Abstract
BK virus reactivation as a result of therapeutic immunosuppression following renal transplant can result in BK polyomavirus nephropathy and renal allograft loss. This is a complex and challenging clinical problem with a range of management options and practices reported in literature. The current standard for early diagnosis and treatment is surveillance by measuring viral DNA in blood using qPCR. Immunosuppression reduction is the cornerstone of effective management but is associated with a risk of acute rejection following treatment.
Prevalence
BK polyoma virus (BKV) is a non-enveloped DNA virus first discovered in the urine of a kidney transplant recipient in 1971.Citation1 Its genome has an early region which codes for the large and small T antigens, a late region which codes for the capsid proteins VP1-3, and agnoprotein, and a non-coding control region (NCCR). BKV strains have six genotypes based on polymorphisms in VP1 and NCCR.Citation2
BKV is widely prevalent in general population with over 80% individuals having antibodies against BK virus.Citation3,Citation4 The most common mode of transmission is through respiratory secretions, resulting in a mild self-limited respiratory infection.Citation5 Viral spread to other organs is believed to be via bloodstream and in immunocompetent individuals, it remains clinically silent in renal tubular epithelium.
“Presumptive” BK Polyoma virus nephropathy (PVN) is defined as persistently high BK viral load in plasma >10,000 copies/mL for four weeks. Renal allograft biopsy remains the gold standard for diagnosing “definite” PVN.Citation6–Citation12 Since the allograft involvement is focal, and the possibility of sampling error is high, two cores containing medulla are required for an adequate biopsy sample.Citation8,Citation9 Intragraft polyomavirus gene expression on renal biopsy has recently been reported as a useful adjunct to the diagnosis of PVN with the potential to differentiate from T-cell-mediated rejection.Citation13 Biopsy proven “definite” PVN has an incidence of 5–6%, with a higher incidence in ABO-incompatible donors and following desensitization in highly sensitized recipients.Citation14–Citation16
The Banff Working Group on Polyomavirus Nephropathy recently published a morphologic classification of definite PVN into three groups, Class I, II, and III, based on polyomavirus load and Banff ci score (interstitial fibrosis) for ease of diagnostic communication and comparative data analysis.Citation17 However, this was a retrospective observational analysis which has not been validated in a mixed population.
Impact
BK-virus-related disease is commonly seen in kidney transplant and hematopoietic stem cell transplant recipients. The cause for reactivation is therapeutic immunosuppression (IS) following transplant.Citation18 BK viruria can be seen in 60% of kidney transplant recipients, while BK viremia is seen in up to 13% kidney transplant recipients, and nephropathy in 10%.Citation19–Citation21 The actual reported incidence varies; however, with the choice of induction IS, maintenance IS, and screening modality used, hence the wide variations in literature. In US, 5.7%– 7.5% of renal allografts are lost to PVN.Citation22
PVN is therefore a serious clinical problem in kidney transplantation. PVN is difficult to treat since there is no BKV-specific anti-viral therapy. Any anti-virals currently in use work poorly and suffer from substantial host toxicity. PVN is treated by stimulating host immune response by IS reduction; however, there is a risk of acute rejection following virus clearance,Citation23 further complicating treatment options since rejection treatment requires escalation of IS which often results in BKV recurrence.
The current standard for management is monitoring for viral DNA using qPCR. Other investigational surveillance tools include monitoring BKV-specific CMIR,Citation24 and donor-derived cell-free DNA (dd-cfDNA). dd-cfDNA is a non-specific marker of injury. Since BKV causes interstitial inflammation and tubulitis, elevated levels of dd-cfDNA have been reported in a study of allograft rejection in kidney transplant in the setting of PVN.Citation25 Since BKV is also known to be associated with development of de novo donor-specific antibodies (DSA),Citation26 elevated dd-cfDNA levels in this infection could actually represent alloantibody-mediated microcirculation injury. Persistent viremia (lasting >140 days) was found to be strongly associated with development of Class II DSAs. The association of Class II DSA with antibody-mediated rejection (ABMR) and graft loss is well known.Citation27
Most studies have found that humoral immune response does not play a significant role in preventing development of PVN.Citation28 Despite the presence of a high level of antibodies, patients with PVN can have high levels of viral load and low CD8+ T cells.Citation29 BKV-specific cell-mediated immune response (CMIR) was demonstrated in normal individuals to be the mechanism responsible for prevention of BKV reactivation in immunocompetent individuals.Citation30 Low levels of BKV-specific interferon-gamma (IFNγ) producing T cells correlate with progression to PVN, while reconstitution of these cells correlates with resolution of nephropathy.Citation31–Citation34 Immune monitoring could help in identifying patients at risk of PVN;Citation34–Citation38 however, this knowledge is still evolving and has not been used in guiding treatment recommendations.
Management Strategies
Risk Factors
The most common factor associated with risk of developing PVN is the intensity of immunosuppression. Donor factors associated with a higher risk include transplanting kidney from BKV seropositive donor to seronegative donor,Citation39,Citation40 number of HLA mismatches, ABO-incompatibility, and ischemia reperfusion injury.Citation6,Citation14,Citation41,Citation42 Recipient factors include old age, male sex, desensitization, and prior kidney transplant with PVN.Citation16,Citation43
Surveillance
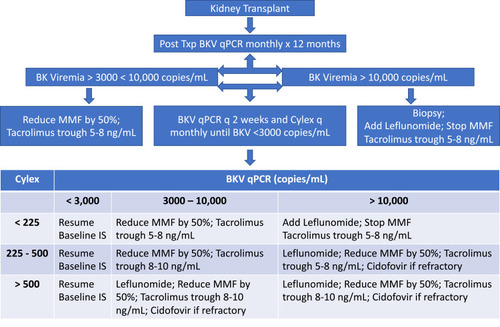
The mainstay of treatment of PVN is immunosuppression reduction. A wide variation in treatment practices is observed based on individual clinician experience. Most centers monitor BKV post-transplant at 3, 6, 9, and 12 months.Citation44 However, with more intense induction regimen or in those with risk factors, it is prudent to perform routine surveillance at monthly intervals in the first 12 months following transplant. This is standard in our center. In addition to following viral loads with qPCR, we also follow ImmuKnow Immune Cell Function Assay (Cylex Inc., Columbia, MD, USA) as an indirect measure of CMIR. Our approach is outlined in .
Immunosuppression Reduction and Antiviral Therapy
For BKV viral load <10,000 copies/mL, IS dose reduction should be considered. For viral loads >10,000 copies/mL, a common initial approach involves calcineurin inhibitor dose reduction by 25–50%. Switching to Cyclosporine A (CsA) has been shown to have some benefit as well.Citation45 Switching from Tacrolimus to CsA is a common approach used in our center in patients with persistent viremia; However, a higher incidence of biopsy-proven acute rejection is seen with this approach.Citation46 Failure of reduction in viral load should prompt reduction of mycophenolate mofetil (MMF) by 50%, or discontinuation of MMF or switching to an mTOR inhibitor.Citation47,Citation48 Switching from MMF to Leflunomide is another option associated with favorable outcomes.Citation49–Citation52 We routinely switch from MMF to Leflunomide in our center; however, the practices vary by center and physician experience. In refractory cases, most common therapeutic option is Cidofovir, use of which is limited by its nephrotoxicity.Citation53–Citation55 Brincidofovir is a prodrug of cidofovir and has also been used with limited success.Citation56,Citation57 IVIG preparations have high titers of neutralizing antibodies to BK virus and can help expedite virus clearance and have been used as a useful adjunctive therapy.Citation58–Citation61 Fluoroquinolones have been tried but failed to show therapeutic benefit.Citation62–Citation64 There is no strong evidence supporting antiviral treatment for PVN;Citation46 however, for patients with persistent BK viremia despite adequate immunosuppression reduction, therapeutic options are outlined in .
Table 1 Anti-Virals for PVN
Conclusion
Due to lack of strong evidence, no strong treatment recommendations can be made; however, it is prudent to start with immunosuppression reduction and add anti-virals for persistent viremia not responding to immunosuppression reduction based on physician experience. Regular monitoring of qPCR remains the cornerstone of early diagnosis and treatment. Novel monitoring strategies being investigated include immune monitoring and ddcf DNA.
Abbreviations
BKV, BK virus; NCCR, non-coding control region; PVN, BK polyoma virus nephropathy; qPCR, quantitative polymerase chain reaction; ddcfDNA, donor-derived cell-free DNA; DSA, donor-specific antibodies; ABMR, antibody-mediated rejection; CMIR, cell-mediated immune response; JCV, JC virus; IFNγ, interferon-gamma; MMF, mycophenolate mofetil; CsA, cyclosporine A; ATP, adenosine triphosphate; ELISPOT, enzyme-linked immunoSpot; IS, Immunosuppression; PML, progressive multifocal leukoencephalopathy.
Disclosure
The authors report no conflicts of interest in this work.
References
- Gardner SD, Field AM, Coleman DV, et al. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1(7712):1253–1257. doi:10.1016/S0140-6736(71)91776-44104714
- Chatterjee M, Weyandt TB, Frisque RJ. Identification of archetype and rearranged forms of BK virus in leukocytes from healthy individuals. J Med Virol. 2000;60(3):353–362. doi:10.1002/(SICI)1096-9071(200003)60:3<353::AID-JMV16>3.0.CO;2-R10630970
- Flaegstad T, Ronne K, Filipe AR, et al. Prevalence of anti BK virus antibody in Portugal and Norway. Scan J Infectious Dis. 1989;21(2):145–147. doi:10.3109/00365548909039961
- Stolt A, Sasnauskas K, Koskela P, et al. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84(Pt 6):1499–1504. doi:10.1099/vir.0.18842-012771419
- Goudsmit J, Wertheim-van Dillen P, van Strien A, et al. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–99. doi:10.1002/jmv.18901002036292361
- Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277–1286. doi:10.1097/01.TP.0000156165.83160.0915912088
- Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3(10):611–623. doi:10.1016/S1473-3099(03)00770-914522260
- Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004;4(12):2082–2092. doi:10.1046/j.1600-6143.2004.00603.x15575913
- Hirsch HH, Randhawa P. AST infectious diseases community of practice. BK virus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S136–S146.20070673
- Sar A, Worawichawong S, Benediktsson H, et al. Interobserver agreement for polyomavirus nephropathy grading in renal allografts using the working proposal from the 10th Banff Conference on Allograft Pathology. Hum Pathol. 2011;42(12):2018–2024. doi:10.1016/j.humpath.2011.03.00821733554
- Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–283. doi:10.1111/ajt.1259024472190
- Masutani K, Shapiro R, Basu A, et al. The Banff 2009 working proposal for polyomavirus nephropathy: a critical evaluation of its utility as a determinant of clinical outcome. Am J Transplant. 2012;12(4):907–918. doi:10.1111/j.1600-6143.2012.03993.x22390378
- Adam BA, Kikic Z, Wagner S, et al. Intragraft gene expression in native kidney BK virus nephropathy versus T cell-mediated rejection: prospects for molecular diagnosis and risk prediction [published online ahead of print, 2020 May 5]. Am J Transplant. 2020. doi:10.1111/ajt.15980
- Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:1320–1327. doi:10.2215/CJN.0077011222626962
- Boan P, Hewison C, Swaminathan R, et al. Optimal use of plasma and urine BK viral loads for screening and predicting BK nephropathy. BMC Infect Dis. 2016;16:342. doi:10.1186/s12879-016-1652-627448566
- Gabardi S, Townsend K, Martin ST, et al. Evaluating the impact of pre-transplant desensitization utilizing a plasmapheresis and low-dose intravenous immunoglobulin protocol on BK viremia in renal transplant recipients. Transpl Infect Dis. 2013;15:361–368. doi:10.1111/tid.1208723647907
- Nickeleit V, Singh HK, Randhawa P, et al. The Banff working group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29:680–693. doi:10.1681/ASN.201705047729279304
- Leung AY, Chan M, Tang SC, et al. Real-time quantitative analysis of polyoma BK viremia and viruria in renal allograft recipients. J Virol Meth. 2002;103(1):51–56. doi:10.1016/S0166-0934(01)00447-5
- Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347(7):488–496. doi:10.1056/NEJMoa02043912181403
- Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immuno-suppression reduction. Am J Transplant. 2005;5(3):582–594. doi:10.1111/j.1600-6143.2005.00742.x15707414
- Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis. 2005;41(3):354–360. doi:10.1086/43148816007533
- Kuypers DR. Management of polyomavirus-associated nephro-pathy in renal transplant recipients. Nat Rev Nephrol. 2012;8(7):390–402. doi:10.1038/nrneph.2012.6422508181
- Van Aalderen MC, Heutinck KM, Huisman C, et al. BK virus infection in transplant recipients: clinical manifestations, treatment options and the immune response. Neth J Med. 2012;70(4):172–183.22641625
- Sharma R, Tzetzo S, Patel S, et al. BK virus in kidney transplant: current concepts, recent advances, and future directions. Exp Clin Transplant. 2016;14(4):377–384. doi:10.6002/ect.2016.003027267780
- Bloom RD, Bromberg JS, Poggio ED, et al. Cell-free DNA and active rejection in kidney allografts. JASN Jul. 2017;28(7):2221–2232. doi:10.1681/ASN.2016091034
- Sawinski D, Forde KA, Trofe-Clark J, et al. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol. 2015;26:966–975. doi:10.1681/ASN.201401011925255921
- Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. doi:10.1111/ajt.1462529243394
- Hariharan S, Cohen EP, Vasudev B, et al. BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am J Transplant. 2005;5(11):2719–2724. doi:10.1111/j.1600-6143.2005.01080.x16212632
- Chen Y, Trofe J, Gordon J, et al. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80(7):3495–3505. doi:10.1128/JVI.80.7.3495-3505.200616537617
- Drummond JE, Shah KV, Donnenberg AD. Cell-mediated immune responses to BK virus in normal individuals. J Med Virol. 1985;17(3):237–247. doi:10.1002/jmv.18901703052999323
- Batal I, Zeevi A, Heider A, et al. Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol. 2008;129(4):587–591. doi:10.1309/23YGPB1E758ECCFP18343786
- Binggeli S, Egli A, Dickenmann M, et al. BKV replication and cellular immune responses in renal transplant recipients. Am J Transplant. 2006;6(9):2218–9;author reply 20. doi:10.1111/j.1600-6143.2006.01460.x
- Prosser SE, Orentas RJ, Jurgens L, et al. Recovery of BK virus large T-antigen-specific cellular immune response correlates with resolution of BK virus nephritis. Transplantation. 2008;85(2):185–192. doi:10.1097/TP.0b013e31815fef5618212622
- Comoli P, Azzi A, Maccario R, et al. Polyomavirus BK-specific immunity after kidney transplantation. Transplantation. 2004;78(8):1229–1232. doi:10.1097/01.TP.0000137932.44791.D315502726
- Comoli P, Binggeli S, Ginevri F, et al. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl Infect Dis. 2006;8(2):86–94. doi:10.1111/j.1399-3062.2006.00167.x16734631
- Comoli P, Cioni M, Basso S, et al. Immunity to polyomavirus BK infection: immune monitoring to regulate the balance between risk of BKV nephropathy and induction of alloimmunity. Clin Dev Immunol. 2013;2013:256923. doi:10.1155/2013/25692324000288
- Schachtner T, Muller K, Stein M, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant. 2011;11(11):2443–2452. doi:10.1111/j.1600-6143.2011.03693.x21831150
- Schachtner T, Stein M, Sefrin A, et al. Inflammatory activation and recovering BKV-specific immunity correlate with self-limited BKV replication after renal transplantation. Transplant Int. 2014;27(3):290–301. doi:10.1111/tri.12251
- Smith JM, McDonald RA, Finn LS, et al. Polyomavirus nephropathy in pediatric kidney transplant recipients. Am J Transplant. 2004;4(12):2109–2117. doi:10.1111/j.1600-6143.2004.00629.x15575916
- Sood P, Senanayake S, Sujeet K, et al. Donor and recipient BKV- specific IgG antibody and post transplantation BKV infection: a prospective single-center study. Transplantation. 2013;95(6):896–902. doi:10.1097/TP.0b013e318282ba8323511214
- Awadalla Y, Randhawa P, Ruppert K, et al. HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am J Transplant. 2004;4(10):1691–1696. doi:10.1111/j.1600-6143.2004.00563.x15367226
- Prince O, Savic S, Dickenmann M, et al. Risk factors for polyoma virus nephropathy. Nephrol Dial Transplant. 2009;24(3):1024–1033. doi:10.1093/ndt/gfn67119073658
- Ramos E, Drachenberg CB, Portocarrero M, et al. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin Transplant. 2002;143–153.
- Dalianis T, Eriksson B-M, Felldin M, et al. Management of BK-virus infection – Swedish recommendations. Infect Dis. 2019;51(7):479–484. doi:10.1080/23744235.2019.1595130
- Chen XT, Li J, Deng RH, et al. The therapeutic effect of switching from tacrolimus to low-dose cyclosporine A in renal transplant recipients with BK virus nephropathy. Biosci Rep. 2019;39(2):BSR20182058. doi:10.1042/BSR2018205830737303
- Gard L, van Doesum W, Niesters HGM, et al. A delicate balance between rejection and BK polyomavirus associated nephropathy; a retrospective cohort study in renal transplant recipients. PLoS One. 2017;12(6):e0178801. doi:10.1371/journal.pone.017880128609473
- Bowman LJ, Brueckner AJ, Doligalski CT. The role of mTOR inhibitors in the management of viral infections: a review of current literature. Transplantation. 2018;102:S50–S59. doi:10.1097/TP.000000000000177729369973
- Bussalino E, Marsano L, Parodi A, et al. Everolimus for BKV nephropathy in kidney transplant recipients: a prospective, controlled study. J Nephrol. 2020. doi:10.1007/s40620-020-00777-2
- Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81(5):704–710. doi:10.1097/01.tp.0000181149.76113.5016534472
- Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352(11):1157–1158. doi:10.1056/NEJM20050317352112515784677
- Williams JW, Mital D, Chong A, et al. Experiences with leflunomide in solid organ transplantation. Transplantation. 2002;73(3):358–366. doi:10.1097/00007890-200202150-0000811884931
- Hirsch HH, Randhawa P. AST infectious diseases community of practice. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):179–188.
- Kadambi PV, Josephson MA, Williams J, et al. Treatment of refractory BK virus-associated nephropathy with cidofovir. Am J Transplant. 2003;3(2):186–191. doi:10.1034/j.1600-6143.2003.30202.x12614296
- Keller LS, Peh CA, Nolan J, et al. BK transplant nephropathy successfully treated with cidofovir. Nephrol Dial Transplant. 2003;18(5):1013–1014. doi:10.1093/ndt/gfg06112686681
- Kuypers DR, Vandooren AK, Lerut E, et al. Adjuvant low-dose cidofovir therapy for BK polyomavirus interstitial nephritis in renal transplant recipients. Am J Transplant. 2005;5(8):1997–2004. doi:10.1111/j.1600-6143.2005.00980.x15996251
- Reisman L, Habib S, McClure GB, et al. Treatment of BK virus-associated nephropathy with CMX001 after kidney transplantation in a young child. Pediatr Transplant. 2014;18:E227–E231. doi:10.1111/petr.1234025174393
- Papanicolaou GA, Lee YJ, Young JW, et al. Brincidofovir for polyomavirus-associated nephropathy after allogeneic hema-topoietic stem cell transplantation. Am J Kidney Dis. 2015;65(5):780–784. doi:10.1053/j.ajkd.2014.11.02025600489
- Randhawa P, Pastrana DV, Zeng G, et al. Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK. Am J Transplant. 2015;15(4):1014‐1020. doi:10.1111/ajt.13083
- Sener A, House AA, Jevnikar AM, et al. Intravenous immu-noglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81(1):117–120. doi:10.1097/01.tp.0000181096.14257.c216421486
- Vu D, Shah T, Ansari J, et al. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Transplant Proc. 2015;47(2):394‐398. doi:10.1016/j.transproceed.2015.01.012
- Moon J, Chang Y, Shah T, et al. Effects of intravenous immunoglobulin therapy and Fc gamma receptor polymorphisms on BK virus nephropathy in kidney transplant recipients [published online ahead of print, 2020 Apr 23]. Transpl Infect Dis. 2020:e13300. doi:10.1111/tid.13300.32323406
- Gabardi S, Waikar SS, Martin S, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5(7):1298–1304. doi:10.2215/CJN.0826110920507960
- Sharma BN, Li R, Bernhoff E, et al. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res. 2011;92(1):115–123. doi:10.1016/j.antiviral.2011.07.01221798289
- Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA. 2014;312(20):2106–2114. doi:10.1001/jama.2014.1472125399012