Abstract
Background
To explore a new combination of thermal treatment and gene therapy for hepatoma, a heat-inducible herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) gene therapy system was developed in which thermal energy generated by Mn0.5Zn0.5Fe2O4 nanoparticles (MZF-NPs) under an alternating magnetic field was used to activate gene expression.
Methods
First, a recombinant eukaryotic plasmid, pHsp 70-HSV-TK, was constructed as a target gene for therapy. This recombinant plasmid was used to transfect SMMC-7721 hepatoma cells and the gene expression was evaluated. Magnet-induced heating was then applied to cells to assess the antihepatoma effects of the polyethylenimine (PEI)-MZF-NPs/pHsp 70-HSV-TK/GCV complex, in vitro and in vivo.
Results
The results showed that cells were successfully transfected with pHsp 70-HSV-TK and that expression levels of HSV-TK remained stable. Both in vitro and in vivo results indicated that the combination of gene therapy and heat treatment resulted in better therapeutic effects than heating-alone group. The rates of apoptosis and necrosis in the combined treatment group were 49.0% and 7.21%, respectively. The rate of inhibition of cell proliferation in the combined treatment group was significantly higher (87.5%) than that in the heating-alone group (65.8%; P<0.01). The tumor volume and mass inhibition rates of the combined treatment group were 91.3% and 87.91%, respectively, and were significantly higher than the corresponding rates of the heating-alone group (70.41% and 57.14%; P<0.01). The expression levels of Stat3 and Bcl-xL messenger RNA and p-Stat3 and Bcl-xL protein in the combined treatment group were significantly lower than those in the other groups (P<0.01). The expression levels of Bax messenger RNA and protein in the recombinant plasmid group were significantly higher than those in the other groups (P<0.01).
Conclusion
It can therefore be concluded that the combined application of heat treatment and gene therapy has a synergistic and complementary effect and that PEI-MZF-NPs can simultaneously act both as a nonviral gene vector and a magnet-induced source of heat, thereby representing a viable approach for the treatment of cancer.
Introduction
The recent development of plasmid technology has partially addressed the concern of the safety of gene therapy, in addition to resolving the technical issue of the efficiency of transfection of exogenous genes.Citation1 Many highly effective cloning vectors have been established for the transfection of various mammalian cell lines deemed appropriate for clinical gene transfection research. Controlling the expression of a target gene in vivo has been demonstrated to be a critical factor for the success of gene therapy.Citation2–Citation4 In current methods of gene therapy, uncontrollable gene expression occurs when target genes are automatically activated by a viral promoter or the vector itself. This overexpression or inappropriate expression can distort the outcome of gene therapy. Moreover, it may cause fatal results, as in the case of insulin gene therapy for diabetes and other gene therapies employing cytokines, such as tumor necrosis factor alpha and interleukin-12.Citation5–Citation7 These issues raise the question of how to effectively control the expression of exogenous genes in vivo, at a level that mimics their natural temporal and spatial expression. Such questions must be answered prior to embarking upon a clinical trial for a particular gene therapy. Among various inducible gene expression methods, those that rely upon external physical factors are more favorable, because target gene expression occurs according to a specified time and spatial order. It has been reported that both γ radiation and the Egr-1 promoter can induce target gene expression.Citation8–Citation10 However, due to bodily injury caused by ionizing radiation, radiation therapy has limited clinical application.
Hyperthermia is a highly recommended, noninvasive, and nontoxic therapy method.Citation11 The application of heat induces a specific promoter, which in turn induces specific target gene expression in a designated tissue.Citation12 Many hyperthermic methods have been used to induce gene expression to date. For example, Huang et alCitation12 demonstrated that in cultured cells, the expression of a heterologous gene with a heat shock protein 70 (Hsp 70) promoter could be elevated to 500- to 1,000-fold greater than background expression by use of a hot water bath (39°C–43°C). However, this method cannot be easily applied to gene therapy in vivo, due to the challenge of heating specific areas within the human body to the desired temperatures.
In 1997, German scholars Jordan et alCitation13 used nanotechnology combined with magnetic induction thermotherapy (magnetic fluid hyperthermia [MFH]) to effectively treat a tumor, thus providing a novel application for MFH. Not only do tumor cells absorb nanomagnetic materials at a high rate (8- to 400-fold greater than normal cells), but they also distribute these materials evenly into descendent cells. Tumor cells containing nanomagnetic particles are easily killed by MFH,Citation14,Citation15 which is also known as intracellular thermotherapy. This method is target-oriented, that is, increased temperatures occur only in tumor tissues with magnetic particles, and does not affect normal tissues not containing magnetic particles.
Along with the development of nanotechnology, studies on nanomagnetic particles as gene transfer carriers have been performed.Citation16–Citation18 In previous research,Citation19,Citation20 we developed a novel heat-inducible gene expression system in which thermal energy generated by Mn–Zn ferrite magnetic nanoparticles (MZF-NPs) under an alternating magnetic field was used to activate gene expression. There were four main advantages to this system: 1) utilization of the relatively low Curie point of MZF-NPs to control the in vivo hyperthermia temperature and therefore achieving safe and effective heat-inducible transgene expression;Citation21,Citation22 2) use of the promoter of HSPs that are conserved among species from bacteria to humans. It has been shown that promoters of some HSPs can activate gene expression by several 1,000-fold in response to moderate hyperthermia;Citation23 3) in addition to the simplicity of preparation of magnetic nanocarriers, there is no associated cellular toxicity, and surface modification confers on them very high biocompatibility;Citation24 4) gene carriers can physically aggregate rapidly at the target site and with the help of mechanical external forces, carrier concentration is significantly increased, thus removing the main barriers to low gene expression efficiency (slow aggregation of the carrier at the target site and insufficient carrier concentration carrier at the target siteCitation25). In conclusion, it is possible to control target gene expression profile via use of the combination of the Hsp 70 promoter and heating.
Based on preliminary findings from our previous studyCitation20 attempts were made to develop a heat-inducible herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) gene therapy system in which thermal energy generated by MZF-NPs under an alternating magnetic field was used to activate gene expression. Out of several new methods of gene therapy that have been explored for the treatment of cancer, the HSV-TK/GCV system demonstrates the highest efficacy for targeted gene therapy.Citation26 In this system, thymidine kinase, a suicide gene expressed by HSV-TK, converts the nontoxic drug precursor GCV into a toxic drug, which kills tumor cells undergoing rapid growth by penetrating cells and inhibiting DNA synthesis. This system also produces a toxic effect on adjacent, untransfected cancer cells, via the bystander effect. In general, if 10% of target cells are transfected and therefore express the gene of interest, the therapeutic toxic effect will extend to the remainder of the tumor tissue via mechanisms related to gap junctions and apoptosis.Citation27,Citation28 Because this class of enzymes does not exist in mammals, the precursor drug exhibits very low toxicity or is nontoxic to mammalian cells.
In this study, we first constructed a recombinant eukaryotic plasmid, pHsp 70-HSV-TK, as a target gene for therapy. Next, this recombinant plasmid was used to transfect SMMC-7721 hepatoma cells in vitro and in vivo. This system was used to explore a new therapy for hepatoma via a combination of thermal treatment and gene therapy, using polyethylenimine (PEI)-coated MZF-NPs (PEI-MZF-NPs) as the gene transfection vector and magnet-induced media simultaneously. Here, we provide evidence that this combined therapy offers a new strategy for the treatment of cancer.
Materials and methods
Plasmids and reagents
pHSV-106, which harbors the herpes simplex virus type I thymidine kinase gene (HSV-TK), was donated by Professor Lu Daru (Life Sciences Institute of Fudan University, Shanghai, People’s Republic of China); pD3SX (11.1 kb), a mammalian expression vector containing the Hsp 70 promoter was obtained from Stressgen Bioreagents Corp. (Victoria, BC, Canada); PEI (average mW 25 kDa, average degree of polymerization 580) and most of the reagents were purchased from Sigma-Aldrich (St Louis, MO, USA); MagnetoFACTOR plates were purchased from Chemicell (Berlin, Germany). Anti-Bax antibody, anti-Bcl-xL antibody, anti-p-Stat3 antibody, and anti-β-actin antibody were purchased from Immuno Way (Newark, DE, USA). Total RNA extraction kit, reverse transcription kit, high-purity gel extraction kit, SYBR® Premix Ex Taq™ polymerase chain reaction (PCR) kit, and DNA marker were obtained from Takara Biotechnology Co. Ltd. (Dalian, Shiga, Japan).
Construction and identification of the eukaryotic expression plasmid pHsp 70-HSV-TK
PCR amplification of HSV-TK segments
HSV-106 plasmids were used as templates, and HSV-TK fragments were amplified by PCR. The restriction enzyme site SalI was introduced into the primers. The primer sequences were as follows: Forward, 5′-GGTCGACCTTGTAGAAGCGCGTATGGC-3′ and Reverse, 5′-CGGTCGACCGGTA TTGTCTCCTTCCGTG-3′.
The SalI restriction enzyme site sequence was GTC GACC and the product length was 1,168 bp.
Construction of recombinant pHsp 70-HSV-TK plasmid
The HSV-TK fragments were collected, amplified, and subcloned into pD3SX at SalI sites. After transformation, the pHsp 70-HSV-TK recombinant plasmid was purified and identified by electrophoresis of the PCR product. PCR primers were:
Forward: 5′-CAGCAGCCTCCGTGGCCTCCAGCATCCGACAAG-3′ (located at pD3SX);
Reverse: 5′-CGGTCGACCGGTATTGTCTCCTTCCGTG-3′.
Identification of recombinant pHsp 70-HSV-TK plasmid by restriction enzyme digestion and DNA sequencing
Recombinant pHsp 70-HSV-TK plasmid was restriction digested by the SalI enzyme, and correct insertion was confirmed by agarose gel electrophoresis. Uncut pHsp 70-HSV-TK plasmid, uncut pD3SX plasmid, and digested pD3SX plasmid served as controls. A 10 µL sample of purified recombinant pHsp 70-HSV-TK plasmid was dispatched to Shanghai Shengong Co. (Shanghai, People’s Republic of China) for complete DNA sequencing.
Preparation and characterization of Mn– Zn ferrite nanoparticles and PEI-coated Mn–Zn ferrite nanoparticles
Mn–Zn ferrite nanoparticles and PEI-MZF-NPs were prepared and characterized as described previously.Citation19,Citation20,Citation24
Cell lines and establishment of tumors in nude mice
SMMC-7721 cells were provided by the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, Shanghai, People’s Republic of China. All experiments involving use of human cells were performed under the approval of the ethics committee of Southeast University, Nanjing, People’s Republic of China. SMMC-7721 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco Life Technologies, New York, NY, USA), supplemented with 10% fetal bovine serum (HyClone, GE Healthcare Life Sciences, Pittsburgh, PA, USA), streptomycin (100 mg/mL), and penicillin (100 U/mL). All cells were incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were passaged, using trypsin/ethylenediaminetetraacetic acid medium, when almost confluent.
BALB/C nude mice (male, 10 weeks of age) were purchased from the Lakes Animal Experimental Centre of the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. Animal experiments were approved by the Animal Care Committee of Jiangsu Province and were performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health.Citation29 All mice were maintained in the Sterile Barrier System of the Medical School, Southeast University, Nanjing, People’s Republic of China.
SMMC-7721 cells (1×107) were subcutaneously transplanted into the right shoulder blades of nude mice. Tumor-bearing mice were randomized for study when tumors grew to 5mm3 in size. These mice were housed in a special pathogen-free animal facility. All animal experiments were carried out in compliance with the national laws related to the conduct of animal experimentation.
Antihepatoma effects in vitro
Testing expression of HSV-TK by reverse transcription-(RT)-PCR
Before transfection, pHsp 70-HSV-TK and PEI-MZF-NPs were diluted separately in serum- and supplement-free medium. Then, 8 µg of pHsp 70-HSV-TK per well were mixed with PEI-MZF-NPs (20 mg/mL) to form a complex, at an N/P ratio of 5, in a final volume of 800 µL of serum- and supplement-free medium. The complexes were incubated at room temperature for 30 minutes. SMMC-7721 cells were seeded in 60 mm dishes at an initial density of 6×105 cells/well in 5 mL of growth medium. After incubation for 18 hours (to reach 80% confluence at the time of transfection), the medium was replaced with 4.2 mL serum-free medium with 800 µL complexes and incubated for a further 15 minutes on a MagnetoFACTOR plate. The medium was exchanged for fresh medium containing serum prior to incubation.
After incubation for 24 hours, MZF-NPs at 10 g/L final concentration were added, followed by exposure of SMMC-7721/TK (thymidine kinase) cells to a high-frequency alternating electromagnetic field (F=230 kHz, I=28 A), and irradiation for 60 minutes; 72 hours later, total RNA was extracted from SMMC-7721/TK cells. As a control, total RNA of the SMMC-7721 parent cells was also extracted. Infected cells that were not exposed to hyperthermia were also used as controls. Each experiment was carried out in triplicate.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA was used as an internal reference gene. All reagents and consumables used to extract RNA were ribonuclease (RNase)-free or were soaked in diethylpyrocarbonate-treated water. Primer sequences are listed in . A two-step RT-PCR method was used to amplify the genes. Gel electrophoresis (2% agarose) was used to identify the correct products.
Table 1 Primer sequences of PCR
Testing expression of HSV-TK by Western blot
Proteins from the aforementioned groups were extracted in lysis buffer (RIPA; Shengong, Shanghai, People’s Republic of China). Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After washing, the membranes were blocked with 5% skimmed milk at room temperature for 1 hour, incubated with anti-TK antibodies (1:200 dilution) or anti-GAPDH antibody (1:1,000, final dilution) at 4°C overnight, then incubated with secondary antibody for 1 hour. Bound antibody was detected using enhanced chemiluminescence (Thermo Pierce ECL Western Blotting Substrate; Thermo Scientific, Rockford, IL, USA) and quantified using Image J software (Gel-Pro analyzer 4.0; Media Cybernetics, Inc., Rockville, IL, USA) Gel-pro Analyzer.
Treatment groups
After transfection as described earlier and incubation for 24 hours, SMMC-7721/TK cells and SMMC-7721 cells were digested, and then cells were seeded in four culture bottles, grouped as: a) the untransfected SMMC-7721 cells group, which served as a blank control; b) the gene transfection-alone group (without heating); c) the thermotherapy-alone group (without transfection); and d) gene therapy combined with heating therapy group (with transfection and heating).
Twenty-four hours later, GCV at 5 µg/mL final concentration was added to the groups (b) and (d). PEI-MZF-NPs at 10 g/L final concentration were added to the groups (c) and (d). An identical volume of DMEM culture medium was added to the blank control group. The thermal therapy bottles (groups [c] and [d]) were exposed to a high-frequency alternating electromagnetic field (F=230 kHz, I=28 A), and irradiated for 60 minutes.
MTT assay of cell growth and proliferation
Incubation of all groups was continued for 48 hours, after which 20 µL of MTT(3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide) solution (5 g/L in phosphate-buffered saline [PBS]) were added to each well, and the cells were further incubated at 37°C for 4 hours. To each well of the 96-well plate, 150 µL of dimethyl sulfoxide were added to solubilize the formazan crystals, followed by reading of the optical density (OD) at 492 nm using a spectrophotometer (Bio-Tek; BioTek Instruments, Inc., Winooski, VT, USA). Readings of six wells were measured for each group. The following formula was used to calculate the cell growth inhibition rate (IR): IR = (1− the experimental group OD/the negative control group OD) ×100%.
Flow cytometry assay
Cells (2×106) were harvested from each group. After washing with PBS, supernatants from each group were removed and cell pellets were resuspended in 100 mL Annexin-V-FLUOS labeling solution containing 2 mL of Annexin-V-FLUOS and 2 mL of propidium iodide (PI). Cells were incubated for 15 minutes without light at 25°C, then analyzed on a flow cytometer (FCM, Vantage SE; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) within 1 hour.
Cell morphology
For electron microscopy, cells (5×106) were collected and centrifuged at 2,500× g for 10 minutes, fixed in 4% glutaraldehyde at 4°C for 72 hours and washed with PBS. They were then fixed in 1% osmium tetroxide, dehydrated stepwise using acetone after washing with PBS, embedded in EPON 812 (Sigma-Aldrich) after permeation, and converged for 12 hours at 35°C, for 24 hours at 45°C, and for 24 hours at 60°C in turn. Finally, cells were prepared as ultrathin sections (60 nm), stained with uranyl acetate and lead citrate, then viewed using a transmission electron microscope (TEM/model Hitachi 750; Hitachi High Technologies Ltd., Tokyo, Japan).
Animal experiments
Experimental groups and treatment
When tumor diameters reached approximately 0.5 cm, mice were divided into four groups of ten mice each: a) the untransfected SMMC-7721 cells group, which served as a blank control; b) the gene transfection-alone group (without heating); c) the thermotherapy-alone group (without transfection); and d) gene therapy combined with heating therapy group (with transfection and heating).
The method of transfection was as follows: pHsp 70-HSV-TK and PEI-MZF-NPs were diluted separately in saline solution. Before transfection, 20 µg of pHsp 70-HSV-TK per nude mouse were mixed with PEI-MZF-NPs magnetic fluid (20 mg/mL) to form a complex, at an N/P ratio of 5, in a final volume of 200 µL of saline. Then, complexes were incubated at room temperature for 30 minutes. Following a multipoint injection strategy, moving clockwise at the 3, 6, 9, and 12 o’clock positions, 200 µL of complexes were injected into the tumors.
The method of hyperthermia treatment was as follows: 0.046 mL of MZF-NPs MF (net magnetite weight: 4.6 mg) was multipointed infiltrative injected into the tumors, which were then subjected to hyperthermia treatment for 60 minutes. The tumor temperature was measured at multiple points using an infrared thermometer (UMI, FISO Technologies Inc., Saint–Foy, QC, Canada).
For groups (b) and (d), gene transfection was performed once per week, while 100 mg GCV/kg intraperitoneal injections were performed every day. For groups (c) and (d), hyperthermia treatment was performed once every 3 days. For group (a), 200 µL of sterile normal saline were injected intraperitoneally every day.
Mice were sacrificed after 6 weeks, and the mass and volume of each tumor were measured. Tumor growth inhibition was evaluated by measuring mass and volume inhibition proportions. Mass inhibition was calculated as (1− relative tumor mass) ×100%, where relative tumor mass was the mean tumor mass of the experimental group divided by the mean tumor mass of the blank control group. Similarly, volume inhibition was calculated as (1− relative tumor volume) ×100%, where relative tumor volume was the mean tumor volume of the experimental group divided by the mean tumor volume of the blank control group.
Expression of HSV-TK in vivo
Total RNA was extracted from the aforementioned groups, both for evaluation of the expression of HSV-TK as described in “Testing expression of HSV-TK by reverse transcription (RT)-PCR” section”. PCR products were separated on 2% agarose gel by electrophoresis and visualized by ethidium bromide staining.
Real-time PCR assay
To quantify the Stat3, Bcl-xl, and Bax messenger RNA (mRNA) expression, quantitative PCR was performed. Real-time PCR was performed using the SYBR® Premix Ex Taq™ PCR kit (Takara Biotechnology Co. Ltd.) on the Applied Biosystems 7300 Real-Time PCR System (Foster, CA, USA). The 20 µL reaction of the SYBR Green assay contained 10 µL of 2× SYBR Premix Ex Taq, 0.4 µL PCR forward primers and 0.4 µL reverse primers, 0.4 µL ROX reference dye (50×), 2 µL cDNA, and 6.8 µL double-distilled H2O. PCR was carried out as follows: one cycle of 95°C for 10 minutes, and 40 cycles of three steps (95°C for 5 seconds, 60°C for 45 seconds, and 72°C for 30 seconds). Data were normalized to GAPDH and calculated using the ΔΔCt method.Citation30 Primer sequences are listed in .
Western blot immunoassay
The p-Stat3, Bcl-xL, and Bax proteins levels were determined by a protein imprinting method (Western blot). Proteins from the aforementioned groups were extracted in lysis buffer (RIPA, shenggong, shanghai People’s Republic of China). Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After washing, the membranes were blocked with 5% skimmed milk at room temperature for 1 hour, incubated with anti-p-Stat3, anti-Bax, or anti-Bcl-xL antibodies (1:1,000 dilution) or anti-β-actin antibody (1:1,000, final dilution) at 4°C overnight, then incubated with secondary antibody for 1 hour. Bound antibody was detected using enhanced chemiluminescence (Thermo Pierce ECL Western Blotting Substrate; Thermo Scientific) and quantified using ImageJ software (Gel-Pro analyzer 4.0; Media Cybernetics, Inc.; Rockville; USA). Data were normalized to loading control and expressed as relative p-Stat3, Bcl-xL, and Bax proteins levels compared to corresponding control.
Immunohistochemistry analysis in vivo
To further examine the heating effects on the tumor, an immunohistochemical analysis of tumors was performed. The tumor sections were first probed with a polyclonal rabbit anti-mouse apoptosis regulator Bax (Bcl-2-associated X protein) antibody (1:400) at 4°C overnight, followed by incubation with biotinylated polyclonal goat anti-rat antibody (1:200), in a humidified chamber for 1 hour, and were then immersed in 0.3% H2O2 in absolute methanol for 15 minutes to block endogenous peroxidase. In succession, the sections were counterstained with hematoxylin and mounted with glass coverslips, and were visualized in an optical microscope.
Statistical analysis
All values are expressed as mean ± standard deviation. Student’s t-test was used to identify significant differences between groups. Values of P<0.05 were considered statistically significant. All tests were performed using SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA).
Results and discussion
Identification of the pHsp 70-HSV-TK plasmid
Identification of pHsp 70-HSV-TK by PCR
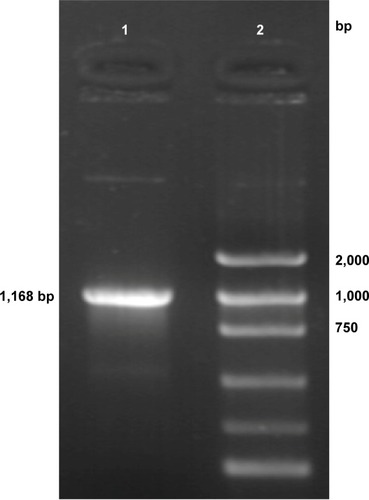
PCR amplification of the HSV-TK gene segment was carried out using pHSV-106 plasmid as a template. The PCR product was electrophoresed on a 10 g/L agarose gel, which resulted in a gene segment of 1,168 bp ().
Figure 1 PCR amplification of HSV-TK gene from the pHSV-106 plasmid.
Notes: Lane 1, HSV-TK gene PCR amplification products; lane 2, marker (each band in turn from bottom to top is 100, 300, 500, 750, 1,000, and 2,000 bp).
Abbreviations: bp, base pair; HSV-TK, herpes simplex virus thymidine kinase; PCR, polymerase chain reaction.
Identification of pHsp 70-HSV-TK by restriction enzyme digestion
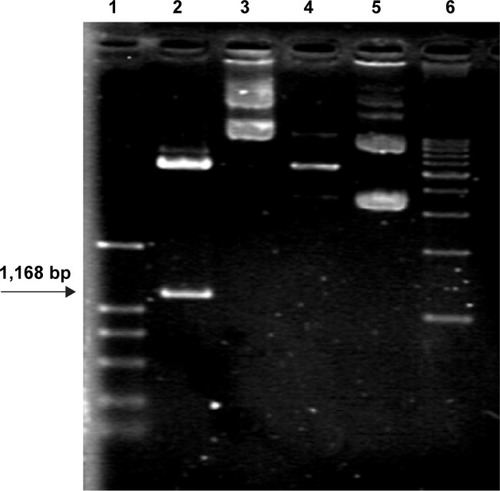
It was expected that digestion of the constructed pHsp 70-HSV-TK gene with SalI would generate a HSV-TK fragment of 1,168 bp. As shown in , agarose gel electrophoresis of pHsp 70-HSV-TK digested with SalI clearly showed a band of ~1,168 bp in lane 2, which corresponded to the recombinant plasmid pHsp 70-HSV-TK.
Figure 2 pHsp 70-HSV-TK was identified by restriction enzyme digestion.
Notes: Lane 1, marker (each band in turn from bottom to top is 100, 300, 500, 750, 1,000, and 2,000 bp); lane 2, pHsp 70-HSV-TK was cut by SalI; lane 3, pHsp 70-HSV-TK was not cut by any restriction endonucleases; lane 4, pD3SX was cut by SalI; lane 5, pD3SX was not cut by any restriction endonucleases; lane 6, marker (each band in turn from bottom to top is 1,000, 2,000, 3,000, 4,000, 5,000, 6,000, 7,000, and 8,000 bp).
Abbreviations: bp, base pair; HSV-TK, herpes simplex virus thymidine kinase.
Identification of pHsp 70-HSV-TK by DNA sequencing
According to the HSV-TK sequence data from DNASTAR and GenBank, the PD3sx-98 was the correct clone, the HSV-TK gene segment was successfully inserted into pD3SX, and the inserted DNA sequence was 100% accurate.
Gene integration of HSV-TK in SMMZ-7721 cells mediated by PEI-MZF-NPs
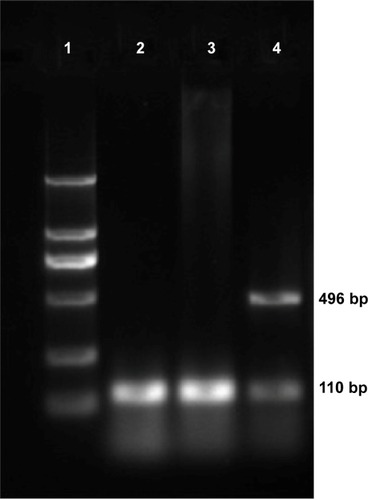
Expression of HSV-TK mRNA in SMMC-7721 cells transfected with PEI-MZF-NPs was evaluated by RT-PCR. The GAPDH gene was used as an internal control to rule out any operational error in the PCR process and to determine the quality of HSV-TK cDNA. As shown in , RT-PCR using mRNA from SMMC-7721 cells transfected and heated display two distinct genetic fragments of 496 bp and 110 kb, which corresponded to the lengths of HSV-TK and GAPDH, respectively. Only a band at 110 kb (corresponding to GAPDH) was detected in the SMMC-7721 cells without transfection. Furthermore, there is also only a band at 110 kb (corresponding to GAPDH) in the transfected SMMC-7721cells that did not receive heating. These results suggested that SMMC-7721 cells were successfully transfected with pHsp 70-HSV-TK mediated by PEI-MZF-NPs, and that the gene expression was activated by MFH.
Figure 3 Gel electrophoresis of HSV-TK amplified by RT-PCR after pHsp 70-HSV-TK transfected into SMMC-7721 cells.
Notes: Lane 1, marker (each band in turn from bottom to top is 100, 300, 500, 750, 1,000, and 2,000 bp); lane 2, infected cells that did not receive hyperthermia with only one strip, 110 bp of GAPDH; lane 3, untransfected SMMC-7721 cells with only one strip, 110 bp of GAPDH; lane 4, infected cells that receive hyperthermia with two clear strips, 496 bp of HSV-TK and 110 bp of GAPDH used as reference gene.
Abbreviations: bp, base pair; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSV-TK, herpes simplex virus thymidine kinase; RT-PCR, reverse transcription-polymerase chain reaction.
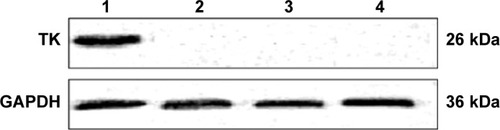
Expression of TK proteins in SMMC-7721 cells transfected with PEI-MZF-NPs was evaluated by Western blot. The GAPDH gene was used as an internal control. As shown in , proteins from SMMC-7721 cells transfected and heated display two distinct band of 26 and 36 kDa, which corresponded to the protein expression of HSV-TK and GAPDH, respectively. Only a band at 36 kDa (corresponding to GAPDH) was detected in the SMMC-7721 cells without transfection. Furthermore, there is also only a band at 36 kDa (corresponding to GAPDH) in the transfected SMMC-7721cells that did not receive heating. These results suggested that SMMC-7721 cells were successfully transfected with pHsp 70-HSV-TK mediated by PEI-MZF-NPs, and that the gene expression was activated by MFH.
Figure 4 Western blot analysis of thymidine kinase (TK) protein expression. TK =26 kDa; GAPDH =36 kDa.
Notes: Lane 1, blank control; lane 2, gene transfection-alone group; lane 3, the thermotherapy-alone group; lane 4, the combined therapy group.
Abbreviation: GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
In vitro therapeutic effects on SMMC-7721 cells
Inhibition of SMMC-7721 cell proliferation was assessed by MTT
The effect of HSV-TK/GCV gene therapy combined with hyperthermia on the proliferation of SMMC-7721 cells for 48 hours was determined by MTT assay. As shown in , cytotoxic effects were increased in the group treated with combined HSV-TK/GCV gene therapy and hyperthermia compared with the other groups, with an IR of 87.5%. By contrast, the IR value for the thermotherapy-alone group was 65.8%. The difference in cytotoxic effect between the HSV-TK/GCV gene therapy combined with hyperthermia group compared with the other groups was statistically significant (P<0.01). These data suggested that the combination of HSV-TK/GCV gene therapy with hyperthermia was more effective in inhibiting cell proliferation than hyperthermia alone.
Table 2 SMMC-7721 cell proliferation inhibition rates of different treatments
As far as the gene therapy-alone group is concerned, the IR value was 1.97%. The difference in cytotoxic effect between the gene therapy-alone group compared with the control group was not statistically significant (P>0.05). These data suggested that the gene therapy-alone group has no cytotoxic effect because the gene expression was not activated by MFH.
Flow cytometric analysis of apoptosis
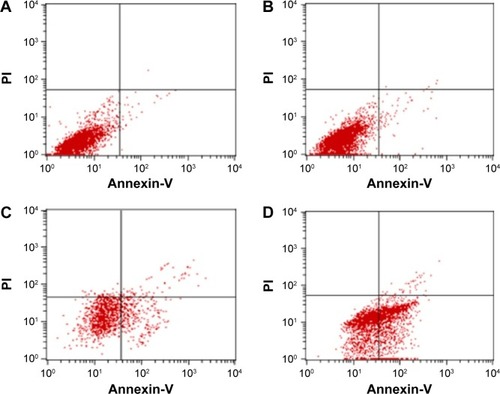
SMMC-7721 cells were stained with Annexin-V and PI and analyzed by flow cytometry to determine whether growth inhibition induced by various treatments was caused by apoptosis or necrosis. Generally speaking, in the early stages of apoptosis, changes occur only at the cell surface. One such alteration is the translocation of phosphatidylserine (PS) from the inner part of the plasma membrane to the outer layer, exposing PS at the cell’s external surface, which can be detected by Annexin-V staining. Since PS is also exposed in necrotic cells according to the loss of membrane integrity, apoptotic cells must be differentiated from necrotic cells. The simultaneous application of a DNA stain, PI, which is used for dye exclusion tests, stains only necrotic cells, thus allowing discrimination between these two cell types.
As shown in and , both apoptotic and necrotic cells were present in all treatment groups, with apoptotic cells being the major constituent. The group in which gene therapy and thermal treatment were combined exhibited the highest efficacy; the rates of apoptosis and necrosis in this group were 49.0% and 7.21%, respectively. By contrast, the rates were 14.49% and 9.62% in the thermotherapy-alone group, 1.53% and 0.11% in the gene therapy-alone group, and 1.04% and 0.07% in the blank control group.
Table 3 The apoptotic and necrotic rate (%) of different therapy groups detected by flow cytometric analysis
Figure 5 Apoptosis and necrosis of SMMC-7721 cells analyzed by flow cytometry after different treatments.
Notes: (A) The blank control group. The AR and NR was, respectively, 1.04% and 0.07%. (B) The gene transfection-alone group. The AR and NR was, respectively, 1.53% and 0.11%. (C) The thermotherapy-alone group. The AR and NR was, respectively, 14.49% and 9.62%. (D) Gene therapy combined with heating therapy group. The AR and NR was, respectively, 49.00% and 7.21%.
Abbreviations: AR, apoptotic rate; NR, necrotic rate; PI, propidium iodide.
Cell ultrastructural examination
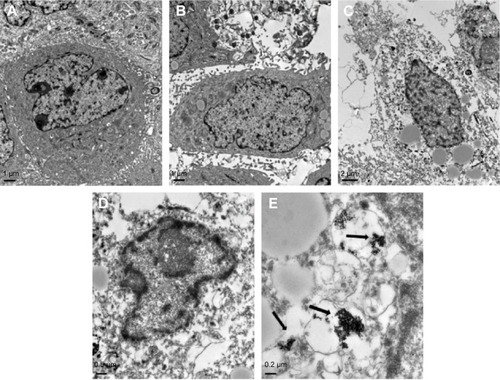
SEM examination revealed that the cells of control group and gene transfection-alone group have rather regular shape with intact nuclei, fine chromatin, and a large, clear nucleolus; and the characteristic features of normal cell surface such as numerous microvilli and lamellipodia extensions are shown in . By contrast, in the pHsp 70-HSV-TK gene combined heat treatment group and thermotherapy-alone group, the cell membrane and organelles disintegrated into pieces. SMMC-7721 cells were observed to exhibit morphological features typical of apoptotic cells, such as chromatin condensation and chromatin margination or cleavage, and apoptotic body formation (). Scattered or flake-gathered high electron density materials are visible in the combined treatment group cells ().
Figure 6 Ultrastructure of SMMC-7721 cells (observed by TEM).
Notes: Sections from the blank control group (A); gene transfection-alone group (B); thermotherapy-alone group (C); and combined therapy group (D and E) are shown. TEM examination revealed that the necrotic areas in C and D were much larger than the intrinsic necrosis of the tumor shown in A and B. The black arrows indicate the magnetic nanoparticles. Magnification: A–C ×6,000; D ×12,000; E ×30,000.
Abbreviation: TEM, transmission electron microscopy.
In vivo therapeutic effects on SMMC-7721 cells
Gene integration of HSV-TK mediated by PEI-MZF-NPs
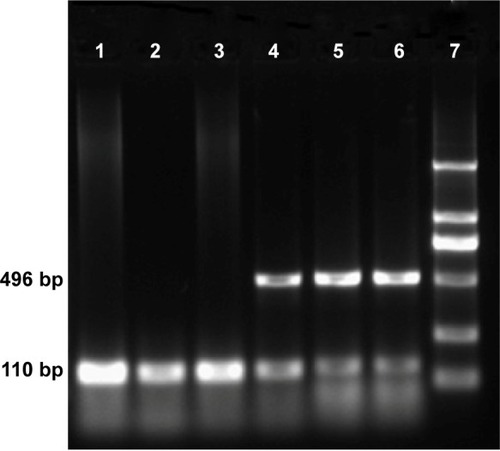
Tumors from all treatment groups were excised 24 hours after hyperthermia treatment, and total RNA was extracted and subjected to RT-PCR. As shown in , two distinct genetic fragments of 496 bp and 110 kb, corresponding to the lengths of HSV-TK and GAPDH, were observed in the gene transfection combined with hyperthermia treatment group, whereas only a band at 110 kb (GAPDH) was observed in the gene transfection-alone group and blank control group. These results suggested that SMMC-7721 cells were successfully transfected with pHsp 70-HSV-TK, mediated by PEI-MZF-NPs, and that gene expression was activated by MFH.
Figure 7 Gel electrophoresis of HSV-TK amplified by RT-PCR after pHsp 70-HSV-TK transfected into SMMC-7721 cells.
Notes: Lane 1, the thermotherapy-alone group with only one strip, 110 bp of GAPDH; lane 2, the gene transfection-alone group with only one strip, 110 bp of GAPDH; lane 3, the blank control group with only one strip, 110 bp of GAPDH; lanes 4–6, gene therapy combined with heating therapy group with two clear strips, 496 bp of HSV-TK and 110 bp of GAPDH used as reference gene; lane 7, marker (each band in turn from bottom to top is 100, 300, 500, 750, 1,000, and 2,000 bp).
Abbreviations: bp, base pair; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSV-TK, herpes simplex virus thymidine kinase; RT-PCR, reverse transcription-polymerase chain reaction.
In vivo antihepatoma effect
To avoid liquid overflow, to ensure fluid dispersion in tumor tissues, and to transfect the maximum possible number of tumor cells, a multipoint injection strategy was applied, in which MZF-NPs or the PEI-MZF-NPs/pHsp 70-HSV-TK complex were injected into each tumor at the 3, 6, 9, and 12 o’clock positions.
As shown in , tumor growth in nude mice exposed to the combination of gene therapy with heat treatment was inhibited to a greater degree than in those treated with MZF magnetic induction heating treatment alone. The tumor volume and mass IR of group (d) were 91.30% and 87.91%, respectively, significantly higher than the 70.41% and 57.14% of group (c). As far as the gene therapy-alone group is concerned, the tumor volume and mass IR were 0.79% and 1.64%, respectively. The difference between the gene therapy-alone groups compared with the control groups was not statistically significant (P>0.05). These data suggested that the gene therapy-alone group has no cytotoxic effect because the gene expression was not activated by MFH.
Table 4 Volume and mass inhibition rates of SMMC-7721 cell in nude mice after different treatments
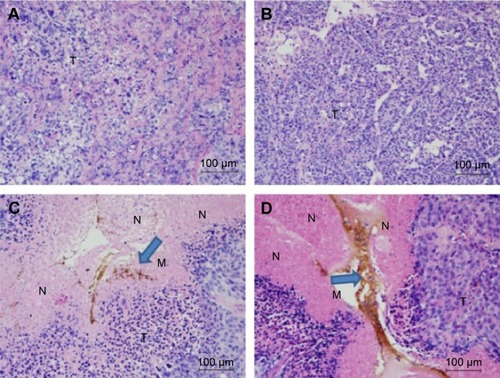
Histological examination revealed that, in both the pHsp 70-HSV-TK gene combined heat treatment group and thermotherapy-alone group, many black nanoparticles accumulated in the stroma of the tumors, with widespread tumor necrosis surrounding the nanoparticles. The necrotic areas of the groups (c) and (d) were larger than those of the control group and gene therapy-alone group ().
Figure 8 Histological changes in xenograft tumors (H & E).
Notes: Sections from the blank control (A); gene transfection-alone group (B); thermotherapy-alone group (C); and combined therapy group (D) are shown. Histological examination revealed that the necrotic areas in C and D were much larger than the intrinsic necrosis of the tumor shown in A and B. The blue arrows indicate the magnetic nanoparticles. Magnification ×200.
Abbreviations: H & E, hematoxylin and eosin; M, materials of Mn0.5Zn0.5Fe2O4 nanoparticles; N, necrosis; T, tumor tissue.
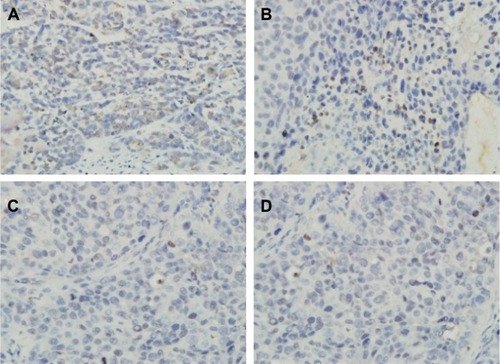
Bax staining assay was performed here to determine the apoptosis of the cells in the tumor after four different treatments (). It indicated that the tumors treated with pHsp 70-HSV-TK gene combined heat treatment group and thermotherapy-alone group have the extensive regions of apoptotic cells (), and the number of apoptotic cells was significantly more than those of the control group and gene therapy-alone group ().
Figure 9 Immunohistochemical staining for Bax.
Notes: Representative immunohistochemical staining with Bax polyclonal antibodies in each group. Apoptosis cells are identified by dark brown (original magnification, ×400). It is evident that the abundance of apoptosis tumor cells is higher in the combined therapy group (A) and the thermotherapy-alone group (B) than in the gene transfection-alone group (C) or blank control (D).
Abbreviation: Bax, Bcl-2 associated X protein.
The previous data indicate that the combination of gene therapy and magnetic induction heating on this type of cancer, using MZF magnetic nanoparticles as the link, can achieve a synergistic effect; that is, 1+1>2. Combined therapy is not simply a combination of thermotherapy and HSV-TK/GCV suicide gene therapy, but requires their synergism and complementarities.
Over the past few years, hyperthermia has a wide-ranging application in the treatment or adjuvant treatment of malignant tumor, but the ability to kill the tumor cells is limited, hence the application of hyperthermia gradually faded away in recent years. However, with the combination of hyperthermia and gene therapy, not only can target gene play a better therapeutic role, but also it is a good method to reduce the side effects caused by the gene therapy. Therefore this combination therapy method will provide a new way for therapy for cancer or other disease.
Heating can induce gene expression: on the one hand, heat-induced gene expression can increase the lethality of hyperthermia; on the other hand, it can decrease the expression of target gene. Hyperthermia can also enhance the effect of gene therapy and play a synergistic antitumor effect; the mechanism may be as follows: 1) High temperature may increase the perfusion of capillary, then the distribution of the drug is changed with local drug concentration increasing. 2) High temperature damages the stability of tumor cells, and increases the permeability of the cell membrane, which make it easy for drug entering the cell, raising the intracellular concentration of the drug. 3) High temperature changes the cytotoxicity of the drug. At 37°C, the drug has no cytotoxicity, but at 40°C it shows significant toxicity. 4) Under effective temperature, the synthesis of HSP that induces drug tolerance is decreased, thereby preventing the occurrence of heat tolerance; this situation has been confirmed in the drugs tolerance experiments such as mitomycin, bleomycin, cisplatin, and so on.Citation31,Citation32 5) Hyperthermia inhibits DNA damage response mediated by DNA polymerase, which inhibits the repairmen of tumor cells with sublethal damage and potentially lethal damage after injury caused by the chemotherapy drugs.Citation33 It can also significantly increase the oxygen content of the tumor, and enhance the treatment sensitivity.Citation34 6) With the combination of hyperthermia and chemotherapy, apoptosis may occur in more tumor cells.Citation35 Demonstrated that under the same time and concentration, hyperthermia combinations with paclitaxel was used to treat liver cancer, thermochemotherapy shows a higher apoptosis rate than the sum of hyperthermia and chemotherapy, the mechanism may be related to the inhibition of the expression of bcl-2 and promoting apoptosis. 7) Hyperthermia can reduce vascular endothelial growth factor synthesized and secreted by tumor, which may lead to the destruction and reduction of tumor angiogenesis.Citation36 Vascular endothelial cells, unlike tumor cells, are not easy to mutate. Vascular endothelial cells rarely establish resistance, and inhibiting tumor angiogenesis to prevent tumor growth also has a magnifying effect.Citation37 8) Blood supply is sufficient in the peripheral portion of the tumor, where the drug is easy to reach, so pharmacologic therapy has advantages on the treatment of peripheral portion. Combining with hyperthermia, the therapeutic effect can cover all tumors.
In summary, in this experiment, we placed promoter Hsp 70 in the upstream of the TK gene, and constructed a eukaryotic expression vector pHsp 70-HSV-TK induced by heat that can be used to gene therapy and increase the expression of HSV-TK gene, thereby the efficiency of killing hepatoma cells are improved. The therapeutic effect of liver cancer is induced by heat and gene therapy, which provide a basis for hyperthermia and gene therapy of liver cancer. This new method of combining gene therapy and hyperthermia is known as gene–hyperthermia therapy, and this method deals with the problems in the practice of hyperthermia or gene therapy alone, which provide a new framework for the treatment of cancer.
Determination of Stat3, Bcl-xL, and Bax expression levels by real-time fluorescent quantitative PCR (real-time PCR) and Western blot immunoassay
Signal transducer and activator of transcription 3 (Stat3) is one of the most important members of the signal transducer and activator of transcriptional family (which includes Stat1–4, Stat5a and Stat5b, and Stat6). Constitutive activation of phosphorylated Stat3 induces the expression of the antiapoptotic genes Bcl-2, Bcl-xL, survivin, Mcl-1, and XIAP, the cell cycle regulation genes cyclin D1 and c-myc and the angiogenesis-related gene vascular endothelial growth factor. This leads to abnormal cell proliferation, malignant transformation, enhanced tumor resistance to apoptosis, promotion of tumor invasion, metastasis, angiogenesis, and enhanced tumor multidrug resistance by downregulation of the expression of antiapoptotic genes such as Bax, Bak, and Bid.Citation38,Citation39 Studies have suggested that the persistent activation of Stat3 plays a key role in resistance to apoptosis of signal transduction pathways in hepatocellular carcinoma.Citation40,Citation41
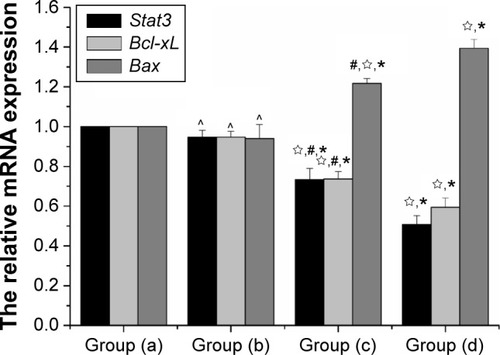
In this study, real-time PCR was used to evaluate the differential expression of Stat3, Bcl-xL, and Bax in different groups. Following normalizing against the house-keeping gene, relative expression of Stat3, Bcl-xL, and Bax was 0.5067±0.045, 0.5933±0.0472, and 1.3933±0.1509 in the gene therapy combined with heating therapy group, respectively. In contrast, it was only 0.9467±0.035, 0.9417±0.0305, and 0.94±0.07 in the gene transfection-alone group, and only 0.7333±0.0568, 0.7367±0.0378, and 1.2167±0.1117 in the thermotherapy-alone group. mRNA expression of Stat3 and Bcl-xL was downregulated, while that of Bax was upregulated in the combination therapy group and the thermotherapy-alone group, compared to the blank control group (P<0.01) (). But, the relative expression of Stat3, Bcl-xL, and Bax in the gene transfection-alone group did not show significant changes compared to the blank control group (P>0.05) ().
Figure 10 The relative mRNA expression of Stat3, Bax, Bcl-xL mRNA was isolated and subjected to quantitative PCR analysis.
Notes: Data were normalized to GAPDH levels, data are reported as mean ± standard deviation (N=10), data represent the mean of ten independent experiments each performed in technical triplicate. Group (a), blank control; group (b), gene transfection-alone group; group (c), the thermotherapy-alone group; group (d), the combined therapy group. *P<0.05, compared with blank control; ☆compared with gene transfection-alone group; #P<0.05, compared with the combined therapy group; ^P>0.05, compared with the blank control group.
Abbreviations: Bax, bcl-2 associated X protein; Bcl-xL, B-cell lymphoma-extra large; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA; PCR, polymerase chain reaction; Stat3, signal transducer and activator of transcription 3.
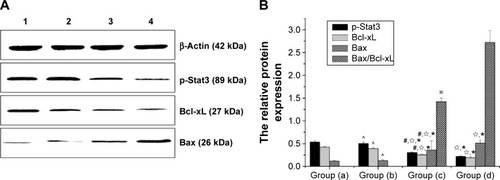
Furthermore, Western blot analysis also manifested that the protein levels of p-Stat3 and Bcl-xL were downregulated, while the level of Bax was upregulated in the recombinant therapy group, compared to the other three groups (P<0.01) (). Meanwhile, we examined whether these therapy methods could alter the balance between proapoptotic Bax and antiapoptotic Bcl-xL proteins at the mitochondrial membrane and which therapy methods caused the greatest impact. As shown in , compared with the control group, groups (c) and (d) all resulted in a decrease in Bcl-xL and an increase in Bax, with an increase in the Bax/Bcl-xL ratio. However, the gene therapy combined with hyperthermia group had the greatest impact, inducing an increase in the Bax/Bcl-xL ratio to 2.716. This indicates that the combined therapy exhibited a rapid response to induce apoptosis of tumor cells.
Figure 11 Western blot analyses of p-Stat3, Bcl-xL, and Bax protein expression. p-Stat3 =89 kDa; Bcl-xL =27 kDa; Bax =26 kDa; β-actin =42 kDa.
Notes: (A) Bax, pStat3, and Bcl-xL analysis by Western blot. Lane 1, blank control; lane 2, gene transfection-alone group; lane 3, the thermotherapy-alone group; lane 4, the combined therapy group. (B) Corresponding optical density ratio of p-Stat3/β-actin, Bcl-xL/β-actin, Bax/β-actin, and Bax/Bcl-xL. Data are reported as mean ± standard deviation (N=10). Group (a), blank control; group (b), gene transfection-alone group; group (c), the thermotherapy-alone group; group (d), the combined therapy group. *P<0.05, compared with blank control; ☆compared with gene transfection-alone group; #P<0.05, compared with the combined therapy group; ^P.0.05, compared with the blank control group; ※P<0.05, compared with the combined therapy group.
Abbreviations: Bax, bcl-2 associated X protein; Bcl-xL, B-cell lymphoma-extra large; Stat3, signal transducer and activator of transcription 3.
Therefore, we proposed that the effects of this combination therapy on liver cancer in nude mice might occur through guanosine triphosphate c oxygen (ganciclovir triphosphate [GCV-TP]) suppression of Stat3 activation, which in turn blocks the Stat3 signal transduction pathway, leading to downregulation of Bcl-xL and upregulation of Bax, thus inhibiting tumor growth.
Conclusion
In this study, we explored a novel combination method of tumor therapy. We successfully transfected SMMC-7721 cells with pHsp 70-HSV-TK, resulting in sustained and stable transgenic expression. Furthermore, MZF-NPs were used as magnetic media for thermotherapy, and an Hsp 70 promoter was employed to strengthen the induction and modulation of HSV-TK gene expression by thermotherapy. Based on MFH, gene therapy and nanotechnology were combined organically. Both in vitro and in vivo experimental results indicated that that combined treatment provides a superior therapeutic effect compared with any of the therapies individually.
This novel combination of two therapeutic methods suggests the feasibility of this potential novel clinical approach to combination treatment of tumors; thus, further research is warranted.
Acknowledgments
This work was supported by the National Key Basic Research Program of China (973 Program) (2013 CB933904), the Natural Science Foundation of Jiangsu Province (BK2012335), and the National Natural Science Foundation of China (81271635, 81071881, and 81201131).
Disclosure
The authors report no conflicts of interest in this work.
References
- HalimNSSAFakiruddinKSAliSAA comparative study of non-viral gene delivery techniques to human adipose-derived mesenchymal stem cellInt J Mol Sci2014159150441506025162825
- CarterJRKeithJHFraserTSEffective suppression of dengue virus using a novel group-I intron that induces apoptotic cell death upon infection through conditional expression of the Bax C-terminal domainVirol J201411111124927852
- ZhaoWSpatzSZhangZNewcastle disease virus (NDV) recombinants expressing infectious laryngotracheitis virus (ILTV) glycoproteins gB and gD protect chickens against ILTV and NDV challengesJ Virol201488158397840624829337
- LengYWuCLiuZRNA-mediated gene silencing in the cereal fungal pathogen Cochliobolus sativusMol Plant Pathol201112328929821356000
- MehtaVAbi-NaderKNCarrDMonitoring for potential adverse effects of prenatal gene therapy: use of large animal models with relevance to human applicationMethods Mol Biol201229132822648778
- RyffelBInterleukin-12: role of interferon-γ in IL-12 adverse effectsClin Immunol Immunopathol199783118209073529
- SigelJESkacelMBergfeldWFThe utility of cytokeratin 5/6 in the recognition of cutaneous spindle cell squamous cell carcinomaJ Cutan Pathol2001281052052411737521
- ManomeYKuniedaTWenPYTransgene expression in malignant glioma using a replication-defective adenoviral vector containing the Egr-1 promoter: activation by ionizing radiation or uptake of radioactive iododeoxyuridineHum Gene Ther1998910140914179681412
- HallahanDEMauceriHJSeungLPSpatial and temporal control of gene therapy using ionizing radiationNat Med1995187867917585181
- TakahashiTNamikiYOhnoTInduction of the suicide HSV-TK gene by activation of the Egr-1 promoter with radioisotopesHum Gene Ther1997878278339143908
- WaltherWSteinUSchlagPMUse of the human MDR1 promoter for heat-inducible expression of therapeutic genesInt J Cancer200298229129611857422
- HuangQHuJKLohrFHeat-induced gene expression as a novel targeted cancer gene therapy strategyCancer Res200060133435343910910053
- JordanAWustPScholzRCellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitroInt J Hyperthermia19961267057228950152
- RenYZhangHChenBMultifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistanceInt J Nanomedicine201172261226922619560
- HuangHSHainfeldJFIntravenous magnetic nanoparticle cancer hyperthermiaInt J Nanomedicine201382521253223901270
- LiuLLiuXXuQSelf-assembled nanoparticles based on the c (RGDfk) peptide for the delivery of siRNA targeting the VEGFR2 gene for tumor therapyInt J Nanomedicine201493509352625114522
- SantosJSousaFQueirozJRhodamine based plasmid DNA nanoparticles for mitochondrial gene therapyColloids Surf B Biointerfaces201412112914024967548
- FargnoliASMuAKatzMGAnti-inflammatory loaded poly-lactic glycolic acid nanoparticle formulations to enhance myocardial gene transfer: an in-vitro assessment of a drug/gene combination therapeutic approach for direct injectionJ Transl Med201412117124934216
- TangQZhangDGuNSynthesis and in vitro study of PEI-coated Mn-Zn ferrite-A novel gene vectorJ Fun Mater20073881268
- TangQZhangDCongXUsing thermal energy produced by irradiation of Mn–Zn ferrite magnetic nanoparticles (MZF-NPs) for heat-inducible gene expressionBiomaterials200829172673267918396332
- KuznetsovAALeontievVGBrukvinVALocal radiofrequency-induced hyperthermia using CuNi nanoparticles with therapeutically suitable Curie temperatureJ Magn Magnetic Mater20073111197203
- ArshakKIAjinaAEganDDevelopment of screen-printed polymer thick film planner transformer using Mn–Zn ferrite as core materialMicroelectron J2001322113116
- ItoAShinkaiMHondaHHeat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapyCancer Gene Ther20018964965411593333
- YuanCYTangQSZhangDSBiocompatibility of Mn0.4Zn0.6Fe2O4 magnetic nanoparticles and their thermotherapy on VX2-carcinoma-induced liver tumorsJ Nanosci Nanotechnol2015151748426328307
- HuthSLausierJGerstingSWInsights into the mechanism of magnetofection using PEI-based magnetofectins for gene transferJ Gene Med20046892393615293351
- HigashiKHazamaSArakiAA novel cancer vaccine strategy with combined IL-18 and HSV-TK gene therapy driven by the hTERT promoter in a murine colorectal cancer modelInt J Oncol20144541412142025051201
- ShaoDLiJXiaoXReal-time visualizing and tracing of HSV-TK/GCV suicide gene therapy by near-infrared fluorescent quantum dotsACS Appl Mater Interfaces2014614110821109024972118
- YinXYuBTangZBifidobacterium infantis-mediated HSV-TK/GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancerCancer Gene Ther2013202778123258087
- National Institutes of HealthGuide for the Care and Use of Laboratory Animals NIH Publication No 86-23, Revised 1996Washington, DCUS Government Printing Office1996
- LitvakKJSchmittgenTDAnalysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) methodMethods200125440240811846609
- WallnerKELiGCEffect of drug exposure duration and sequencing on hyperthermic potentiation of mitomycin-C and cisplatinCancer Res19874724934953098412
- MasunagaSINishimuraYHiraokaMEfficacy of mild temperature hyperthermia in combined treatments for cancer therapyThermal Med2007233103112
- OeiALVriendLEMCrezeeJEffects of hyperthermia on DNA repair pathways: one treatment to inhibit them allRadiat Oncol201510116526245485
- LuoHXuMZhuXLung cancer cellular apoptosis induced by recombinant human endostatin gold nanoshell-mediated near-infrared thermal therapyInt J Clin Exp Med2015868758876626309527
- SaediTAGharfourianSJafarlouMBerberis vulgaris fruit crude extract as a novel anti-leukaemic agentJ Biol Regul Homeost Agents201529239539926122228
- WaniKDKaduBSMansaraPSynthesis, characterization and in vitro study of biocompatible Cinnamaldehyde functionalized magnetite nanoparticles (CPGF NPs) for hyperthermia and drug delivery applications in breast cancerPLoS One201499e10731525268975
- DvorakHFVascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapyJ Clin Oncol200220214368438012409337
- BrombergJFWrzeszczynskaMHDevganGStat3 as an oncogeneCell199998329530310458605
- NetchiporoukELitvinovIVMoreauLDeregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progressionCell Cycle201413213331333525485578
- YcoLPMoczGOpoku-AnsahJWithaferin A inhibits STAT3 and induces tumor cell death in neuroblastoma and multiple myelomaBiochem Insights2014711325452693
- LinWLLaiDYLeeYJAntitumor progression potential of morusin suppressing STAT3 and NFκB in human hepatoma SK-Hep1 cellsToxicol Lett2015232249049825476160
