Abstract
Diabetic nephropathy (DN) is one of the microvascular complications of the kidney arising commonly from type 1 diabetes mellitus (T1DM), and occasionally from type 2 diabetes mellitus (T2DM). Microalbuminuria serves as an early indicator of DN risk and a predictor of its progression as well as cardiovascular disease risk in both T1DM and T2DM. Although microalbuminuria remains the gold standard for early detection of DN, it is not a sufficiently accurate predictor of DN risk due to some limitations. Thus, there is a paradigm shift to novel biomarkers which would help to predict DN risk early enough and possibly prevent the occurrence of end-stage kidney disease. These new biomarkers have been broadly classified into glomerular biomarkers, tubular biomarkers, biomarkers of inflammation, biomarkers of oxidative stress, and miscellaneous biomarkers which also include podocyte biomarkers, some of which are also considered as tubular and glomerular biomarkers. Although they are potentially useful for the evaluation of DN, current data still preclude the routine clinical use of majority of them. However, their validation using high-quality and large longitudinal studies is of paramount importance, as well as the subsequent development of a biomarker panel which can reliably predict and evaluate this renal microvascular disease. This paper aims to review the predictive role of these biomarkers in the evaluation of DN.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Diabetic nephropathy (DN) is one of the renal microvascular complications which is commonly due to type 1 diabetes mellitus (T1DM), and occasionally due to type 2 diabetes mellitus (T2DM).Citation1–Citation3 It is a clinical syndrome comprising the following features: persistent albuminuria (or albuminuria excretion rate of >300 mg/d or 200 μg/min) recorded at least twice within a 3- to 6-month interval, progressive reduction in glomerular filtration rate (GFR), and hypertension.Citation3 The evolution of DN occurs over a period of 10–20 years, beginning from microalbuminuria and progressing to end-stage kidney disease (ESKD).Citation1 Thus, overt DN is very rare in the pediatric age group,Citation4 although some previous reports have confirmed its occurrence in childhood.Citation5–Citation7 Notably, patients with DN and T1DM almost always present with other signs of diabetic microvascular disease such as retinopathy and neuropathy; in fact, retinopathy usually precedes the onset of overt DN in these patients.Citation2
Glomerular hyperfiltration occurs in the early stage of DN resulting in microalbuminuria. As the disease progresses, macroalbuminuria sets in and is followed by deterioration in renal function and ESKD, which may eventually require renal replacement therapy.Citation8 Microalbuminuria therefore serves not only as an indicator of DN risk but also as a strong predictor of its progression, and a predictor of cardiovascular disease risk in both T1DM and T2DM.Citation2 For instance, 80% of T1DM patients with microalbuminuria will end up with overt nephropathy (macroalbuminuria) within 10–15 years, 50% of whom will develop ESKD within 10 years and 75% within 20 years if there are no appropriate therapeutic interventions.Citation9 However, subsequent studies have disputed these previous observations.Citation10–Citation12 It is now projected that the rate of progression from microalbuminuria to macroalbuminuria over a 5- to 10-year period is about 15–30%, although as high as 45% in patients with less than 15 years of diabetes duration but much lower than the initially estimated figure of 80%.Citation13
Although microalbuminuria remains the gold standard marker for early detection of DN, it is not a sufficiently accurate predictor of DN risk given some limitations. For example, not all diabetics with microalbuminuria will end up with ESKD,Citation8,Citation14 and 30% of them may actually have normoalbuminuria,Citation15 while several biomarkers of glomerular or tubular dysfunction can precede microalbuminuria, suggesting that microalbuminuria is present once significant renal injury has already occurred.Citation16 Nevertheless, its predictive accuracy can be enhanced by other parameters such as a painstaking family history, consideration of absolute versus categorical urine albumin excretion (UAE) values, more frequent UAE estimates, ambulatory blood pressure monitoring, precise GFR measurements, diabetic retinopathy assessments, and plasma lipid profile.Citation13 Interestingly, there is now a paradigm shift to novel biomarkers which would help to predict DN risk early enough, and possibly prevent the occurrence of ESKD. This paper aims to review the predictive role of these biomarkers in the evaluation of DN.
Pathogenesis of DN: overview of molecular mechanisms
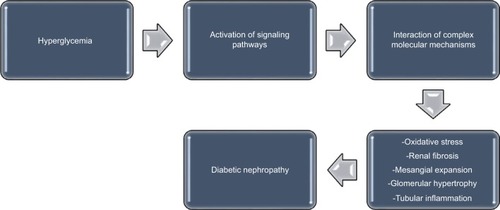
Recent observations indicate that several pathways are activated during the development of diabetes mellitus; these pathways individually or collectively modulate the induction and progression of DN.Citation17 Although the pathogenesis of DN is multifactorial, the mechanisms that propel its development remain largely unclear.Citation17,Citation18 During the course of DN, the functional derangement and structural remodeling of the kidney, triggered by hyperglycemic injury, are linked to changes in several cellular events and activation of signaling pathways.Citation19
These pathways interact in a cascade of complex molecular mechanisms, resulting in the major pathogenic components of DN, which consist of renal fibrosis, mesangial expansion, glomerular hypertrophy, oxidative stress, and tubular inflammation ().Citation17 Some biomarkers are linked to these pathways. For instance, the renin–angiotensin–aldosterone system is associated with inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL) 1β,Citation20 while substantial evidence suggests that angiotensin IICitation21–Citation26 and aldosteroneCitation27–Citation29 are major mediators in the pathogenesis of DN. Furthermore, the role of protein kinase C (PKC) in the induction and progression of DN through a complex mechanism involving its isoforms (PKC-α, PKC-β, and PKC-ε) has been well reported.Citation30–Citation33 These isoforms have been implicated as mediators of renal fibrosis and mesangial expansion through upregulation of vascular endothelial growth factor (VEGF) expression in mesangial cells, as well as transforming growth factor-β (TGF-β), type IV collagen, laminin, and fibronectin in the glomeruli. Even as hyperglycemia-induced expression of some NADPH oxidase subunits in mesangial cells occurs in a PKC-dependent fashion,Citation34,Citation35 NADPH oxidase-driven renal oxidative stress stimulates mesangial expansion and albuminuria by increasing the expression of fibronectin and collagen-1 in the kidney, TGF-β1 being the main “fibrogenic” cytokine in vivo.Citation36,Citation37
Finally, inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-18 are all involved in the development and progression of DN, as inflammation also plays a crucial role in the process.Citation38 Remarkably, elevated levels of circulating TNF receptors in patients with T2DM have been linked with progression to ESKD,Citation39 while urinary TNF-α excretion has been associated with severity of glomerular and tubulointerstitial injury in patients with T2DM.Citation40
Albuminuria as the conventional biomarker
Ideally, a little quantity of albumin is filtered in the glomerulus, and is accompanied by its near-complete reabsorption in the tubules.Citation41 Thus, increased UAE is accepted as a well-established biomarker of glomerulopathy,Citation16 as well as tubulopathy because in the latter, there is reduced reabsorption of the filtered albumin.Citation42
Since albuminuria is an important component of DN, it is important to establish the definition of the different degrees of UAE. Normoalbuminuria refers to UAE of <30 mg/day or 20 μg/min, while microalbuminuria and macroalbuminuria refer to UAE of 30–300 mg/day or 20–200 μg/min, and >300 mg/day or 200 μg/min, respectively.Citation43
Because baseline albuminuria is the strongest predictor of ESKD and cardiovascular morbidity in T2DM,Citation44,Citation45 the American Diabetes Association has recommended screening with yearly UAE in all patients with T2DM, commencing at the time of diagnosis.Citation46
Remarkably, microalbuminuria constitutes a risk factor for chronic kidney disease and ESKD, and also serves as a powerful predictor of cardiovascular morbidity and mortality in diabetic subjects.Citation47–Citation49 Nevertheless, about 30% of diabetics with renal dysfunction have normoalbuminuria,Citation15 while the cardiovascular and renal morbidity is increased in the “high normal” range of UAE.Citation49–Citation52
With these observations and the previously mentioned drawbacks, the use of albuminuria as the conventional biomarker for early detection of DN is thus becoming untenable. Moreover, recent evidence indicates that a remarkable number of patients with macroalbuminuria can revert to normoalbuminuria, while the concept of non-albuminuric DN is well documented, reflecting the fact that patients with diabetes mellitus can present with a reduction in GFR without progressing from normo- to macroalbuminuria.Citation53 As a result, other biomarkers (reflective of glomerular or tubular injury) are now considered as more reliable, alternative predictors of DN risk. Since tubular lesions occur early in DN, markers of tubular injury will particularly be more useful than albuminuria in the early prediction of disease.
Recently, haptoglobin has been identified as another alternative dependable biomarker. By including the haptoglobin/creatinine ratio to a model using the albumin/creatinine ratio to predict early decline in renal function, some investigators reported an improvement in its predictive performance.Citation54 Thus, it was concluded that the haptoglobin/creatinine ratio may be useful to predict patients with T2DM at risk of nephropathy prior to the onset of macroalbuminuria or reduced GFR.
Novel biomarkers in DN
Biomarkers of glomerular injury, tubular injury, inflammation, and oxidative stress precede albuminuria in some patients and thus may be useful for the early prediction of DN, although most of them still require validation.Citation55,Citation56 Because of the diversity of these novel biomarkers, different methods of classification have been documented for clarity.Citation56
Some authors have classified the biomarkers according to both their origin and the already-identified pathologic processes impairing the nephron such as kidney injury, oxidative stress, and inflammation: biomarkers of renal dysfunction, inflammatory biomarkers (cytokines and chemokines), and oxidative stress biomarkers.Citation16 Others had proposed a classification which grouped the biomarkers into three categories: glomerular, tubular, and other proteins.Citation57
For the purpose of this review, a combination of these classifications will be used to discuss these markers (). It is equally important to note that an overlap exists in the categorization; some biomarkers of inflammation can also be regarded as glomerular markers, while some others that are grouped as miscellaneous biomarkers can also be seen as tubular or glomerular markers.
Table 1 Classification of the novel biomarkers
Glomerular biomarkers
These biomarkers include transferrin, immunoglobulin G (IgG), ceruloplasmin, type IV collagen, laminin, glycosaminoglycans (GAGs), lipocalin-type prostaglandin D synthase (L-PGDS), fibronectin, podocytes-podocalyxin, and VEGF.Citation56 Podocalyxin and VEGF are essentially considered as podocyte biomarkers.
Interestingly, newer approaches which can be used to identify potential novel biomarkers of DN have also been described; these include urinary microRNAs which are short noncoding mRNAs that regulate gene expression and urine proteomics, highlighting a possible role for epigenetic factors in the development of the disease.Citation58 For instance, in a review to assess the correlation between changes in various mRNAs and progression of DN, some authors noted that mRNA-377, mRNA-192, mRNA-216/217, and mRNA-144 were elevated in body fluids of patients with DN, while mRNA-21 and mRNA-375 were decreased. They therefore suggested the plausibility of using urine-specific mRNAs as novel biomarkers for the diagnosis of early stages of DN.Citation59 For glomerular biomarkers, urinary transferrin has been demonstrated as a more reliable marker of glomerular injury than albuminuria (). As a major serum iron-binding protein, transferrin conveys iron in its ferric forms to proliferative cells.Citation16 Some reports indicate that prior to the development of microalbuminuria, urinary transferrin excretion appeared higher in diabetic subjects than in their healthy controls.Citation60–Citation62 In fact, urinary transferrin has been considered as a more sensitive biomarker of glomerular injury in diabetes mellitus.Citation63–Citation65 This is because patients with diabetes mellitus have increased likelihood of presenting with urinary transferrinuria than with albuminuria, while albumin/transferrin ratio is significantly lower in diabetic patients with normoalbuminuria and microalbuminuria than in those with macroalbuminuria.Citation66 In addition, transferrinuria predicts the development of microalbuminuria in patients with T2DM who have normoalbuminuria.Citation67,Citation68 Moreover, in macroalbuminuric diabetic patients, urinary transferrin excretion is positively correlated with UAE.Citation69–Citation71 However, transferrinuria also occurs in primary glomerulonephritis, as well as systemic diseases which secondarily affect the glomerulus, thus underscoring its lack of specificity to DN.Citation72
Table 2 Summary of some of the novel biomarkers for diabetic nephropathy
Urinary IgG is another related biomarker. The immunoglobulin is an anionic plasma protein which crosses the glomerulus with difficulty, but appears in the urine concomitantly with elevated values of urinary transferrin, urinary ceruloplasmin, and urinary orosomucoid before the onset of microalbuminuria, which suggests the ability of increased elimination of urinary IgG to predict microalbuminuria in DM patients.Citation73 For urinary ceruloplasmin, this copper-transporting serum protein is filtered with difficulty through the glomerular barrier because it is negatively charged like IgG.Citation74 Similar to urinary transferrin and urinary IgG, urinary ceruloplasmin can predict DN earlier than albuminuria in T2DM patients; in fact, the values of the three markers can be elevated concomitantly.Citation75 Both laminin and type IV collagen are components of glomerular basement membrane, although the latter is also a component of mesangial matrix. Since elevated urinary levels of type IV collagen occur in normoalbuminuric patients with T1DM, the biomarker can also be used as an early predictor of DN,Citation75 and has actually been considered to be a specific indicator of early DN.Citation76
Moreover, some studies indicate that urinary excretion of type IV collagen in T2DM is related to UAE,Citation77–Citation79 while other investigators have shown that T2DM patients with evidence of kidney disease present with a significantly higher type IV collagen/albumin ratio compared to their nondiabetic counterparts with nephropathy.Citation77,Citation78 Again, these findings support the possible use of urinary type IV collagen in the differentiation of DN from nondiabetic nephropathy. Finally, urinary type IV collagen has also been found to show more sensitivity than albuminuria in the detection of renal injury in patients with T2DM,Citation75 although other authors have reported that as many as one third of patients with microalbuminuria do not have increased urinary type IV collagen excretion.Citation80 Remarkably, several studies have shown that urinary laminin excretion is higher in patients with diabetes mellitus in comparison to their healthy controls, even prior to the onset of microalbuminuria.Citation78,Citation81,Citation82 To buttress the discriminatory role of this biomarker in diabetic and nondiabetic kidney disease, it has been reported that patients with T2DM who show evidence of nephropathy present with significantly higher laminin/albumin ratio compared to patients with nondiabetic nephropathy.Citation78
Urinary fibronectin is probably another useful biomarker of DN, but its relevance compared to albuminuria needs to be validated by further studies. Nevertheless, as an intrinsic component of the glomerular extracellular matrix, the urinary excretion of this high-molecular-weight protein is noted to be higher in subjects with diabetes mellitus compared to their controls, with a significant difference observed only in patients with macroalbuminuria.Citation83,Citation84 Further evidences pointing to its possible predictive role in DN include its higher excretion in diabetic subjects with microalbuminuria than in those with normoalbuminuria,Citation83 the correlation of its urinary levels with the progression of biopsy-proven glomerular diffuse lesions,Citation85 and the correlation of its degradation products with UAE.Citation86
Urinary GAGs are increased in DM patients with normoalbuminuria,Citation87 and are associated with other tubular markers such as Tamm–Horsfall protein, which expresses a distal tubular dysfunction in patients with diabetes mellitus.Citation88 GAGs are also present at the level of the tubular basement membrane. Finally, L-PGDS is a biomarker related to lesions of the glomerular capillary walls and reflects their increased permeability. Although it is basically considered to predict renal lesions, it is less relevant as an early biomarker of DN.Citation89
Tubular biomarkers
Notably, tubulointerstitial lesions are also associated with glomerular injury during DN.Citation90 Interestingly, tubular biomarkers have shown that tubular dysfunction can be present early in DN, occasionally preceding glomerular injury. This observation underscores the fact that tubular biomarkers are early predictors of DN compared to microalbuminuria and other glomerular biomarkers. Examples of tubular biomarkers include neutrophil gelatinase-associated lipocalin (NGAL), α-1-microglobulin, kidney injury molecule 1 (KIM-1), N-acetyl-β-D-glucosaminidase (NAG), cystatin C, and liver-type fatty acid-binding protein (L-FABP).
A recent review of diagnostic markers which can detect DN at an early stage shows that urinary tubular markers like NAG, α-1-microglobulin, L-FABP, as well as other makers such as nephrin and angiotensinogen may be elevated before the appearance of microalbuminuria in T2DM.Citation91
Similarly, elevated urinary NGAL is present in normoalbuminuric diabetic patients, as well as used to assess tubular lesions in the disease,Citation92 and has been shown to precede microalbuminuria in T1DM.Citation93,Citation94 In addition, high values of this biomarker were noted in T2DM patients with normoalbuminuria which rose progressively in patients with microalbuminuria and macroalbuminuria.Citation95 Thus, urinary NGAL in T2DM patients likely has a role in predicting the evolution of disease.Citation96
Regarding α-1-microglobulin, it is normally filtered through the glomerulus and reabsorbed in the proximal tubule; tubular dysfunction thus impairs its reabsorption resulting in its urinary excretion. A study has shown that normoalbuminuric T2DM patients had elevated urinary levels of this biomarker, given that tubular injury precedes the onset of microalbuminuria, making it a more sensitive and an earlier urinary biomarker.Citation97 Besides, urinary α-1-microglobulin in early stages of diabetes mellitus could also have a role in predicting DN,Citation98 and has been identified as an inexpensive biomarker for the early diagnosis of DN.Citation99
There appears to be disparate findings about the sensitivity of NAG as a biomarker for the early prediction of DN. While some authors reported that NAG can be considered an early tubular biomarker as well as the most sensitive biomarker for detecting early renal injury in diabetic patients,Citation100,Citation101 others failed to demonstrate any clinical significance as an early biomarker of DN.Citation102 Nevertheless, as a sensitive tubular biomarker, increased urinary NAG can precede the appearance of microalbuminuria in T1DM.Citation103
For cystatin C, its urinary excretion suggests tubular injury as it is elevated early in diabetes and prediabetic nephropathy, together with NGAL.Citation104 Besides the predictive role of urinary cystatin C for the progression of DN,Citation105 its serum and urinary levels are useful biomarkers for assessing early nephropathy in T2DM.Citation106
Although KIM-1, as tubular transmembrane glycoprotein, is a well-known sensitive biomarker for acute kidney injury,Citation107 increased urinary levels have been reported in normoalbuminuric T2DM patients, indicating lesions of the proximal tubule in early stages of the disease. Moreover, microalbuminuric patients have higher urinary KIM-1 levels than those with normoalbuminuria.Citation108 Another tubular biomarker with good predictive ability for diabetic kidney disease is urinary L-FABP. Elevated levels of this biomarker were found in normoalbuminuric T1DM patients; urinary L-FABP has a predictive role with respect to the evolution toward microalbuminuria, and of microalbuminuria toward macroalbuminuria.Citation109 Similarly, patients with normoalbuminuric T2DM also presented high levels of urinary L-FABP, this protein being considered as a useful biomarker for diagnosing early DN,Citation110 as well as an independent predictor of the progression of DN.Citation111
Biomarkers of inflammation
Biomarkers of inflammation such as TNF-α and IL-1β, which are cytokines involved in the onset and progression of DN, also play predictive roles in the DN.Citation112,Citation113 A study by a group of researchers was the first to suggest that these proinflammatory cytokines could participate in the development of DN.Citation114 In this study, macrophages incubated with glomerular basement membranes from diabetic rats produced significantly greater levels of IL-1β and TNF-α than macrophages incubated with membranes of normal nondiabetic rats.Citation114 However, scientific attention has been directed more at the implications of TNF-α in the context of DN. TNF-α is reported to exert cytotoxic effect on glomerular, mesangial, and epithelial cells, and may trigger direct renal injury.Citation115,Citation116 In addition, increased urinary TNF-α excretion and elevated TNF-α levels in renal interstitial fluid have been observed to precede a significantly increased albuminuria in experimental murine models.Citation117 Similarly, the findings from clinical studies indicate a direct and significant relationship between urinary protein excretion and serum TNF-α in diabetic patients with normal renal function and microalbuminuria on one hand, as well as in patients with overt nephropathy and ESKD on the other hand.Citation118,Citation119 Notably, the fact that urinary TNF-α levels are elevated in diabetic patients who have increased UAE, coupled with the significant rise of urinary TNF-α excretion as DN progresses, strongly supports the prospect of using this cytokine as a biomarker for predicting DN.Citation113
Other biomarkers of inflammation, which are also glomerular markers, include IL-18, interferon gamma-induced protein (IP-10), monocyte chemoattractant protein 1 (MCP-1), granulocyte colony-stimulating factor (G-CSF), eotaxins, RANTES (regulated on activation, normal T cell expressed and secreted) or CCL-5, and orosomucoid. IL-18 is a proinflammatory cytokine originating from mononuclear cells. Its serum and urinary levels have been reported to correlate positively with albumin excretion rate, while its serum levels also correlate positively with carotid intima-media thickness in patients with T2DM, and thus may be a predictor of DN progression, as well as cardiovascular diseases.Citation120,Citation121 IP-10 and MCP-1 are other proinflammatory cytokines whose serum levels are significantly increased in subjects with T2DM.Citation121 Moreover, serum and urinary levels of these two proinflammatory cytokines were found to be positively correlated with albumin excretion rate and intima-media thickness, suggesting that microinflammation may be a common risk factor for DN and atherosclerosis in T2DM.Citation121 Serum and urinary G-CSF levels are also increased in the early stages of DN,Citation122 while urinary RANTES (or CCL-5) and eotaxin excretion rates were noted to be significantly higher in hyperfiltering than in normofiltering T1DM patients.Citation123 Like RANTES which is a chemotactic cytokine, eotaxins are a chemotactic cytokine subfamily of eosinophil chemotactic proteins consisting of eotaxin-1 (CCL-11), eotaxin-2 (CCL-24), and eotaxin-3 (CCL-26).Citation124 The high urinary levels of eotaxin and RANTES, from hyperfiltration in the diabetic kidney, occur as result of high intraglomerular pressure which causes renal inflammation, inflammation being a component of DN.Citation123
Finally, some investigators reported high IL-8 and MCP-1 levels in early and late stages of DN in T2DM patients, respectively,Citation125 while another study on T2DM patients with normoalbuminuria and microalbuminuria reported higher values of IL-8, IP10, MCP-1, G-CSF, eotaxin, and RANTES in patients with microalbuminuria than in those with normoalbuminuria.Citation126 Urinary orosomucoid has been noted to be high in T1DM patients with normoalbuminuria than in controls, while the levels increase in patients with microalbuminuria and macroalbuminuria.Citation127 In addition, T2DM patients were reported to present with increased urinary orosomucoid, in parallel with the excretion of IgG, ceruloplasmin, and transferrin.Citation128
Biomarkers of oxidative stress
A typical example of a biomarker of oxidative stress is urinary 8-oxo-7,8-dihydro-2-deoxyguanosine (8oHdG). This marker is produced secondary to oxidative DNA damage, and appears in the urine without being metabolized.Citation129 Remarkably, urinary 8oHdG has been reported as a useful clinical marker to predict the development of DN in diabetic patients given the observation of a significant progression of DN in the patients with higher excretion of the biomarker in urine compared with their counterparts with moderate or lower excretion of the molecule.Citation130
Miscellaneous biomarkers
These include some tubular markers such as urinary heart fatty acid-binding protein and urinary retinol-binding protein, podocyte biomarkers such as podocalyxin, nephrin, and VEGF, as well as urinary proteomics and urinary advanced glycation end products (AGEs). These podocyte biomarkers are also regarded as glomerular markers. Nephrin is a slit diaphragm protein whose expression is reduced in late proteinuric phase of experimental DN, suggesting that reduced nephrin expression may be a determinant of glomerular hyperpermeability in DN.Citation131,Citation132 Since podocyte dysfunction occurs in diabetes mellitus and DN is actually considered a podocytopathy, nephrinuria has been reported in some T1DM patients before the onset of microalbuminuria,Citation133 as well as in some normoalbuminuric T2DM patients.Citation134 Furthermore, another podocyte biomarker like urinary VEGF has been found to be increased in T2DM patients with microalbuminuria and macroalbuminuria,Citation135 while higher levels of podocalyxin were seen in diabetic patients with microalbuminuria than in diabetic patients with normoalbuminuria.Citation136 Finally, some authors have reported that high values of urinary AGEs were seen in 50% of the T2DM patients with normoalbuminuria and in 85% of those with microalbuminuria.Citation137
Conclusion
Albuminuria is the conventional biomarker for the detection and prediction of DN and cardiovascular risk in T1DM and T2DM, although it has several limitations. Thus, there is now a focus on novel biomarkers which have higher sensitivity and specificity for earlier detection of DN, as well as for a more precise prediction of its progression to ESKD.
Although these biomarkers are potentially useful for the evaluation of diabetic kidney disease, current data still preclude the routine clinical use of majority of them. Nevertheless, the research trajectory on novel biomarkers for DN should take the course of persistent efforts at their discovery, their validation using high-quality and large longitudinal studies, and subsequent development of a biomarker panel which can reliably predict and evaluate this renal microvascular disease.
Acknowledgments
The author acknowledges the invaluable information obtained from the studies by Arora and Singh,Citation17 Cohen-Bucay and- Viswanathan,Citation43 and Gluhovschi et alCitation56 during the preparation of the manuscript.
Disclosure
The author reports no conflicts of interest in this work.
References
- KoulouridisEDiabetic nephropathy in children and adolescents and its consequences in adultsJ Pediatr Endocrinol Metab200114Suppl 5S1367S1377
- UwaezuokeSNPrevention of diabetic nephropathy in children and adolescents: how effective are the current strategies?Int J Diabetol Vasc Dis Res2015 S5:00115
- DeferrariGRepettoMCalviCCiabattoniMRossiCRobaudoCDiabetic nephropathy: from micro- to microalbuminuriaNephrol Dial Transplant199813Suppl 8S11S15
- DanneTKordonouriOHövenerGWeberBDiabetic angiopathy in childrenDiabet Med19971412101210259455928
- MaghribiHAbu-OdehAEarly diabetic nephropathy in a pediatric patientJRMS20061315153
- FrancisJRoseSJRaafatFMilfordDVEarly onset of diabetic nephropathyArch Dis Child19977765245259496190
- DeClueTJCamposADiabetic nephropathy in a prepubertal diabetic femaleJ Pediatr Endocrinol19947143468186823
- RemuzziGSchieppatiARuggenentiPNephropathy in patients with type 2 diabetesN Engl J Med2002346151145115111948275
- American Diabetes AssociationPosition statement: diabetic nephropathyDiabetes Care199922Suppl 1S66S69
- CaramoriMLFiorettoPMauerMThe need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient?Diabetes20004991399140810969821
- PerkinsBAFicocielloLHSilvaKHFinkelsteinDMWarramJHKrolewskiASRegression of microalbuminuria in type 1 diabetesN Engl J Med2003348232285229312788992
- GiorginoFLaviolaLCavallo PerinPSolnicaBFullerJChaturvediNFactors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications StudyDiabetologia20044761020102815170497
- CaramoriMLFiorettoPMauerMEnhancing the predictive value of urinary albumin for diabetic nephropathyJ Am Soc Nephrol200617233935216394108
- AdlerAIStevensRJManleySEBilousRWCullCAHolmanRRUKPDS GROUPDevelopment and progression of nephropathy in type 2 diabetes: the United Kingdom prospective diabetes study (UKPDS 64)Kidney Int200363122523212472787
- AnJHChoYMYuHGThe clinical characteristics of normoalbuminuric renal insufficiency in Korean type 2 diabetic patients: a possible early stage renal complicationJ Korean Med Sci200924SupplS75S8119194567
- MathesonAWillcoxMDPFlanaganJWalshBJUrinary biomarkers involved in type 2 diabetes: a reviewDiabetes Metab Res Rev201026315017120222150
- AroraMKSinghUKMolecular mechanisms in the pathogenesis of diabetic nephropathy: an updateVascul Pharmacol201358425927123313806
- WadaJMakinoHInflammation and the pathogenesis of diabetic nephropathyClin Sci (Lond)2013124313915223075333
- TavridouAGeorgoulidouARoumeliotisAAssociation of plasma adiponectin and oxidized low-density lipoprotein with carotid intima-media thickness in diabetic nephropathyJ Diabetes Res2015201550726526064982
- MatavelliLCHuangJSiragyHM(Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammatoryClin Exp Pharmacol Physiol201037327728219769609
- EgidoJVasoactive hormones and renal sclerosisKidney Int19964925785978821847
- FeliersDGorinYGhosh-ChoudhuryGAbboundHEKasinathBSAngiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen speciesAm J Physiol Renal Physiol20062904927936
- NakamuraSNakamuraIMaLVaughanDEFogoABPlasminogen activator inhibitor-1 (PAI-1) expression is regulated by the angiotensin type 1 receptor in vivoKidney Int200058125125910886570
- Ruiz-OrtegaMRupérezMEstebanVAngiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseasesNephrol Dial Transplant2006211162016280370
- KangYSParkYGKimBKAngiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytesJ Mol Endocrinol200636237738816595708
- GiacchettiGSechiLARilliSCareyRMThe renin-angiotensin-aldosterone system, glucose metabolism and diabetesTrends Endo-crinol Metabol2005163120126
- FujisawaGOkadaKMutoSSpironolactone prevents early renal injury in streptozotocin-induced diabetic ratsKidney Int20046641493150215458443
- TairaMTobaHMurakamiMSpironolactone exhibits direct renoprotective effects and inhibits renin-angiotensin-aldosterone system in diabetic ratsEur J Pharmacol20085891–326427118582458
- GuoCMartinez-VasquezDMendezGPMineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitusEndocrinology2006147115363537316901964
- Thallas-BonkeVThorpeSRCoughlanMTInhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathwayDiabetes200857246046917959934
- YaoLWangJMaoYZhuHDengAZhuZDifferent expressions of protein kinase C-alpha, beta I and beta II in glomeruli of diabetic nephropathy patientsJ Huazhong Univ Sci Technology Med Sci2006266651653
- XiaLWangHMunkSReactive oxygen species, PKC-beta I and PKC-zeta mediate high glucose-induced vascular endothelial growth factor expression in mesangial cellsAm J Physiol Endocrinol Metab20072935E1280E128817711990
- KoyaDJirousekMRLinYWIshiiHKubokiKKingGICharacterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-β, extracellular matrix components and prostanoids in the glomeruli of diabetic ratsJ Clin Invest199710011151269202063
- GorinYBlockKHernandezJNox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidneyJ Biol Chem200528047396163962616135519
- XiaLWangHGoldbergHJMunkSFantusIGWhitesideCIMesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expressionAm J Physiol Renal Physiol20062902F345F35616131649
- AsabaKTojoAOnozatoMLEffects of NADPH oxidase inhibitor in diabetic nephropathyKidney Int20056751890189815840036
- ZugmaierGPaikSWildingGTransforming growth factor beta 1 induces cachexia and systemic fibrosis without an antitumor effect in nude miceCancer Res19915113359035942054795
- MoriwakiYYamamotoTShbutaniYElevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathyMetabolism200352560560812759891
- NiewczasMAGohdaTSkupienJCirculating TNF receptors 1 and 2 predict ESRD in type 2 diabetesJ Am Soc Nephrol201223350751522266663
- NavarroJFMoraCMurosMGarciaJUrinary tumour necrosis-alpha excretion independently correlates with clinical markers of glomerular and tubulo-interstitial injury in type 2 diabetic patientsNephrol Dial Transplant200621123428343416935891
- HaraldssonBNyströmJDeenWMProperties of the glomerular barrier and mechanisms of proteinuriaPhysiol Rev200888245148718391170
- BirnHChristensenEIRenal albumin absorption in physiology and pathologyKidney Int200669344044916514429
- Cohen-BucayAViswanathanGUrinary markers of glomerular injury in diabetic nephropathyInt J Nephrol2012201214698722645683
- de ZeeuwDRamjitDZhangZRenal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAALKidney Int20066991675168216572114
- de ZeeuwDRemuzziGParvingHHAlbuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathyCirculation2004110892192715302780
- American Diabetes AssociationStandards of medical care in diabetes-2014Diabetes Care201437Suppl 1S14S8024357209
- NinomiyaTPerkovicVde GalanBEADVANCE Collaborative GroupAlbuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetesJ Am Soc Nephrol20092081813182119443635
- DinneenSFGersteinHCThe association of microalbuminuria and mortality in none-insulin-dependent diabetes mellitus: a systematic overview of the literatureArch Int Med199715713141314189224218
- RuggenentiPRemuzziGTime to abandon microalbuminuria?Kidney Int20067071214122216871239
- GersteinHCMannJFYiQHOPE Study InvestigatorsAlbuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individualsJAMA2001286442142611466120
- KlausenKBorch-JohnsenKFeldt-RasmussenBVery low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetesCirculation20041101323515210602
- WachtellKIbsenHOlsenMHAlbuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE studyAnn Int Med20031391190190614644892
- CurrieGMcKayGDellesCBiomarkers in diabetic nephropathy: present and futureWorld J Diabetes20145676377625512779
- BhensdadiaNMHuntKJLopes-VirellaMFVeterans Affairs Diabetes Trial (VADT) study groupUrine haptoglobin levels predict early renal functional decline in patients with type 2 diabetesKidney Int20138361136114323536133
- MorescoRNSangoiMBDe CarvalhoJATatschEBochiGVDiabetic nephropathy: traditional to proteomic markersClin Chim Acta2013421173023485645
- GluhovschiCGluhovschiGPetricaLUrinary biomarkers in the assessment of early diabetic nephropathyJ Diabetes Res20162016462612527413755
- HongCYChiaKSMarkers of diabetic nephropathyJ Diabetes Complications199812143609442815
- LeeSYChoiMEUrinary biomarkers for early diabetic nephropathy: beyond albuminuriaPediatr Nephrol20153071063107525060761
- YangYXiaoLLiJKanwarYSLiuFSunLUrine miRNAs: potential biomarkers for monitoring progression of early stages of diabetic nephropathyMed Hypotheses201381227427823683774
- NaritaTHosobaMMiuraTLow dose of losartan decreased urinary excretions of IgG, transferrin, and ceruloplasmin without reducing albuminuria in normoalbuminuric type 2 diabetic patientsHorm Metab Res200840429229518548390
- CheungCKCockramCSYeungVTSwaminathanRUrinary excretion of transferrin by non-insulin-dependent diabetics: a marker for early complications?Clin Chem1989358167216742758634
- MartinPWaltonCChapmanCBodanskyHJSticklandMHIncreased urinary excretion of transferrin in children with type 1 diabetes mellitusDiabet Med19907135401688749
- O’DonnellMJMartinPFlorkowskiCMUrinary transferrin excretion in type 1 (insulin-dependent) diabetes mellitusDiabet Med1991876576611833118
- BernardAMAmorAAGoemaere-VannesteJMicrotransferrinuria is a more sensitive indicator of early glomerular damage in diabetes than microalbuminuriaClin Chem198834919201921
- O’DonnellMJMartinPCavanDIncreased urinary transferrin excretion in exercising normoalbuminuric insulin-dependent diabetic patientsAnn Clin Biochem199128Pt 54564601720297
- McCormickCPKonenJCShihabiZKMicrotransferrinuria and microalbuminuria: in the diabetic humanClin Physiol Biochem1989825358
- KazumiTHozumiTIshidaYIncreased urinary transferrin excretion predicts microalbuminuria in patients with type 2 diabetesDiabetes Care19992271176118010388985
- NaritaTHosobaMKakeiMItoSIncreased urinary excretions of immunoglobulin G, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetesDiabetes Care200629114214416373913
- SasakiAOikawaSToyotaTMicroalbuminuria is closely related to diabetic macroangiopathyDiabetes Res Clin Pract1999441354010414938
- BernardAAmorAOGoemare-VannesteJUrinary proteins and red blood cell membrane negative charges in diabetes mellitusClin Chim Acta199019032492622253403
- MartinPTindallHHarveyJNHandleyTMChapmanCDaviesJAGlomerular and tubular proteinuria in type 1 (insulin-dependent) diabetic patients with and without retinopathyAnn Clin Biochem199229Pt 32652701376979
- YaqoobMMcClellandPPatrickAWStevensonAMasonHBellGMTubular damage in microalbuminuric patients with primary glomerulonephritis and diabetic nephropathyRen Fail199517143497770643
- NaritaTSasakiHHosobaMParallel increase in urinary excretion rates of immunoglobulin G, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patientsDiabetes Care20042751176118115111541
- WangCLiCGongWLouTNew urinary biomarkers for diabetic kidney diseaseBiomarker Res201319
- KotajimaNKimuraTKandaTType IV collagen as an early marker for diabetic nephropathy in non-insulin-dependent diabetes mellitusJ Diabetes Complications2000141131710925061
- TanYYangYZhangZZhangXLiuYUrinary type IV collagen: a specific indicator of incipient diabetic nephropathyChin Med J (Engl)2002115338939411940372
- KadoSAokiAWadaSUrinary type IV collagen as a marker for early diabetic nephropathyDiabetes Res Clin Pract1996311–31031088792108
- BanuNHaraHOkamuraMEgusaGYamakidoMUrinary excretion of type IV collagen and laminin in the evaluation of nephropathy in NIDDM: comparison with urinary albumin and markers of tubular dysfunction and/or damageDiabetes Res Clin Pract199529157678593760
- SthaneshwarPChanSPUrinary type IV collagen levels in diabetes mellitusMalays J Pathol2010321434720614725
- IijimaTSuzukiSSekizukaKFollow-up study on urinary type IV collagen in patients with early stage diabetic nephropathyJ Clin Lab Anal19981263783829850190
- HaiyashiYMakinoHOtaZSerum and urinary concentrations of type IV collagen and laminin as a marker of microangiopathy in diabetesDiabet Med1992943663701600709
- MiyakeHNagashimaKYagiHOnigataKUrinary laminin P1 as an index of glycemic control in children with insulin-dependent diabetes mellitusDiabetes Res1992233131138
- TakahashiMIncreased urinary fibronectin excretion in type II diabetic patients with microalbuminuriaNihon Jinzo Gakkai Shi19953763363427666599
- FageruddJAGroopPHHonkanenETeppoAMGrönhagen-RiskaCUrinary excretion of TGF-β1, PDGF-BB and fibronectin in insulin-dependent diabetes mellitus patientsKidney Int19975163S195S197
- KanauchiMNishiokaHHashimotoTDohiKDiagnostic significance of urinary transferrin in diabetic nephropathyNihon Jinzo Gakkai Shi199537116496548583702
- KubokiKTadaHShinKOshimaYIsogaiSRelationship between urinary excretion of fibronectin degradation products and proteinuria in diabetic patients, and their suppression after continuous subcutaneous heparin infusionDiabetes Res Clin Pract199321161668253024
- UetaITakamatsuKHashimotoKUrinary glycosaminoglycans in patients with incipient diabetic nephropathyNihon Jinzo Gakkai Shi199537117237699949
- TorffvitOUrinary sulphated glycosaminoglycans and Tamm-Horsfall protein in type 1 diabetic patientsScand J Urol Nephrol199933532833210572998
- UeharaYMakinoHSeikiKUradeYL-PGDS Clinical Research Group of KidneyUrinary excretions of lipocalin-type prostaglandin D synthase predict renal injury in type-2 diabetes: a cross-sectional and prospective multi-center studyNephrol Dial Transplant200924247548218799608
- MiseKHoshinoJUenoTPrognostic value of tubulointerstitial lesions, urinary N-acetyl-β-d-glucosaminidase, and urinary β2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathyClin J Am Soc Nephrol201611459360126801478
- FisehaTUrinary biomarkers for early diabetic nephropathy in type 2 diabetic patientsBiomark Res2015311626146561
- BolignanoDLacquanitiACoppolinoGNeutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patientsKidney Blood Press Res2009322919819321980
- Yürük YıldırımZNayırAYılmazAGedikbasıABundakRNeutrophil gelatinase-associated lipocalin as an early sign of diabetic kidney injury in childrenJ Clin Res Pediatr Endocrinol20157427427926777038
- LacquanitiADonatoVPintaudiB“Normoalbuminuric” diabetic nephropathy: tubular damage and NGALActa Diabetol201350693594223754672
- de CarvalhoJATatschEHausenBSUrinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetesClin Biochem201649323223626519090
- YangYHHeXJChenSRWangLLiEMXuLYChanges of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up studyEndocrine2009361455119390997
- HongCYHughesKChiaKSNgVLingSLUrinary alpha1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in SingaporeDiabetes Care200326233834212547859
- WainaiHKatsukawaFTakeiIMaruyamaHKataokaKSarutaTInfluence of glycemic control and hypertension on urinary microprotein excretion in non-insulin-dependent diabetes mellitusJ Diabetes Complications199152–3160161
- ShoreNKhurshidRSaleemMAlpha-1microglobulin: a marker for early detection of tubular disorders in diabetic nephropathyJ Ayub Med Coll Abbottabad20102245355
- PatelDNKaliaKEfficacy of urinary N-acetyl-β-D glucosaminidase to evaluate early renal tubular damage as a consequence of type 2 diabetes mellitus: a cross-sectional studyInt J Diabetes Dev Ctries201535Suppl 3449457
- AssalHSTawfeekSRasheldEAEl-LebedyDThabetEHSerum cystatin C and tubular urinary enzymes as biomarkers: a renal dysfunction in type 2 diabetes mellitusClin Med Insights Endocrinol Diabetes2013671323966807
- AmbadeVSinghPSomaniBLBasannarDUrinary N-acetyl β-glucosaminidase and γ-glutamyl transferase as early markers of diabetic nephropathyIndian J Clin Biochem2006212142148
- JonesAPLockSGriffithsKDUrinary N-acetyl-β-glucosaminidase activity in type I diabetes mellitusAnn Clin Biochem199532Pt 158627762951
- GargVKumarMMahapatraHSChitkaraAGadpayleAKSekharVNovel urinary biomarkers in pre-diabetic nephropathyClin Exp Nephrol201519589590025634253
- KimSSSongSHKimIJUrinary cystatin C and tubular proteinuria predict progression of diabetic nephropathyDiabetes Care201336365666123093662
- JeonYKKimMRHuhJECystatin C as an early biomarker of nephropathy in patients with type 2 diabetesJ Korean Med Sci201126225826321286018
- BonventreJVKidney injury molecule-1: a translational journeyTrans Am Clin Climatol Assoc201412529329925125746
- PetricaLVladAGluhovschiGProximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional studyPLoS One2014911e11253825397960
- NielsenSESugayaTHovindPBabaTParvingH-HRossingPUrinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patientsDiabetes Care20103361320132420185732
- Kamijo-IkemoriASugayaTYasudaTClinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patientsDiabetes Care201134369169621273494
- PanduruNMForsblomCSaraheimoMFinnDiane Study GroupUrinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetesDiabetes Care20133672077208323378622
- ElmarakbyAASullivanJCRelationship between oxidative stress and inflammatory cytokines in diabetic nephropathyCardiovasc Ther2012301495920718759
- NavarroJFMoraCDiabetes, inflammation, proinflammatory cytokines, and diabetic nephropathyScientificWorldJournal2006690891716906324
- HasegawaGNakanoKSawadaMPossible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathyKidney Int1991406100710121762301
- OrtizAGonzález-CuadradoSBustosCTumor necrosis factor as a mediator of glomerular damageJ Nephrol199582734
- BertaniTAbbateMZojaCTumor necrosis factor induces glomerular damage in rabbitAm J Pathol198913424194302916653
- KalantariniaKAwasASSiragyHMUrinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic ratsKidney Int20036441208121312969138
- NavarroJFMoraCMacaMGarcaJInflammatory parameters are independently associated with urinary albumin excretion in type 2 diabetes mellitusAm J Kidney Dis2003421536112830456
- NavarroJFMoraCRiveroAUrinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administrationAm J Kidney Dis199933345846310070909
- NakamuraAShikataKHiramatsuMSerum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetesDiabetes Care200528122890289516306550
- KajitaniNShikataKNakamuraANakatouTHiramatsuMMakinoHMicroinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetesDiabetes Res Clin Pract201088217117620138680
- PerlmanASChevalierJMWilkinsonPSerum inflammatory and immune mediators are elevated in early stage diabetic nephropathyAnn Clin Lab Sci201545325626326116588
- HarRScholeyJWDanemanDThe effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitusDiabetologia20135651166117323412605
- Van CoillieEVan DammeJOpdenakkerGThe MCP/eotaxin subfamily of chemokinesCytokine Growth Factor Rev1999101618610379912
- TashiroKKoyanagiISaitohAUrinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL8), and renal injuries in patients with type 2 diabetic nephropathyJ Clin Lab Anal20021611411835523
- LiuJZhaoZWillcoxMDXuBShiBMultiplex bead analysis of urinary cytokines of type 2 diabetic patients with normo- and microalbuminuriaJ Immunoassay Immunochem201031427928921113841
- JiangHGuanGZhangRIncreased urinary excretion of orosomucoid is a risk predictor of diabetic nephropathyNephrology200914333233719143942
- ZoppiniGTargherGChoncholMPredictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney functionClin J Am Soc Nephrol20127340140822282481
- WuLLChiouCCChangPYWuJTUrinary 8OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabeticsClin Chem Acta20043391–219
- HinokioYSuzukiSHiraiMSuzukiCSuzukiMToyotaTUrinary excretion of 8-oxo-7,8-dihydro-2-deoxyguanosine as a predictor of the development of diabetic nephropathyDiabetologia200245687788212107732
- KellyDJAaltonenPCoxAJExpression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of antiproteinuric therapiesNephrol Dial Transplant20021771327133212105259
- LanghamRGKellyDJCoxAJProteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibitionDiabetologia200245111572157612436341
- PätäriAForsblomCHavanaMTaipaleHGroopPHHolthöferHNephrinuria in diabetic nephropathy of type 1 diabetesDiabetes200352122969297414633858
- NgDPTaiBCTanENephrinuria associates with multiple renal traits in type 2 diabetesNephrol Dial Transplant20112682508251421196468
- KimNHKimKBKimDLPlasma and urinary vascular endothelial growth factor and diabetic nephropathy in type 2 diabetes mellitusDiabet Med200421654555115154937
- ShojiMKobayashiKTakemotoMSatoYYokoteKUrinary podocalyxin levels were associated with urinary albumin levels among patients with diabetesBiomarkers201521216416726635084
- TurkNMornarAMrzljakVTurkZUrinary excretion of advanced glycation end products in patients with type 2 diabetes and various stages of proteinuriaDiabetes Metab200430218719215223992