Abstract
The aim of the study was to investigate a porous nanohydroxyapatite/polyamide 66 (n-HA/PA66) scaffold material that was implanted into muscle and tibiae of 16 New Zealand white rabbits to evaluate the biocompatibility and osteogenesis and osteoinductivity of the materials in vivo. The samples were harvested at 2, 4, 12 and 26 weeks respectively, and subjected to histological analysis. At 2 weeks, the experiment showed that osteogenesis was detected in porous n-HA/PA66 composite and the density of new bone formation was similar to the surrounding host bone at 12 weeks. The study indicated that three-dimensional pore structures could facilitate cell adhesion, differentiation and proliferation, and help with fibrovascular and nerve colonization. In conclusion, porous n-HA/PA66 scaffold material could be a good candidate as a bone substitute material used in clinics due to its excellent histocompatibility, osteoconductivity and osteoinductivity.
Introduction
In bone tissue engineering, scaffolds serve as the matrices of tissue formation, and play a pivotal role in osseointegration and tissue integration. After dental extractions, periodontal disease or trauma factors, alveolar bone resorption alters both the height and thickness of the remaining alveolar ridge.Citation1 Bone grafting is frequently used to augment bone healing with numerous approaches to reconstructing or replacing skeletal defects. Autologous bone graft remains the most effective grafting material because it provides the three elements required for bone regeneration: osteoconduction, osteoinduction, and osteogenic cells. But there are some problems for allogeneic and autologous bone grafts such as limited availability and increased trauma. Allogeneic bone grafting can sometimes induces immunologic rejection and transmission of potential pathogens such as the human immunodeficiency virus (HIV) and the hepatitis virus. Therefore, the search for an ideal artificial bone substitute material has been a hot issue for bone repair and reconstruction.
Nanohydroxyapatite (n-HA) is an ideal bioactive material whose composition and crystal structure is very close to the natural bone, and can directly bond to bone tissue in vivo. It has been developed in a variety of forms such as substitute and regeneration material because of its excellent osteoconductivity, osteoinductivity, abrasion resistance, corrosion resistance and stable chemical properties. But this is exactly what limits its application, because ideal scaffolds should support cell migration and bone growth, in accordance with the rate of material degradation.Citation2,Citation3 On the other hand, its brittleness and poor performance of mechanical stability limit its use for the regeneration of load bearing bone defects.
Bone is one of the biomineralization materials which are comprised of collagen, apatite and water, one third of which is inorganic and two thirds of which is organic. Polyamide (PA) has good biocompatibility with human tissue, probably due to its similarity to collagen protein in chemical structure. It has been widely used in the biomaterials application of surgical sutures for nearly half a century. Especially important, PA also exhibits excellent mechanical properties.Citation4 But the degradation of it is difficult to control. To overcome these shortages, the novel porous nanohydroxyapatite/polyamide 66 (n-HA/PA66) which is synthesized from hydroxyapatite and polyamide is foamed by thermal pressing and injection molding techniques. High porosity and proper pore size may facilitate cell seeding, survival, growth, differentiation and proliferation. Therefore, the aim of this research is to investigate the tissue reaction to evaluate the histocompatibility and osteogenesis of the materials in vivo.
Materials and methods
Materials and characterization
Porous n-HA/PA66 scaffold materials were synthesized from n-HA and polyamide, foamed by thermal pressing and injection molding techniques. The bending strength, tensile strength and compressive strength of the composite with 64.5 wt% n-HA content were 95, 79, and 117 MPaCitation5 respectively, which was close to the natural bone (80–100, 60–120, 50–140 MPa separately).Citation6 The elastic modulus of the composite was 5.6 GPa which is similar to natural bone (3–25 GPa). Therefore, the composite prepared can match well to human bone.Citation5
Surgery
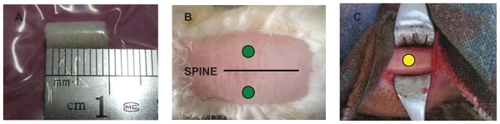
Sixteen New Zealand white rabbits (average weight between 2.0 kg and 2.5 kg) were obtained from State Key Laboratory, Chengdu, China, and were divided by random treatment into four groups of four each. The rabbits were anesthetized by intravenous injection of 3% sodium pentobarbital (1 ml/kg) and operated on under sterile conditions. As seen in , middle skin incision was performed on the middle of the back, and two discs of porous n-HA/PA66 samples (5 mm in diameter and 10 mm in length) were inserted into the muscular pocket and sutured wounds. At the same time, the samples (2 mm in diameter and 4 mm in length) were implanted into the bone defect which matched the sample size in the tibiae. The rabbits were sacrificed at 2, 4, 12, and 26 weeks respectively. The specimens were submitted to histological assessment.
Ethical approval
All animal procedures were carried out according to the guidelines of the Animal Ethics Committee of Sichuan University.
Results
Macroscopic observation
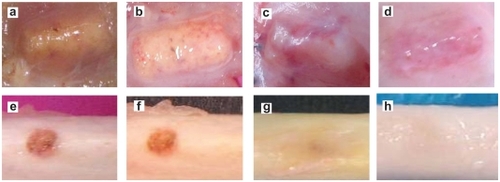
No rabbits died or were infected in any of the groups. Food intake and weight bearing in each animal subject gradually improved. The wounds healed uneventfully with no postoperative infection or material displacement. Macroscopic observation revealed that muscular tissue bundled the samples tightly with no suppuration or necrosis (). As time went by, porous material integrated with bony tissue into a whole. There was no infection or immunologic rejection either. ().
Intramuscular implantation
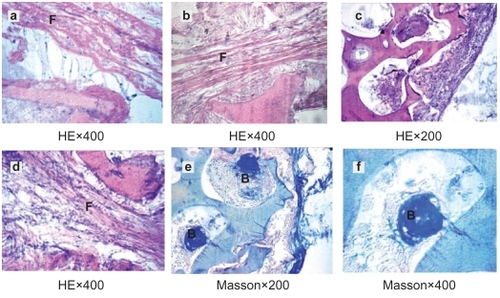
The host reaction was reflected at the site of implantation by histological assessment.Citation7 At 2 weeks, material was surrounded by fibrous connective tissue which was comprised of approximately 10 layers of loose cells. The main reaction was that the fibroblast bundled the scaffold to form a fibrous capsule, because the organism considered it foreign.Citation8 Small vessels and fibroblasts with a few lymphocytes and neutrophils infiltrated into the pores but no macrophages were detected, indicating the porous material had an excellent histocompatibility (). About 4 weeks later, the fibrous connective tissue gradually became thinner and denser. Collagen fiber formatted and crept into the pores (). At 12 weeks, the fibrous capsule wall was comprised of a great number of collagen fibers, and fibrocytes with no inflammatory cells existed (). Finally, reengineered cells were basically stable at 26 weeks (). Bone lacuna was clearly observed and new bone formed in the interconnected porosity increased, which indicated the n-HA/PA66 material with porosity and proper pore size facilitated cell adhesion, differentiation, and proliferation. It proved that porous n-HA/PA66 scaffold had excellent tissue compatibility and osteoinduction.
Endosseous implantation
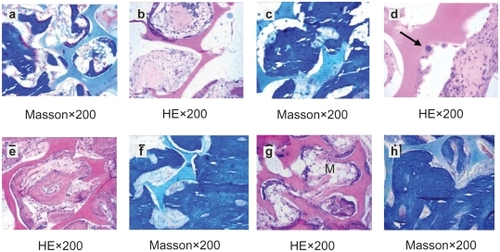
No immunologic rejection was observed throughout the experiment. Pores were filled with loose connective tissue and small vessels at 2 weeks. Osseous tissue gradually crept into the interconnected porosity and connected with the material directly, without fibrous tissue. The amount of osteoid tissue was illustrated in the pore structure (). As we know, fibrous tissue existing at the interface between material and osseous tissue is a primary cause of implantation failure. After 4 weeks, osseous tissue formation augmented and became more mature. Bone trabecula enlarged showed active osteogenesis. Ossein graduated into woven bone and calcific nodules in the interconnected porosity (). Osteoclasts adhered to the surface of the pores’ structure which contributed to the bioresorption of the material (). At 12 weeks, needle-like bone trabecula connected with each other. New bone formatted in the pores and gradually linked one to another, and connected with materials directly (). At 26 weeks, interconnected porosity filled completely with new bone tissue, which integrated into a whole, and showed good osteoinductivity and osteocompatibility. Marrow cavity was also generated at that time ().
Discussion
Bone was considered as a nanocomposite material which was comprised of collagen, apatite and water, one third of which was inorganic and two thirds of which was organic. Artificial bone grafting repaired bone defects as substitutes in the implantation, and not only had a good biocompatibility but also combined with natural bone as in osseointegration. It was necessary to provide support, voids and enhance biologic repair of bone defects. Strategies for development of biological substitutes capable of mimicking homeostasis were based on an understanding of the basic events in the healing of bone defects. Artificial bone substitutes had an ideal osteoconduction due to its three-dimensional structure and the related process of growth of capillaries, perivascular tissue and osteoprogenitor cells of the host into the graft. Bone substitutes used in the past (eg, medical metal materials, macromolecule materials, and bio-ceramic materials) could not bond to osseous tissue tightly, mostly due to the mechanical retention of the composites. Porous n-HA/PA66 scaffold material had better biological activity with high specific surface area, since the three-dimensional structure had a large contact region which facilitated cell adhesion, differentiation and proliferation, and benefited the metabolization. Kim indicated that nano-HA particles had better bioactivity and osteoinductivity than micro ones.Citation9 In addition, the implantation environment was beneficial for osteoinduction because there was sufficient blood supply in the muscular tissue where there were some undifferentiated mesenchymal cells around vessels. Therefore, we observed bone lacuna clearly, and bone formed in the interconnected porosity when samples were implanted in muscle.
We evaluated porosity as a main indicator of three-dimensionally porous material, which related to the quantity and rapidity of osteogenesis. The pore size was a critical factor to osseointegration.Citation10 Hulbert proposed 100 μm was the minimum porosity which allowed bone to creep in, and 150 μm was the best.Citation11 Vaz considered that 500 μm was a more suitable diameter for bone cells.Citation12 Scaffold for tissue engineering should possess a three-dimensionally porous structure with porosity no less than 70% and pore size ranging from 50 to 900 μm.Citation13 Pores provide an ideal structure to induce cell adhesion, differentiation and proliferation, which not only benefits nutrient transportation, but also helps with fibrovascular and nerve colonization. However, the degree of porosity and diameter of pore size influenced the mechanical property of the scaffold. Consequently, to provide a lasting stress-absorbent core framework with transplants for bone tissue regeneration, we prepared an innovative n-HA/PA66 scaffold with relatively low porosity but better mechanical properties. Pore size of porous n-HA/PA66 scaffold material used in this study was 300–500 μm, porosity was 65%–75%. Macropores and micropores were captured from scanning electron microscope photographs of the porous scaffold. Mean size of macropores ranged from 300 to 500 μm, and on the wall of them there were micropores whose pore size was less than 50 μm.Citation5 The pore size and inner surface area was important for the growth of bone tissue into the scaffold, the bigger the inner surface area the more the cells planting in it and the faster the tissue and organ formation. Macropores and micropores worked together to facilitate tissue ingrowth. In addition, the pores’ structure was propitious to form mechanical locking within, which was good for enhancing the combination of bone tissue and scaffold materials and creating a reliable retention. Pore structure also reduced the rigidity of the materials that were closed to the elastic modulus of the organization, which ensured the interface was stable. Cambium crept into the pores as time went by, not only reducing the brittleness of the materials, but also improving the bending strength – though the strength of the porous scaffold was low in the early days of planting.
Conclusions
The innovative bone tissue engineering porous n-HA/PA66 scaffold material provided a three-dimensional microstructure to accelerate bone tissue, fibrovascular and nerves colonization. According to the histological experiment results, we indicated that porous n-HA/PA66 scaffold material could be a good candidate as a bone substitute due to its excellent histocompatibility, osteoconductivity and osteoinductivity.
Disclosure
The authors report no conflicts of interest in this work.
References
- MecallRARosenfeldAThe influence of residual ridge resorption patterns on fixture placement and tooth positonInt J Periodontics Restorative Dent19911118232071325
- StrocchiRBone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbitsJ Oral Implantol20022827327812498535
- CouriCJMazeGIHinksonDWByranHDeborahVMedical grade calcium sulfate hemihydrate versus expanded polytetrafluoroethylene in the treatment of mandibular class II furcationsJ Periodontol200273111352135912479641
- SpringerINGFleinerBJepsenSAcilYCulture of cells gained from temporomandibular joint cartilage on non-absorbable scaffoldsBiomaterials200122182569257711516090
- JieWYubaoLTissue engineering scaffold material of nano-apatite crystals and polyamide compositeEur Polym J200440509515
- VerheyenCCPMde WijnJRvan BlitterswijkCADe GrootKEvaluation of hydroxylapatite/poly (L-lactide) composites: mechanical behaviorJ Biomed Mater Res199226127712961331112
- UngersbockAPohlerOPerrenSMEvaluation of the soft tissue interface at titanium implants with different surface treatments: experiment study on rabbitsBio-Med Mat Eng199444317325
- BehlingCASpectorMQuantitative characterization of cells at the interface of long-term implants of selected polymersJ Biomed Mater Res19862056536663711138
- KimHMFuruyaTKokuboTMiyazakiTNakamuraTBrownSClarkeIWilliamsPComposition of apatite produced in simulated body fluidsBioceramicsStafa-Zurich, SwitzerlandTrans Tech Publications200114621624
- GreenDWWalshDMannSOreffoROCThe potential of biomimesis in bone tissue engineering: lessons from the design and synthesis of invertebrate skeletonsBone200230681081512052446
- HulbertSFMorrisonSJKlawitterJJTissue reaction to three ceramics of porous and non-porous structuresJ Biomed Mater Res1972653473744116127
- VazLLopesABAlmeidaMPorosity control of hydroxyapatite implantsJ Mater Sci Mater Medicine1999104239242
- WangYKimHJVunjak-NovakovicGKaplanDLStem cellbased tissue engineering with silk biomaterialsBiomaterials200627366064608216890988