Abstract
Since pharmaceuticals cannot be used in space until liver and kidney dysfunctions are corrected, and with invariable malabsorption, it appears there is no alternative other than to use subcutaneous magnesium (Mg) replacements in the presence of deficiencies and use of gene therapy. I suggest beginning with the correction of as many as four gene deficiencies: atrial natriuretic peptide (ANP), nitric oxide (NO), vascular endothelial growth factor (VEGF), and erythropoietin (EPO), all as well as Mg related to perfusion and angiogenesis. There is no evidence of significant lunar radiation levels in the absence of a solar storm. It could then be determined whether this has resulted in correction of liver and kidney dysfunction. If this persists, serial additions of gene therapy will be required determining the effect of each individual gene trial on organ function. Microgravity and endothelial gaps with leaks trigger reduced plasma volume. Partial correction by use of a plasma volume substitute and development of a delivery device may reduce complexity of gene therapy. Research would be conducted both on Earth and in microgravity, with the development of subcutaneous pharmaceuticals and Mg, and a space walk-reliable subcutaneous silicon device, given that no replenishable subcutaneous device is presently available. A three-pronged approach provides a plan for the next 50 years: A. complete correction of a Mg deficit; B. partial replacement with plasma volume substitutes, and C. multiple gene factor strategy.
The most sacred of the duties of a government [is] to do equal and impartial justice to all its citizens.
Introduction
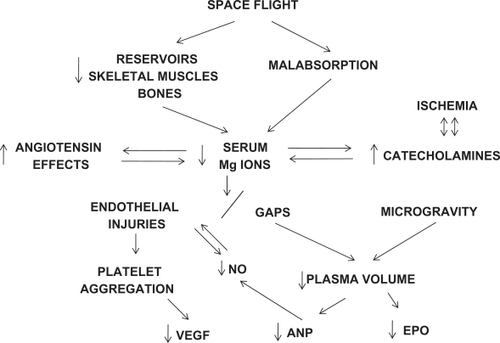
It has been 50 years since humans ventured into space. Will the next 50 years bring solutions to the vascular hazards resulting from both microgravity and hypokinesia (restricted movements)? Some animals that live all of their lives in caves cannot adjust to living outside the cave. Are humans spared the restrictions of nature? During space flight, the endothelium is vulnerable to injuries complicating oxidative stress mechanisms with inflammation, triggered by catecholamine, angiotensin, and endothelin elevations, along with reductions in magnesium (Mg) ions, atrial natriuretic peptide (ANP), and nitric oxide (NO), with resulting vicious cycles. With impaired angiogenesis secondary to a Mg deficit,Citation1 and with impaired perfusion of the liver and kidneys and, in turn, insufficient metabolism and excretion of pharmaceuticals, how can an acute myocardial infarction, for example, be treated after perhaps 20 months of space flight? It has been notedCitation1 that the pharmacokinetic and pharmacodynamics of medications may well be influenced by the effects of microgravity, and emphasized the effects of microgravity on blood flow in the liver. During the 438-day Mir mission, the cosmonaut’s cyclic guanosine monophosphate (cGMP) was not detectable after five months in space and did not return to premission levels until three months after return from his space flight. cGMP is a second messenger of both NO and ANP.Citation2,Citation3 In the presence of dust inhalation, in such habitats as Apollo 15, combined with space flight and endothelial dysfunction/injury,Citation4–Citation6 these factors can lead to extraordinary elevations of stress test hypertension, even in missions of less than two weeks’ duration and in the absence of significant exposure to radiation.Citation1 Techniques to prevent inhalation of dust in the environment may eventually be successful, first by removing the space suit before entry and backing into the habitat or module. In addition, ultrafine dust particles (<100 nm) might be removed from the space suit by electrostatic precipitators. Even without dust exposure, very prolonged space flight may lead to renovascular hypertension with evidence of self-limiting proteinuria, impairment in renal concentrating ability, and elevations of serum creatinine.Citation1 In addition, with space flight, there is reduced diurnal blood pressure variation which portends kidney disease. Indeed, on earth, the endothelial repair process is somewhat impaired after the age of 30 years, and these repair mechanisms are further impaired with space flight.Citation1,Citation4,Citation5 Pharmaceuticals cannot be used, other than for emergencies, because of invariable malabsorption, and potential impairment in hepatic and renal function, so that those in a space flight would not be able to metabolize or excrete pharmaceuticals satisfactorily, if the mission was sufficiently prolonged. Furthermore, at this time, there is no suitable, replenishable, subcutaneous silicon device to administer medications. In addition, some pharmaceuticals deteriorate during space flight, but why this occurs is unknown. Despite decreased sensitivity of serum Mg, it has been reportedCitation1 that significantly decreased serum Mg levels (P < 0.0001) after shuttle missions. Both too muchCitation4 and too littleCitation5 exercise will decrease these Mg levels. In the early weeks of a mission, there is a 1%–2% loss of bone per month and loss of skeletal muscle mass, both of which are storage sites for Mg, with further loss from malabsorption. Mg is required for at least 300 enzymatic reactions, including those which metabolize drugs. Mg is also an antioxidant and calcium blocker.Citation1
All the enzymes that metabolize vitamin D require Mg. Vitamin D, which is depleted in space, is required for bone metabolism and protects the cardiovascular system. Since deficiencies of vitamin D occur in one-third to one-half of otherwise healthy adults, and are conductive to elevations of vessel constrictors and in turn, hypertension, variations in vitamin D levels both prior to and during a mission may account at least partially for the discrepancies in space flight blood pressures. Reductions, no change, and significant elevations of blood pressures have all been reported.Citation1 A request through the Freedom of Information Act (FOIA) in 2007 showed that Irwin possibly had endothelial dysfunction prior to his Apollo 15 mission, and that from one month before lift-off, his resting blood pressure was as high as 145/110 mmHg. A subsequent request for FOIA stress test data regarding Conrad (Apollo 12) and Shepard (Apollo 14) was of no value because the National Aeronautics and Space Administration (NASA) was unable to provide sufficient data for comparison with those from Irwin. Furthermore, NASA will only provide data for astronauts who are deceased.
Gene therapy
Although the entire body is in jeopardy during a space flight, obviously it would be absurd to suggest that for a very long mission, replacement of a large portion of the genome would be required. However, it is reasonable to begin by using gene therapy to correct four deficiencies, ie, ANP, NO, erythropoetin (EPO), and vascular endothelial growth factor (VEGF),Citation7–Citation10 the latter being triggered by space flight, with thrombocytopenia and diminished VEGF expression complicating space flight insulin resistance, and Mg deficit playing a role.Citation1 Thrombocytopenia may be induced by platelet adhesions and aggregation, which is prevented by NO,Citation2,Citation10 and platelets are the primary source of VEGF.Citation1 Red blood cells, which function as antioxidants, are reduced by about 10% even on short missions, along with invariable dehydration with, in addition, decreased thirst and invariable reductions of plasma volume, for example, which are reduced by 10% after a nine-day mission. Radiation was not a factor on Apollo missions.Citation11 As for gene therapy, a recent editorial suggests that prospects for continuing advancement of gene therapy to wider applications remain strong.Citation12 However, it is apparent that because the molecular mechanisms responsible for angiogenesis are very complex, a “multiple factor strategy” will be required.Citation13
Mg deficiency during a space flight plays an important role in renal disease. An experimental Mg deficiency has been shown to cause hypertrophy of the juxtaglomerular apparatus, resulting in aldosterone secretion, which in turn increases Mg loss and creates vicious cycles. These vicious cycles can develop from Mg ion deficits, with resultant catecholamine elevations and an increased angiotensin effect. The latter can contribute to an upregulated intrarenal renin-angiotensin system with hypertension (see ). However, if renal insufficiency occurs, Mg replacement will have to be closely monitored.Citation1 Focusing on correction of peripheral vascular complications during space flight, for example, in the presence of a 40% reduction of calf blood flow in microgravity and doubling of calf vascular resistance,Citation1 the results with intramuscular gene transfer of VEGF have been encouraging, with an increase in collateral vessels around the injection sites during a nine-month follow-up study.Citation14,Citation15
Figure 1 Proposed mechanisms for space flight-related vascular complications requiring magnesium repacement and possible correction of at least four gene deficiencies.
What has been the space flight experience with delivery of gene therapy systemically? Gene therapy was first suggested a decade ago for treating astronauts with space-induced anemia.Citation16 More recent earth-based studies suggest that space flight-gene therapy may be useful for correcting ANP deficiencies of >40% after 7–12 space flight days.Citation1 Space flight deficiencies in ANP may play an important role, because on earth, dust inhalation does not cause the degree of hypertension Irwin experienced.Citation17 A decrease in renal blood flow may be significantly reversed following NO synthase gene transfer, with improvement in renal function.Citation1,Citation10 It is clear that with gene therapy we are only at the beginning, with possibly decades of gene therapy research ahead, involving studies both on earth and at the International Space Station. No meaningful research seems to have been conducted with gene therapy to offset the vascular complications of space flight. There are numerous problems with gene therapy, first and foremost, finding a suitable vector. The focus has been primarily in search of a virus vector which would penetrate into the nucleus without causing, eg, immune reactions, severe hypotension, or shock.Citation7,Citation10 Transfection efficacy has been found to be only temporary, and there may be pathologic overgrowth of endothelial cells.Citation8 Furthermore, McCarthy et alCitation10 have emphasized that reproducibility of positive results has yet to be demonstrated conclusively. It is important to stress that there is no evidence from Berry’s lunar studies that the various Apollo complications were triggered by significant radiation exposure,Citation11 nor is there evidence at this time that the deterioration of some pharmaceuticals during space flight is radiation-related. However, during a solar proton storm the levels would be much higher. On earth, with head-down-tilt bed rest studies, the concentration of cGMP is also decreased.Citation2 Therefore, one can assume that the impairment in liver and kidney function may be entirely due to vascular insults.Citation1 This must be corrected, and pharmaceuticals, other than for symptomatic treatment or emergencies, cannot be administered with any degree of safety.
In cardiac tissue, hypokinesia and space flight studies in experimental animals have shown endothelial projections and narrowing of small vessels with occlusions.Citation1 Gaps, with endothelial projections, can cause plasma to leak from vessels.Citation18 This intensifies loss of plasma volume from microgravity, beginning soon after lift-offCitation1 (see ). The invariable plasma volume reduction in microgravity triggers anemia associated with reduced EPO levels.Citation19 In addition to correcting anemia, EPO may contribute to angiogenesis along with VEGF, NO, ANP, and Mg.Citation1,Citation7–Citation10,Citation13,Citation15,Citation19–Citation21 It has been shown that serum EPO, suppressed immediately after launch, remained low during a nine-day shuttle mission.Citation19 Correction of EPO deficiency has also been shown to be cardioprotective. However, there is a risk of both hypertension and thrombosis with chronic exposure to EPO gene therapy.Citation21
On the other hand, although gene therapy with both NO and ANP can lead to hypotension if the expression of gene therapy is not tightly regulated to the target site,Citation1 the risk of inducing shock may be greater with ANP because it directly inhibits the renin–angiotensin–aldosterone axis and the sympathetic nervous system. Because ANP is significantly reduced, partially as a result of reduction in plasma volume, and Mg is probably necessary for both ANP synthesis and release, this Mg deficiency must be corrected first, using a yet to be developed subcutaneous silicon device, which can be replenished and would be reliable during strenuous effort, as with space walks.Citation22 It is proposed that, after reversal of a Mg deficitCitation1,Citation22–Citation24 it is worthwhile correcting, as a start, deficiencies of as many as four genes, ie, ANP, NO, VEGF, and EPO (see ). If this is successful, as indicated by restoration of both liver and kidney function, subcutaneous pharmaceuticals can be used.
Table 1 Rationale for gene therapy trials, limitations, expected benefits with potential correction of at least four gene deficiencies
There is limited experience with both subcutaneous MgCitation22 and subcutaneous pharmaceuticals at this time. These will require research at the International Space Station. If there is still no evidence that organ dysfunction has been offset, then additional genes, specifically those enhancing perfusion, will have to be administered, one by one, and will require decades of research. Because reduced plasma volume is an important trigger for deficiencies of both EPO and ANP, it seems reasonable to postulate that development of a plasma volume substituteCitation25 and a subcutaneous delivery device might reduce the complexity of this proposed gene therapy requiring a multifactorial strategy. Furthermore, at least partial restoration of plasma volume may reduce the potential for hypotension and shock (see ). Epstein et alCitation13 have stressed how complicated such a process can be in duplicating angiogenesis, determining the number of factors required, and determining the appropriate sequence and concentration.
Finally, it is conceivable that a centrifuge could be developed which would duplicate exactly 1G for travel in transit, but the problem would remain of construction of habitats and tunnels, and exploration for long periods on the surface in a microgravity environment. Furthermore, without gene therapy, it seems apparent that man will not be able to colonize the Moon, Mars, an asteroid, or beyond.
Acknowledgements
Dedicated to the memory of Dr Mildred Seelig, magnesium expert and my mentor.
Disclosure
The author reports no conflict of interest in this work.
References
- RoweWJPotential renovascular hypertension, space missions, and the role of magnesiumInt J Nephrol Renovasc Dis20092515721694921
- RosslerANoskovVLaszloZPolyakowVVHinghoffer-SzalkayHGPermanent depression of plasma cGMP during long-term space flightPhysiol Res200150839011300230
- CostaMAElesgarayRCaniffiCFelletAMacLaughlinMArranzCRole of nitric oxide as a key mediator on cardiovascular actions of atrial natriuretic peptide in spontaneously hypertensive ratsAm J Physiol Heart Circ Physiol2010298H778H78619783776
- RoweWJExtraordinary unremitting endurance exercise and permanent injury to normal heartLancet19923407127141355810
- RoweWJInterplanetary travel and permanent injury to normal heartActa Astronautica19974071972211543600
- RoweWJThe Apollo 15 space syndromeCirculation1998971191209443446
- SchillingerKJTsaiSYTaffetGERegulatable atrial natriuretic peptide gene therapy for hypertensionProc Natl Acad Sci U S A2005102137891379416162668
- JinHLLiuMLKimHARole of the oxygen-dependent degradation domain in a hypoxia-inducible gene expression system in vascular endothelial growth factor gene therapySpine200934E952E95820010384
- RinschCRegulierEDeglonNDalleBBeuzardYAebischerPAGene therapy approach to regulated delivery of erythropoietin as a function of oxygen tensionHum Gene Ther19978188118899382954
- McCarthyHOCoulterJARobsonTHirstDGGene therapy via inducible nitric oxide synthase: A tool for the treatment of a diverse range of pathological conditionsJ Pharm Pharmacol200860999101718644193
- BerryCAMedical legacy of ApolloAerosp Med1974104610574153403
- KohnDBCandottiFGene therapy fulfilling its promiseN Engl J Med200936051852119179320
- EpsteinSEFuchsSZhouYFBaffourRKornowskiRTherapeutic interventions for enhancing collateral development by administration of growth factors: Basic principles, early results and potential hazardsCardiovasc Res20014953254211166266
- RoweWJAs with spaceflight, a magnesium deficit may be responsible for both peripheral vascular dysfunction and kidney diseaseAm J Cardiol20101051203120420381679
- KimHJJangSYParkJIVascular endothelial growth factor-induced angiogenic gene therapy in patients with peripheral artery diseaseExp Mol Med20043633634415365252
- OhiSDeveloping protocols for recombinant adeno-associated virus-mediated gene therapy in spaceJ Gravit Physiol200076768
- RoweWJExtraordinary hypertension after a lunar missionAm J Med2009122e119854309
- McDonaldDMThurstonGBalukPEndothelial gaps as sites for plasma leakage in inflammationMicrocirculation1999672210100186
- de SantoNGCirilloMKirschKAAnemia and erythropoietin in space flightsSemin Nephrol20052537938716298259
- YamaharaKItohHChunTHSignificance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regenerationProc Natl Acad Sci U S A20031003404340912621153
- MehtaJLErythropoietin in cardioprotection: Does it have a future or is it all in the past?Cardiovasc Res20087954955018583337
- RoweWJThe case for a subcutaneous magnesium product and delivery device for space missionsJ Am Coll Nutr200423S525S528
- BernardiniDNasulewicAMazurAMaierJAMagnesium and microvascular endothelial cells: A role in inflammation and angiogenesisFront Biosci2005101177118215769616
- BeckingGCHepatic drug metabolism in iron-, magnesium-, and potassium-deficient ratsFed Proc19763524802485824159
- Lundsgaard-HansenPCollinsJADavid-WestASUse of plasma volume substitutes and plasma in developing countriesBull World Health Organ1983617226188548