Abstract
Refractory ascites can occur in patients with various conditions. Although several procedures based on the reinfusion of ascitic fluid have been reported after the failure of bed rest, salt and water restriction, diuretics, intravenous administration of albumin, and repeated paracentesis, these procedures are performed for ascitic fluid removal without dialytic effect. In this study, a flow control reinfusion of ascites during hemodialysis (HD) was performed to demonstrate the efficacy of this method in a lupus patient with massive refractory ascites and respiratory and acute renal failure (ARF). The alleviation of ascites and ARF attests to the success of the flow control reinfusion of ascites during HD. This procedure can control the rate of ascites and body fluid removal simultaneously during HD using the roller pump. In conclusion, with a normal coagulation profile, the procedure of flow control reinfusion of ascites during HD is an effective alternative treatment for the alleviation of refractory ascites with renal failure.
Keywords:
Introduction
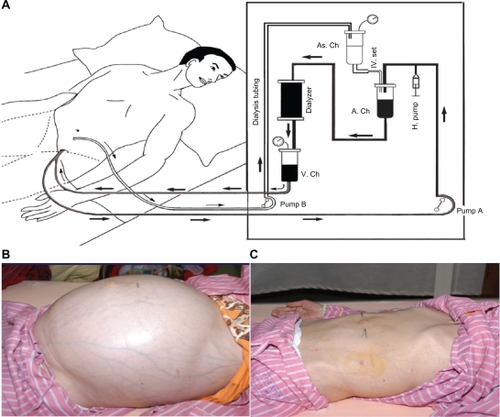
Refractory ascites can occur in patients with conditions such as liver cirrhosis, congestive heart failure, nephrotic syndrome, and lupus serositisCitation1 and in many cases cause abdominal discomfort and respiratory distress to the patient. The treatments for refractory ascites, eg, bed rest, salt and water restriction, diuretics, intravenous administration of albumin, and repeated paracentesis, are usually unsatisfactory. Several procedures based on the reinfusion of ascitic fluid have been performed for ascitic fluid removal.Citation2–Citation8 This report introduces the procedure flow control reinfusion of ascites into the dialyzer during hemodialysis (HD) (), by which a 34-year-old lupus patient with massive ascites, respiratory distress, and acute renal failure (ARF), who did not respond to diuretics, repeated paracentesis with intravenous albumin infusion, and HD, was successfully treated. Prophylactic fresh frozen plasma infusion, sequential ultrafiltration, and reduced dialysate temperature during HD in previous treatments did not prevent intradialytic hypotension. The patient’s symptoms were remedied by seven sessions of flow control reinfusion of ascites during HD ().
Figure 1 A) A paracentesis pigtail catheter was inserted into the abdominal cavity to draw out the ascites into the arterial chamber to be mixed with the blood by the flow control roller pump (pump B) during hemodialysis. This procedure allows for control of the flow of ascite reinfusion by the flow control roller pump (pump B) and the amount of body fluid removal by the ultrafiltration rate of the dialyzer simultaneously. B) The abdomen was distended by massive ascites. The superficial veins were also engorged before continuous infusion of ascites into the dialyzer to mix with blood. C) After ascite removal by ascite reinfusion into dialyzer, the distended abdomen and engorged veins disappeared.
Case
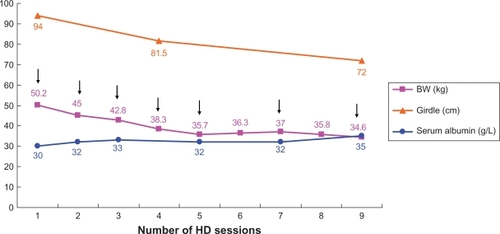
A 34-year-old female with systemic lupus erythematosus was admitted due to progressive lower leg edema, massive ascites, and nausea after treatment of bed rest, salt and water restriction, diuretics, intravenous administration of albumin and methylprednisolone, and repeated paracentesis for 2 months. On physical examination, her blood pressure was 127/82 mm Hg, pulse rate 84 beats/min, respiratory rate 20 breaths/min, and body temperature 37°C. Her abdomen was markedly distended and her lower extremities were severely edematous. The results obtained in laboratory investigations were as follows: white blood cells (WBCs) 5.8 × 109/L (normal range 4.5–11 × 109/L), hemoglobin 89 g/L (normal range 120–160 g/L), platelets 220 × 109/L (normal range 150–350 × 109/L), serum blood urea nitrogen (BUN) 13.5 mmol/L (normal range 2.5–7 mmol/L), creatinine 170 μmol/L (normal range 50–110 μmol/L), albumin 19 g/L (normal range 37–53 g/L), alanine aminotransferase 0.24 μkat/L (normal range 0–0.66 μkat/L), aspartate aminotransferase 0.60 μkat/L (normal range 0.08–0.76 μkat/L), C-reactive protein 0.286 mg/dL (normal range 0–0.5 mg/dL), complement 3 (C3) 0.4 g/L (normal range 0.8–1.5 g/L), complement 4 (C4) 0.1 g/L (normal range 0.2–0.4 g/L), and double-strand DNA (dsDNA) 263 IU/mL (normal range <30 IU/mL). Urine protein excretion was 4.86 g/day. Chest X-ray showed massive bilateral pleural effusion. The WBC count of ascitic fluid was 10/mm3. The serum–ascites albumin gradient was 1.4 mg/dL. The results of bacteria culture, acid-fast stain, malignant cell, and tuberculosis– polymerase chain reaction of ascites were negative. The Doppler of the main portal vein, inferior vena cava, major portal branches, and hepatic vein were patent without obstruction. The abdominal computed tomography scan revealed massive ascites (). The echocardiogram showed no pericardial effusion and preserved left ventricle systolic function. Unfortunately, progressive massive ascite accumulation gave rise to breathing difficulty and decreased urine output, despite an increase in serum albumin from 19 g/L to 28 g/L after the aforementioned treatments for 1 month at the hospital. The serum BUN and creatinine levels were increased to 30.5 mmol/L and 720 μmol/L from 13.5 mmol/L and 170 μmol/L, respectively. Disseminated intravascular coagulation (DIC) was within a normal range. Her lupus activity did not respond to plasma exchange, methylprednisolone 1000 mg pulse therapy, oral prednisolone 60 mg daily, or cyclosporine 100 mg twice daily. Her renal failure, leg edema, and refractory ascites could not be controlled by HD due to frequent intradialytic hypotension, which could not be corrected by prophylactic fresh frozen plasma infusion, sequential ultrafiltration, and reduction in dialysate temperature during HD. Therefore, continuous flow control reinfusion of ascites into a dialyzer during HD was designed to alleviate the ascites. A paracentesis pigtail catheter was inserted into the abdominal cavity and connected to a sterile three-way stopcock. When ascite reinfusion was planned, a set of dialysis tubing was connected with the sterile three-way stopcock to draw out the ascites at the speed of 12–15 mL/min (720–900 mL/h) into the dialyzer to mix with the blood by the roller pump during HD. The mixed blood and ascites were then channeled into the systemic circulation during a 4-hour HD session (). The ultrafiltration rate of the dialyzer was maintained at around 1 L/h to allow fluid removal from the blood and infused ascites in each 4-hour HD session. After seven sessions of continuous reinfusion of ascites into the dialyzer during HD, ∼20 L of ascites were drawn out. The body weight and abdominal girth decreased from 50.2 kg to 37 kg and from 92 cm to 72 cm, respectively (). The serum albumin level increased from 30 to 35 g/L (). There was no intradialytic hypotension, fever, gastrointestinal bleeding, or DIC. Her respiratory distress and general condition also improved after the procedures. The levels of complements C3 and C4 also increased from 0.4 g/dL to 0.7 g/dL and from 0.1 g/dL to 0.13 g/dL, respectively. After the ascites subsided, the paracentesis pigtail catheter was removed. A piece of omentum was obtained by peritoneoscopy for the evaluation of the cause of refractory ascites. The pathologic finding of the omentum was chronic inflammation with cell infiltration, congestion, and fibrosis ().
Figure 2 A) Contrast-enhanced CT of abdomen revealed massive ascites. B) Contrast-enhanced CT taken after modified hemodialysis for 10 days. The scan at the same level as (A) reveals the disappearance of ascites.
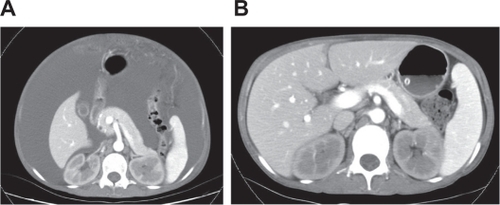
Figure 3 The changes in body weight, abdominal girdle, and serum albumin after seven sessions of continuous infusion of ascites into the dialyzer during hemodialysis over 9 days.
In the following 60 months, the serum creatinine level was around 120 μmol/L on immunosuppressive agents. There were no further episodes of refractory ascites. The patient also had an increase in muscle mass.
Discussion
In our patient, liver cirrhosis, congestive heart failure, carcinomatous peritonitis, and bacterial peritonitis-related ascites were excluded by serial examinations. The refractory ascites caused by lupus serositis was most likely due to the high level of dsDNA, low serum level of C3/C4, and inflammatory omentum ().
The patient’s refractory ascites, leg edema, and respiratory distress improved after seven sessions of continuous infusion of ascites into the dialyzer during HD after failure of other treatments, including bed rest, salt and water restriction, diuretics, intravenous administration of albumin, repeated paracentesis, sequential ultrafiltration, and reduced dialysate temperature during HD. The success of this treatment showed that the removed body fluid was replaced by the drawn out ascites at a speed of 12–15 mL/min (720–900 mL/h) concurrently during HD while ultrafiltration was set at around 1 L/h. There were 2.88–3.6 L of ascitic fluid from the abdomen infused into the dialyzer and a net 0.4–1.12 L of fluid removed from the systemic circulation during each 4-hour HD session. A serum albumin level increase from 28 g/L to 35 g/L after seven sessions of continuous reinfusion was due to the infused ascites and protein into the systemic circulation during HD (). The increase in serum level of albumin might have also pulled the interstitial fluid into the intravascular space via the increase in serum oncotic pressure.Citation5,Citation7,Citation9 Leg edema, pleural effusion, and refractory ascites were ameliorated by small negative fluid balance and increased serum albumin level during HD.
In this case, ARF occurred, along with the progressively worsening ascites and respiratory distress, but improved after the refractory ascites subsided. This implied that ARF was mostly related to the refractory ascite-induced hypovolemia and unstable hemodynamic status.
Ascite reinfusion has been reported in chronic HD patients with cirrhotic, lupus, or nephrogenic ascites.Citation2–Citation8,Citation10–Citation13 In this case, ascitic fluid was infused into the dialyzer under flow control by the roller pump, and the fluid was removed from the systemic circulation simultaneously during HD. This procedure of flow control allowed adequate fluid removal without intradialytic hypotension.
Several procedures based on the reinfusion of ascitic fluid have been reported in chronic HD patients with cirrhotic, lupus, or nephrogenic ascites, eg, intravenous reinfusion of concentrated ascites, extracorporeal ultrafiltration of ascite fluid with peritoneal reinfusion, ascitic fluid concentration with blood reinfusion during HD, and peritoneovenous shunt.Citation2–Citation8,Citation10–Citation13 In comparison with those procedures, the flow control reinfusion of ascites during HD is rapid, easier, and effective. Ascitic fluid does not need to be concentrated before reinfusion. This procedure and McGill et al’s methodCitation13 could simultaneously control the flow of ascite reinfusion by the flow control roller pump and the amount of body fluid removal by the ultrafiltration rate of dialyzer. The serum albumin level was increased after the infusion of protein-rich ascites into the systemic circulation during HD. The elevated serum level of albumin pulls the interstitial fluid into the intravascular space via increased serum oncotic pressure.
With the successful outcome of this case report, we believe that flow control reinfusion of ascites during HD is an effective alternative treatment for the alleviation of refractory ascites in patients with renal failure, who are unresponsive to bed rest, salt and water restriction, diuretics, intravenous administration of albumin, repeated paracentesis, sequential ultrafiltration, and reduced dialysate temperature during HD. Although not observed in this case, the potential adverse effects, such as fever, gastrointestinal bleeding, DIC, peritonitis, or septicemia, by the intravenous or intra-abdominal infusion of ascites should be kept in mind.Citation14,Citation15 The activation of fibrinolytic activity of ascites due to the relative plasminogen activator inhibitor type-1 deficiency and enhanced basal tissue plasminogen activator has been reported.Citation16–Citation19 Therefore, clotting tests should be carried out before the procedure is initiated, and if the patient’s blood and ascites denote a fibrinolytic activity, the procedure should be considered hazardous and probably contraindicated.
In conclusion, the alleviation of ascites and ARF attest to the success of the procedure of continuous infusion of ascites into the dialyzer during HD. With normal coagulation function, it is an effective alternative treatment of refractory ascites, especially in patients with renal failure or who are unresponsive to bed rest, salt and water restriction, diuretics, intravenous administration of albumin, repeated paracentesis, sequential ultrafiltration, and reduced dialysate temperature during HD.
Acknowledgements
This case report was supported by a grant (NSC 96-2314-B-075-020-MY3, NSC96-2314-B-010-028-MY2) from the National Science Council and by the Szu-Yuan Research Foundation of Internal Medicine, Republic of China. We thank Dr Sandy Cho and Ming-Yu Lai for providing valuable suggestions and English correction.
Disclosure
The authors report no conflicts of interest in this work.
References
- WilkinsKWJrHoffmanGSMassive ascites in systemic lupus erythematosusJ Rheumatol19851235715744045854
- OkadaKTakahashiSHiguchiTMaedaHLong-term effect of intravenous reinfusion of unmodified autogenous peritoneal fluid combined with hemodialysis in a patient with dialysis-related ascitesNephron19936534744758290005
- BrunoSBorzioMRomagnoniMComparison of spontaneous ascites filtration and reinfusion with total paracentesis with intravenous albumin infusion in cirrhotic patients with tense ascitesBMJ19923046843165516581633517
- AlbalateMLópez GarcíaMDVázquezAConcentrated ascitic fluid reinfusion in cirrhotic patients: a simplified methodAm J Kidney Dis19972933923989041215
- DaimonSYasuharaSSagaTTokunagaSChikakiHDanKEfficacy of extracorporeal ultrafiltration of ascitic fluid as a treatment of refractory ascitesNephrol Dial Transplant19981310261726239794570
- HwangJCChenJAFungHYHemodialysis alternative with ascites ultrafiltration for an end-stage renal failure patient associated with tense ascites secondary to decompensated liver cirrhosisAm J Kidney Dis19962868999038957043
- CatalanoCFabbianFdi LandroDReinfusion and concentration of ascitic fluid during hemodialysis in a cirrhotic uremic patientAm J Kidney Dis19983211641679669439
- LeveenHHChristoudiasGIpMLuftRFalkGGrosbergSPeritoneo-venous shunting for ascitesAnn Surg197418045805914415019
- NichollsAJPlattsMMTrigerDRRegular reinfusion of ascites during haemodialysis in a patient with amyloidosisBr Med J (Clin Res Ed)19832876394726
- AsakimoriYYoriokaNKumagaiJKawanishiHTsuchiyaSDirect infusion of ascites into the blood circuit during hemodiafiltration in uremic patients with cirrhosisInt J Artif Organs200023423223610832656
- LiuJZhangCZhuXShangSLiWGuQIntravenous infusion ascitic fluid during hemodialysis: a study of 108 treatments in 13 uremic casesArtif Cells Blood Substit Immobil Biotechnol199927215316210092936
- TouamMOrozcoRFumeronCGaneaADrüekeTGrünfeldJP[Refractory ascites in hemodialysis: treatment by paracentesis–reinjection during dialysis]. [article in French]Nephrologie2001221252811280038
- McGillRLBakosJRMarcusRJAscites reinfusion dialysis (ARD) for renal failure with refractory ascitesClin Nephrol200462537437915571183
- MoultPJParbhooSPSherlockSClinical experience with the Rhône–Poulenc ascites reinfusion apparatusPostgrad Med J1975515985745761234345
- LévyVGOpolonPPauleauNCaroliJTreatment of ascites by reinfusion of concentrated peritoneal fluid–review of 318 procedures in 210 patientsPostgrad Med J1975515985645661234342
- AgarwalSJoynerKAJrSwaimMWAscites fluid as a possible origin for hyperfibrinolysis in advanced liver diseaseAm J Gastroenterol200095113218322411095345
- BuøLKarlsrudTSDyrhaugGThe fibrinolytic system in human ascitesScand J Gastroenterol19953011110111078578171
- FergusonJWHelmyALudlamCWebbDJHayesPCNewbyDCHyperfibrinolysis in alcoholic cirrhosis: relative plasminogen activator inhibitor type 1 deficiencyThromb Res2008121567568017870147
- Scott-CoombesDMWhawellSAVipondMNCrnojevicLThompsonJNFibrinolytic activity of ascites caused by alcoholic cirrhosis and peritoneal malignancyGut1993348112011228174965