Abstract
In Argentina, hemolytic uremic syndrome (HUS) constitutes the most frequent cause of acute renal failure in children. Approximately 2%–4% of patients die during the acute phase, and one-third of the 96% who survive are at risk of chronic renal sequelae. Little information is available about the direct effect of Shiga toxin type 2 (Stx2) on the onset of proteinuria and the evolution of toxin-mediated glomerular or tubular injury. In this work, rats were injected intraperitoneally with recombinant Escherichia coli culture supernatant containing Stx2 (sStx2; 20 μg/kg body weight) to induce HUS. Functional, immunoblotting, and immunohistochemistry studies were carried out to determine alterations in slit diaphragm proteins and the proximal tubule endocytic system at 48 hours post-inoculation. We detected a significant increase in microalbuminuria, without changes in the proteinuria values compared to the control rats. In immunoperoxidase studies, the renal tubules and glomerular mesangium showed an increased expression of transforming growth factor β1(TGF-β1). The expression of megalin was decreased by immunoperoxidase and the cytoplasm showed a granular pattern of megalin expression by immunofluorescence techniques. Western blot analysis performed in the renal cortex from sStx2-treated and control rats using anti-nephrin and anti-podocalyxin antibodies showed a decreased expression of these proteins. We suggest that the alterations in slit diaphragm proteins and megalin expression could be related to the development of microalbuminuria in response to lethal doses of Stx2.
Introduction
In Argentina, hemolytic uremic syndrome (HUS) constitutes the most frequent cause of acute renal failure in children. HUS is a disease associated with infection by Shiga toxin (Stx)-producing Escherichia coli (STEC), defined by thrombotic microangiopathy, hemolytic anemia, thrombocytopenia, and acute renal failure (ARF). Approximately 2%–4% of patients die during the acute phase, and one-third of the 96% who survive are at risk of chronic renal sequelae.Citation1
Although glomerular lesions observed in HUS involve the presence of thrombotic microangiopathy, little information is available about the direct effect of Shiga toxin type 2 (Stx2) on the onset of proteinuria and the evolution of toxin-mediated glomerular injury.
Visceral glomerular epithelial cells or podocytes are currently considered to play an important role in the physiology of the glomerular filtration barrier and consequently in the pathogenesis of glomerular diseases associated with proteinuria and nephrotic syndrome.Citation2
The foot processes of podocytes are connected by a continuous membrane-like structure called the slit diaphragm.Citation3
It has been reported that nephrin, a gene product of NPHS1, might be a core protein of the slit diaphragm.Citation4 Although the molecular function of nephrin is still poorly understood, recent studies have indicated that nephrin acts as a signaling moleculeCitation5 and has an intimate relationship with filamentous actin.Citation5,Citation6
The external surface of podocytes is covered with a sialic acid-rich glycocalyx known as podocalyxin (PC).Citation7 PC is the target of injury in many glomerular diseases that affects the shape of foot processes and reduces the expression of components of the slit diaphragm. One of the consequences of the dysfunction of podocytes may be the development of albuminuria.
Although albuminuria is an important marker for the onset and progression of renal diseases,Citation10 the mechanism by which albuminuria is caused still remains a topic of debate. Recent studies have investigated the tubular role in the post-glomerular processing of albumin on the onset of albuminuria showing a possible role of TGF-β.Citation11 Recently, evidence has suggested that the renal tubular injury observed in HUS is also induced by the direct action of Stx2 on tubular epithelial cells.Citation12 We have previously reported a model of HUS in rats inoculated with lethal doses of Stx2 with alterations similar to those described in humans with HUS.Citation13 Recently, we have characterized in the same model the early tubular response to the effect of Stx2 and detected that tubular cells develop an immunophenotype change induced by TGF-β, the first step in the evolution of epithelial-to-mesenchymal transition and tubule-interstitial fibrosis. Taking into account these results, it is feasible that tubular functions are altered, such as protein reabsorption. Functional studies in rat kidney suggest that megalinCitation15 is involved in albumin endocytosisCitation16 in proximal tubules regulated by TGF-β, affecting the level of urinary albumin excretion.
Taking all the above into account, the aim of our study was to determine the glomerular and tubular response to lethal doses of Stx2 in rats. We focused our study on modifications of the slit diaphragm and protein tubular endocytosis.
Materials and methods
Adult male Sprague Dawley rats (150 ± 3 g body weight) were obtained from the animal facility of the School of Pharmacy and Biochemistry, University of Buenos Aires, Argentina. The rats were housed individually under controlled conditions of light/dark, humidity, and temperature, with food and water available ad libitum.
Experimental protocols
Rats were randomly divided into two groups of six rats each. Stx2 injury was induced as previously described.Citation13 Briefly, rats from the experimental group (Stx2-treated rats) were injected intraperitoneally with recombinant Escherichia coli culture supernatant (sStx2) containing Stx2 (LD50: 20 μg/kg body weight. The animals died between 48 and 72 hours after administration). Control rats were inoculated with the same volume of culture supernatant that did not contain Stx2. sStx2 having a cytotoxicity of 2 × 104 CD50/mL (1 CD50 = 40 pg/mL) was obtained as previously described.Citation17 The LPS content in the control and experimental supernatant, determined using the HEK-Blue LPS Detection Kit (Invitrogen, San Diego, CA), was 70 ng LPS/μg Stx2 protein.
Renal functions
Rats were kept in metabolic cages for 48 hours with free access to water and food and under a controlled light/dark cycle and temperature to collect the urine volume. Blood samples were obtained from rats by cardiac puncture prior to sacrifice. Creatinine and urea plasma concentrations were assessed using a commercial kit (Wiener Lab, Rosario, Argentina). Proteinuria (pyrogallol red-molybdate [colorimetric], Alcyon 300I analyzer, Abbott Laboratories, Chicago, IL) and microalbuminuria (nephelometry IMMAGE, Beckman Coulter, Brea, CA) were also measured.
Light microscopy
Rats were anesthetized (100 μg ketamine and 10 μg diazepam/g body weight, intraperitoneally) and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). Kidneys were removed and tissues were fixed in formol buffer 10% in PBS 0.1 M (pH 7.4). The tissue sections were dehydrated and included in paraffin. Sagittal cuts (5 μm) were made with a microtome (Leica RM 2125; Leica Gmbh, Wetzlar, Germany) and mounted on 2% silane-coated slides. The slides were stained with hematoxylin-eosin and observed by light microscopy (Nikon Eclipse 200; Nikon, Tokyo, Japan).
Immunofluorescence
The slides were preincubated with no immune rabbit serum in PBS (1:50) at room temperature for 30 minutes, followed by incubation with a polyclonal anti-nephrin or polyclonal anti-PC (1:50, Alpha Diagnostic, San Antonio, TX) and polyclonal anti-megalin (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) antibodies overnight in a wet chamber at 4°C. After several rinses in PBS, the slides were incubated with an anti-rabbit IgG antibody conjugated with fluorescein (1:200, Santa Cruz Biotechnology) for 1 hour at room temperature in a wet chamber. Finally, all the slides were mounted on a mixture containing PBS:glycerol (1:3), and observed in an epifluorescent microscope (Nikon Eclipse E200, Nikon, Tokyo, Japan). Negative controls were performed without primary antibodies.
Immunoperoxidase
Samples were blocked with endogenous peroxidase with H2O2 0.3% for 10 minutes. Later, the slides were preincubated with no immune rabbit serum, diluted in PBS (1:50) at room temperature for 30 minutes and incubated with the primary antibodies: anti-megalin (1:50, Santa Cruz Biotechnology) or anti-TGF-β1 (1:100, Santa Cruz Biotechnology) in a humidity chamber at 4°C overnight. The immunoperoxidase technique was then performed following the protocol from the RTU Vectastain Kit (Vector, Peterborough, UK). The antigen was revealed by diaminobenzidine (DAB, Vector). Finally, the sections were counterstained and mounted for observation. Negative controls were performed without primary antibodies.
Measurement of nephrin, PC, and megalin expressions
Ten separate fields of each section stained by immunofluorescence using nephrin and PC antibodies and immunohistochemistry using anti-megalin antibody were scanned at 200× magnification, put into digital form and analyzed using imaging software (Image Pro Plus 5.1 Media Cybernetics, Silver Spring, MD).
Western Blot
The renal cortex was micro-dissected and homogenized in buffer (10 mM triethanolamine, 250 mM sucrose, pH 7.6) with proteinase inhibitors. The protein concentration was determined with the BCA Protein Assay Kit (Pierce Biotechnology Inc, Rockford, IL). Samples of 100 μg were electrophoresed using 12.5% gels and electroblotted. Blots were incubated with rabbit polyclonal anti-nephrin or anti-PC antibodies (1:1000) and horseradish peroxidase-conjugated goat antirabbit IgG antibody (1:3000, Bio-Rad, Hercules, CA).
To determine the uniformity of loading, protein blots were probed with monoclonal anti-β-actin antibody (1:1000, Sigma-Aldrich, St Louis, MO). Band intensities were measured using Quantity One densitometry software package (Bio-Rad). Protein bands were normalized to their respective β-actin bands. The immunoblot analysis was carried out on three different tissue preparations from three independent experiments.
Statistical analysis
Data shown are mean ± SEM. The statistical significance between two mean values obtained for two different experimental conditions was calculated using Student’s t-test. A value of P < 0.05 was considered significant. Statistical analysis was performed using GraphPad InStat (v 3.6; GraphPad Software, Inc, La Jolla, CA).
Results
A significant increase in creatinine (1.8 ± 0.3 vs 0.35 ± 0.2 mg/dL, P < 0. 01, n = 12) and urea (263 ± 8.1 vs 7.9 ± 6.8 mg/dL, P < 0.01, n = 12) plasma concentrations was observed in experimental rats at 48 hours post-sStx2 treatment as compared with control rats. No changes were detected in the urine volume of the two groups of rats (16.7 ± 3 vs 21.3 ± 2 mL/d, n = 12). Detection of proteinuria and microalbuminuria 48 hours after Stx2 treatment in the urine collected for 24 hours in metabolic cages showed a significant increase in microalbuminuria (1.40 ± 0.32 vs 0.60 ± 0.10, P < 0.01, n = 12) without changes in the proteinuria values with respect to the control rats ().
Table 1 Renal functions at 48 hours post-treatment
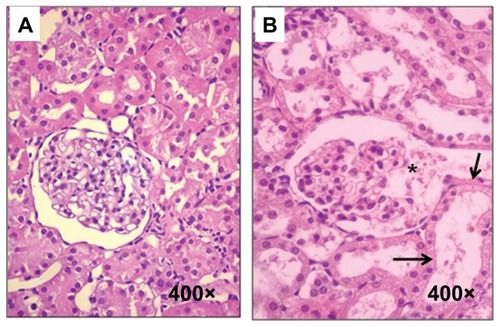
The hematoxylin-eosin stain showed tubular necrosis and mesangiolysis ().
Figure 1 Histological sections from control and experimental rats. (A) Control rats, (B) sStx2-treated rats showing mesangiolysis (black asterisk) and tubular necrosis (black arrow).
Abbreviation: sStx2, supernatant Shiga toxin type 2.
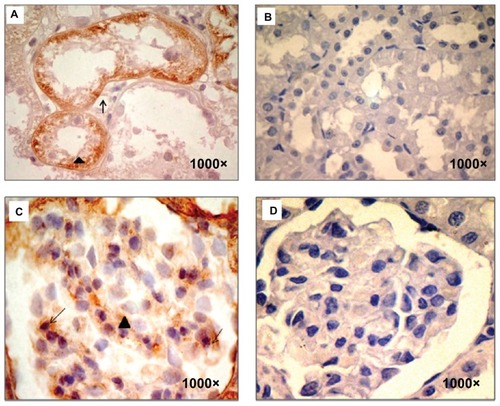
Using immunoperoxidase studies, renal tubules showed increased expression of TGF-β1 in the basolateral membrane and cytoplasm as well as in the podocytes and glomerular mesangium from sStx2-treated rats at 48 hours ().
Figure 2 TGF- β1 immunodetection. (A) sStx2-treated rats showing TGF-β1 expression in the basolateral membrane (black arrow) and the cytoplasm (black arrowhead) of renal tubules, (B and D) control rats showed no stain for TGF-β1, (C) sStx2-treated rats showing expression in the mesangium (black arrowhead) and podocytes (black arrow).
Abbreviation: sStx2, supernatant Shiga toxin type 2.
The expression of megalin in sStx2-treated rats was decreased by immunoperoxidase (). The cytoplasm showed a granular pattern of megalin expression () by immunofluorescence techniques.
Figure 3 Megalin immunodetection. sStx2-treated rats showing decreased expression of megalin in proximal tubules (A and B) (black arrows), (C) (white arrow); (D–F), control rats; (G–I), negative controls without primary antibody; (C, F and H), immunofluorescence; (A, B, D, E, and G), immunoperoxidase. (I) the number of tubules positive for megalin by immunoperoxidase were counted and the percentage measured using a grid superimposed on the image. Megalin was significantly decreased with respect to the controls (21.8% ± 4.7% vs 94.2% ± 2.9%; P < 0.01, n = 4).
Abbreviation: sStx2, supernatant Shiga toxin type 2.
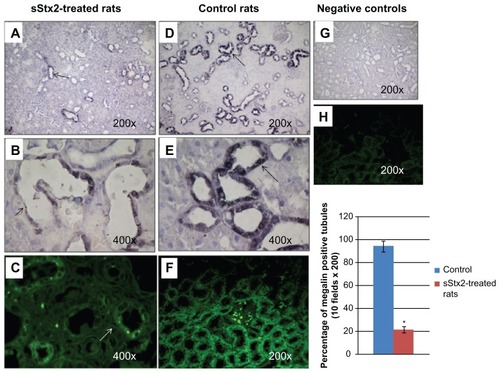
The number of tubules positive for megalin was counted and the percentage measured using a grid superimposed on the image. Expression of megalin (21.8% ± 4.7% vs 94.2% ± 2.9%; P < 0.01, n = 4) was significantly decreased with respect to the controls ().
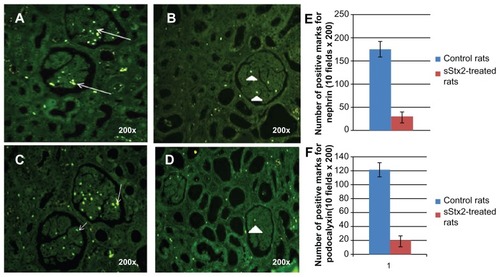
The immunofluorescence studies of glomeruli from sStx2-treated rats at 48 hours showed a decreased expression of nephrin () and PC in podocytes (). Morphometric quantification detected a significant decrease in the expression of nephrin (32.16 ± 10.6 vs 176.33 ± 16.6; P < 0.01, n = 6) and PC (19.16 ± 7.9 vs 122.16 ± 10.6; P < 0.01, n = 6) with respect to the controls ().
Figure 4 Nephrin and PC immunodetection. (A) nephrin expression (white arrows) in control rats; (B) sStx2-treated rats showing a decreased expression of nephrin (white arrowhead); (C) PC expression in control rats (white arrowhead); (D) a marked decreased expression of PC in sStx2-treated rats. The number of positive marks of nephrin and PC was counted in ten fields of 200× using a grid superimposed on the image. (E) Expressions of nephrin (176.33 ± 16.6 vs 32.16 ± 10.6; P < 0.01, n = 6) and (F) PC (122.16 ± 10.6 vs 19.16 ± 7.9; P < 0.01, n = 6) were significantly decreased with respect to the controls.
Abbreviations: sStx2, supernatant Shiga toxin type 2; PC, podocalyxin.
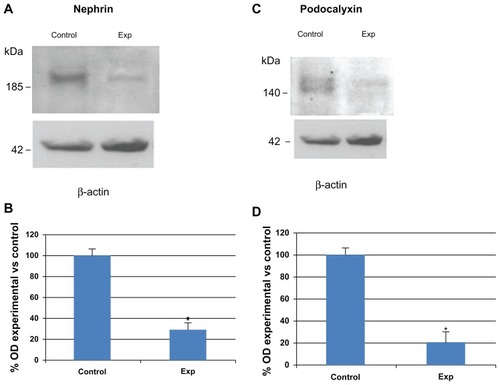
Western blot analysis performed on the renal cortex from sStx2-treated and control rats using anti-nephrin and anti-PC antibodies confirmed the results observed by immunofluorescence showing decreased expression in the 185 kDa band corresponding to nephrin () and 140 kDa band corresponding to PC ().
Figure 5 Nephrin and PC immunodetection by Western blot in renal cortex. (A) representative blots of nephrin; (C) representative blots of PC; (B and D) bar graphs representing average protein levels normalized to the internal control β-actin. Note that the 185 kDa band corresponding to nephrin and the 140 kDa band corresponding to PC were decreased in sStx2-treated rats as compared to controls.
Discussion
The current study demonstrates for the first time that the early response of podocytes to the effect of Stx2 is related to the decreased expression of nephrin and PC proteins as observed by Western blot and immunohistochemistry studies. These results are associated with the decrease and alteration observed in the pattern of megalin expression in the proximal tubule. The decrease in megalin expression could be involved in a development of microalbuminuria.
Functional studies carried out in the present work are in agreement with our previous findings.Citation13 We showed that Stx2-treated rats developed increases in creatinine and urea plasma concentrations, which are markers of renal dysfunction. Urine volume showed similar results.
The present study demonstrates that Stx2-treated rats develop microalbuminuria at 48 hours post-treatment, without changes in the proteinuria values. It is known that approximately 2%–4% of patients with HUS die during the acute phase, and that one-third of the 96% who survive are at risk of chronic sequelae such as proteinuria.Citation1,Citation18 Microalbuminuria has been identified as a predictor of progressive renal disease in various clinical settings.Citation19–Citation21
It has been described that, during HUS, renal endothelial and podocyte cells are very sensitive to the action of Stx2Citation22 and that lipopolysaccharide from Escherichia coli often triggers albuminuria and glomerular nephritis. It has been found that in inflammatory diseases there is an increase in plasma interleukin-1β (IL-1β) and TNF-α concentrations related to a transient downregulation of nephrin.Citation23
We thus suggest that this mechanism could be involved in the development of microalbuminuria since we have previously observed that the cytotoxic effect of Stx2 holotoxin is enhanced by IL-1β, TNF-α, and LPS.Citation24
It is known that under physiological conditions the slit diaphragm may maintain this stability in response to changes in filtration pressure. This would require constant reorganization of the podocyte foot process and the slit diaphragm components, but the mechanisms underlying the turnover of slit diaphragm proteins are largely unknown. Recent studies have provided evidence that podocytes have a key function in the development of albuminuria.Citation25,Citation26
Abnormal uptake of plasma proteins by podocytes has been described as a mechanism developed after TGF-β1 upregulation.Citation27 In agreement with this we detected TGF-β1 expression in podocytes and mesangium.
sStx2-treated rats showed a significantly decreased expression of nephrin and PC slit diaphragm proteins at 48 hours post-sStx2 inoculation. In agreement with our results, Wiggins et al demonstrated that reduced expression of components of the slit diaphragm, such as nephrin and PC, and podocyte loss are associated with proteinuria and glomerulosclerosis.Citation28 Moreover, in experimental models of nephrosis with proteinuria, it has been shown that the connection of PC to the actin cytoskeleton is disrupted.Citation29,Citation30
In studying the transport mechanism involved in protein reabsorption in proximal tubules, we detected a modified pattern expression of megalin and TGF-β1. Megalin expression was decreased whereas TGF-β1 was detected in the basolateral membranes and cytoplasm of proximal tubules from sStx2-treated rats by immunohistochemistry. Albumin endocytosis in a cell culture model (proximal-tubule-derived opossum kidney [OK] cells) is mediated by megalin/cubulin and regulated by TGF-β1 by a downregulation of the system affecting the binding, internalization, and intracellular trafficking of the ligand albumin. Citation31 Previously, we reported the immunophenotype changes in tubular cells damaged by Stx2 by TGF-β1 in rats.Citation14
We suggest that megalin deficiency is associated with the increased expression of TGF-β1 that produces the immunophenotype change of tubular cells, and the presence of microalbuminuria at 48 hours post-sStx2 inoculation.
We thus suggest that the inflammatory state developed during response to Stx2 could be related to the modifications in the slit diaphragm structure and in the albumin endocytosis system associated with an increase in the active form of TGF-β1.
Early identification of patients at risk of progressive nephropathy would enable health providers to intervene and thereby slow or halt the development of progressive renal insufficiency.Citation32
Further studies are necessary to detect the intrinsic mechanism involved in the molecular regulation of renal protein management during the evolution of HUS.
Acknowledgments
The University of Buenos Aires (ME95, M827, M470), the National Council of Research (CONICET, PIP 344), and the ANPCYT (PICT-642, 118) supported this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- RepettoHAEpidemic hemolytic-uremic syndrome in childrenKidney Int1997526170817199407523
- BarisoniLSchnaperHWKoppJBAdvances in the biology and genetics of the podocytopathies. Implications for diagnosis and therapyArch Pathol Lab Med2009133220121619195964
- RodewaldRKarnovskyMJPorous substructure of the glomerular slit diaphragm in the rat and mouseJ Cell Biol19746024234334204974
- KestilaMLenkkeriUMannikkoMPositionally cloned gene for a novel glomerular protein – nephrin – is mutated in congenital nephrotic syndromeMol Cell1998145755829660941
- BenzingTSignaling at the slit diaphragmJ Am Soc Nephrol20041561382139115153549
- YuanHTakeuchiESalantDJPodocyte slit-diaphragm protein nephrin is linked to the actin cytoskeletonAm J Physiol Renal Physiol20022824F585F59111880318
- MichaelAFBlauEVernierRLGlomerular polyanion alteration in aminonucleoside nephrosisLab Invest19702366496574098608
- KershawDBThomasPEWharramBLMolecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endotheliumJ Biol Chem19952704929439294467493982
- KerjaschkiDDharkeyDJFarquharMGIdentification and characterization of podocalyxin-the major sialoprotein of the renal glomerular epithelial cellJ Cell Biol1984984159115966371025
- JerumsGPanagiotopoulosSTsalamandrisCAllenTJGilbertREComperWDWhy is proteinuria such an important risk factor for progression in clinical trialsKidney Int Suppl199763S87S929407431
- RussoLMOsickaTMBrammarGCCandidoRJerumsGComperWDRenal processing of albumin in diabetes and hypertension in rats: possible role of TGF-beta1Am J Nephrol2003232617012481143
- Pistone CreydtVSilbersteinCZottaEIbarraCCytotoxic effect of Shiga toxin-2 holotoxin and its B subunit on human renal tubular epithelial cellsMicrobes Infect20068241041916242986
- ZottaELagoNOchoaFRepettoHAIbarraCDevelopment of an experimental hemolytic uremic syndrome in ratsPediatr Nephrol200823455956718253762
- OchoaFLagoNRGerhardtEIbarraCZottaECharacterization of Stx2 tubular response in a rat experimental model of hemolytic uremic syndromeAm J Nephrol201032434034620733289
- ChristensenEINielsenSMoestrupSKSegmental distribution of the endocytosis receptor gp330 in renal proximal tubulesEur J Cell Biol19956643493647656901
- CuiSVerroustPJMoestrupSKChristensenEIMegalin/gp330 mediates uptake of albumin in renal proximal tubuleAm J Physiol19962714 Pt 2F900F9078898021
- Pistone CreydtVFernandez MiyakawaMMartínFZottaESilbersteinCIbarraCShiga toxin 2B subunit inhibits net fluid absorption in human colon and elicits fluid accumulation in rat colon loopsBraz J Med Biol Res200437679980815264022
- TarrPIEscherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infectionClin Infect Dis1995201187727633
- LewisJBMicroalbuminuria: Accuracy of economicsAm J Kidney Dis19983235245289740175
- PanayiotouBNMicroalbuminuria: pathogenesis, prognosis and managementJ Int Med Res19942241812017958379
- BakrisGLMicroalbuminuria: prognostic implicationsCurr Opin Nephrol Hypertens1996532192238737856
- PsotkaMAObataFKollingGLShiga toxin 2 targets the murine renal collecting duct epitheliumInfect Immun20097795996919124603
- TakanoYYamauchiKHayakawaKTranscriptional suppression of nephrin in podocytes by macrophages: role of inflammatory cytokines and involvement of the PI3K/Akt pathwayFEBS Lett2007581342142617239861
- CreydtVPSilbersteinCZottaEIbarraCCytotoxic effect of Shiga toxin-2 holotoxin and its B subunit on human renal tubular epithelial cellsMicrobes Infect20068241041916242986
- AsanumaKYanagida-AsanumaETakagiMKodamaFTominoYThe role of podocytes in proteinuriaNephrology (Carlton)200712Suppl 3S15S2017995522
- RinaldiSAdembriCGrechiSGaudioARLow-dose hydrocortisone during severe sepsis: effects on microalbuminuriaCrit Care Med20063492334233916850006
- AbbateMZojaCMorigiMTransforming growth factor-b1 is up-regulated by podocytes in response to excess intraglomerular passage of proteinsAm J Pathol200216162179219312466133
- WigginsRCThe spectrum of podocytopathies: a unifying view of glomerular diseasesKidney Int2007711205121417410103
- PavenstadtHKrizWKretzlerMCell biology of the glomerular podocytePhysiol Rev200383125330712506131
- SchmiederSNagaiMOrlandoRATakedaTFarquharMGPodocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cellsJ Am Soc Nephrol20041592289229815339978
- GekleMKnausPNielsenRTransforming growth factor-β1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cellsJ Physiol2003552Pt 247148114561830
- HilgersKFDotschJRascherWMannJFTreatment strategies in patients with chronic renal disease: ACE inhibitors, angiotensin receptor antagonists, or both?Pediatr Nephrol200419995696115278690