Abstract
In order to study the effects of iron oxide (IO) nanoparticles on Staphylococcus aureus, IO nanoparticles were synthesized via a novel matrix-mediated method using polyvinyl alcohol (PVA). The IO nanoparticles were characterized by transmission electron microscopy and dynamic light scattering. Further, S. aureus were grown in the presence of three different IO nanoparticle concentrations for four, 12, and 24 hours. Live/dead assays were performed and the results provide evidence that IO/PVA nanoparticles inhibited S. aureus growth at the highest concentration (3 mg/mL) at all time points.
Introduction
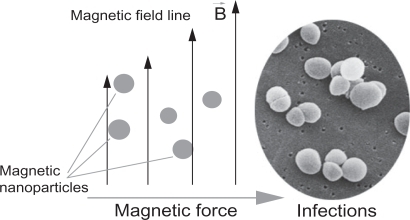
Iron oxide (IO) has been widely used in biomedical research because of its biocompatibility and magnetic properties.Citation1,Citation2 Nanoparticles of IO, with sizes less than 100 nm, have been developed as contrast agents for magnetic resonance imaging (MRI),Citation3,Citation4 as hyperthermia agents,Citation5,Citation6 and as carriers for targeted drug delivery to treat several types of cancer.Citation7,Citation8 It is further believed that through the use of magnetic nanoparticles, an optimal drug delivery system can be developed by using an external magnetic field to direct such nanoparticles to desirable sites (such as implant infection) for immediate treatmentCitation9 ().
Figure 1 A simplified design for a magnetic drug delivery system in which magnetic nanoparticles coated with drugs are directed to infection sites by an external magnetic field.
Staphylococcus aureus is one of the most common human pathogens, and leads to many types of infection.Citation10 This bacterium is responsible not only for local infections, such as wounds or postoperative infection, but also for prosthetic infection (such as through the use of catheters, endotracheal tubes, and other biomaterials).Citation11–Citation13 S. aureus is also known to possess an increasing ability to resist antibioticsCitation14–Citation16 (such as penicillin, methicillin, tetracycline, erythromycin, and vancomycin). Thus, it is necessary to find an alternative treatment (perhaps without the use of antibiotics) for S. aureus infection that is directed to the site of infection, localized, and difficult for bacteria to formulate resistance.
Along this line, some have hypothesized that reactive oxygen species (ROS) generated by Fe3O4 nanoparticles could kill bacteria without harming non-bacterial cells.Citation17 Specifically, Pareta et al cultured osteoblasts (bone-forming cells) with IO nanoparticles (at a concentration of 4.25 mg/mL) and found that cell density was greatly enhanced in the presence of IO nanoparticles compared with cells cultured without nanoparticles.Citation9
Polyvinyl alcohol (PVA) is a polymer commonly used in biomedical applications because of its biocompatibility.Citation18 IO nanoparticles stabilized by PVA were developed in several studies focused on targeted drug delivery.Citation19–Citation21 In the present study, PVA was used as a matrix to deliver nonagglomerated nanoparticles, as suggested by previous researchers.Citation22,Citation23 The objectives of this study were to design, synthesize, and characterize PVA/IO nanoparticles and investigate the bactericidal activity of such nanoparticles when cultured with S. aureus.
Materials and methods
Iron oxide nanoparticle synthesis
IO nanoparticles were synthesized in situ via a matrix-mediated method using PVA similar to previously suggested processes.Citation24,Citation25 Briefly, an aqueous solution of PVA (Acros Organics, Geel, Belgium) was mixed with equal volumes of ferrous/ferric aqueous solutions under ambient conditions. The iron-loaded PVA gels were soaked in stoichiometric amounts of warm aqueous solutions of NaOH. The resulting ferrosoferric hydroxide dehydrates gave brownish precipitations in the polymer solution.
Characterization of iron oxide/PVA
A droplet of IO nanoparticles was placed on a transmission electron microscopy (TEM) copper grid and allowed to dry. The imaging was carried out at 100 kV on a Philips EM420 TEM (Philips, Amsterdam, The Netherlands) and size calculations were carried out with ImageJ (version 1.42q; NIH, Bethseda, MD).
To measure the hydrodynamic size of the nanoparticles, 100 μL of the particle solution was diluted with 1.5 mL of water and placed into a cuvette of a Zetasizer-nano instrument (Malvern, Philadelphia, PA). Experiments were conducted in triplicate to obtain an average number-size distribution. The same apparatus was used to measure zeta potentials.
X-ray diffraction (XRD) on dried IO nanoparticle powders was performed on a Siemens D500 (Siemens, Berlin, Germany) within a 2θ range of 20–80 degrees using Cu Kα radiation.
The magnetic properties of the dried nanoparticles were obtained using vibrating sample magnometry (VSM, LakeShore 7040) at room temperature.
S. aureus culture
S. aureus were obtained in frozen form from the American Type Culture Collection (ATCC; ATCC 25923). The bacteria were thawed on ice for 20 minutes before being plated on an agar plate. The plate was dried before incubation for 16 hours in a standard cell culture environment (37°C, 5% CO2, and 95% air). A single colony of S. aureus was selected using a 10 μL loop (Sigma, St. Louis, MO) and inoculated into centrifuge tubes containing 5 mL of tryptic soy broth. Bacteria in centrifuge tubes were then incubated at 37°C under agitation at 200 rpm for another 16 hours. At that point, the bacteria solution was diluted in tryptic soy broth to an optical density of 0.52 at 562 nm using a microplate reader (Spectra-Max300; Molecular Devices, Sunnyvale, CA). According to the standard curve correlating bacteria number with optical density, this value was equivalent to 5 × 106 cells/mL. The cells were further diluted in tryptic soy broth to 5 × 104 cells/mL before being added to a new centrifuge tube at 3 mL/tubes.
Concentrated IO nanoparticles in solution were added to bacteria tubes at different doses [30 μg/mL (low dose), 300 μg/mL (medium dose), and 3 mg/mL (high dose)]. A tube of bacteria without nanoparticles served as a control. The IO solution was also added to tubes containing only tryptic soy at the same concentration as above and this served as a particle control. Bacteria were then incubated under agitation for four hours, 12 hours, and 24 hours before a 200 μL bacteria solution was transferred to a 96-well plate for optical density readings at 562 nm using a microplate reader.
S. aureus live/dead assay
After four, 12, and 24 hours of incubation, 100 μL of the bacteria suspension was transferred into a 96-well plate. A live/dead assay was performed according to manufacturer’s instructions (Live/Dead BacLight, L7007; Invitrogen, Carlsbad, CA). Briefly, two solutions containing SYTO 9 dye and propidium iodide were mixed and diluted with double distilled water before being added to a bacteria solution at 100 μL/well. The plate was incubated at room temperature in the dark for 15 minutes. Fluorescence intensities for live cells (excitation: 485 nm, emission: 530 nm) and dead cells (excitation: 485 nm, emission: 630 nm) were measured using a fluorescence microplate reader (SpectraMax M5; Molecular Devices). The two intensities were divided and reported as the ratio of live/dead bacteria.
Statistical analysis
All experiments were conducted in triplicate and repeated at least three times. Differences between means were determined using a Student’s t-test.
Results and discussion
Nanoparticle synthesis and characterization
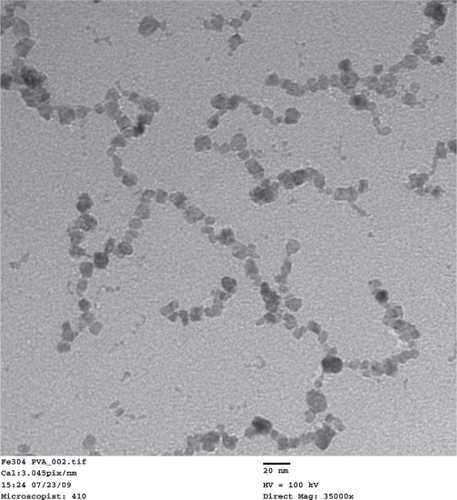
TEM images of the synthesized PVA-coated IO nanoparticles () showed that the size of the nanoparticles was 9 nm ± 4 nm. The nanoparticles formed necklace-like chains with a typical length of approximately 100–200 nm. A similar formation was reported in an earlier study, in which IO nanoparticles were believed to precipitate along the polymer chain of PVA.Citation22 The nanoparticle solution was a clear brownish color and no major types of aggregation were visible after months from synthesis.
Figure 2 Transmission electron microscope images of the synthesized IO/PVA nanoparticles. Scale bar = 20 nm.
Abbreviations: IO, iron oxide; PVA, polyvinyl alcohol.
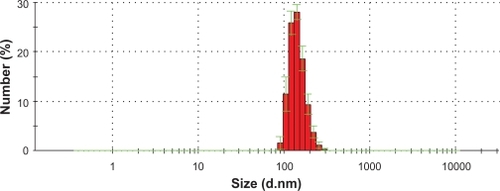
The hydrodynamic diameter measurement results () showed that with the PVA coating, the IO chain-like particles had an average size of 140 nm.
Figure 3 IO nanoparticle size distribution as measured by dynamic light scattering.
Abbrevation: IO, iron oxide.
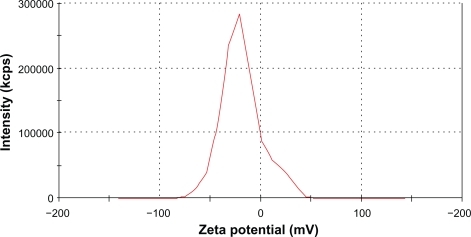
The measured average zeta potential was −19 mV (). This low value suggested that the nanoparticle solution was stable mostly because of steric repulsion but not electrostatic repulsion. The long hydrophilic chains of the PVA associated with water molecules, thus, preventing agglomeration.
Figure 4 Zeta potential of IO nanoparticles in an aqueous solution was measured by a Malvern Zetasizer-nano instrument. Average zeta potential was −19.09 mV.
Abbreviation: IO, iron oxide.
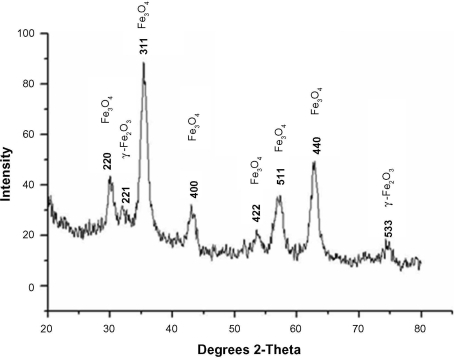
The XRD pattern confirmed that the final product was a mixture of Fe3O4 and γ-Fe2O3 (). The existence of γ-Fe2O3 was common and was because of the oxidation of Fe3O4 during synthesis.Citation26
Figure 5 X-ray diffraction results of synthesized nanoparticles confirmed the existence of magnetite (Fe3O4) and maghemite γ-Fe2O3.
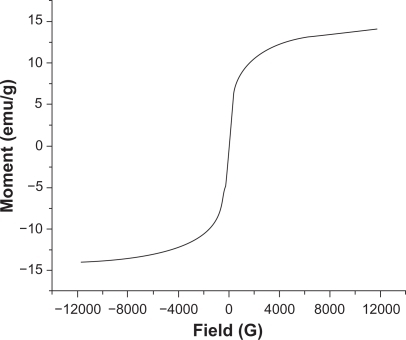
According to the VSM results (), the negligible coercivity of IO nanoparticles showed properties of superparamagnetic materials, meaning that these nanoparticles do not retain any magnetism after removal of a magnetic field. The high magnetization and superparamagnetic properties are highly desirable for biomedical applications because larger magnetic particles form aggregates after exposure to a magnetic field.Citation2 The saturation magnetization of the synthesized IO nanoparticles was 15 emu/g. As expected, because of the PVA coating saturation, magnetization of nanoparticles was lower than that of bulk phase magnetite (∼90 emu/g).
Bactericidal activity of PVA-coated IO
After four hours of incubation, the optical density measurements () showed that there was no significant difference in bacteria numbers in the presence of IO nanoparticles. However, and more importantly, the results from the live/dead assay demonstrated that after four hours, the ratio of live/dead bacteria was significantly lower in the solution with the highest dose (3 mg/mL) of IO nanoparticles compared with the control sample as well as the low and medium dose samples; the same trend was observed after 12 hours and 24 hours ().
Figure 7 Optical density reading of bacteria in IO/PVA solution after four hours, 12 hours, and 24 hours. The results are mean ± SEM. (n = 3). All densities are significantly (*P < 0.01) greater at 12 and 24 hours compared with the four-hour time point.
Abbreviations: IO, iron oxide; PVA, polyvinyl alcohol.
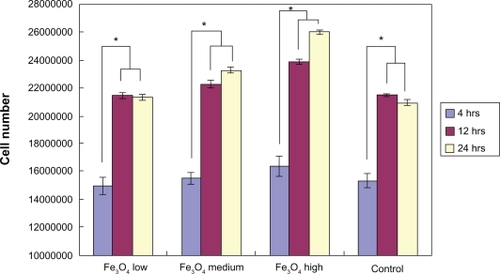
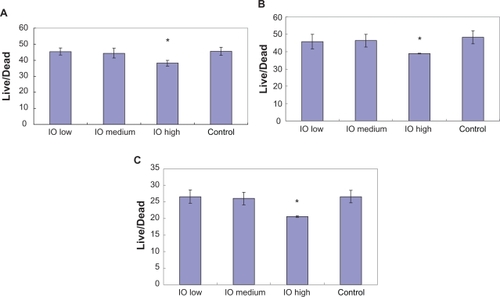
Figure 8 Live/dead assay results showed lower live/dead ratios in the presence of the high dose (3 mg/mL) IO/PVA nanoparticle solution after incubation for A) four hours, B) 12 hours, and C) 24 hours. Data = mean ± SEM; n = 3. *P < 0.05 compared with control sample and samples with low (30 mg/mL) and medium doses (300 mg/mL) of IO nanoparticles.
Abbreviations: IO, iron oxide; PVA, polyvinyl alcohol.
There are several factors that caused the presently studied IO nanoparticles to be bactericidal. The main mechanism by which antibacterial drugs and antibiotics work is via oxidative stress generated by ROS.Citation27 ROS, including superoxide radicals (O2−), hydroxyl radicals (−OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2), can cause damage to proteins and DNA in bacteria.Citation28 Park et al also demonstrated an antibacterial activity from silver metals because of ROS generation.Citation29 In this case, metal oxide Fe3O4 could be the source that created ROS leading to the inhibition of S. aureus. A similar process was described by Keenan et al in which Fe2+ reacted with oxygen to create hydrogen peroxide.Citation30 This H2O2 consequently reacted with ferrous irons via the Fenton reaction and produced hydroxyl radicals which are known to damage biological macromolecules.Citation17
Other research has demonstrated that the small size of nanoparticles can also contribute to bactericidal effects. For example, Lee et al reported that the inactivation of Escherichia coli by zero-valent iron nanoparticlesCitation31 could be because of the penetration of the small particles (sizes ranging from 10–80 nm) into E. coli membranes. Nano-Fe0 could then react with intracellular oxygen, leading to oxidative stress and eventually causing disruption of the cell membrane. Several other studies on ZnO and MgO nanoparticles also concluded that antibacterial activity increased with decreasing particle size.Citation32–Citation34
In this study, the concentration of nanoparticles was a major contribution to S. aureus activity inhibition. A similar concentration-dependent behavior was observed by Kim et al when they investigated the antimicrobial effects of Ag and ZnO nanoparticles on S. aureus and E. coli.Citation32,Citation35 In a study of bactericidal effects of IO nanoparticles on S. epidermidis, Taylor et al also reported concentration-dependent bacteria inhibition.Citation36 Briefly, S. epidermidis density progressively decreased at time points of 12, 24, and 48 hours when incubated with 100 μg/mL, 1 mg/mL, and 2 mg/mL IO.
It is also important to note that IO nanoparticles do not negatively influence all cells. Specifically, osteoblast (bone-forming cell) proliferation was enhanced in the presence of Fe2O3 nanoparticles (at 4.25 mg/mL, the same order of magnitude which inhibited bacteria in this study).Citation9 Such results showed that IO nanoparticles could have a dual therapeutic function which can enhance bone growth and inhibit bacteria infection. Lastly, this present study provided evidence that with an appropriate external magnetic field, IO magnetic particles may be directed to kill bacteria as needed throughout the body.
Conclusions
Stable IO/PVA nanoparticles were successfully synthesized. The particles were characterized with TEM, dynamic light scattering, XRD and VSM. A live/dead assay showed that at the highest dose of iron oxide (3 mg/mL), the growth of S. aureus was inhibited significantly compared with the control samples. Further studies should investigate the bactericidal effect of iron oxide nanoparticles on other types of bacteria for potentially widening such antibacterial applications.
Acknowledgements
The authors thank the Hermann Foundation, Vietnam Education Foundation, and the Indo-US Center for Biomaterials for Healthcare for funding.
Disclosure
The authors report no conflict of interest in this work.
References
- GuptaAKGuptaMSynthesis and surface engineering of iron oxide nanoparticles for biomedical applicationsBiomaterials200526183995402115626447
- BerryCCCurtisASGFunctionalisation of magnetic nanoparticles for applications in biomedicineJ Phys D Appl Phys200336R198R206
- Beets-TanRGHVan EngelshovenJMAGreveJWMHepatic adenoma and focal nodular hyperplasia: MR findings with superparamagnetic iron oxide-enhanced MRIClin Imaging19982232112159559234
- BabesLDenizotBTanguyGLe JeuneJJJalletPSynthesis of iron oxide nanoparticles used as MRI contrast agents: A parametric studyJ Colloid Interface Sci1999212247448210092379
- ChanDCFKirpotinDBBunnPASynthesis and evaluation of colloidal magnetic iron oxides for the site-specific radiofrequency-induced hyperthermia of cancerJ Magn Mag Mat199312213374378
- Gonzales-WeimullerMZeisbergerMKrishnanKMSize-dependent heating rates of iron oxide nanoparticles for magnetic fluid hyperthermiaJ Magn Mag Mat20093211319471950
- ChertokBMoffatBADavidAEIron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumorsBiomaterials200829448749617964647
- KohlerNSunCWangJZhangMMethotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cellsLangmuir200521198858886416142971
- ParetaRATaylorEWebsterTJIncreased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticlesNanotechnology20081926265101
- GrinholcMSzramkaBKurlendaJGraczykABielawskiKPBactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependentJ Photochem Photobiol A Chem20089015763
- MichaelCHWarrenKRKellyPFStaphylococcus aureus adhesion to bone matrix and bone-associated biomaterialsFEMS Microbiol Lett1999173227928410227156
- MariuszGGrzegorzWJuliannaKEvaluation of biofilm production and prevalence of the icaD gene in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains isolated from patients with nosocomial infections and carriersFEMS Immunol Med Microbiol200750337537917537178
- HarrisLGTosattiSWielandMTextorMRichardsRGStaphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(-lysine)-grafted-poly(ethylene glycol) copolymersBiomaterials200425184135414815046904
- JevonsMP“Celbenin”-resistant StaphylococciBMJ196115219124125
- HiramatsuKVancomycin-resistant Staphylococcus aureus: A new model of antibiotic resistanceLancet Infect Dis20011314715511871491
- ChambersHFThe changing epidemiology of Staphylococcus aureus?Emerg Infect Dis20017217818211294701
- TouatiDIron and oxidative stress in bacteriaArch Biochem Biophys200037311610620317
- AlbornozCJacoboSEPreparation of a biocompatible magnetic film from an aqueous ferrofluidJ Magn Mag Mat200630511215
- ChastellainMPetriAHofmannHParticle size investigations of a multistep synthesis of PVA coated superparamagnetic nanoparticlesJ Colloid Interface Sci2004278235336015450454
- XuCTejaASContinuous hydrothermal synthesis of iron oxide and PVA-protected iron oxide nanoparticlesJ Supercritical Fluids2008448591
- Petri-FinkAChastellainMJuillerat-JeanneretLFerrariAHofmannHDevelopment of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cellsBiomaterials200526152685269415585272
- PardoeHChua-anusornWSt. PierreTGDobsonJStructural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcoholJ Magn Mag Mat2001225124146
- SchöpfBNeubergerTSchulzeKMethodology description for detection of cellular uptake of PVA coated superparamagnetic iron oxide nanoparticles (SPION) in synovial cells of sheepJ Magn Mag Mat20052931411418
- SinhaANayarSMurthyGVSJoyPARaoVRamachandraraoPBiomimetic synthesis of superparamagnetic iron oxide particles in proteinsJ Mater Res200318613091313
- SinhaANayarSNathBKDasDMukhopadhyayPKMagnetic field induced synthesis and self-assembly of super paramagnetic particles in a protein matrixColloids Surf B Biointerfaces200543171115885991
- CannasCGatteschiDMusinuAPiccalugaGSangregorioCStructural and magnetic properties of Fe2O3 nanoparticles dispersed over a silica matrixJ Phys Chem B19981024077217726
- KohanskiMADwyerDJHayeteBLawrenceCACollinsJJA common mechanism of cellular death induced by bactericidal antibioticsCell2007130579781017803904
- SiesHOxidative stress: oxidants and antioxidantsExp Physiol19978222912959129943
- ParkHJKimJYKimJSilver-ion-mediated reactive oxygen species generation affecting bactericidal activityWater Res20094341027103219073336
- KeenanCRSedlakDLFactors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygenEnviron Technol200842412621267
- LeeCKimJYLeeWINelsonKLYoonJSedlakDLBactericidal effect of zero-valent iron nanoparticles on Escherichia coliEnviron Technol2008421349274933
- ZhangLJiangYDingYPoveyMYorkDInvestigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids)J Nanopart Res200793479489
- YamamotoOInfluence of particle size on the antibacterial activity of zinc oxideInter J Inorg Mater200137643646
- MakhlufSDrorRNitzanYAbramovichYJelinekRGedankenAMicrowave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocideAdv Funct Mater2005151017081715
- KimJSKukEYuKNAntimicrobial effects of silver nanoparticlesNanomed Nanotechnol Biol Med20073195101
- TaylorENWebsterTJThe use of superparamagnetic nanoparticles for prosthetic biofilm preventionInt J Nanomedicine2009414515219774113