitivity.Citation128 Therefore, the major tasks in the development of bioassays would be achieving a high sensitivity as well as specificity in the detection of biomarkers through nanotechnology.
Highly sensitive PSA detection through nanotechnology
As shows, the vast majority of studies have developed various nanotechnology-based bioassays for the detection of PSA in serum, whereas only a few studies have utilized the other body fluids. The larger number of studies with serum may be attributed to the longer history of serum as a bodily fluid for diagnosis. The first nanotechnological approach for prostate cancer screening was serum detection of PSA with a cantilever.Citation146 The sensitivity was recorded in terms of the limit of detection, and it was as low as 200 pg/mL. The principle of this bioassay method is by measuring the difference in oscillation frequency between the PSA bound and unbound cantilevers.
Table 1 PSA and related nanotechnology-based bioassays
Of all the developed nanotechnology-based bioassays, the electrochemical assay is the most popular method and has also achieved the highest sensitivity with <0.9 fg/mL in its detection limit and 1 ng/mL being the highest. The most common material for this method is carbon nanotubes (CNT), which is the typical material for electric sensing systems. CNT is an excellent signal transducer for achieving a high sensitivity. Biomedical functionalization with the PSA antibody (anti-PSA) conjugation onto the surface of the material allowed for the detection of protein PSA in serum samples. The captured PSA alters the current that runs through the CNT giving rise to detection.Citation147–Citation159 Electrochemiluminescence assays took advantage of this in conjugation with the nature of titanium nanotubes to achieve a low detection limit of 1 fg/mL.Citation160
The most popular nanomaterial is gold either as nanoparticle or as a nanopore. Only this material has been utilized across all the different body fluids, indicative of its popularity in nanotechnology applications.Citation151,Citation156,Citation157,Citation161–Citation172 However, its most popular form of use is as a nanoparticle. These nanomaterials are fabricated to obtain a high surface area to volume ratio to maximize the amount of antibodies loaded onto the material.
Although gold nanoparticles (GNPs) have been used the most in the electrochemical assay, the trend for PSA detection is to use CNT.Citation148,Citation151–Citation154,Citation157,Citation173–Citation175 This trend is most likely for proof of principle of the developed bioassays because CNT is primarily used for academic purposes rather than for practical use. GNP was the second most used nanomaterial, an indicative of effort to develop more sensitive PSA detection methods in serum.Citation151,Citation157,Citation161–Citation166,Citation171,Citation172 Unlike the CNTs, GNPs are used across various methodologies (). GNP is an excellent material for conjugation via various methods, thus it can load a high amount of targeting moieties that capture biomarkers, dye conjugates, or catalysts using its high surface area to volume ratio.
Nanotechnology toward non-PSA biomarker detection
Non-PSA biomarkers are not only protein biomolecules but also nucleic acids and other metabolites. Nucleic acids are the building blocks of DNA or RNA strands. Studies listed in show that the nucleic acids are microRNAs, a type of RNAs.Citation182,Citation183
Table 2 Non-PSA biomarkers and related nanotechnology-based bioassays
These nanotechnology-based bioassays for non-PSA biomarker detection point toward a practical use. None of these biomarkers are a good reference point for the proof of principle. The only thing that matters with these new prostate biomarkers is whether the bioassays are capable of capturing the biomarkers for detection. Effective detection ability with sufficient sensitivity and specificity in urine requires nanotechnology to achieve noninvasive initial screening. Notably, there are more studies about the detection of new non-PSA markers in urine. In response to clinical researchers, a number of studies have tried to detect non-PSA biomarkers in urine with some of the recently discovered prostate cancer biomarkers.Citation184–Citation189 PSMA, endoglin, and ANXA3 are the most promising biomarkers among the new ones, and the fact that they are tested with bioassays indicates how the studies are intended to show the practicality of the bioassays for initial screening.
The non-PSA biomarkers vary in type and require a different way of capturing the molecules. As with most nanomaterials, immunoassays are the most popular form of assay that is functionalized with specific antibodies against targeting biomarkers. All the studies used traditional nanomaterials as shown in . However, a novel material was used for the detection of PSMA. This nanomaterial is a nanowire that is conjugated with engineered bacteriophage M13. The unconventional nanomaterials are also used to develop new alternative assays. Rather than using traditional nanomaterials such as gold nanomaterials, quantum dots, and magnetic nanoparticles, some studies have utilized and exploited graphene and virus-based nanomaterials to achieve a more sensitive detection.Citation186,Citation187 The two interesting studies demonstrated the use of M13-PEDOT polymer nanowires to detect PSMA using an electrochemical assay. Another interesting study used a polymer-based nanomaterial which was a single-walled CNT. The nanotube detected miR-141, a microRNA known as a novel prostate cancer biomarker. Like the assay method used in the studies with M13-PEDOT polymer, the electrochemical assay is used for miR-141 detection in serum.Citation183,Citation186,Citation187
A unique study performed multiplex detection by using a microarray method with GNPs. This study does not present a limit of detection with the biomarkers but instead presents a unique aspect of the assay with the sensitivity and specific ity for prostate cancer diagnosis. Unlike all the other studies that employed nanotechnology shown in , , and , it has a clinical implication on how it can be a promising tool for future prostate cancer diagnoses.Citation190
Table 3 Device platforms for the nanotechnology-based bioassays
Another notable fact from is that nanotechnology-based assays can be applied for multiplexed detection. Nanotechnology is capable of detecting multiple biomarkers simultaneously. The use of a panel of prostate cancer biomarkers has been suggested for future diagnosis of prostate cancer. However, no single study has demonstrated multiplexed detection of other body fluids.Citation148,Citation182,Citation190
Considering there are many DNA biomarkers for prostate cancer, more studies are warranted for a nanotechnological approach for biomarkers. Since almost all known DNA biomarkers exhibit epigenetic alteration, a sensitive nanoprobe is required. Such as alteration as a biomarker candidate for prostate cancer would add another layer of reliability to biomarker detection. Having diversified biomarkers would lead to a more accurate initial screening.
Nanotechnology into device platform
Nanotechnology-based bioassays of PSA and non-PSA biomarkers have been developed to achieve a higher sensitivity and specificity. The aforementioned techniques are built into devices to materialize new sensing platforms (). The development of the device is a convergence of nanotechnology and other sensor technologies. The main focus is to utilize nanomaterials in conjugation with traditional sensors to develop a new nanomaterial-bearing sensing device platform. This approach has led to higher sensitivity and specificity toward the detection of the prostate biomarkers, which have not been achieved without nanomaterials. The efforts to improve the properties of the devices that are intended for the detection of biomarkers from body fluids other than serum is because other body fluids – such as urine – require a higher sensitivity. However, serum is the most tested bodily fluid. One of the device platforms that yielded one of the highest sensitivities is the field-effect transistor, which mainly utilized CNT as sensing nanomaterial.Citation148,Citation152,Citation178,Citation183,Citation191 It is interesting to note that the sensitivity differs dramatically depending on the sensing nanomaterial used. Compared to CNT, polysilicon nanowires demonstrated outstanding sensitivity of ~5 fg/mL of limit of detection.Citation149 A similar device, microgapped interdigitated enzymatic assay, achieves even higher sensitivity with a 0.9 fg/mL limit of detection.Citation155 Electrical sensing devices seem to be the most popular sensing method. An interesting aspect of this device is that the distance between the two electrodes is in the micrometer range, and there is a report of a nanogapped microelectrode array.Citation155,Citation198 Optical-based sensing devices such as immunochromatographic strips and quartz crystal microbalance (QCM) exhibit a fair sensitivity in comparison to the electrical-based sensing devices.Citation1,Citation4,Citation9,Citation167–Citation169,Citation184
Conclusion
Beginning from the early 2000s, nanotechnology has emerged as a therapeutic and diagnostic tool for the treatment and diagnosis of prostate cancer, and more studies have been accumulating since. Complications from unreliable initial screenings gave a need for researching the development of panels of novel prostate cancer biomarkers for better initial screening. Many reports have been published about the discovery of novel biomarkers. However, for the detection of some of the novel biomarkers, the initial screening requires a bioassay with a higher sensitivity and specificity while keeping it noninvasive. In response to these needs, studies have employed nanotechnology to bring forth novel bioassay development. Each of the studies has demonstrated noninvasive detection of their bioassays in one of the body fluids.
Nanotechnology is of particular interest because when we shrink the sizes of the existing materials down to the nanoscale, new distinct physical properties start to appear. As we have harnessed the properties of bulk materials, the new physical properties of nanomaterials can also be harnessed for various applications such as biomedicine. The studies aforementioned have exploited these new properties to overcome the limitations that we face with prostate cancer initial screening by significantly improving the sensitivity and specificity of biomarker detection.
Nanotechnology has proven its potential in prostate cancer biomarker detection. Nanotechnology-based bioassays have demonstrated a much higher detection sensitivity compared to the conventional enzyme-linked immunosorbent assay (ELISA) method. The higher sensitivity has much benefit because it means a stable detection – even of a trace of a biomarker. Higher sensitivity, therefore, allows for biomarker detection via urine instead of serum. The current initial screening of prostate cancer requires a blood sample for the PSA assay. It usually takes more than a drop to obtain and requires a syringe with a needle to acquire. Since this kind of operation needs a professional to carry out, the conventional method is still not enough to be patient friendly. Furthermore, the conventional method needs to be carried out by a professional, and thus, it cannot be a point-of-care for the general population to use. If we are able to take advantage of what nanotechnology can offer, it would be a great advancement in patient care because it is highly anticipated to minimize both overdiagnosis and underdiagnosis of cancer – due to its excellent specificity and sensitivity, respectively – and could monitor the disease for people at risk of recurrence after recovery.
To overcome the limitations of conventional initial screening methods, a significant number of studies proposed their nanotechnology-based bioassays. Most of the bioassays have measured biomarkers – PSA and beyond-PSAs – from serum. They outnumber the studies tested from other body fluids. Of the studies with serum, the majority of them chose PSA as the targeting biomarker. It is quite interesting to note that some studies are not aware of what the clinical community demands and what the exact problem with current initial screening with PSA detection is. This misunderstanding should be avoided to make further research more effective. Thus, the communication between the clinical research and nanotechnology engineering fields must be up to date at all times.
PSA has been a good reference to compare with other bioassays that have a measured PSA level. Using PSA detection as the proof of principle for their developed bioassays will not be as advantageous as before. The complications of the initial screenings turned out to be due to PSA itself being an unreliable biomarker rather than the low sensitivity of the enzyme-linked immunosorbent assay method. As the notion has grown that it is unlikely to find a perfect biomarker to diagnose a disease, PSA might still be used as one of the panel of prostate cancer biomarkers. To establish other prostate cancer biomarkers into the panel, testing the bioassay detection of the novel biomarkers is needed. However, compared to PSA, other biomarkers have less of a chance to get tested in the development of nanotechnology-based bioassays.
Clinical trials are important for the development of the bioassays in order to make them clinically relevant. Finding the cutoff value for a diagnosis or initial screening is another problem. Clinical researcher must have their attention on these nanotechnologies for the development of a more effective initial screening strategy.
Another important clinical implication is that nanotechnology-based bioassays can also contribute to monitoring the stage progression of the disease. Dynamic changes of biomarkers occur as prostate cancer develops and advances. Thus, detecting different biomarkers that represent particular stages of the disease may add an additional benefit to an accurate diagnosis. It has been reported that benign and malignant tumors must be differentiated and subjected to different treatments. Attempting to treat a benign tumor can only exacerbate the diseased state while undertreatment can also bring consequences. In that regard, aggressive stratification of the disease’s stages is one of the urgent needs. To achieve this stratification, developing diagnostic tools capable of discerning the different stages of the disease could significantly improve the quality of life of the diagnosed patients by delivering an accurate treatment.
Finally, the development of nanotechnology-based bioassays is a highly interdisciplinary area that will require a robust multidisciplinary approach. Active collaboration between clinicians and experts in nanotechnology is a must in order to take development toward commercialization. It would be highly favorable for future investors and policy officials to put their interest in this area of research.
Review criteria
Pubmed and Web of Science were used in search, and the keywords were “seminal” OR “serum” OR “urine” AND “prostate cancer” AND “nano*”.
Acknowledgments
We thank Suchang Mun for his graphical art contribution for this review. We are thankful of the financial support from the project grants: CTP (Convergence Technology Project at Korea Institute of Science and Technology #2V03990) and TRC (Translational Research Center with Asan Medical Center #2E25502).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin20156552925559415
- FerlayJSteliarova-FoucherELortet-TieulentJCancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012Eur J Cancer2013491374140323485231
- HumphreyPAGleason grading and prognostic factors in carcinoma of the prostateMod Pathol20041729230614976540
- GleasonDFHistologic grading and staging of prostatic-carcinomaAm J Surg Pathol19815193193
- AmisESJrBigongiariLRBluthEIPretreatment staging of clinically localized prostate cancer. American College of Radiology. ACR Appropriateness CriteriaRadiology2000215suppl70370811037488
- ChungMSLeeSHLeeDHChungBHEvaluation of the 7th American Joint Committee on cancer TNM staging system for prostate cancer in point of classification of bladder neck invasionJpn J Clin Oncol20134318418823225909
- DashASandaMGYuMTaylorJMFeckoARubinMAProstate cancer involving the bladder neck: recurrence-free survival and implications for AJCC staging modification. American Joint Committee on CancerUrology20026027628012137826
- HanMWalshPCPartinAWRodriguezRAbility of the 1992 and 1997 American Joint Committee on Cancer staging systems for prostate cancer to predict progression-free survival after radical prostatectomy for stage T2 diseaseJ Urol2000164899210840430
- IyerRVHanlonALPinoverWHHanksGEOutcome evaluation of the 1997 American Joint Committee on cancer staging system for prostate carcinoma treated by radiation therapyCancer1999851816182110223577
- TaylorSHMerrimanKWSpiessPEPistersLInadequacies of the current American Joint Committee on cancer staging system for prostate cancerCancer200610655956516369982
- ZaorskyNGLiTDevarajanKHorwitzEMBuyyounouskiMKAssessment of the American Joint Committee on Cancer staging (sixth and seventh editions) for clinically localized prostate cancer treated with external beam radiotherapy and comparison with the National Comprehensive Cancer Network risk-stratification methodCancer20121185535554322544661
- MooreSKuhrikMKuhrikNSheaLScreening for prostate cancer: PSA blood test, rectal examination, and ultrasoundUrol Nurs1992121061071382318
- CrawfordEDDeAntoniEPPSA as a screening test for prostate cancerUrol Clin North Am1993206376467505971
- HalperinECScreening for prostate cancer utilizing the prostate-specific antigen (PSA)N C Med J1993544434467694161
- MarksLSFradetYDerasILPCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsyUrology20076953253517382159
- HaeseAde la TailleAvan PoppelHClinical utility of the PCA3 urine assay in European men scheduled for repeat biopsyEur Urol2008541081108818602209
- MeariniEAntognelliCDel BuonoCThe combination of urine DD3(PCA3) mRNA and PSA mRNA as molecular markers of prostate cancerBiomarkers20091423524319489685
- PepePAragonaFPCA3 score vs PSA free/total accuracy in prostate cancer diagnosis at repeat saturation biopsyAnticancer Res2011314445444922199313
- PerdonàSCavadasVDi LorenzoGProstate cancer detection in the “grey area” of prostate-specific antigen below 10 ng/mL: head-to-head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3Eur Urol201159818720947244
- MelicharBPSA, PCA3 and the philosophy of prostate cancer managementClin Chem Lab Med20135170771223518451
- ShinoharaKImproving cancer detection by prostate biopsy: the role of core number and siteNat Clin Pract Urol2006352652717031375
- ElabbadyAAKhedrMMExtended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason scoreEur Urol2006494953 discussion 5316314035
- CampodonicoFCasaricoAGavazziLCancer detection with TRUS-guided 10-core biopsy of the prostate. an institutional assessment at the first, repeated and surgical specimen biopsyArch Ital Urol Androl200678394316929600
- SinghHCantoEIShariatSFImproved detection of clinically significant, curable prostate cancer with systematic 12-core biopsyJ Urol20041711089109214767277
- NarayanPJajodiaPSteinRCore biopsy instrument in the diagnosis of prostate cancer: superior accuracy to fine needle aspirationJ Urol19911457957972005704
- SpaanderPJMostofiKBlomJBiopsy techniques and cytology in prostate cancerProg Clin Biol Res19903571571582217459
- LeeFLittrupPJMcLearyRDNeedle aspiration and core biopsy of prostate cancer: comparative evaluation with biplanar transrectal US guidanceRadiology19871635155203550883
- GreenLDigital rectal examination screening for prostate cancerJAMA19932701315 author reply 1315-68360960
- GerberGSChodakGWDigital rectal examination in the early detection of prostate cancerUrol Clin North Am1990177397442219574
- ChodakGWKellerPSchoenbergHWAssessment of screening for prostate cancer using the digital rectal examinationJ Urol1989141113611382709500
- Torp-PedersenSTLittrupPJLeeFMettlinCEarly prostate cancer: diagnostic costs of screening transrectal US and digital rectal examinationRadiology19881693513543140290
- ChodakGWKellerPSchoenbergHRoutine screening for prostate cancer using the digital rectal examinationProg Clin Biol Res198826987983393557
- GhaiSToiARole of transrectal ultrasonography in prostate cancerRadiol Clin North Am2012501061107323122038
- KostakopoulosAPikraminosDLissiotisPMalachiasGStavropoulosNIKontothanasisDThe contribution of transrectal ultrasonography in the early diagnosis of prostate cancerActa Urol Belg19926057631384290
- WilsonTMGuthmanDACurrent status of transrectal ultrasonography in the detection of prostate cancerOncology (Williston Park)199157378 discussion 831828689
- HernandezADSmithJAJrTransrectal ultrasonography for the early detection and staging of prostate cancerUrol Clin North Am1990177457572219575
- FujinoAScardinoPTTransrectal ultrasonography for prostatic cancer. II. The response of the prostate to definitive radiotherapyCancer1986579359403510709
- PeelingWBGriffithsGJEvansKTRobertsEEDiagnosis and staging of prostatic cancer by transrectal ultrasonography. A preliminary studyBr J Urol19795156556993975
- HubenRPMurphyGPTransrectal ultrasonography of the prostate and prostate cancer – an updateAppl Pathol198531982053916055
- ResnickMIWillardJWBoyceWHTransrectal ultrasonography in the evaluation of patients with prostatic carcinomaJ Urol19801244824847420589
- CarpentierPJSchroederFHBlomJHTransrectal ultrasonography in the followup of prostatic carcinoma patientsJ Urol19821287427467143595
- CarpentierPJSchroderFHTransrectal ultrasonography in the followup of prostatic carcinoma patients: a new prognostic parameter?J Urol19841319039056708224
- FujinoAScardinoPTTransrectal ultrasonography for prostatic cancer: its value in staging and monitoring the response to radiotherapy and chemotherapyJ Urol19851338068103886938
- PontesJEEisenkraftSWatanabeHOheHSaitohMMurphyGPPreoperative evaluation of localized prostatic carcinoma by transrectal ultrasonographyJ Urol19851342892913894694
- RifkinMDTransrectal prostatic ultrasonography: comparison of linear array and radial scannersJ Ultrasound Med19854152579244
- DevonecMDiagnostic value of transrectal ultrasonography in prostatic cancerProg Clin Biol Res1987243B1103309977
- RorvikJServollEHalvorsenOJTransrectal ultrasonography in the staging of localized prostatic carcinoma. A pilot studyScand J Urol Nephrol19922615191631502
- MottetNLehmannMCicorelliSTransrectal ultrasonography in prostatic cancer: interexaminer variability of interpretationEur Urol1997321501549286644
- PrakashVSMohanGCKrishnaiahSVTen-core versus 16-core transrectal ultrasonography guided prostate biopsy for detection of pro-static carcinoma: a prospective comparative study in Indian populationProstate Int2013116316824392441
- FowlerJEJrMillsSEOperable prostatic carcinoma: correlations among clinical stage, pathological stage, Gleason histological score and early disease-free survivalJ Urol198513349523964879
- WooSKaplanIRoachMBagshawMFormula to estimate risk of pelvic lymph node metastasis from the total Gleason score for prostate cancerJ Urol19881403873398155
- PartinAWYooJCarterHBThe use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancerJ Urol19931501101147685418
- WolfJSJrShinoharaKCarrollPRNarayanPCombined role of transrectal ultrasonography, Gleason score, and prostate-specific antigen in predicting organ-confined prostate cancerUrology1993421311377690168
- NarayanPGajendranVTaylorSPThe role of transrectal ultrasound-guided biopsy-based staging, preoperative serum prostate-specific antigen, and biopsy Gleason score in prediction of final pathologic diagnosis in prostate cancerUrology1995462052127542823
- ThickmanDSpeersWCPhilpottPJShapiroHEffect of the number of core biopsies of the prostate on predicting Gleason score of prostate cancerJ Urol19961561101138648769
- FernandesETSundaramCPLongRSoltaniMErcoleCJBiopsy Gleason score: how does it correlate with the final pathological diagnosis in prostate cancer?Br J Urol1997796156179126095
- JeongIGLimJHYouDIncremental value of magnetic resonance imaging for clinically high risk prostate cancer in 922 radical prostatectomiesJ Urol20131902054206023791890
- PilepichMVKrallJMal-SarrafMAndrogen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomized comparative trial of the Radiation Therapy Oncology GroupUrology1995456166237716842
- WalshPCImmediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators GroupBr J Urol1997792352469052476
- BollaMGonzalezDWardePImproved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelinN Engl J Med19973372953009233866
- HillBKyprianouNSequencing hormonal ablation and radiotherapy in prostate cancer: a molecular and therapeutic perspective (Review)Oncol Rep200291151115612375010
- HugginsCHodgesCVStudies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941J Urol200216891212050481
- StuderUEHauriDHanselmannSImmediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88J Clin Oncol2004224109411815483020
- MessingEMManolaJYaoJEastern Cooperative Oncology Group Study EST 3886Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomyLancet Oncol2006747247916750497
- GleaveMKlotzLTanejaSSThe continued debate: intermittent vs continuous hormonal ablation for metastatic prostate cancerUrol Oncol200927818619111804
- EllisRJKaminskyDAZhouEHTen-year outcomes: the clinical utility of single photon emission computed tomography/computed tomography capromab pendetide (Prostascint) in a cohort diagnosed with localized prostate cancerInt J Radiat Oncol Biol Phys201181293420961696
- SodeeDBConantRChalfantMPreliminary imaging results using In-111 labeled CYT-356 (Prostascint) in the detection of recurrent prostate cancerClin Nucl Med1996217597678896922
- ShrevePDGrossmanHBGrossMDWahlRLMetastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucoseRadiology19961997517568638000
- SanzGRiojaJZudaireJJBerianJMRichterJAPET and prostate cancerWorld J Urol20042235135215503049
- ReskeSNBlumsteinNMNeumaierBImaging prostate cancer with 11C-choline PET/CTJ Nucl Med2006471249125416883001
- MintzAPET/CT in prostate cancer: an unmet clinical needOncology (Williston Park)2014281065106625738198
- JadvarHPSMA PET in prostate cancerJ Nucl Med20155681131113225977465
- de KoningHJSchroderFHPSA screening for prostate cancer: the current controversyAnn Oncol19989129312969932158
- McCarthyMPSA screening said to reduce prostate-cancer deaths, or does it?Lancet1998351156310326552
- CatalonaWJSmithDSRatliffTLMeasurement of prostate-specific antigen in serum as a screening-test for prostate-cancerN Engl J Med1991324115611611707140
- BrettonPRProstate-specific antigen and digital rectal examination in screening for prostate cancer: a community-based studySouth Med J1994877207237517580
- CatalonaWJRichieJPAhmannFRComparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 menJ Urol1994151128312907512659
- ThompsonIMPaulerDKGoodmanPJPrevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliterN Engl J Med20043502239224615163773
- SchroderFHvan der Cruijsen-KoeterIde KoningHJVisANHoedemaekerRFKranseRProstate cancer detection at low prostate specific antigenJ Urol200016380681110687982
- TumaRSNew tests for prostate cancer may be nearing the clinicJ Natl Cancer Inst201010275275420498428
- SchröderFHHugossonJRoobolMJERSPC InvestigatorsScreening and prostate-cancer mortality in a randomized European studyN Engl J Med20093601320132819297566
- AndrioleGLCrawfordEDGrubbRL3rdBuysSSProstate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-upJ Natl Cancer Inst201210412513222228146
- Al-ShyoukhIYuFFengJSystematic quantitative characterization of cellular responses induced by multiple signalsBMC Syst Biol201158821624115
- HrkachJVon HoffDMukkaram AliMPreclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profileSci Transl Med20124128ra39
- WongPKYuFShahangianAChengGSunRHoCMClosed-loop control of cellular functions using combinatory drugs guided by a stochastic search algorithmProc Natl Acad Sci U S A20081055105511018356295
- DawidczykCMKimCParkJHState-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicinesJ Control Release2014187C13314424874289
- FerrariMCancer nanotechnology: opportunities and challengesNat Rev Cancer2005516117115738981
- CondonADesigned DNA molecules: principles and applications of molecular nanotechnologyNat Rev Genet2006756557516770339
- SilvaGANeuroscience nanotechnology: progress, opportunities and challengesNat Rev Neurosci20067657416371951
- Cancer nanotechnology: small, but heading for the big timeNat Rev Drug Discov2007617417517396287
- McCarthyNNanotechnology: tag teamsNat Rev Cancer20111153721753792
- SchroederAHellerDAWinslowMMTreating metastatic cancer with nanotechnologyNat Rev Cancer201212395022193407
- SrikanthMKesslerJANanotechnology-novel therapeutics for CNS disordersNat Rev Neurol2012830731822526003
- ErricoANew technology: nanotechnology targets cancer cellsNat Rev Clin Oncol20131066724129354
- HarrisonCNanotechnology: biological proteins knock nanoparticles off targetNat Rev Drug Discov20131226423493078
- SmithDMSimonJKBakerJRJrApplications of nanotechnology for immunologyNat Rev Immunol20131359260523883969
- Axiak-BechtelSMUpendranALattimerJCGum arabic-coated radioactive gold nanoparticles cause no short-term local or systemic toxicity in the clinically relevant canine model of prostate cancerInt J Nanomedicine201495001501125378926
- GaoXLuoYWangYProstate stem cell antigen-targeted nanoparticles with dual functional properties: in vivo imaging and cancer chemotherapyInt J Nanomedicine201274037405122888241
- GobinAMMoonJJWestJLEphrinA1-targeted nanoshells for photothermal ablation of prostate cancer cellsInt J Nanomedicine2008335135818990944
- SatoAItchoNIshiguroHMagnetic nanoparticles of Fe3O4 enhance docetaxel-induced prostate cancer cell deathInt J Nanomedicine201383151316023990723
- TaylorRMSillerudLOPaclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a PSMA-dependent mannerInt J Nanomedicine201274341435222915856
- WuXDingBGaoJSecond-generation aptamer-conjugated PSMA-targeted delivery system for prostate cancer therapyInt J Nanomedicine201161747175621980237
- WuXTaiZZhuQStudy on the prostate cancer-targeting mechanism of aptamer-modified nanoparticles and their potential anticancer effect in vivoInt J Nanomedicine201495431544025473281
- YuanLLiuCChenYZhangZZhouLQuDAntitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer modelInt J Nanomedicine201384339435024235831
- MouliSKZhaoLCOmaryRAThaxtonCSLymphotropic nanoparticle enhanced MRI for the staging of genitourinary tumorsNat Rev Urol20107849320084078
- MaLHeSHuangJCaoLYangFLiLMaximizing specificity and yield of PCR by the quantum dot itself rather than property of the quantum dot surfaceBiochimie20099196997319442702
- RazzakMNanotechnology opens up new realm of detection in GBMNat Rev Clin Oncol201310423183633
- ParkHHwangMPLeeKHImmunomagnetic nanoparticle-based assays for detection of biomarkersInt J Nanomedicine201384543455224285924
- ParkHHwangMPLeeJWChoiJLeeKHHarnessing immunomagnetic separation and quantum dot-based quantification capacities for the enumeration of absolute levels of biomarkerNanotechnology20132428510323787774
- HwangMPLeeJWLeeKELeeKHThink modular: a simple apoferritin-based platform for the multifaceted detection of pancreatic cancerACS Nano201378167817423988226
- LeeJWSongJHwangMPLeeKHNanoscale bacteriophage biosensors beyond phage displayInt J Nanomedicine201383917392524143096
- ChoiJParkSStojanovićZFacile solvothermal preparation of monodisperse gold nanoparticles and their engineered assembly of ferritin-gold nanoclustersLangmuir201329156981570324283573
- GallowayJFWinterALeeKHQuantitative characterization of the lipid encapsulation of quantum dots for biomedical applicationsNanomedicine201281190119922197728
- LeeKHGallowayJFParkJQuantitative molecular profiling of biomarkers for pancreatic cancer with functionalized quantum dotsNanomedicine201281043105122306154
- ParkHLeeJWHwangMPLeeKHQuantification of cardiovascular disease biomarkers via functionalized magnetic beads and on-demand detachable quantum dotsNanoscale201358609861523893124
- DrabovichAPSaraonPJarviKDiamandisEPSeminal plasma as a diagnostic fluid for male reproductive system disordersNat Rev Urol20141127828824709963
- ThomasCESextonWBensonKSutphenRKoomenJUrine collection and processing for protein biomarker discovery and quantificationCancer Epidemiol Biomarkers Prev20101995395920332277
- BarrattJTophamPUrine proteomics: the present and future of measuring urinary protein components in diseaseCMAJ200717736136817698825
- ZhouMLucasDAChanKCAn investigation into the human serum “interactome”Electrophoresis2004251289129815174051
- LopezMFMikulskisAKuzdzalSHigh-resolution serum proteomic profiling of Alzheimer disease samples reveals disease-specific, carrier-protein-bound mass signaturesClin Chem2005511946195416081505
- AyacheSPanelliMMarincolaFMStroncekDFEffects of storage time and exogenous protease inhibitors on plasma protein levelsAm J Clin Pathol200612617418416891190
- DearJWStreetJMBaileyMAUrinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signallingProteomics2013131572158023129434
- FliserDNovakJThongboonkerdVAdvances in urinary proteome analysis and biomarker discoveryJ Am Soc Nephrol2007181057107117329573
- NolenBMLokshinAEThe advancement of biomarker-based diagnostic tools for ovarian, breast, and pancreatic cancer through the use of urine as an analytical biofluidInt J Biol Markers20112614115221928247
- BroedbaekKWeimannAStovgaardESPoulsenHEUrinary 8-oxo-7,8-dihydro-2′-deoxyguanosine as a biomarker in type 2 diabetesFree Radic Biol Med2011511473147921820047
- KuJHGodoyGAmielGELernerSPUrine survivin as a diagnostic biomarker for bladder cancer: a systematic reviewBJU Int201211063063622353238
- RigauMOlivanMGarciaMThe present and future of prostate cancer urine biomarkersInt J Mol Sci201314126201264923774836
- AndersonNLAndersonNGThe human plasma proteome: history, character, and diagnostic prospectsMol Cell Proteomics2002184586712488461
- SchachtMJGarnettJEGrayhackJTBiochemical markers in prostatic-cancerUrol Clin North Am1984112532676203205
- WinnesMLissbrantEDamberJEStenmanGMolecular genetic analyses of the TMPRSS2-ERG and TMPRSS2-ETV1 gene fusions in 50 cases of prostate cancerOncol Rep2007171033103617390040
- DemichelisFFallKPernerSTMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohortOncogene2007264596459917237811
- ClarkJMersonSJhavarSDiversity of TMPRSS2-ERG fusion transcripts in the human prostateOncogene2007262667267317043636
- YoshimotoMJoshuaAMChilton-MacneillSThree-color FISH analysis of TMPRSS2/ERG fusions in prostate cancer indicates that genomic microdeletion of chromosome 21 is associated with rearrangementNeoplasia2006846546916820092
- ChinnaiyanAMRole of the TMPRSS2-ERG gene fusion in prostate cancerNeoplasia200810177U2318283340
- CerveiraNRibeiroFRPeixotoATMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesionsNeoplasia2006882683217032499
- LapointeJKimYHMillerMAA variant TMPRSS2 isoform and ERG fusion product in prostate cancer with implications for molecular diagnosisMod Pathol20072046747317334351
- NamRKSugarLMKlotzLHVenkateswaranVSethAExpression of TMPRSS2: ERG gene fusion in prostate cancer cells is an important prognostic factor for cancer progressionJ Urol2007177467468
- SollerMJElfvingPLundgrenRPanagopolsIConfirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancerGenes Chromosomes Cancer20064571771916575875
- WangJHCaiYRenCXIttmannMExpression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancerCancer Res2006668347835116951141
- PernerSDemichelisFBeroukhimRTMPRSS2: ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancerCancer Res2006668337834116951139
- IljinKWolfMEdgrenHTMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogrammingCancer Res200666102421024617079440
- HermansKGvan MarionRvan DekkenHJensterGvan WeerdenWMTrapmanJTMPRSS2: ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancerCancer Res200666106581066317108102
- PernerSMosqueraJMDemichelisFTMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasionAm J Surg Pathol20073188288817527075
- KannanKCoarfaCRajapaksheKCDKN2D-WDFY2 is a cancer-specific fusion gene recurrent in high-grade serous ovarian carcinomaPLoS Genet201410e100421624675677
- HesselsDSchalkenJAThe use of PCA3 in the diagnosis of prostate cancerNat Rev Urol2009625526119424173
- WuGDatarRHHansenKMThundatTCoteRJMajumdarABioassay of prostate-specific antigen (PSA) using microcantileversNat Biotechnol20011985686011533645
- WangHZhangYYuHLabel-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticlesAnal Biochem201343412312723201390
- ChikkaveeraiahBVBhirdeAMalhotraRPatelVGutkindJSRuslingJFSingle-wall carbon nanotube forest arrays for immuno-electrochemical measurement of four protein biomarkers for prostate cancerAnal Chem2009819129913419775154
- HuangYWWuCSChuangCKReal-time and label-free detection of the prostate-specific antigen in human serum by a polycrystalline silicon nanowire field-effect transistor biosensorAnal Chem2013857912791823898965
- KokkinosCEconomouAPetrouPSKakabakosSEMicrofabricated tin-film electrodes for protein and DNA sensing based on stripping voltammetric detection of Cd(II) released from quantum dots labelsAnal Chem201385106861069124131278
- KavosiBSalimiAHallajRAmaniKA highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocompositeBiosens Bioelectron201452202824016535
- KimJPLeeBYLeeJHongSSimSJEnhancement of sensitivity and specificity by surface modification of carbon nanotubes in diagnosis of prostate cancer based on carbon nanotube field effect transistorsBiosens Bioelectron2009243372337819481922
- PaniniNVMessinaGASalinasEFernandezHRabaJIntegrated microfluidic systems with an immunosensor modified with carbon nanotubes for detection of prostate specific antigen (PSA) in human serum samplesBiosens Bioelectron2008231145115118162392
- SalimiAKavosiBFathiFHallajRHighly sensitive immunosensing of prostate-specific antigen based on ionic liquid-carbon nanotubes modified electrode: application as cancer biomarker for prostate biopsiesBiosens Bioelectron20134243944623235113
- HuangYWangTHJiangJHShenGLYuRQProstate specific antigen detection using microgapped electrode array immunosensor with enzymatic silver depositionClin Chem20095596497119264854
- PandeyBDemchenkoAVStineKJNanoporous gold as a solid support for protein immobilization and development of an electrochemical immunoassay for prostate specific antigen and carcinoembryonic antigenMicrochim Acta20121797181
- TianJNHuangJLZhaoYCZhaoSLElectrochemical immunosen-sor for prostate-specific antigen using a glassy carbon electrode modified with a nanocomposite containing gold nanoparticles supported with starch-functionalized multi-walled carbon nanotubesMicrochim Acta20121788188
- KumarVSrivastavaSUmraoSYadavRKNathGSumanaGNanostructured palladium-reduced graphene oxide platform for high sensitive, label free detection of a cancer biomarkerRSC Adv2014422672273
- QuBChuXShenGYuRA novel electrochemical immunosensor based on colabeled silica nanoparticles for determination of total prostate specific antigen in human serumTalanta20087678579018656659
- TianCYZhaoWWWangJXuJJChenHYAmplified quenching of electrochemiluminescence from CdS sensitized TiO2 nanotubes by CdTe-carbon nanotube composite for detection of prostate protein antigen in serumAnalyst20121373070307522624149
- ChangYFHungSHLeeYJDiscrimination of breast cancer by measuring prostate-specific antigen levels in women’s serumAnal Chem2011835324532821591802
- GaoZXuMHouLChenGTangDMagnetic bead-based reverse colorimetric immunoassay strategy for sensing biomoleculesAnal Chem2013856945695223806145
- GrubishaDSLipertRJParkHYDriskellJPorterMDFemtomo-lar detection of prostate-specific antigen: an immunoassay based on surface-enhanced Raman scattering and immunogold labelsAnal Chem2003755936594314588035
- ChenZLeiYChenXWangZLiuJAn aptamer based resonance light scattering assay of prostate specific antigenBiosens Bioelectron201236354022542926
- HuangLReekmansGSaerensDProstate-specific antigen immunosensing based on mixed self-assembled monolayers, camel antibodies and colloidal gold enhanced sandwich assaysBiosens Bioelectron20052148349016076438
- KongXLQiHZhouHXRenLLDengCYLiFRA novel sensitive immunoassay by nucleic acid barcode dot and its application in the detection of prostate-specific antigenClin Chem Lab Med20104827928320001442
- SatoISagiMIshiwariANishijimaHItoEMukaiTUse of the “SMITEST” PSA card to identify the presence of prostate-specific antigen in semen and male urineForensic Sci Int2002127717412098528
- SatoIBarniFYoshiikeMApplicability of Nanotrap Sg as a semen detection kit before male-specific DNA profiling in sexual assaultsInt J Legal Med200712131531916583248
- SatoIKojimaKYamasakiTRapid detection of semenogelin by one-step immunochromatographic assay for semen identificationJ Immunol Methods200428713714515099762
- YuhiTNagataniNEndoTResin-based micropipette tip for immunochromatographic assays in urine samplesJ Immunol Methods2006312546016624320
- GeSGYuJHJiaoXLChenDRUltrasensitive electrochemiluminescence immunoassay for protein specific detection based on dendrimer-encapsulated gold nanoparticles labelsJ Inorg Organomet Polym Mater20132311131121
- ThaxtonCSElghanianRThomasADNanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomyProc Natl Acad Sci U S A2009106184371844219841273
- LiaoKTChengJTLiCLLiuRTHuangHJUltra-sensitive detection of mutated papillary thyroid carcinoma DNA using square wave stripping voltammetry method and amplified gold nanoparticle biomarkersBiosens Bioelectron2009241899190419010660
- Gonzalez-GarciaMBFernandez-SanchezCCosta-GarciaAColloidal gold as an electrochemical label of streptavidin-biotin interactionBiosens Bioelectron20001531532111219743
- DequaireMDegrandCLimogesBAn electrochemical metalloimmunoassay based on a colloidal gold labelAnal Chem2000725521552811101226
- LiWGeSWangSYanMGeLYuJHighly sensitive chemiluminescence immunoassay on chitosan membrane modified paper platform using TiO2 nanoparticles/multiwalled carbon nanotubes as labelLuminescence20132849650223355319
- ChoiHKLeeJHRole of magnetic Fe3O4 graphene oxide in chemiluminescent aptasensors capable of sensing tumor markers in human serumAnal Methods2013569646968
- BrimanMArtukovicEZhangLChiaDGoodglickLGrunerGDirect electronic detection of prostate-specific antigen in serumSmall2007375876217352433
- LiWPElectropolymerized poly(3,4-ethylendioxythiophene)/graphene composite film and its application in quantum dots electro-chemiluminescence immunoassayJ Inorg Organomet Polym Mater201323719725
- KermanKEndoTTsukamotoMChikaeMTakamuraYTamiyaEQuantum dot-based immunosensor for the detection of prostate-specific antigen using fluorescence microscopyTalanta2007711494149919071481
- HuhtinenPSoukkaTLovgrenTHarmaHImmunoassay of total prostate-specific antigen using europium(III) nanoparticle labels and streptavidin-biotin technologyJ Immunol Methods200429411112215604021
- AlhasanAHKimDYDanielWLScanometric MicroRNA array profiling of prostate cancer markers using spherical nucleic acid-gold nanoparticle conjugatesAnal Chem2012844153416022489825
- TranHVPiroBReisbergSTranLDDucHTPhamMCLabel-free and reagentless electrochemical detection of microRNAs using a conducting polymer nanostructured by carbon nanotubes: application to prostate cancer biomarker miR-141Biosens Bioelectron20134916416923743328
- KimYJRahmanMMLeeJJUltrasensitive and label-free detection of annexin A3 based on quartz crystal microbalanceSensor Actuat B Chem2013177172177
- SrinivasanBLiYJingYXingCSlatonJWangJPA three-layer competition-based giant magnetoresistive assay for direct quantification of endoglin from human urineAnal Chem2011832996300221417448
- ArterJADiazJEDonavanKCYuanTPennerRMWeissGAVirus-polymer hybrid nanowires tailored to detect prostate-specific membrane antigenAnal Chem2012842776278322339784
- MohanKDonavanKCArterJAPennerRMWeissGASub-nanomolar detection of prostate-specific membrane antigen in synthetic urine by synergistic, dual-ligand phageJ Am Chem Soc20131357761776723614709
- ZitkaOKrizkovaSKrejcovaLMicrofluidic tool based on the antibody-modified paramagnetic particles for detection of 8-hydroxy-2′-deoxyguanosine in urine of prostate cancer patientsElectrophoresis2011323207322022012838
- ZitkaOCerneiNHegerZMicrofluidic chip coupled with modified paramagnetic particles for sarcosine isolation in urineElectrophoresis2013342639264723775886
- O’RourkeDJDiJohnsonDACaiazzoRJJrAutoantibody signatures as biomarkers to distinguish prostate cancer from benign prostatic hyperplasia in patients with increased serum prostate specific antigenClin Chim Acta201241356156722146597
- LernerMBD’SouzaJPazinaTHybrids of a genetically engineered antibody and a carbon nanotube transistor for detection of prostate cancer biomarkersACS Nano201265143514922575126
- GaoXZhangHLiYSuXMn-doped ZnSe d-dots-based alpha-methylacyl-CoA racemase probe for human prostate cancer cell imagingAnal Bioanal Chem20124021871187722241581
- HuoQLitherlandSASullivanSHallquistHDeckerDARivera-RamirezIDeveloping a nanoparticle test for prostate cancer scoringJ Transl Med2012104422404986
- FangCChenZLiLXiaJBarcode lateral flow immunochromato-graphic strip for prostate acid phosphatase determinationJ Pharm Biomed Anal2011561035104021880451
- ChandaNShuklaRKattiKVKannanRGastrin releasing protein receptor specific gold nanorods: breast and prostate tumor avid nano-vectors for molecular imagingNano Lett200991798180519351145
- HarisinghaniMGBarentszJHahnPFNoninvasive detection of clinically occult lymph-node metastases in prostate cancerN Engl J Med20033482491249912815134
- TongSZhouXZhouCA strategy to decorate porous polymer monoliths with graphene oxide and graphene nanosheetsAnalyst20131381549155723350066
- FanYChenXTriggADTungCHKongJGaoZDetection of microRNAs using target-guided formation of conducting polymer nanowires in nanogapsJ Am Chem Soc20071295437544317411036
