Abstract
The prevalence of childhood steroid-resistant nephrotic syndrome (SRNS) ranges from 35% to 92%. This steroid resistance among Nigerian children also reflects underlying renal histopathology, revealing a rare minimal-change disease and a varying burden of membranoproliferative glomerulonephritis and focal segmental glomerulosclerosis (FSGS). FSGS tends to progress to end-stage kidney disease, which requires dialysis and/or renal transplantation. While knowledge of the molecular basis of NS is evolving, recent data support the role of mutant genes that otherwise maintain the structural and functional composition of the glomerular filtration barrier to account for many monogenic forms of FSGS. With the advent of next-generation sequencing, >39 genes are currently associated with SRNS, and the number is likely to increase in the near future. Monogenic FSGS is primarily resistant to steroids, and this foreknowledge obviates the need for steroids, other immunosuppressive therapy, and renal biopsy. Therefore, a multidisciplinary collaboration among cell biologists, molecular physiologists, geneticists, and clinicians holds prospects of fine-tuning the management of SRNS caused by known mutant genes. This article describes the genetics of NS/SRNS in childhood and also gives a narrative review of the challenges and opportunities for molecular testing among children with SRNS in Nigeria. For these children to benefit from genetic diagnosis, Nigeria must aspire to have and develop the manpower and infrastructure required for medical genetics and genomic medicine, leveraging on her existing experiences in genomic medicine. Concerted efforts can be put in place to increase the number of enrollees in Nigeria’s National Health Insurance Scheme (NHIS). The scope of the NHIS can be expanded to cater for the expensive bill of genetic testing within or outside the structure of the National Renal Care Policy proposed by Nigerian nephrologists.
Introduction
Nephrotic syndrome (NS) is the commonest chronic glomerular disease in children characterized by heavy proteinuria.Citation1–Citation3 This is accompanied by hypoalbuminemia, hyperlipidemia, and generalized edema.Citation1–Citation3 Clinical features can include acute life-threatening conditions, such as hypovolemia, acute kidney injury, hypercoagulation, thromboembolism, and infection.Citation4,Citation5 When NS does not respond to treatment, it also progresses to chronic kidney disease (CKD) and end-stage KD (ESKD).Citation6
The annual incidence of NS in the US and EuropeCitation7 is estimated at seven in 100,000; however, the overall incidence of NS is difficult to estimate in Africa.Citation8 The limited data in Africa is a result of a lack of pediatric nephrologists, poor access to diagnostic tools, such as electron and immunofluorescence microscopes, weak capacity for renal biopsies, and inadequate reporting of renal diseases.Citation8 Cumulatively, these challenges make NS undiagnosed, underdiagnosed, and underreported in Africa.
In a broad classification, childhood NS can be secondary, congenital, infantile, and idiopathic.Citation3 Secondary NS refers to etiology that is not intrinsic to the kidney.Citation3,Citation9 The causes of secondary NS include 1) autoimmune and vasculitic diseases, such as Henoch–Schönlein purpura, systemic lupus erythematosus, and antineutrophil cytoplasmic antibody–associated vasculitis, 2) infectious diseases, such as congenital syphilis, malaria, HIV, and hepatitis B and C, 3) malignancy, 4) environmental and drug exposure, such as heroin and mercury, and 5) systemic diseases, such as diabetes mellitus, among many other causes.Citation3,Citation9 While congenital NS (CNS) manifests in the first 3 months of life, infantile NS is seen in children from 3 months to 12 months of life.Citation3,Citation9 When NS is not congenital, infantile, or secondary, it is said to be idiopathic NS (INS).Citation3
Variants of INS include membranous nephropathy, membranoproliferative glomerulonephritis (MPGN), C3 glomerulonephritis, IgA nephropathy, and diffuse mesangial proliferation. However, the histological classifications of INS are either minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS).Citation3,Citation10
Under light microscopy, the glomerulus in MCD appears normal; however, electron microscopy reveals effacement of podocyte foot processes. Light microscopy findings in FSGS include segmental destruction of glomerular capillaries and fusion between Bowman’s capsule and the sclerosed segments. Electron-microscopy findings are however similar to MCD.Citation9,Citation10
Clinically, INS can be classified as a steroid-sensitive NS or steroid-resistant NS (SRNS), as the response to steroids correlates with histological subtype and prognosis.Citation11,Citation12 Primary SRNS is lack of remission in proteinuria despite 4 weeks’ treatment with prednisolone at a daily dose of 60 mg/m2 followed by 40 mg/mCitation2 on alternate days for 4 weeks.Citation9,Citation12 Secondary SRNS refers to the development of steroid resistance in a child with a previous steroid-sensitive disease.Citation9,Citation12 Remission in proteinuria is commonly described as 3 consecutive days of zero or trace proteinuria during steroid treatment for NS.Citation9,Citation12
In the Western hemisphere, MCD is found on renal biopsy in the vast majority (80%) of preadolescent children with INS.Citation11,Citation12 Response to steroids has been reported in 90% of MCD cases and only 30% of FSGS cases.Citation11–Citation14 Also, children with FSGS tend to progress to ESKD requiring a renal transplant, with a risk of recurrence of 30%–50% in the first transplanted kidney and a higher risk of recurrence in subsequent renal grafts.Citation15
In Africa, MCD NS with good treatment outcome to steroids also predominates in temperate regions.Citation16–Citation19 In tropical Africa and from the 1960s to 1980s, steroid-resistant non-MCD, including quartan malaria nephropathy, was the dominant renal histopathology type.Citation20–Citation23 However, in the years after 1989, proliferative glomerulonephritis, MCD, and FSGS took predominance.Citation20,Citation24–Citation26
In Nigeria, geographical variations put steroid resistance at 35%–92% for children with NSCitation27–Citation37 and 17.2%–54.8% for children with INS.Citation32,Citation35,Citation37 This steroid resistance parallels the underlying renal histopathology, which reveals a rarity of MCD, but a varying burden of MPGN, focal mesangial proliferative lesion, and FSGS.Citation27,Citation32,Citation34,Citation37,Citation38 It would also appear that there is a transition from quartan malaria nephropathy through MPGN to FSGS in Nigeria,Citation23,Citation30,Citation38–Citation40 a finding that is also consistent with an emerging burden of FSGS among children in Europe, the US, and Asia.Citation41–Citation45
While FSGS suggests steroid unresponsiveness, histo-pathological diagnosis of FSGS is also fraught with the challenge of underdiagnosis from single renal biopsies, because of its focal nature and the small cores of biopsies.Citation7,Citation9,Citation46–Citation48 In addition, since FSGS has the propensity for deep juxtamedul-lary glomeruli, FSGS lesions may be missed readily when only cortical glomeruli are biopsied.Citation7,Citation9,Citation46–Citation48 Larger samples of glomeruli are thus necessary for the histological diagnosis of FSGS.Citation49,Citation50 Differential staining patterns for synaptopodinCitation51 and dystroglycanCitation52 may also be useful in distinguishing MCD by its response to steroids.Citation47
Unfortunately, large-scale efforts at studying steroid responsiveness in children with NS have been only among Caucasians. These studies include PodoNet (European descent),Citation53 NephroS (European descent),Citation54 NEPTUNECitation55 (North America), CHILDNEPH (Canada),Citation56 and INSIGHTCitation57 (Canada). It remains arguable that the generalizability of the findings of these studies to African children will be fraught with limitations inherent in the ethnic differences.
The proposed Human Heredity and Health (H3) Africa Kidney Disease Research Network (H3A-KDRN) will provide information on a cohort of children with NS from Ghana, Nigeria, Tanzania, South Africa, and Cameroon.Citation58 The H3A-KDRN will determine if clinical, infectious, and genetic factors account for variability in initial steroid response, steroid resistance, frequent relapse/steroid dependence, and CKD-progression course (over 4 years) in childhood NS.Citation58 Africa and indeed Nigeria provide a great opportunity to study diversities in human genetics by virtue of their widely varied population, climate, diet, and exposure to infectious diseases.Citation59
While the H3A-KDRN findings are being awaited, this paper seeks to review the prospects of having the capacity for genetic testing as part of a standard of care in the management of NS/SRNS in Nigerian children. Based on the existing resource-constrained health-care system in Nigeria, this article gives a narrative review of the challenges against the prospects of genetic testing, and also highlights the opportunities available for genetic testing for the management of SRNS among Nigerian children. The article will be valuable to all clinicians caring for children with NS/SRNS in developing countries who may be having similar challenges and opportunities for genomic medicine as exist in Nigeria.
Focal segmental glomerulosclerosis and steroid-resistant nephrotic syndrome
FSGS is the most common cause of SRNS and a significant cause of ESRD.Citation3,Citation47,Citation48,Citation60 FSGS ensues from podocyte injury by many pathogenic mechanisms that result in loss of selectivity of the glomerular filtration barrier (GFB).Citation47,Citation48 These underlying pathogenic mechanisms have also been used in its classification.Citation47,Citation48
FSGS is classified into primary (idiopathic) and secondary forms.Citation48,Citation61 The secondary forms include 1) virus-associated forms (HIV1, cytomegalovirus, parvovirus B19, and Epstein–Barr virus, and other parasites, including Plasmodium, Schistosoma mansoni and filariasis),Citation62,Citation63 2) drug-induced forms (IFNα, -β, or -γ therapy, bisphosphonates, lithium, heroin, sirolimus, doxorubicin, and daunomycin),Citation64–Citation68 3) forms mediated by adaptive structural–functional responses (ie, conditions associated with increased total kidney glomerular filtration rate like congenital cyanotic heart disease, sickle-cell anemia, obesity, androgen abuse, sleep apnea, and high-protein diet, and conditions associated with reduced renal mass, including prematurity and/or small for gestation age, renal anomalies, reflux nephropathy, and acute kidney injury),Citation69–Citation74 and 4) familial/genetic forms.Citation48,Citation61
The primary/idiopathic form is a diagnosis of exclusion after ruling out secondary forms of FSGS. The pathogenesis of primary FSGS probably involves a circulating factor.Citation75–Citation79 While it is unclear what may be the circulating factor, possible candidates include CLCF1, ApoA1b (an isoform of ApoA1), anti-CD40 antibody, and suPAR.Citation75–Citation79 Widespread foot-process effacement on electron microscopy characterizes podocyte injury in primary FSGS, and it is commonly associated with nephrotic-range proteinuria (sometimes massive), reduced plasma albumin levels, and hyperlipidemia.Citation47,Citation80 Primary FSGS also tends to respond to immunosuppressive treatment.Citation80 Electron-microscopy features of podocytopathy in adaptive FSGS are segmental effacement of foot processes, and present clinically with normal serum albumin.Citation47,Citation80,Citation81 The management of secondary FSGS requires the elimination of the causative agent, reducing hemodynamic pressure on glomeruli (eg, weight loss), and instituting antiproteinuric strategies.Citation80
Since the discovery of mutations in nephrin (NPHS1) by Kestilä et al in 1998 as the cause of podocyte dysfunction in CNS,Citation81 over 36 other genes have been described for genetic FSGS.Citation83,Citation84 The term “genetic FSGS” has been coined based on the discovery of genes that if mutated can cause mono-genic forms of FSGS in humans.Citation84 While details of genetic FSGS will be discussed subsequently in the next section, it is important to introduce Apol1, which is emerging as a peculiar form of primary FSGS in populations of African descent.Citation47,Citation85,Citation86 Apol1 FSGS progresses more rapidly to ESRD, but also has the prospect of having therapy targeted at Apol1 variant–driven molecular pathways.Citation85,Citation86
Clinical implications of genetic forms of nephrotic syndrome
Researchers over the years have enhanced efforts at finding ways of predicting steroid responsiveness in individuals with NS.Citation87–Citation89 This knowledge will enable clinicians to spare children that do not respond to steroids, unwarranted side effects of steroids, and at the same time provide a timely alternative management plan to include the use of second-line immunosuppressive agents.Citation87–Citation89 It will also obviate the need for renal biopsy and enable clinicians to prioritize early renal transplantation for children that do not respond to immunosuppressive therapies.Citation87–Citation89 There is a low risk of disease reoccurrence post-transplant when a genetic aetiology has been confirmed.Citation90 On the other hand, the risk of post-transplant disease is high in idiopathic NS as the circulating factors settle and damage the new allograft.Citation90 While living related transplant donors may be acceptable in recessive mutations, it excludes these donors in cases of dominant mutations.Citation89 Carriers of WT1 mutations are monitored closely for Wilms tumor and gonadoblastoma.Citation89 Familial genetic counseling advocates for prenatal diagnosis in autosomal-dominant patients of childbearing age.Citation89 Interestingly, concerted research efforts have so far identified some noninvasive biomarkers of SRNS. These biomarkers include some elevated urinary factors (suPAR, urinary CD80) and some elevated serum factors (DBP, prealbumin, NGAL, fetuin A, and α2 macroglobulin).Citation88,Citation89,Citation91–Citation95
However, limitations in the clinical application of SRNS biomarkers include unavailability of these biomarkers in most laboratories, requirement for longitudinal studies to establish its validity as a noninvasive predictor of steroid unresponsiveness,Citation88,Citation89 and lack of specificity of some biomarkers (urinary fetuin A and NGAL) requiring that the SRNS to be of longstanding duration to cause proximal tubular megalin dysfunction (megalin endocytosis reabsorbs these filtered proteins back into the bloodstream).Citation96,Citation97 In addition, studies that identified these biomarkers were not powered sufficiently in their sample sizes and were not multicenter studies.Citation88 This limits the generalizability of the clinical usefulness of these biomarkers.Citation88,Citation91
Genetics of nephrotic syndrome
From the discovery in 1998 of mutations in nephrin (NPHS1) among a subset of CNS,Citation82 many other mutations affecting genes that affect the integrity of the GFB have been found to account for many secondary genetic forms of FSGS.Citation83,Citation84 With newer sequencing techniques, over 36 genes are currently associated with SRNS,Citation84,Citation98–Citation104 with great prospects for new discoveries.
The GFB is made up of three layers that functionally prevent plasma proteins from passing into the urine. These layers are the endothelium, glomerular basement membrane, and visceral epithelial cells, also known as podocytes.Citation84,Citation105 Podocytes are highly differentiated neuron-like cells made up of a cell body, with primary, secondary, and tertiary cellular processes that wrap around the capillaries, leaving slits between themCitation83,Citation84 ().Citation84 The integrity of the GFB is lost in NS, and the identification of single-gene causes of SRNS has revealed proteins, each of which is an indispensable component of glomerular function, with consequent proteinuria and FSGS.Citation105 However, studies of familial NS/FSGS found that virtually all the gene mutations causing NS/FSGS were localized to podocytes in hereditary SRNS.Citation106–Citation112 This gave rise to the concept of podocytopathy being the underlying pathology in most cases of NS, especially FSGS.Citation113 The known podocyte genes do not explain >20%–30% of hereditary NS; however, they do explain 57%–100% of familial and infant-onset NS compared with 10%–20% of sporadic cases.Citation83
Figure 1 Proteins involved in single-gene causes and pathogenic pathways of SRNS.
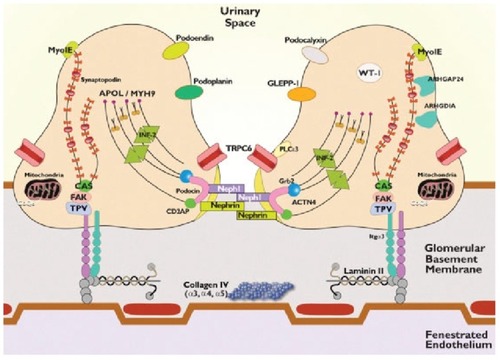
Mutations affect both functional and structural proteins that are involved in several vital pathways including those involved in slit diaphragm structure and function, podocyte actin cytoskeletal organization, co-enzyme Q biosynthesis, lysosomal pathways, and adhesion to the GBM. ().Citation84 Genes responsible for SRNS/genetic FSGS are also inherited in autosomal-recessive and -dominant fashion and are either isolated disorders or a component of a multisystem genetic disorder (see for the list of autosomal and determinant genes).Citation84
Table 1 Steroid resistant nephrotic syndrome
The main mutations causing autosomal-recessive NS are in nephrin (NPHS1), podocin (NPHS2), and PLCE1, while the main autosomal-dominant NS mutations include those in INF2, TRPC6, WT1, and ACTN4.Citation14 Genetic risk factors of MYH9 and APOL1 (associated with pathological features of FSGS in African–Americans) are increasingly being found to be similar to those of FSGS and complex inheritance patterns of FSGS.Citation114,Citation115
In recessive mutations, presentation occurs in childhood, history of NS is often negative, parents of index patients are mostly healthy heterozygous carriers, and there is no ancestral history of the disease.Citation104 However, in dominant disease, it occurs in adulthood, one of the parents of the index patient is most probably affected, and the disease may have been passed on from generation to generation (with the exception of spontaneous mutations or incomplete penetration).Citation104 Dominant mutations also have important clinical implications, as disease-causing mutations in the related donor have to be excluded before acceptance for planned living related donor kidney transplantation, as SRNS just may not yet have manifested.Citation84
Approach to genetic testing in steroid-resistant nephrotic syndrome
Gbadegesin et alCitation14 suggested pertinent questions that warrant answering before a genetic test is requested for. Will this result assist in establishing the diagnosis? Will the test result modify the patient’s treatment or improve prognosis? Will the test result provide information to predict risk among other family members? Within the context of monogenic SRNS, the answers to these questions are “Yes”.
To answer the first question, about 53 monogenic genes have already been identified as being responsible for SRNS/FSGS.Citation86,Citation98–Citation104 In addition, certain phenotypic characteristics like age of onset of disease also exist that can suggest the type of mutations in individuals with SRNS.Citation98,Citation116 Early-childhood onset of disease is associated with mutations in the recessive genes NPHS1, LAMB2, or PLCE1, whereas others, such as NPHS2, cause onset in later childhood.Citation98,Citation116 With the exception of WT1, mutations in dominant SRNS genes (ACTN4, TRPC6, INF2, ANLN, and ARHGAP24) cause adult-onset SRNS.Citation98 In “multiple allelism” (ie, within the same gene, specific mutations may determine a range for age of onset of SRNS that is dependent on the specific mutation), R138Q NPHS2 mutations cause onset in early childhood,Citation117,Citation118 whereas the mutation R229Q in compound heterozygosity with specific second mutations causes adult-onset SRNS.Citation119
Even when genetic testing can be expensive, the result of genetic testing in SRNS alters the management of the patient or better informs a discussion of likely outcomes and prognosis. First, immunosuppressive therapy can be avoided in some patients who will not benefit from it, likewise renal biopsy. Treatment with coenzyme Q10 may be indicated for children with gene mutations of coenzyme Q10 biosynthesis (COQ2, COQ6, ADCK4, or PDSS2). Likewise, steroids or cyclosporine A may benefit patients with recessive mutations in PLCE1,Citation104,Citation120 and cyclosporine A can also induce steroid responsiveness in some individuals with NPHS1 and NPHS2 mutations.Citation121 Second, while the recurrence rate of FSGS in familial/idiopathic SRNS is reported to be ~30% (as high as 75% in those who progress to ESRD in 3 years).Citation122,Citation123 Contrariwise, an estimated 0–2.5% recur after renal transplantation, making recurrence rare in genetic forms of FSGS. This encourages an earlier plan for renal transplantation, with the assurance that recurrence of FSGS in the posttransplanted kidney is rare.Citation124–Citation127 Third, selection of donors for patients with genetic FSGS among family members will require careful screening considering the risks of the donor. In fact, studies have reported cases of FSGS in the remaining kidney among donors after organ donation.Citation128 Depending on the Mendelian mode of inheritance as either a recessive or dominant gene, the index patient with SRNS can be a predictor of risk in other family members as enumerated previously.Citation104
However, it is equally important to highlight the limitations of genetic testing for SRNS. Only a small proportion of genetic tests (<2%) for NS are likely to identify a clinically relevant mutation.Citation14 Considering the costs, genetic testing for SRNS requires proper selection.Citation14 Likewise, a negative test does not exclude the diagnosis, as many cases of genetic NS or FSGS are heterogeneous in nature.Citation14 Besides, most of the genetic testing has been among non-African children,Citation83,Citation98–Citation104 where allelic variations may occur.Citation128 The currently known genes associated with SRNS account for only 20%–30% of hereditary and 10%–20% of sporadic cases.Citation128 It is suggested that ethnicity plays a role in the differences in disease burden across countries, but differences among response to steroids and other treatment modalities among specific ethnic groups are not well understood.Citation128
Hitherto, Lovric et alCitation104 have suggested genetic testing for children with SRNS/FSGS <25 years of age, as there is a higher probability of detecting a mutant gene for steroid unresponsiveness. However, going by the comprehensive study of Santin et al among patients with NS, family history of NS and early-onset disease were the risk factors associated with mutations.Citation129 Although no consensus exists, the current evidence provided by Santin et al and other epidemiological data, an appropriate, simple, cost-effective, and informed approach to genetic testing for SRNS-related mutations can be determined on the basis of current evidence in scenarios with 1) family history of SRNS, 2) congenital or infantile onset (<1 year) of SRNS, 3) onset of SRNS before 25 years of age, 4) lack of response to immunosuppressive drugs, 5) histological findings of idiopathic FSGS or diffuse mesangial sclerosis, 6) syndromic manifestations, 7) impaired renal function or renal failure, and 8) certain ethnic groups.Citation14,Citation130,Citation131
Clinical testing vs research-based testing
Clinical genetic testing refers to determining the mutations responsible for a monogenic disease. This can only be conducted by a certified clinical genetic testing laboratory that issues results for clinical decision-making (eg, Clinical Laboratory Improvement Amendments certification).Citation104 However, research laboratories continue to remain relevant, as they are needed to continue to discover unidentified rare and novel monogenic genes. Even when they have the capacity to identify disease-causing mutations, research laboratories cannot provide results with clinical judgment, except when such results are subjected to Clinical Laboratory Improvement Amendments certification.Citation104
Gene panels
Gene-panel analysis (GPA) is currently the most advantageous approach in terms of cost and efficacy for mutation detections.Citation98,Citation132,Citation133 In GPA, known exon primers that identify mutations are utilized.Citation104 Panels with gene sequences of disorders are utilized.Citation104 Sanger sequencing is used.Citation84 The advantages of GPA are in its cheapness, fastness, and the fact that positive results can be interpreted easily without extensive bioinformatics.Citation104 However, a negative result may not indicate a lack of mutations, as mutations may exist in noncoding regions of the genes or mutations in new genes.Citation104
Whole-exome sequencing
Whole-exome sequencing (WES) involves the use of an advanced technology of “next-generation sequencing” to sequence the whole human exome.Citation103,Citation134 This technology will likely decrease the need for GPA and become the preferred approach in the future.Citation14 The major advantage of WES is that it has a higher yield, as its results are restricted to candidate genes and it can also detect mutations in novel genes.Citation14 The latter further expands the body of knowledge on the genetic basis of NS and therapeutic targets.Citation14 Currently, the use of WES is limited to research laboratories, and results require robust bioinformatic support for interpretation.Citation14 In addition, WES may inadvertently detect mutations that are irrelevant to the disease under investigation.Citation135
Whole-genome sequencing
Whole-genome sequencing (WGS) sequences the entire genome utilizing parallel DNA-enrichment technology including noncoding and highly variable segments.Citation14,Citation104 WGS is the most extensive sequencing technique and is very expensive.Citation104 In monogenic disease, mutations outside the exons bear no direct role in protein synthesis; WGS may thus appear to have no comparative advantage over WES.Citation104 However, WGS also identifies promotor and deep intronic mutations relating to protein expression and RNA splicing and is useful in monogenetic disease caused by mutations occurring outside the exons.Citation137 For example, in Frasier syndrome where mutations exist in the KTS (Splice insertion) site of the WT1 gene, (not in the exons per se), causing a defective WT1 protein structure.Citation90
Genetic counseling in steroid-resistant nephrotic syndrome and/or genetic FSGS
Genetic counseling is a communication process that involves explanation of the contribution of genetics to health, trans mission risks of a trait, and modalities to manage the condition or its transmission. Counselors are required to possess the skills to communicate in a neutral, nondirective manner and support the individual and family to cope with decisions made.Citation137,Citation138
Genetic counseling includes the services of a genetic counselor, geneticist, and cell biologist or molecular physiologist that work in a clinical research laboratory.Citation137,Citation138 While a pediatric nephrologist can serve the role of a genetic counselor, it must be noted that their role is not to advise or recommend, but to provide information and support for the patient/family members to decide.
In the context of SRNS, the first step is to establish the diagnosis of SRNS as defined previously.Citation9,Citation21 This is the most crucial step in any genetic consultation. It will include comprehensive history-taking, obtaining a detailed family history that lists the patient’s relatives (including abortions, stillbirths, and deceased persons) with their sex, age, and state of health, up to and including third-degree relatives. It involves gathering information on prenatal, pregnancy, and delivery histories. The counselor should know about the latest available genetic information concerning SRNS, and there should be an avenue for communicating the latest information on SRNS. A thorough physical examination of the affected individual should be conducted. All this information will dictate the type of genetic testing that will be requested for the patient. The counseling sessions must include education on the specific genetic mutation, knowledge on the diagnosis of the particular condition, the natural history of the condition, the genetic aspects of the condition, the risk of recurrence and prevention, therapies, and referral. It will include calculating, qualifying, and quantifying the risk of the SRNS in proper context. Knowledge of the known genes for SRNS will guide in counseling about the age of onset and its mode of inheritance.
A satisfactory counseling session must have answered the possible envisaged genetic mutation, the possible etiology or inheritance pattern, the natural history of the genetic mutation, the best mode of therapy available, the mode of inheritance of the disorder, and the risk of developing and/or transmitting it. The technique of genetic counseling also entails presenting the information clearly in a sympathetic and appropriate manner. Counselors are expected to listen patiently and be receptive to patients’ and caregivers’ perspectives on issues carefully, being receptive to the fears and aspirations of the family. The environment should be comfortable for the patient, offer confidentiality, and be at a time that allows for detailed uninterrupted discussions. Communication should be in simple terms and medical terms should be avoided, and if necessary should be well explained. All questions should be honestly and exhaustively addressed.
Steroid-resistant nephrotic syndrome among Nigerian children
Although a lot of studies have been done on NS in Nigerian children, very few have actually reported on their responsiveness to steroid therapy. Nevertheless, geographical variations put steroid resistance at 35%–92% for children with NSCitation24,Citation27–Citation37 and 17.2%–54.8% for children with INS.Citation32,Citation35,Citation37 These rates of steroid resistance also parallel the underlying histopathology, which reveals a rarity of MCD, but studies have reported MPGN in northwest Nigeria,Citation27 focal mesangial proliferative lesion in north–central Nigeria,Citation34 and FSGS in south–southCitation32 and southwest Nigeria.Citation37,Citation38
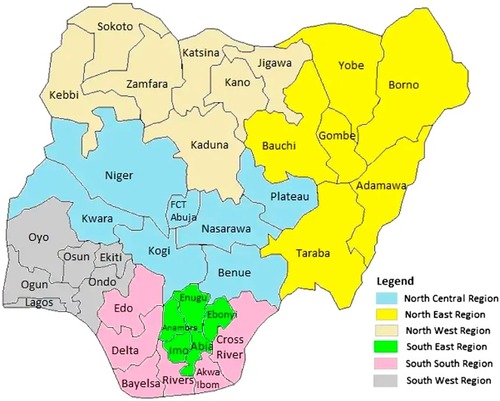
In fact, it would appear that there is a transition from quartan malaria glomerulopathy through MPGN to FSGS in southwest Nigeria,Citation23,Citation30,Citation38 a finding also consistent with an emerging burden of FSGS among children in Europe, the US, Asia, and South Africa.Citation41–Citation45 There is little information on childhood NS in northeast Nigeria, a finding that also signifies a dearth of pediatric nephrologists in that geopolitical region of Nigeria. See for the 6 geopolitical zones in Nigeria.
Figure 2 Map of Nigeria’s states and zones.
Although the lack of electron and immunofluorescence microscopes may have contributed to the rarity of MCD and MPGN, respectively, improvements in the National Malaria Control Programme and increased accessibility to vaccines against viral hepatitis B could also account for the decline in secondary NS and the propensity to have nonminimal (ie, FSGS) disease outcome.Citation34,Citation37,Citation38 Going by the recent publication of Pelletier et al,Citation141 which studied the risks of recurrence of NS following kidney transplantation, it can also be extrapolated that the majority of Nigerian children with FSGS as an underlying histopathological finding may also fare better than those with MCD. Pelletier et al reported that the rate of recurrence in the initial native kidney was 76% among children with MCD, 40% among FSGS, and 0 in patients with monogenic NS.Citation141
Although few Nigerian studiesCitation28–Citation38 have mentioned whether steroid resistance is primary or secondary, the unadjusted logistic regression of Pelletier et al revealed that initial steroid responsiveness was associated with higher odds of recurrence in renal grafts. The challenges of Nigerian children with NS and/or SRNS are enormous.Citation34,Citation37 There are problems of lack of funding for diagnosis, lack of resources for frequent hospitalizations from complications of SRNS and steroid therapy, unaffordability of renal biopsies, high costs of dialysis, unavailability of and lack of funding for procuring second-line immunosuppressive therapies (ie, cyclophosphamide, cyclosporine, mycophenolate) and ancillary medication, and prospects for renal transplantation also being perennially bleak.
Parents and/or caregivers often cannot sustain the management of these children for a long period.Citation34,Citation37 Eventually, frustration, despair, and despondency set in, which often make parents seek alternative medicines, including herbal concoctions and other traditional modes of treatment.Citation34,Citation37 Nephrotic children are soon lost to follow-up, with a majority progressing to ESKD because of continuous exposure of kidneys to uncontrolled nephrotoxic proteinuria.Citation34,Citation37
A good preventive approach to SRNS in Nigeria would be first to document the prevalence and pattern of SRNS. Unfortunately, like other CKDs, this has been hampered by a dearth of epidemiological studies, poor definitions of what SRNS is, and the lack of a renal registry.Citation142 While efforts at controlling glomerular diseases from infectious agents like hepatitis B and C, malaria, and syphilis are yielding positive results among children in Nigeria,Citation34,Citation37,Citation38 these children are still at risk of INS.Citation20
In light of the foregoing prospective, well-structured, highly powered epidemiological and genetic studies are in dire need in dire Nigeria to describe the pattern of NS in these children, who will be unresponsive to steroids.Citation28–Citation38 Hopefully, the H3A-KDRN will be able to answer these research questions in Nigeria and other African countries.Citation58
Challenges and opportunities for genetic investigation of childhood nephrotic syndrome in Nigeria
The challenges facing genetic investigations for NS/SRNS in Nigeria can be captured under two main prongs. The first is inadequate human resources, and the second is inadequate financial resources
Inadequate human resources
This presupposes that there is accurate information about the diagnosis of childhood NS and/or SRNS that may be needing genetic counseling and genetic testing. There lies the first challenge, as many cases of NS are probably missed because of lack of capacity to diagnose NS/SRNS in the first place.
The current population of Nigeria in 2018 is about 182 million (projected from a 3.5% growth rate from the 2006 census),Citation143 and the country has about 72,000 medical doctors registered with the Medical and Dental Council of Nigeria, among which about half (~40,000 doctors) are practicing in Nigeria.Citation144 This gives an approximate unacceptable physician:patient ratio of 1:2,500, as against the World Health Organization’s recommendation of 1:600.Citation144 Nigeria has about 400 (adult and pediatric) nephrologists, giving an approximate 0.6 nephrologists per million population.Citation145,Citation146 In general and in actual fact, all the potential threats to the nephrology workforce recognized earlier by Sharif et alCitation145 are also applicable to Nigeria’s setting. These threats include but are not limited to declining interest in postgraduate nephrology training among doctors, the rising costs of medical education and specialist training, an aging workforce, and increasing incidence and prevalence of CKD and ESKD.Citation145
This ugly fact of inadequate manpower needed to diagnose and manage renal diseases in Nigeria (including NS/SRNS) is compounded by a system that loses its trained hands to “brain drain” as doctors migrate to North America and Europe.Citation147–Citation149 According to the UK General Medical Council database, the numbers of Nigerian doctors settling in the UK doubled in 2016 compared to 2006, with over 5,000 registered to practice in the UK.Citation147 A recent poll in 2018 revealed that the majority of Nigerian doctors (88%) were seeking to emigrate.Citation147 This cut across all cadres of doctors, including junior, midlevel, and senior doctors in all institutions. The same survey revealed the reasons for the continuous brain drain to include high taxes and deduction from salary (98%), low work satisfaction (92%), poor salaries and emoluments (91%), and the huge knowledge gap that exists in medical practice abroad (47%).Citation147 The few available doctors in Nigeria are also maldistributed, and the referral system is at most inefficient. To illustrate this, a recent survey observed that the northeast region of Nigeria had only one pediatrician for every 700,000 children.Citation150
The concern about the quality of care children with NS are receiving in Nigeria was made manifest by a recent publication that revealed that the management of SRNS was significantly different from best-available evidence.Citation151 From a study questionnaire administered to heads of pediatric nephrology units, it was found that remarkable variations existed in the dose duration of steroids used for NS across many centers. In addition, 12% rarely requested a kidney biopsy for SRNS.Citation49 Therefore, if challenges remain in the diagnosis and treatment of SRNS, it is plausible that knowledge on the genetics of NS (and the need for genetic investigations in SRNS) will be nonexistent. So far, only one attempt has been made at genetic evaluation among Nigerian children with NS.Citation152 In that study, reported by Anochie et al in Port-Harcourt, southern Nigeria, two siblings with familial FSGS were genetically investigated.Citation152 The two siblings — a 4-year-old male and a 15-year-old female — were from a nonconsanguineous family with renal biopsy–confirmed FSGS who presented with NS.Citation152 The mutational analysis performed on the family by sequencing both exons of NPHS2, WT1, and APOL1 showed an absence of common mutations in these genes in the two children. The sequencing analysis was also done outside Nigeria. While the sample was small, the authors suggested that there may be different and unidentified gene mutations responsible for FSGS in indigenous African children.Citation152
Nigeria lacks the manpower and infrastructure for medical genetics.Citation153 Patients with these disorders are largely managed by pediatricians who may be interested in medical genetics, but who have not received formal professional training in genetics. Training opportunities for medical genetics are unavailable within Nigeria. While a few facilities can conduct DNA extraction and even fewer with capacity for sequencing, most genetic studies are conducted outside Nigeria.Citation153
Nigeria still lacks a functioning renal registry.Citation154 This is partly due to inadequate knowledge and skills for documenting the array of KDs and also to the lack of capacity for renal histopathology and its interpretation. The flawed renal data occasioned by different epidemiological and technical differences in describing renal diseases and pathologies among the available data sources may also explain the lack of a renal registry in Nigeria. Also in Nigeria, like in many developing countries, the focus of most governments is still on infectious diseases, as the lack of data on renal burden fails to convince governments of a paradigm shift toward allocating sufficient funding for prevention and care of renal diseases.
Inadequate financial resources
Genetic testing for NS is not cheap, and health financing in Nigeria is still largely by patients paying out of pocket. For example, only 3.7% of gross domestic product was attributed to health expenditure in 2014, and only 0.9% of that was public-sector funding.Citation155 The need to pay out of pocket should also be interpreted against the fact that the minimum wage for Nigerians is ₦18,000 per month (since 2011), up from ₦5,500 per month in 2004.Citation156 This ₦18,000 (ie, US$50 at a conversion rate of ₦360 naira to $1) translates to $1.6 per day, and about 152 million Nigerians live on <$2 a day, representing about 80% of the country’s population, according to the African Development Bank in 2018.Citation157
Genetic testing is not cheap. It costs $600–$3,500 to sequence 26–30 SRNS genes.Citation104 This is however expected to reduce, as WES becomes more applicable with the discovery of more genes.Citation14,Citation84 Even in developed economies, insurance coverage for genetic testing in NS is not universal.Citation14 Not all insurers currently reimburse for genetic testing in NS, as this is not yet considered the standard of care.Citation14 Therefore, if developed countries are still finding it difficult to pay for genetic testing, it remains a bigger challenge for Nigeria, whose health system is currently being underfunded.
Although a national health-insurance scheme (NHIS) was launched in 2005,Citation158 participation is still not mandatory and has very low coverage (<10%). Therefore, most health expenses are borne out of pocket. For renal diseases, NHIS covers only six sessions of acute hemodialysis among its enrollees.Citation158 Also, Nigeria enacted the National Health Act on October 31, 2014 to set standards for health-care delivery.Citation160 This act did not offer much attention to renal care. As such, thus far neither the NHIS nor the act have directly addressed the care of renal diseases, not to mention emerging genetic services or genomic medicine of the 21st century.
Strengths and opportunities
In Nigeria, a well-designed, highly powered prospective study is needed to document the genetic predisposition to SRNS involving clinical, electron-microscopy, and immunofluorescence histopathology of SRNS among ethnically black sub-Saharan children. It will allow for baseline data with which comparisons of genetic mutations and varying alleles of those genetic mutations can be made with children of other races. Hopefully, the H3A-KDRN will be able to answer these research questions among Nigerian children with NS/SRNS.Citation58 If a high burden of genetic mutation is demonstrated for Nigerian children with SRNS, it will argue for the inclusion of genetic investigation as the standard of care for these children and for inclusion of its cost as a mandatory part of renal care under the proposed Nigerian Renal Care Policy (NRCP), discussed subsequently.
In Nigeria, the practice of medical genetics may be rudimentary, but it is not in a state of nothingness.Citation153 The first clinical and cytogenetic outfit was set up in the early 1970s in Ibadan, Nigeria.Citation153 The unit offered clinical and research studies on chromosomal trisomies and other dysmorphic disorders.Citation160–Citation164 As such, medical reports of genetic studies were already available in Nigeria in the early 1970s. In those early days, case reports of genetic disorders were published.Citation165,Citation166 Adeyokunnu reported the incidence of trisomy 21 as one in 865 live births.Citation161,Citation167 Nigeria also has immense experience accrued from the medical genetics of sickle-cell disease (SCD). SCD is a common genetic disorder of hemoglobin synthesis that affects 2%–3% of newborns.Citation154 Up to 22%–25% of the population has the trait.Citation168–Citation172 Most secondary health facilities screen for SCD by hemoglobin electrophoresis at 9 months.Citation173 In a few health centers, capacity also exists for HPLC for screening newborns for SCD.Citation153 Most knowledge of medical genetics by Nigerian health workers is clinically demonstrable by their ability to explain the genetics of SCD and their ability to provide genetic counseling for couples seeking to get married. Their understanding of genetics can also be seen in their proficiency in explaining the risk of having SCD in offspring of couples with sickle-cell traits. Presently, a prenatal diagnosis of SCD is also available at the Sickle Cell Center in Lagos, Nigeria.Citation153 The Sickle Cell Foundation of Nigeria also has the capacity to perform PCR–restriction-fragment length polymorphism analysis for the sickle-cell mutation.Citation153
There is also experience in molecular genetics in complex metabolic disorders, such as hypertension, obesity, and diabetes, going back to the 1990s.Citation174–Citation187 Nigerians were also included in population testing for genetic risk factors for malaria and genetic disorders of orofacial clefts, congenital deafness, and congenital heart defects.Citation189–Citation195 Nigerian clinicians are also participating in the National Human Genome Research Institute Atlas of Human Malformation Syndromes in Diverse Populations.Citation196,Citation197
The H3A-KDRN is jointly funded by the National Institutes of Health and the Wellcome Trust and has invested in several major grants to African investigators for genomic research, capacity building, and improving infrastructure for genome research in Africa.Citation198 The H3A-KDRN seeks to determine the association of APOL1 variants with different rates of CKD in many African settings, including Nigeria, and potential environmental interaction. It will also incorporate causative genetic studies among familial SRNS children aged 15 years and above.Citation199 The present proposal seeks to study a wider age-range of children (1–18 years) with nonsyndromic familial or sporadic steroid-resistant INS in an ethnically diverse young population in Nigeria.
From the foregoing, it is apparent that Nigeria is not a novice in the field of human genetics and genomic medicine. Lessons learned from earlier experiencesCitation153–Citation200 can be brought to bear in developing the much-needed infrastructure for genomic medicine (genetic and molecular laboratories for genotyping, sequencing, and bioinformatics) and in building the necessary manpower (genetic counselors, geneticists, cell biologists, and molecular physiologists) required for genetic investigation of SRNS among Nigerian children.
Ultimately, for children with ESKD from SRNS requiring renal transplantation, opportunities already exist for renal transplantation in Nigeria.Citation201 In 2000, St Nicholas Hospital, a private hospital in Lagos, performed the first kidney transplant in Nigeria.Citation201 Since then, more Nigerian hospitals have transplanted kidneys with Obafemi Awolowo University Teaching Hospital Complex, Ife, Osun state in 2002 being the first among public tertiary health institutions to perform renal transplantation.Citation200,Citation201 The hospital complex also has a standard immunology laboratory for proper human leukocyte-antigen typing and tissue cross-matching.Citation201
Apart from the challenges of cost and infrastructure, other issues relating to organ donation are being taken care of under the National Health Act of 2014. Citation159 The act deals with the illegality of harvesting tissues or organs from a person who cannot give consent or taking tissue from someone aged <18 years.Citation159,Citation200 The act recognizes that transplantation must be carried out in an authorized hospital with full written authorization of the head of the hospital.Citation159 In 2005, the Nigerian Association of Nephrology proposed the NRCP.Citation202 The structure of the NRCP included defining and establishing renal care centers, preventive nephrology, funding of renal care, monitoring and evaluation, establishment of a National Kidney Institute, establishment of the renal registry, legislation on an organ-procurement and -transplant program, and developing a robust relationship among states, local-government councils, and federal authorities.Citation202 Specifically, the NRCP has not as yet made provision for the financing of medical genetics or genomic medicine related to renal diseases in Nigeria. Genomic medicine can be incorporated in the structure of the renal care centers, starting from the tertiary teaching hospitals. The manpower required for genetic analysis can also be developed within the same framework.
An opportunity also exists to expand the scope of the NHIS to cover the exorbitant price of genetic testing within the framework of funding for CKDs, including childhood NS. Another strategy will include advocacy to the Nigerian government to make concerted efforts in stemming the tide of the so-called brain drain by addressing the reasons for the needless emigration of Nigerian doctors, as discussed previously. Nigeria also needs to train more medical doctors to meet the demands of her teeming population. Training and retraining in pediatric nephrology can begin to be the focus of continuing medical education by the Nigerian Medical Council. The capacity to recognize childhood NS and the capacity to know when to refer cases of SRNS to tertiary health centers cannot be overemphasized. Creating and maintaining a registry of children with KD as a composite part of the national renal registry is also much needed.
Conclusion
Nigeria has more than over 500 indigenous ethnic groups,Citation203 thus bolstering the out-of-Africa theory that posits that humans evolved in Africa.Citation204,Citation205 Nigeria presents potential genetic variations in genetic causes of NS/SRNS. With prevalence rates of renal diseases among Nigerian children on hospital admission ranging from 1.1% to 4.5%, NS is also the commonest renal disorder.Citation28,Citation36,Citation206–Citation209 With emerging evidence that monogenic FSGS/NS has zero risk of recurrence,Citation141 knowing the genetic diagnosis of SRNS is expected to revolutionize the approach to the management of SRNS in childhood. Therefore, the diagnosis of genetic NS will obviate the need for renal biopsy and the unwarranted exposure to immunosuppressive therapy and its attendant complications. Nigeria can leverage its existing experience in genomic medicine to start to include genetic testing for SRNS as part of a standard of care. The NHIS scope can be expanded to include paying for the expensive bill of genetic testing within the structure of the proposed NRCP. The NRCP, while still a “roaring tiger on paper”, is bound to improve the care of Nigerians with renal diseases once the policy is put to practical use. As Nigerian nephrologists forge ahead in advocacy for implementation of the NRCP, with expected goodwill from the political establishment, the comprehensive care package contained in the NRCP will soon start to save millions of Nigerians with renal disorders. As the request for genetic testing expands, clinicians will be willing to undergo subspecialty training in human genetics and genomic medicine. As more funding opportunities become available for new research projects, training of manpower and infrastructural development would also become progressively available. The advantages of individualized precision medicine that a genetic diagnosis of SRNS portends are too glaring for Nigerian children not also to partake in the 21st century.
Acknowledgments
We are grateful to Professor Rasheed Gbadegesin of Duke University Medical Center, Durham, NC, USA for making some articles available for us in the course of this work.
Disclosure
This work was not carried out in the presence of any personal, professional, or financial relationships that could be construed as a conflict of interest. We report no conflicts of interest.
References
- NiaudetPSteroid–responsive idiopathic nephrotic syndrome in childrenAvnerDEHarmonEWNiaudetPediatric Nephrology5th edPhiladelphia, PALippincott William & Wilkin2004543573
- HaraldssonBNyströmJDeenWMProperties of the glomerular barrier and mechanisms of proteinuriaPhysiol Rev200888245148718391170
- LaneJCLangmanCBPediatric nephrotic syndrome Available from: https://emedicine.medscape.com/article/982920-overviewAccessed August 12, 2017
- HodsonEMWongSCWillisNSCraigJCInterventions for idiopathic steroid-resistant nephrotic syndrome in childrenCochrane Database Syst Rev2016239
- RheaultMNWeiCCHainsDSWangWKerlinBASmoyerWEIncreasing frequency of acute kidney injury amongst children hospitalized with nephrotic syndromePediatr Nephrol201429113914724037143
- WongWIdiopathic nephrotic syndrome in New Zealand children, demographic, clinical features, initial management and outcome after twelve-month follow-up: results of a three-year national surveillance studyJ Paediatr Child Health200743533734117489822
- EddyAASymonsJMNephrotic syndrome in childhoodThe Lancet20033629384629639
- CochatPMouraniCExantusJPediatric nephrology in developing countriesMed Trop200969543547
- ReesLBroganPABockenhauerDWebbNJAGlomerular Diseases in: PaediatricNephrology2nd edOxfordOxford University Press2012192216
- DownieMLGalliboisCParekhRSNooneDGNephrotic syndrome in infants and children: pathophysiology and managementPaediatr Int Child Health201737424825828914167
- Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosisA report of the International study of kidney disease in childrenKidney Int1978132159165713276
- BarnettHLEdelmanncmGreiferIThe primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International study of kidney disease in childrenJ Pediatr19819845615647205481
- GipsonDSMassengillSFYaoLManagement of childhood onset nephrotic syndromePediatrics2009124274719651590
- GbadegesinRAWinnMPSmoyerWEGenetic testing in nephrotic syndrome–challenges and opportunitiesNat Rev Nephrol20139317918423321566
- CochatPFargueSMestralletGDisease recurrence in paediatric renal transplantationPediatr Nephrol200924112097210819247694
- van BiljonGNephrotic syndrome in children – Studies from South Africa, An update on glomerulopathies – Clinical and treatment aspectsPrabhakarSInTech2011439454978-953-307-673-7 Available from: https://www.intechopen.com/books/an-updateon-glomerulopathies-clinical-andtreatment-aspects/nephrotic-syndrome-in-children-studies-from-southafricaAccessed3 January, 2018
- ElzoukiAYAminFJaiswalOPPrevalence and pattern of renal disease in eastern LibyaArch Dis Child19835821061096830287
- ElzoukiAYAminFJaiswalOPPrimary nephrotic syndrome in Arab childrenArch Dis Child19845932532556712274
- SouilmiFHoussainiTAlaouiHIndications and results of renal biopsy in children: a single-center experience from MoroccoSaudi J Kidney Dis Transpl201526481081526178566
- OlowuWAAdemolaAAjiteABSaadYMChildhood nephrotic syndrome in tropical Africa: then and nowPaediatr Int Child Health201737425926828949280
- GillesHMHendrickseRGPossible aetiological role of Plasmodium malariae in nephrotic syndrome in Nigerian childrenLancet19601712880680713850078
- GillesHMHendrickseRGNephrosis in Nigerian children. Role of Plasmodium malariae, and effect of antimalarial treatmentBr Med J196325348273113947894
- HendrickseRGGillesHMThe nephrotic syndrome and other renal diseases in children in western NigeriaEast Afr Med J19634018620113953715
- OlowuWAAdelusolaKAAdefehintiOOyetunjiTGQuartan malaria-associated childhood nephrotic syndrome: now a rare clinical entity in malaria endemic NigeriaNephrol Dial Transplant201025379480119861316
- OkoroBAOkaforHUNephrotic syndrome in Nigerian children with homozygous sickle cell diseaseEast Afr Med J199774128198219557431
- AdemolaADAsinobiOOOladokunREKidney disease in hospitalised HIV positive children in Ibadan, South West NigeriaAfr J Med Med Sci201241222123023185922
- AbdurrahmanMBAikhionbareHABabaoyeFAClinicopathlogical features of childhood nephrotic syndrome in northern NigeriaQ J Med1990278563576
- EkeFUEkeNNRenal disorders in children: a Nigerian studyPediatr Nephrol1994833833867917872
- MoIAbiodunPOEpidemiology and clinicopathologic characteristics of childhood nephrotic syndrome in Berlin-City, NigeriaJ Pakistan Med Assoc1998488235238
- AsinobiAOGbadegesinRAAdeyemoAAThe predominance of membranoproliferative glomerulonephritis in childhood nephrotic syndrome in Ibadan, NigeriaWest Afr J Med199918320320610593158
- BaOOkaforHUPattern of childhood renal disorders in EnuguNig J Paediatrics1999261418
- AnochieIEkeFOkpereaChildhood nephrotic syndrome: change in pattern and response to steroidsJ Nat Med Assoc2006981219771981
- AsinobiAOGbadegesinRAOgunkunleOOIncreased steroid responsiveness of young children with nephrotic syndrome in NigeriaAnn Trop Paediatr200525319920316156985
- AdedoyinOTGbeleeHODAdeniyiAChildhood nephrotic syndrome in IlorinNiger J Paediatr20012836872
- OlowuWAAdelusolaKAAdefehintiOChildhood idiopathic steroid resistant nephrotic syndrome in southwestern NigeriaSaudi J Kid Dis Transpl2010215979990
- MoIGeOIbadinMOOfovweGEPattern of renal diseases in children in mid-western zone of NigeriaSaudi J Kid Dis Transpl200314539544
- LadapoTAEsezoborCILesiFEHigh steroid sensitivity among children with nephrotic syndrome in southwestern NigeriaInt J Nephrol2014201435064025140253
- AsinobiAOAdemolaADOkoloCAYariaJOTrends in the histopathology of childhood nephrotic syndrome in Ibadan Nigeria: preponderance of idiopathic focal segmental glomerulosclerosisBMC Nephrol201516121326670137
- AdeniyiAHendrickseRGHoubaVSelectivity of proteinuria and response to prednisolone or immunosuppressive drugs in children with malarial nephrosisLancet1970176486446484190632
- HendrickseRGAdeniyiAEdingtonGMQuartan malarial nephrotic syndrome. Collaborative clinicopathological study in Nigerian childrenLancet197217761114311494113056
- SrivastavaTSimonSDAlonUSHigh incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhoodPediatr Nephrol1999131131810100283
- GulatiSSharmaAPSharmaRKGuptaAChanging trends of histopathology in childhood nephrotic syndromeAm J Kidney Dis199934464665010516344
- Bonilla-FelixMParraCDajaniTChanging patterns in the histopathology of idiopathic nephrotic syndrome in childrenKidney Int19995551885189010231451
- KariJAChanging trends of histopathology in childhood nephrotic syndrome in Western Saudi ArabiaSaudi Med J200223331732111938425
- FillerGYoungEGeierPCarpenterBDrukkerAFeberJIs there really an increase in non-minimal change nephrotic syndrome in children?Am J Kidney Dis20034261107111314655180
- GulatiSSharmaAPSharmaRKGuptaAGuptaRKDo current recommendations for kidney biopsy in nephrotic syndrome need modifications?Pediatr Nephrol200217640440812107803
- RosenbergAZKoppJBFocal segmental glomerulosclerosisClin J Am Soc Nephrol201712350251728242845
- KiffelJRahimzadaYTrachtmanHFocal segmental glomerulosclerosis and chronic kidney disease in pediatric patientsAdv Chronic Kidney Dis201118533233821896374
- CorwinHLSchwartzMMLewisEJThe importance of sample size in the interpretation of the renal biopsyAm J Nephrol19888285893394725
- SchachterADComputational simulation of renal biopsy accuracy in focal segmental glomerulosclerosisPediatr Nephrol200621795395716773406
- Wagrowska-DanilewiczMDanilewiczMSynaptopodin immunoexpression in steroid-responsive and steroid-resistant minimal change disease and focal segmental glomerulosclerosisNefrologia200727671071518336100
- GiannicoGYangHNeilsonEGFogoABDystroglycan in the diagnosis of FSGSClin J Am Soc Nephrol20094111747175319808230
- TrautmannABodriaMOzaltinFSpectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohortClin J Am Soc Nephrol201510459260025635037
- DingWYKoziellAMccarthyHJInitial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrenceJ Am Soc Nephrol20142561342134824511128
- GadegbekuCAGipsonDSHolzmanLBDesign of the nephrotic syndrome Study network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approachKidney Int201383474975623325076
- SamuelSScottSMorganCThe Canadian childhood nephrotic syndrome (CHILDNEPH) project: overview of design and methodsCan J Kidney Health Dis201411725960884
- HussainNZelloJAVasilevska-RistovskaJThe rationale and design of insight into nephrotic syndrome: investigating genes, health and therapeutics (insight): a prospective cohort study of childhood nephrotic syndromeBMC Nephrol2013142523351121
- NIH Awards by Location and Organization - NIH Research Portfolio Available from: https://report.nih.gov/award/index.cfm?ot=&fy=2017&state=86&ic=NIDDK&fm=&orgid=&distr=&rfa=&pid=Accessed 4 March, 2018
- CampbellMCTishkoffSAAfrican genetic diversity: implications for human demographic history, modern human origins, and complex disease mappingAnnu Rev Genomics Hum Genet20089140343318593304
- BenoitGMachucaEHeidetLHereditary kidney diseases: highlighting the importance of classical Mendelian phenotypesAnn N Y Acad Sci20101214839820969579
- D’AgatiVDFogoABBruijnJAJennetteJCPathologic classification of focal segmental glomerulosclerosis: A working proposalAm J Kidney Dis20044336838214750104
- MarrasDBruggemanLAGaoFTanjiNMansukhaniMMCaraARossMDGusellaGLBensonGD’AgatiVDHahnBHKlotmanMEKlotmanPEReplication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathyNat Med2002852252611984599
- ChandraPKoppJBViruses and collapsing glomerulopathy: A brief critical reviewClin Kidney J201361523372939
- MarkowitzGSAppelGBFinePLFenvesAZLoonNRJagannathSKuhnJADratchADD’AgatiVDCollapsing focal segmental glomerulosclerosis following treatment with high dose pamidronateJ Am Soc Nephrol2001121164117211373339
- SakarcanAThomasDBO’ReillyKPRichardsRWLithium induced nephrotic syndrome in a young pediatric patientPediatr Nephrol20021729029211956885
- LetavernierEBrunevalPMandetCDuongVan Huyen JPPe´raldiMNHelalINoe¨lLHLegendreCHigh sirolimus levels may induce focal segmental glomerulosclerosis de novoClin J Am Soc Nephrol2007232633317699432
- ChoMEHurleyJKKoppJBSirolimus therapy of focal segmental glomerulosclerosis is associatedwith nephrotoxicityAm J Kidney Dis20074931031717261434
- MohamedNGoldsteinJSchiffJJohnRCollapsing glomerulopathy following anthracycline therapyAm J Kidney Dis20136177878123219112
- MorganCAl-AklabiMGarcia GuerraGChronic kidney disease in congenital heart disease patients: A narrative review of evidenceCan J Kidney Health Dis2015227
- AygunBMortierNASmeltzerMPHankinsJSWareREGlomerular hyperfiltration and albuminuria in children with sickle cell anemiaPediatr Nephrol2011261285129021559933
- WickmanCKramerHObesity and kidney disease: Potential mechanismsSemin Nephrol201333142223374890
- HerlitzLCMarkowitzGSFarrisABSchwimmerJAStokesMBKunisCColvinRBD’AgatiVDDevelopment of focal segmental glomerulosclerosis afteranabolic steroid abuseJ Am Soc Nephrol20102116317219917783
- HanlyPJAhmedSBSleep apnea and the kidney: Is sleep apnea a risk factor for chronic kidney disease?Chest20141461114112225288001
- IkezumiYSuzukiTKarasawaTYamadaTHasegawaHNishimuraHUchiyamaMLow birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosisAm J Nephrol20133814915723920104
- KomatsuKFrohlichEDOnoHOnoYNumabeAWillisGWGlomerular dynamics and morphology of aged spontaneously hypertensive rats. Effects of angiotensin-converting enzyme inhibitionHypertension1995252072137843770
- SavinVJSharmaMZhouJGennochiDFieldsTSharmaRMcCarthyETSrivastavaTDomenJTormoAGauchatJFRenal and hematological effects of CLCF-1, a B-Cell stimulating cytokine of the IL-6 familyJ Immunol Res2015714964
- Lopez-HellinJCantarellCJimenoLSanchez-FructuosoAPuig-GayNGuiradoLVilari~noNGonzalez-RonceroFMMazuecosALauzuricaRBurgosDPlumedJSJacobs-CachaCJimenezCFernandezAFernandez-AlvarezPTorregrosaVNietoJLMeseguerAAlonsoAGREAT Study GroupA form of apolipoprotein a-I is found specifically in relapses of focal segmental glo-merulosclerosis following transplantationAm J Transplant20131349350023205849
- DelvilleMSigdelTKWeiCLiJHsiehSCFornoniABurkeGWBrunevalPNaesensMJacksonAAlachkarNCanaudGLegendreCAnglicheauDReiserJSarwalMMA circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantationSci Transl Med20146256ra136
- WeiCElHindi SLiJFornoniAGoesNSageshimaJMaiguelDKarumanchiSAYapHKSaleemMZhangQNikolicBChaudhuriADaftarianPSalidoETorresASalifuMSarwalMMSchaeferFMorathCSchwengerVZeierMGuptaVRothDRastaldiMPBurkeGRuizPReiserJCirculating urokinase receptor as a cause of focal segmental glomerulosclerosisNat Med20111795296021804539
- LeporiNZandLSethiSFernandez-JuarezGFervenzaFCClinical and pathological phenotype of genetic causes of focal segmental glomerulosclerosis in adultsClin Kidney J201811217919029644057
- DeegensJKJDijkmanHBPMBormGFPodocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosisKidney Int2008741568157618813290
- KestilaMPositionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndromeMol Cell199815755829660941
- Tae-SunHaGenetics of hereditary nephrotic syndrome: a clinical reviewKorean J Pediatr2017603556328392820
- RheaultMNGbadegesinRAThe Genetics of Nephrotic SyndromeJ Ped Gen201651152410.1055/s-0035-1557109 2146–4596
- KoppJBNelsonGWSampathKJohnsonRCGenoveseGAnPFriedmanDBriggsWDartRKorbetSMokrzyckiMHKimmelPLLimouSAhujaTSBernsJSFrycJSimonEESmithMCTracht-manHMichelDMSchellingJRVlahovDPollakMWinklerCAAPOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathyJ Am Soc Nephrol2011222129213721997394
- KasembeliANDuarteRRamsayMMosianePDickensCDix-PeekTLimouSSezginENelsonGWFogoABGoetschSKoppJBWinklerCANaickerSAPOL1 risk variants are strongly associated with HIV-associated nephropathy in black South AfricansJ Am Soc Nephrol2015262882289025788523
- NeuhausTJFayJDillonMJTrompeterRSBarrattTMAlternative treatment to corticosteroids in steroid sensitive idiopathic nephrotic syndromeArch Dis Child1994715225267726612
- BennettMRPleasantLTHaffnerCMaQHaffeyWDYingJWagnerMGreisKDDevarajanPA Novel Biomarker Panel to Identify Steroid Resistance in Childhood Idiopathic Nephrotic SyndromeBio Insights201712111
- Tasic1VGucevZPolenakovicMSteroid resistant nephrotic syndrome-genetic considerationSec of Med Sci2015XXXVI3
- PrestonRStuartHMLennonRGenetic testing is steroid-resistant nephrotic syndrome: why, who, when and how?Pediatric Nephr201934195210
- UwaezuokeSNThe role of novel biomarkers in childhood idiopathic nephrotic syndrome: a narrative review of published evidenceInt J Nephrol Renovascular Dis20171012312
- BennettMRPiyaphaneeNCzechKMitsnefesMDevarajanPNGAL distinguishes steroid sensitivity in idiopathic nephrotic syndromePediatr Nephrol201227580781222200895
- WeiCEl HindiSLiJCirculating urokinase receptor as a cause of focal segmental glomerulosclerosisNat Med201117895296021804539
- NourbakhshNMakRHSteroid-resistant nephrotic syndrome: past and current perspectivesPediatr Health Med Therap20178293
- ReiserJWeiCTumlinJSoluble urokinase receptor and focal segmental glomerulosclerosisCurr Opin Nephrol Hypertens201221442843222569341
- MatsuiIHamanoTMikamiSRetention of fetuin-A in renal tubular lumen protects the kidney from nephrocalcinosis in ratsAm J Physiol Renal Physiol2013304F751F76023344571
- RussoLMSandovalRMMcKeeMThe normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic statesKidney Int20077150451317228368
- SadowskiCELovricSAshrafSA single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndromeJ Am Soc Nephrol2015261279128925349199
- BierzynskaASoderquestKKoziellAGenes and podocytes - new insights into mechanisms of podocytopathyFront Endocrinol20145226
- EbarasiLAshrafSBierzynskaADefects of CRB2 cause steroidresistant nephrotic syndromeAm J Human Genet20159615316125557779
- MiyakeNTsukaguchiHKoshimizuEBiallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant nephrotic syndromeAm J Human Genet20159755556626411495
- BraunDASadowskiCEKohlSMutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndromeNat Genet20164845746526878725
- GeeHYZhangFAshrafSKANK deficiency leads to podocytes dysfunction and nephrotic syndromeJ Clin Invest20151252375238425961457
- LovricSAshrafSTanWHildebrandtFGenetic testing in steroid-resistant nephrotic syndrome: when and how?Nephrol Dial Transplant2016311802181326507970
- GentzonHGbadegesinRATranslating genetic findings in hereditary nephrotic syndrome: the missing loopsAm J Physiol Renal Physiol20153091F24F2810.1152/ajprenal.00683.201425810439
- AbrahamsonDRHudsonBGStroganovaLSt BorzaDBJohn PL. Cellular origins of type IV collagen networks in developing glomeruliJ Am Soc Nephrol2009201471147919423686
- AkileshSSuleimanHYuHStanderMCLavinPGbadegesinRAntignacCPollakMKoppJBWinnMPShawASArhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosisJ Clin Invest20111214127413721911940
- CaridiGTrivelliASanna-CherchiSPerfumoFGhiggeriGMFamilial forms of nephrotic syndromePediatr Nephrol20102524125219066979
- ClementLCAvila-CasadoCMaceCSoriaEBakkerWWKerstenSChughSSPodocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndromeNat Med20111711712221151138
- KitamuraATsukaguchiHHiramotoRShonoADoiTKagamiSIijimaKA familial childhood-onset relapsing nephrotic syndromeKidney Int20077194695117290294
- MaloneAFPhelanPJHallGCetincelikUHomstadAAlonsoASJiangRLindseyTBWuGSparksMASmithSRWebbNJKalraPAAdeyemoAAShawASConlonPJJennetteJCHowellDNWinnMPGbadegesinRARare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosisKidney Int2014861253125925229338
- PavenstadtHKrizWKretzlerMCell biology of the glomerular podocytePhysiol Rev20038325330712506131
- BuscherAKWeberSEducational paper: the podocytopathiesEur J Pediatr20121711151116022237399
- KoppJBMYH9 is a major-effect risk gene for focal segmental glomerulosclerosisNat Genet2008401175118418794856
- GenoveseGAssociation of trypanolytic ApoL1 variants with kidney disease in African AmericansScience201032984184520647424
- HildebrandtFHeeringaSFSpecific podocin mutations determine age of onset of nephrotic syndrome all the way into adult lifeKidney Int20097566967119282856
- RufRGLichtenbergerAKarleSMPatients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndromeJ Am Soc Nephrol20041572273214978175
- HinkesBVlangosCHeeringaSSpecific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndromeJ Am Soc Nephrol20081936537118216321
- ToryKMenyhardDKWoernerSMutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndromeNat Genet20144629930424509478
- TasicVGenetics of Steroid Resistant Nephrotic syndromeIPNA teaching course in pediatric nephrology17-192015 Yerevan, Armenia
- KlaassenIOzgorenBSadowskiCEMollerKvan HusenMLehnhardtAResponse to cyclosporine in steroid-resistant nephrotic syndrome: discontinuation is possiblePediatr Nephrol201530914778325903641
- CosioFGCattranDCRecent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantationKidney Int20179130431427837947
- HicksonLJGeraMAmerHKidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrenceTransplantation2009871232123919384172
- HofstraJMLainezSvan KuijkWHMNew TRPC6 gain -of-function mutation in a non-consanguineous Dutch family with late-onset focal segmental glomerulosclerosisNephrol Dial Transplant2013281830183823291369
- JungraithmayrTCHoferKCochatPScreening for NPHS2 mutations may help predict FSGS recurrence after transplantationJ Am Soc Nephrol20112257958521355056
- WeberSGribouvalOEsquivelELNPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrenceKidney Int20046657157915253708
- ConlonPJLynnKWinnMPSpectrum of disease in familial focal and segmental glomerulosclerosisKidney Int1999561863187110571795
- ChanchlaniRParekhRSEthnic Differences in Childhood nephroticSyndrome Front Pediatr43910.3389/fped.2016.00039.27148508
- SantínSClinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndromeClin J Am Soc Nephrol201161139114821415313
- JoshiSAndersenRJespersenBRittigSGenetics of steroid-resistant nephrotic syndrome: a review of mutation spectrum and suggested approach for genetic testingActa Paediatr20131028445623772861
- BierzynskaAMcCarthyHJSoderquestKSenESColbyEDingWYGenomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease managementKidney Int20179193794728117080
- HalbritterJBaumMHynesAMFourteenmonogenic genes account for 15%of nephrolithiasis/nephrocalcinosisJ Am Soc Nephrol20152654355125296721
- HalbritterJDiazKChakiMHigh-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencingJ Med Genet20124975676723188109
- TeerJKMullikinJCExome sequencing: the sweet spot before whole genomesHum Mol Genet201019R145R15120705737
- ConradDFVariation in genome-wide mutation rates within and between human familiesNat Genet20114371271421666693
- MeytsIBoschBBolzeABoissonBItanYBelkadiAPedergnanaVMoensLPicardCCobatABossuytXAbelACasanovaJ-LExome and genome sequencing for inborn errors of immunityJ Allergy Clin Immunol201613895796927720020
- LeeBGenetic counseling20th edKliegmanRMStantonBFStGeme JWSchorNFNelson Textbook of Paediatrics PhiladelphiaSaunders2016582583
- Steps in genetic counselling briefoutline.eachinganatomy.blogspot.com/2013/02/Steps-Genetic-Counseling.html
- BhimmaRCoovadiaHMAdhikariMNephrotic syndrome in South African children: changing perspectives over 20yearsPediatr Nephrol1997114294349260239
- South West States in Nigeria [webpage on the Internet]Olawale J2018 Available from: https://www.legit.ng/1117167-south-west-states-nigeria.htmlAccessed April 17, 2019
- PelletierJHKumarKREngenRRecurrence of nephrotic syndrome following kidney transplantation is associated with initial native kidney biopsy findingsPediatr Nephrol201833101773178029982878
- YirsawBDChronic kidney disease in sub-Saharan Africa: Hypothesis for research demandAnn Afr Med2012111192022406674
- PopulationNigeria’s ticking time bomb Available from: https://www.blueprint.ng/population-nigerias-ticking-time-bomb/Accessed 6th May 2018
- Unease over dearth of physicians in Nigeria’s health sector Available from: https://leadership.ng/2018/09/17/unease-over-dearth-of-physicians-in-nigerias-health-sector/Accessed 1st October 2018
- SharifMElsayedMEStackAGThe global nephrology workforce: emerging threats and potential solutionsClinl Kidney J201691122
- 3 in 10 Nigerian adults have kidney disease, says nephrologists Available from: https://www.dailytrust.com.ng/3-in-10-nigerian-adults-have-kidney-disease-says-nephrologists.htmlAccessed 2nd February 2018
- Banke-ThomasAThe emigration of doctors from Nigeria is not today’s problem, it is tomorrow’s15102018 Available from: https://www.naijiant.com/guestcolumn/the-emigration-of-doctors-from-nigeria-is-not-todays-problem-it-is-tomorrows/Accessed 1st November 2018
- Uchenunu-IbehONigerian doctors seek work opportunities abroad372018 Available from: https://leadership.ng/2018/07/03/88-ofNigerian-doctors-seek-work-0opportunities-abroad/Accessed 3rd August 2018
- NwakanmaA2982018The brain drain on Nigeria’s health sector spells imminent crisis Available from: https://thenerveafrica.com/21483/the-brain-drain-on-nigerias-health-sector-spells-imminent-crisis/Accessed 20th March 2019
- EkureENEsezoborCIBalogunMRPaediatrician workforce in Nigeria and impact on child healthNig J Paediatr20134021128
- EsezoborCIAsinobiAOOkaforHUAkuseRGbadegesinRNational survey found that managing childhood nephrotic syndrome in Nigeria varied widely and did not comply with the best evidenceActa Paediatr201852210.1111/apa.14409
- AnochieICEkeFUOkpereANFamilial focal segmental glomerulosclerosis (FSGS) in a Nigerian family and exclusion of mutations in NPHS2,WT1 and APOL1West Afr J Med201231273623468032
- AdeyemoAAAmoduOKEkureEEOmotadeOOMedical genetics and genomic medicine in NigeriaMol Genet Genomic Med2018631432129871027
- MakindeF15th February 2017 Stop sending renal patients abroad, minister tells doctors https://punchng.com/stop-sending-renal-patients-abroad-minister-tells-doctors/Accessed 13th February 2018
- BhardwajVFACT-CHECK: Does Nigeria have the lowest health budget in Africa?1652016 https://www.premiumtimesng.com/features-and-interviews/203494-fact-check-nigeria-lowest-health-budget-africa.htmlAccessed 4th March 2018
- Nigerian labour law https://en.wikipedia.org/wiki/Nigerian_labour_lawAccessed 6th April 2018
- AmaefuleE152 million Nigerians live on less than 2$/day-AfDB622018 https://punchng.com/152-million-nigerians-live-onless-than-2day-afdbAccessed 6th May 2018
- National Health Insurance Scheme https://www.nhis.gov.ng/home/Assessed 25th October 2018
- EnabuleleOEnabuleleJENigeria’s National Health Act: An assessment of health professionals’ knowledge and perceptionNiger Med J201657526026527833244
- AdeyokunnuA AThe genetics of omphaloceleClin Genet19812032367307324
- AdeyokunnuAAThe incidence of Down’s syndrome in NigeriaJ Med Genet198219277279 10.1136/jmg.19.4.2776214633
- AdeyokunnuAASpectrum of bone dysplasias in African children: Ibadan Nigerian experienceProg Clin Biol Res19821044274407163284
- AdeyokunnuAAThe incidence of Turner’s syndrome in Ibadan, NigeriaAJMMS1982113105112
- AdeyokunnuAAAdeniyiAThe Beckwith-Wiedemann syndrome in Nigerian infants (exomphalos, macroglossia and gigantism)East Afr Med J19815896846907318721
- DadaTODystrophia myotonica in Nigerian familyEast Afr Med J1973502132284730256
- FamilusiJ BJaiyesimiFOjoCOAttahEDBHereditary anhidrotic ectodermal dysplasia: studies in a Nigerian familyArc Dis Child197550642
- AdeyokunnuA AAutosomal trisomy 18 and 13 syndromes in Ibadan, NigeriaAJMMS198328189
- AkinyanjuOOA profile of sickle cell disease in NigeriaAnn N Y Acad Sci19895651261362672962
- FlemingAFStoreyJMolineauxLIrokoEAAttaiEDAbnormal haemoglobins in the Sudan savanna of Nigeria.I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survivalAnn Trop Med Parasitol197973168172
- JelliffeDBHumphreysJThe sickle-cell trait in western Nigeria; a survey of 1,881 cases in the YorubaBr Med J195223405406
- KaineWNUdeozoIOIncidence of sickle cell trait and Anaemia in Igbo Preschool ChildrenNig J Paediatr198188789
- NwogohBAdewoyinA SIheanachoOEBazuayeG NPrevalence of haemoglobin variants in Benin City, NigeriaAnn Biomed Sci2012116064
- OmotadeOOKayodeCMFaladeSLIkpemeSAdeyemoAAAkinkugbeFMRoutine screening for sickle cell haemoglobinopathy by electrophoresis in an infant welfare clinicWest Afr J Med19981791949715113
- RotimiCMorrisonLCooperROyejideCEffiongELadipoMAngiotensinogen gene in human hypertension. Lack of an association of the 235T allele among African AmericansHypertension1994245915947960018
- AndersonJLBunkerCHAstonCEKambohMIRelationship of two apolipoprotein B polymorphisms with serum lipoprotein and lipid levels in African blacksHuman Biol1997697938079353975
- KaufmanJSDurazo-ArvizuR ARotimiCNMcGeeDLCooperRSObesity and hypertension prevalence in populations of African originEpidemiol19967398405
- McKenzieCASinsheimerJSAdeyemoAACoxRDSouthamLHugillASNP haplotypes in the angiotensin I-converting enzyme (ACE) gene: Analysis of Nigerian family data using gamete competition modelsAnn Hum Genet20056922723215720304
- RotimiCNCooperRSAtamanSLOsotimehinBKadiriSMunaWDistribution of anthropometric variables and the prevalence of obesity in populations of west African origin: The International Collaborative Study on Hypertension in Blacks (ICSHIB)Obesity19953S295105
- RotimiCCooperROgunbiyiOMorrisonLLadipoMTewksburyDWardRHypertension, serum angiotensinogen, and molecular variants of the angiotensinogen gene among NigeriansCirculation1997209523482350
- RotimiCPurasACooperRMcFarlane-AndersonNForresterTOgunbiyiOPolymorphisms of reninangiotensin genes among Nigerians, Jamaicans, and African AmericansHypertension1996275585638613203
- RotimiCNDunstonGMBergKAkinseteOAmoahAOwusuSIn search of susceptibility genes for type 2 diabetes in West Africa: The design and results of the first phase of the AADM studyAnn Epidemiol200111515811164120
- RotimiCNChenGAdeyemoAAFurbert-HarrisPParish-GauseDZhouJA genome-wide search for type 2 diabetes susceptibility genes in West Africans: The Africa America Diabetes Mellitus (AADM) StudyDiabetes20045383884110.2337/diabetes.53.3.83814988271
- AdeyemoAATekola-AyeleFDoumateyAPBentleyARChenGHuangHEvaluation of genome wide association study associated type 2 diabetes susceptibility loci in sub Saharan AfricansFront Genet2015246335
- ChenGDoumateyAPZhouJLeiLBentleyARTekola-AyeleFGenome-wide analysis identifies an african-specific variant in SEMA4D associated with body mass indexObesity201725479480028296344
- KangSJChiangCWPalmerCDTayoBOLettreGButlerJLGenome-wide association of anthropometric traits in African- and African-derived populationsHum Mol Genet2010192725273820400458
- NandakumarPLeeDRichardMATekola-AyeleFTayoBOWareERare coding variants associated with blood pressure variation in 15914 individuals of African ancestryJ Hypertens2017351381138928234671
- RederNPTayoBOSalakoBOgunniyiAAdeyemoARotimiCCooperRSAdrenergic alpha-1 pathway is associated with hypertension among Nigerians in a pathwayfocused analysisPLoS ONE20127e3714510.1371/journal.pone.003714522615923
- AmoduOKOlaniyanSAAdeyemoAATroye-BlombergMOlumesePEOmotadeOOAssociation of the Sickle Cell Trait and the ABO Blood Group with clinical severity of malaria in southwest NigeriaActa Tropica2012123727722503377
- OlaniyanSAAmoduOKBakareAATroye-BlombergMOmotadeOORockettKAMalariaGEN ConsortiumTumour necrosis factor alpha promoter polymorphism, TNF-238 is associated with severe clinical outcome of falciparum malaria in Ibadan southwest NigeriaActa tropica2016161626727178813
- OlaniyanSAAmoduOKYindomLMConwayDJAkaPBakareAAOmotadeOOKiller-cell immunoglobulin-like receptors and falciparum malaria in southwest NigeriaHum Immunol20147581682124929143
- ClarkeGMRockettKKivinenKHubbartCJeffreysAERowlandsKMalariaGEN ConsortiumCharacterisation of the opposing effects of G6PD deficiency on cerebral malaria and severe malarial anaemiaeLife20176e1508528067620
- ButaliAMosseyPAAdeyemoWLEsheteMAGainesLAEvenDNovel IRF6 mutations in families with Van Der Woude syndrome and popliteal pterygium syndrome from sub-Saharan AfricaMol Genet Genomic Med20142325426024936515
- EsheteMALiuH LiAdeyemoWLGowansLJJMosseyPALoss-of-function GRHL3 variants detected in African patients with isolated cleft palateJ Dent Res2018971414828886269
- GowansLJJOseniGMosseyPAAdeyemoWLEsheteMABuschTDNovel GREM1 variations in sub-Saharan African patients with cleft lip and/or cleft palateCleft Palate-Cran J2018555736742
- BademciGLasisiAYarizKOMontenegroPMenendezIVinuezaRNovel domain-specific POU3F4 mutations are associated with X-linked deafness: Examples from different populationsBMC Med Genet201516910.1186/s12881-015-0149-225928534
- KoretzkyMBonhamVLBerkmanBEKruszkaPAdeyemoAMuenkeMHullSCTowards a more representative morphology: Clinical and ethical considerations for including diverse populations in diagnostic genetic atlasesGenet Med201618111069107426963283
- MuenkeMAdeyemoAKruszkaPAn electronic atlas of human malformation syndromes in diverse populationsGenet Med201618111085108726938780
- H3Africa ConsortiumResearch capacity. Enabling the genomic revolution in AfricaScience20143441346134824948725
- OsafoCRajiYRBurkeDTayoBOTiffinNMoxey-MimsMMHuman Heredity and Health (H3) in Africa kidney disease research network: a focus on methods in subsaharan AfricaClin J Am Soc Nephrol2015101222798726138261
- AjayiS ORajiYSalakoB LEthical and legal issues in renal transplantation in NigeriaSaudi J Kidney Dis Transpl201627125826787578
- Gbenga-MustaphaOWe can do kidney transplant2562013 Available from: http://thenationonlineng.net/we-can-do-kidney-transplantAccessed 15th January 2018
- The Nigerian Association of NephrologyProposed National Renal Care Policy Available from: http://nanephrology.org.ng/index.php/education/proposed-national-renal-care-policyAccessed 14th December 2017
- NigeriaAbout the country Available from: https://en.wikipedia.org/wiki/NigeriaAccessed 4th March 2018
- StringerCGalway-WithamJPalaeoanthropology: On the origin of our speciesNature2017546765721221428593955
- StringerCWhy we are not all multiregionalists nowTrends Ecol Evol201429524825124702983
- EzeonwuBOkikeCOguonuTNwankwoOPattern of renal diseases in children admitted into the paediatric ward of Federal Medical Centre AsabaAfr J Paed Nephrol20141811
- EtukISAnahMUOchigboSOEyongMPattern of pediatric renal disease in inpatients in Calabar, NigeriaTrop Doct20063625617034716
- OchekeIEOkoloSNBode- ThomasFAgabaEIPatterns of childhood renal disorders in Jos, Nigeria: A preliminary reportJ Med Trop201012525
- IkpemeEEDixon-UmoOTPaediatric renal diseases in Uyo, Nigeria: a 10-year reviewAfr J Paed Nephrol20141127
