Abstract
Balkan endemic nephropathy (BEN) is a chronic kidney disease that affects persons living in the Balkans. Despite the unique geographical specificity of this disease, its etiology has remained unclear. Even if a positive family history of BEN has been identified, it is still uncertain how the disease develops in offspring. In this paper, we examine clinical mechanisms related to the onset of BEN in individuals who have a parental history of BEN to identify early detection of the disease and formulate interventions. We conducted a 5-year prospective study, using markers in years one and three to predict new cases of BEN in year five. New cases of BEN were defined based on three criteria: parental history of BEN, reduced kidney size, and reduced kidney function. Incident cases were divided into (1) probable, (2) definite, and (3) combined labeled total incidence. We evaluated parental history in relation to BEN and tested the potentially intervening effects of kidney length, kidney cortex width, β2-microglobulin, C-reactive protein, and creatinine clearance, using path analyses. The findings of the path analyses suggested that parental history of BEN had both direct and indirect effects. The direct effect was significant for all three modes of parental history (biparental, maternal, and paternal; odds ratios 71.5, 52.3, and 50.1, respectively). The indirect effects of maternal BEN acted via kidney length and creatinine clearance. Biparental BEN was mediated by (1) kidney length and creatinine clearance, and (2) creatinine clearance alone. Paternal BEN had three indirect effects: (1) through kidney length and creatinine clearance, (2) via kidney cortex width and creatinine clearance, and (3) via kidney cortex width only. In conclusion, a family history of BEN led to reduced kidney length and cortex width, and a decline in creatinine clearance, which in turn predicted the onset of BEN.
Introduction
Balkan endemic nephropathy (BEN) is a renal disease with an unknown etiology.Citation1,Citation2 It solely targets the Balkan population.Citation3–Citation5 BEN is not diagnosed in children but is discovered in victims when they reach their 50s, suggesting a long latency period. A family history of BEN has been demonstrated to increase the risk of this disease.Citation6,Citation7 Additionally, decreased kidney sizes, reduced creatinine clearance rate (CCr), and increased urine excretion, especially of β2-microglobulin, have been illustrated to be related to BEN.Citation2,Citation8
Existing literature and prior studies have demonstrated that increased β2-microglobulin and reduced kidney length were associated with both BEN patients and their healthy offspring.Citation9–Citation15 Moreover, increased C-reactive protein (CRP) and narrow kidney cortex width were related to a parental history of BEN (PHB) before the disease manifestation. A reduced CCr was connected to both BEN patients and offspring, and it was inversely correlated with kidney sizes in BEN patients.Citation9,Citation13,Citation15 Even when an affected family has been verified, it is still uncertain how the disease develops in offspring. We hypothesized that kidney length and cortex width, CCr, CRP, or β2-microglobulin may be intervening factors between PHB and BEN development. If we can identify morphological and functional changes that lead to BEN in individuals with higher risk due to PHB, we may be able to target specific interventions.
To respond to this challenge, it is necessary to evaluate the effect and direction of biological factors on developmental BEN. One option pursued in this study was firstly to track clinical changes over time in adult offspring of BEN patients, and secondly to identify links in the relationship between PHB and onset of BEN. This would allow us to distinguish whether such changes were in the pathway of the developmental BEN or were only bystanders. We used path analysis to evaluate the various links. This method enabled us to study direct and indirect association among PHB, clinical markers, and incidence of BEN simultaneously as a web of associations.Citation16
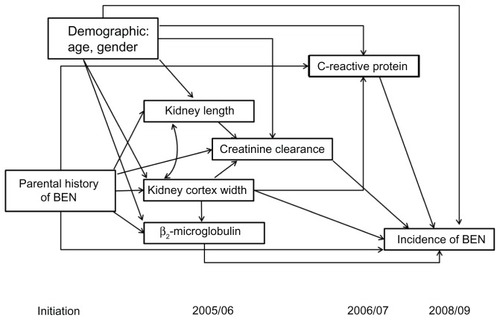
To our knowledge, no study has investigated the pathways between PHB and the incidence of BEN. Moreover, out of multiple possible markers, we have not identified those that link PHB and onset of BEN. Therefore, a hypothetical path model () was constructed, bridging the links between PHB and new cases of BEN, based upon the literature and prior studies. We tested whether or not PHB, which leads to new onset of BEN in offspring, is mediated by kidney size, function, or serum levels of CRP. If effects of PHB were mediated, then intervening variables would be identified. If none of the tested morphological and functional changes statistically significantly link PHB with the onset of BEN, we would conclude that no potential mediators could be detected.
Materials and methods
Study design and population
A follow-up study in healthy adult offspring from families with and without BEN was conducted in Bulgaria and is described in detail elsewhere.Citation10,Citation17 Briefly, 104 adult offspring with a PHB were recruited from a hospital database in Vratza, where BEN is still endemic. Ninety-seven adult offspring of non-BEN parents were frequency-matched with regard to sex and 10-year age groups and served as the control group. Both study groups were enrolled and examined for the first time in 2003–2004 and reexamined four times between 2004 and 2008. In 2005–2006, 18 participants were newly recruited with offspring of BEN patients. The institutional review boards of the National Center of Public Health Protection, Sofia, Bulgaria, and of the University of South Carolina approved the study. Participants provided written consent.
Data collection
Face-to-face interviews were conducted asking participants about their birth date and family history of BEN. Both kidneys’ sizes were measured using ultrasound. The longest dimension and the smallest thickness of kidney parenchyma were determined. Average length and cortex width of both kidneys were calculated. β2-microglobulin was measured in an aliquot of the hour-one urine sample, and details have been described elsewhere.Citation10 CCr was estimated from serum creatinine values by using the Cockcroft–Gault formulaCitation18 and then normalized to body surface area. Serum CRP was measured with Immulite (Siemens, Tarrytown, NY) chemiluminescent immunometric assay.Citation17
Definition of incidence of BEN
Definite cases of BEN required (1) PHB, (2) reduced kidney size (kidney length < 100 mm, kidney cortex width < 11 mm), and (3) reduced kidney functions (serum creatinine > 124 μmol/L for males and > 106 μmol/L for females; creatinine clearance using the Cockcroft–Gault formula < 60 mL/min; and β2-microglobulin > 0.2 mg/g creatinine). Probable BEN cases were defined as those with PHB, reduction in either kidney length or cortex width, and reduction in at least one kidney function marker with repeated measurements. Both groups were combined as the incidence of BEN. The criteria we applied were central criteria used in previous studies.Citation2,Citation6,Citation8
Statistical analysis
Descriptive statistics were applied to characterize the study population. To test the prediction of BEN in 2008–2009, age and clinical markers, comprising kidney length and cortex width, CCr, β2-microglobulin in 2003–2004 and 2005–2006, and CRP in 2006–2007, were used as continuous variables. Due to a skewed distribution, β2-microglobulin and CRP were log-transformed. In the path analysis, the independent variable ‘PHB’ was analyzed in two ways: (1) as the specific status of PHB (biparental, maternal, parental, and non-BEN); and (2) as the dichotomous outcome (yes/no). An ordinal BEN classification (definite, probable, and non-BEN) was used as outcome variable. To determine the agreement between repeated measurements, we estimated the intraclass correlation coefficient (ICC).Citation19
To test whether kidney sizes, functions, or CRP mediate the path from PHB while controlling for age and sex, path analyses were applied using Mplus 6 (Muthén and Muthén, Los Angeles, CA).Citation20 This method distinguishes three types of effects: direct, indirect, and total. Direct effects indicate the impact of a risk factor on an outcome not mediated by other variables. In Mplus 6, the indirect effect is calculated by the product of the coefficient method.Citation21 The total effect of a risk factor is the sum of direct and indirect statistical links.Citation16
Our results showed that PHB perfectly predicted the occurrence of BEN and caused the maximum likelihood estimate for the effect of PHB infinitely. Penalized maximum likelihood estimation could be used to resolve this problem.Citation22 However, since it is not provided in Mplus 6, to ease the computing burden, one pseudo-BEN case was generated by a random selection among the non-BEN subjects with no parental history.
Estimates in Mplus 6 for incidence of BEN are shown as logit coefficients in the path analyses and are transformed to odds ratios (ORs = exp [logit coefficient]) to explain the strength of the association. Regarding kidney length and cortex width, CCr, CRP, and β2-microglobulin, the estimation of coefficients are linear regression coefficients.Citation20 Per unit increase in the independent variable, the effect is estimated by multiplying the estimated path coefficient with the unit increase in the dependent variable.
Mplus 6 provides the Akaike information criterion and Bayesian information criterion as indicators of the model fit; the smaller the model indicators, the better the fit.Citation20 Selecting a model involves simultaneously fitting a series of linear regressions and logistic regressions and then choosing the model for the best fit.
Results
A total of 219 participants were enrolled in this study, and 177 (80.8%) remained in the last year of follow-up. Over a 5-year period, 38 (17.4%) offspring with a parental history of BEN developed the disease, of which 6.8% and 10.5% developed definite and probable BEN, respectively, whereas offspring without a parental history of BEN did not develop BEN.
Participants with definite BEN were older, had the smallest kidney length and cortex width, and presented high β2-microglobulin levels (). Moreover, about 50% of participants with definite BEN had a maternal history of BEN. Definite BEN was equally frequent in women and men. In the paternal BEN group, more probable BEN cases were found. Women were slightly more likely to develop probable BEN ().
Table 1 Characteristics of participants stratified by incidence of BEN in year 2005–2006
Regarding rank correlations of age and clinical markers, most were moderately correlated with age and each other (P < 0.05, ). Maternal BEN was correlated with kidney length (P = 0.009). A high agreement between measurements of kidney length and cortex width in left and right kidneys (ICC = 0.99) over the course of two repeated assessments was detected (ICC = 0.98, data not shown). Regarding kidney function (CCr and β2-microglobulin), the ICCs over the course of two repeated examinations were 0.99 for both markers.
Table 2 Rank correlation coefficients (Spearman) of parental history, age, and clinical markers
Model with PHB (yes/no) as predictor
Statistically significant path coefficients for this model are presented in . Single-headed arrows in the path diagrams demonstrate the effect of a PHB on outcome variables (kidney length, kidney cortex width, CCr, or incidence of BEN). Single-headed arrows with a solid line represent a direct effect while those with a dotted line demonstrate an indirect effect. The curved line with arrows at each end indicates a correlation between variables. The value on each line represents the path coefficient.
Figure 2 Direct and indirect path linking parental Balkan endemic nephropathy (BEN) and the incidence of BEN.
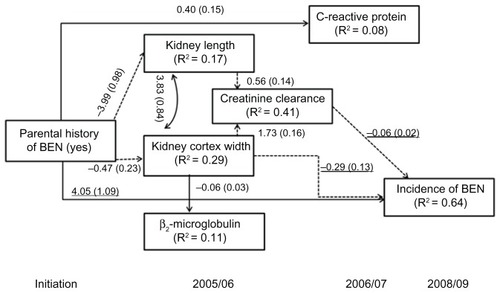
Participants with a PHB were associated with small kidney length and cortex width and increased CRP level. A reduction of the kidney cortex width of 10 mm increased β2-microglobulin by about 6 μg/g creatinine. There were positive associations between CCr and both kidney length and cortex width in all participants. No association was identified between PHB and β2-microglobulin or between CRP and either kidney size or function. Kidney cortex width, CCr, and PHB were directly related to the occurrence of BEN. Offspring with a PHB were 57 times more likely to develop BEN than persons without a PHB (exponential of 4.05, ). A reduction of kidney cortex width of 1 mm increased the risk of having BEN (OR = 0.74; exponential of −0.29). Furthermore, a decline in CCr was associated with the occurrence of BEN (OR = 0.94; exponential of −0.06). No direct associations between CRP, kidney length, or β2-microglobulin and new cases of BEN were detected. We also found that PHB was indirectly associated with the occurrence of BEN through decreased kidney length or kidney cortex width and reduced CCr. Another pathway was mediated by kidney cortex width alone. When all variables were included, 64.3% of the incidence of BEN was explained.
Model with specific status of PHB as predictor
When examining the model using the specific PHB, we found that short kidney length and increased CRP level were related to participants with a maternal history of BEN (). Increased CCr and CRP levels in participants were related to a biparental history of BEN. No association was identified between any PHB and β2-microglobulin or between CRP and either kidney size or function.
Figure 3 Direct and indirect path linking a specific status of parental Balkan endemic nephropathy (BEN) and the incidence of BEN.
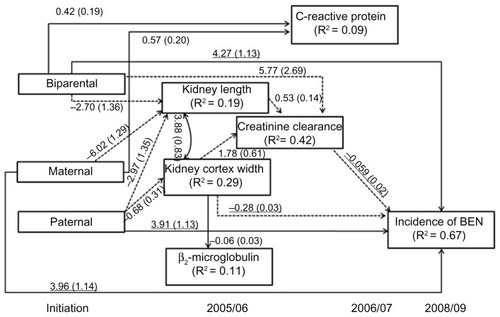
Statistically significant direct associations were observed between the incidence of BEN and all three PHBs, indicating that a major part of the effect of PHB on the onset of BEN was not mediated by clinical markers. Those offspring with a biparental BEN status were 71.5 times more likely to develop BEN than those without a PHB (OR = exponential of 4.27, ). Participants with a maternal history of BEN were 52.3 times more likely to develop BEN, while those with a paternal history of BEN were 50.1 times more likely to develop the disease than those without a PHB (OR = exponential of 3.91). A reduction of kidney cortex width of 1 mm increased the risk of having BEN (OR = 0.75; exponential of −0.28). Also, an increase in CCr was associated with lower occurrence of BEN (OR = 0.94; exponential of −0.059). No direct associations between CRP, kidney length, or β2-microglobulin and new cases of BEN were detected.
There were several indirect impacts of PHB on the onset of BEN (). We found that maternal history of BEN had an indirect effect on BEN through reduced kidney length, which then affected CCr. Paternal history of BEN had three indirect effects on developing BEN: mediated by kidney length and CCr, by kidney cortex width and CCr, and by kidney cortex width alone. The indirect paths of biparental history on BEN were mediated by two clinical markers (kidney length and CCr) and CCr only. Overall, the model accounted for 67.3% of the variability in the incidence of BEN.
For both models in and , age and sex were not associated with the occurrence of BEN; however, an increase in age and being female were statistically significantly associated with almost all clinical markers. Thus, both factors indirectly affected BEN via CCr or kidney cortex width. All models fit well, with the smallest of the Akaike information criterion and Bayesian information criterion. The goodness of fit was high for all models.
Discussion
This paper aimed to examine whether the association between PHB and incidence of BEN was mediated by such clinical markers as kidney sizes, kidney functions, and serum CRP. The results showed that PHB to a large extent directly influenced new occurrence of BEN. However, the effect of PHB on new cases was partially mediated through kidney sizes, kidney cortex width, and CCr. Maternal history influenced the occurrence of new cases via kidney length and CCr. Biparental BEN had two pathways: through kidney length and CCr, and through CCr only. There were three indirect associations between paternal history of BEN and new onset: (1) small kidney length resulting in decreased CCr, (2) small kidney cortex width and decline in CCr, and (3) declined kidney cortex width. The web of associations also indicated that CCr was an important intervening factor between parental history of BEN and new cases; however, CRP and β2-microglobulin did not act as intervening variables.
Our findings are unlikely to have resulted from a selection bias, as a high follow-up proportion of participants remained at the end of study (81%). Information bias, if any, was minimal, since identical diagnosis criteria of BEN were applied to detect the outcome during the follow-up period, and there was a high agreement of measurements of clinical markers over time, such as kidney dimensions, function, and CCr.
We found that short kidney length was indirectly associated with BEN through decreased CCr. Kidney cortex width directly influenced the onset of BEN and was also mediated through CCr. Our results demonstrated that kidney sizes had a direct effect (kidney cortex width) and an indirect effect (kidney length). These findings support autopsy studies of BEN patients indicating cortical sclerosing atrophy in the early stage of BEN.Citation23,Citation24
In agreement with the literature, a decrease of CCr directly increased the risk of BEN.Citation9,Citation25 Other studies have also reported that β2-microglobulin excretion was higher in BEN patients and their offspring,Citation10,Citation12 yet we did not detect an association between β2-microglobulin, parental history, and the development of BEN. One explanation for this difference is that β2-microglobulin may not be a part of the pathological development but only a bystander or may be increased after the disease manifests, resulting from a reduced kidney cortex width.
Unexpectedly, CRP was neither directly nor indirectly related to BEN. However, we found that CRP was associated with parental history, agreeing with a prior study.Citation17 Since a PHB is an important risk factor for BEN, it is possible that other inflammatory pathways are involved in the development of BEN. Further studies on inflammatory markers are warranted.
The present study focuses on PHB instead of family history, since PHB is less likely to create information biases compared to the collection of the history of BEN in grandparents or other relatives. As expected, PHB had a direct effect on the onset of BEN, indicating that PHB is an important risk factor.Citation26,Citation27 Participants with biparental history were more likely to develop BEN than those with a maternal or paternal history.Citation1
The association between PHB and incidence of BEN seems to have a variety of different pathways (). Maternal BEN acted through kidney length, paternal BEN acted via kidney length and kidney cortex width, and biparental BEN indirectly affected the incidence of BEN via kidney length and CCr. It is likely that kidney sizes and CCr are significant mediators between parental status and development of BEN. Further studies specifying the web of associations between parental BEN, risk factors, and the incidence of BEN are needed.
It has been documented in healthy persons that kidney sizes are associated with renal parenchymal mass and the total number of nephrons and that their number decreases with age,Citation28,Citation29 resulting in the reduction of kidney cortex width and decrease in kidney function.Citation30 In the case of BEN, it is possible that offspring with BEN may have a small kidney size at birth due to a reduction in the number of nephrons,Citation31 suggesting a maternal influence as demonstrated before.Citation10 This assumption is based on the idea that the onset of BEN occurs in childhood, has a long latency period, and then clinically manifests itself at a later age.Citation4 There is a need to determine kidney size of BEN offspring at birth or early in life and then follow them up frequently.
Since 64.3% of the incidence of BEN was explained, our findings support the hypothesized model. However, this does not exclude the possibility of other causal models and additional risk factors. For example, other clinical markers and possible exposures to environmental factors could be included in future path models to improve the explanation of BEN. Given that a reduction of kidney length, kidney cortex width, and CCr played a central role in mediating parental status of BEN with new occurrence of BEN in offspring, it is important to intervene by preventing a reduction of these markers due to other risk factors such as smoking and obesity.Citation32–Citation34
Conclusion
Our findings provide evidence elucidating the relationship between parental history of BEN (PHB) and incidence of BEN. First, we demonstrated that parental history has both a direct and an indirect effect. Second, we found several path links between PHB and occurrence of BEN: (1) maternal BEN acting via kidney length, (2) paternal BEN acting via kidney length or cortex width, and (3) biparental BEN acting via kidney length or CCr. Third, we demonstrated that β2-microglobulin excretion and CRP were bystanders and not involved in the pathway. Our results suggest the need for additional studies to corroborate our findings and to explore further the effect of additional factors on the development of BEN.
Acknowledgments
This research was supported by NIH Research Grant R01 TW06192, funded by the Fogarty International Center and the National Institute of Environmental Health Sciences, National Institutes of Health, USA, awarded to the National Center of Public Health Protection, Sofia, Bulgaria. The authors thank Susan Davis for suggestions on this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- TonchevaDDimitrovTStojanovaSEtiology of Balkan endemic nephropathy: a multifactorial disease?Eur J Epidemiol19981443893949690758
- StefanovicVJelakovicBCukuranovicRDiagnostic criteria for Balkan endemic nephropathy: proposal by an international panelRen Fail200729786788017994457
- TanchevYDorossievDThe first clinical description of Balkan endemic nephropathy (1956) and its validity 35 years laterIARC Sci Publ199111521281820335
- DimitrovTBalkan endemic nephropathy in BulgariaFacta Univ Ser Med Biol200291714
- MarícIBukvícDBogdanovícMCross-sectional study in the Balkan endemic nephropathy village of Vreoci (Serbia)BANTAO J20064258
- StefanovicVCosynsJBalkan NephropathyOxfordOxford University Press2004
- StefanoviVPolenakoviMFifty years of research in Balkan endemic nephropathy: where are we now?Nephron Clin Pract20091122c51c5619390202
- Schi l lerAGusbeth-TatomirPPavlovicNFer lugaDSpasovskiGCovicABalkan endemic nephropathy: a still unsolved puzzleJ Nephrol200821567368018949721
- BukviDMariIArsenoviAJankoviSDjukanoviLPrevalence of Balkan endemic nephropathy has not changed since 1971 in the Kolubara region in SerbiaKidney Blood Press Res20073011712317396036
- DimitrovPTsolovaSGeorgievaRClinical markers in adult offspring of families with and without Balkan Endemic NephropathyKidney Int200669472372916407881
- HanjangsitKDimitrovPKarmausWReduced kidney size in adult offspring of Balkan endemic nephropathy patients and controls: a prospective studyAm J Med Sci201034029410220555250
- StefanovicVMitic-ZlatkovicMCukuranovicRMiljkovicPPavlovicNVlahovicPβ2-microglobulin in patients with Balkan nephropathy and in healthy members of their familiesKidney Int Suppl19914034S21S261762327
- DjukanovicLBukvicDMaricICreatinine clearance and kidney size in Balkan endemic nephropathy patientsClin Nephrol20046138438615224801
- RadonicMRadosevicZClinical features of Balkan endemic nephropathyFood Chem Toxicol19923031891921618441
- ArsenovicABukvicDTrbojevicSMaricIDjukanovicLDetection of renal dysfunctions in family members of patients with Balkan endemic nephropathyAm J Nephrol2005251505415731549
- BollenAKStucture Equations with Latent VariablesNew York, NYWiley-Interscience Publication1989
- KarmausWDimitrovPSimeonovVTsolovaSBatumanVOffspring of parents with Balkan Endemic Nephropathy have higher C-reactive protein levels suggestive of inflammatory processes: a longitudinal studyBMC Nephrol2009101019400955
- CockcroftDWGaultMHPrediction of creatinine clearance from serum creatinineNephron197616131411244564
- ArmstrongBWhiteESaracciRPrinciples of exposure measurement in epidemiologyArmstrongBWhiteESaracciRMonographs in Epidemiology and Biostatistics21New York, NYOxford University Press1992
- MuthénLMuthénBMplus User’s Guide6th edLos Angeles, CAMuthén and Muthén2010
- MacKinnonDLockwoodCBrownCWangWHoffmanJThe intermediate endpoint effect in logistic and probit regressionClin Trials20074549917942466
- HeinzeGSchemperMA solution to the problem of separation in logistic regressionStat Med200221162409241912210625
- FerlugaDHvalaATrnacevicSPathology of Balkan endemic nepropathy: a correlation with established kidney disease entitiesFacta Univ Ser Med Biol2002918287
- SavinMBumbašireviVDjukanoviLPetroniVThe significance of apoptosis for early diagnosis of Balkan nephropathyNephrol Dial Transplant200116Suppl 63011568234
- Mitic-ZlatkovicMCukuranovicRLecicNStefanovicVUrinary creatinine excretion in children from families with Balkan endemic nephropathy: evidence for genetic predisposition to the diseasePathol Biol (Paris)200048655455710965533
- TonchevaDDimitrovTGenetic predisposition to Balkan endemic nephropathyNephron19967245645698730422
- MarinkovicDCvjeticaninSPopulation-genetic study of Balkan endemic nephropathy in SerbiaRuss J Genet2007438942946
- MaertensSVan Den NoortgateNKidney in old ageActa Clin Belg2008631818386760
- SchlangerLChapter 4: Kidney senescenceAmerican Society of Nephrology. Geriatric Nephrology Curriculum200917
- HekmatniaAYaraghiMSonographic measurement of absolute and relative renal length in healthy Isfahani adultsJ Res Med Sci20049254
- LacklandDMechanisms and fetal origins of kidney diseaseJ Am Soc Nephrol2005169253116093446
- CulletonBFLarsonMGWilsonPWFCardiovascular disease and mortality in a community-based cohort with mild renal insufficiencyKidney Int19995662214221910594797
- HarounMKJaarBGHoffmanSCRisk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, MarylandJ Am Soc Nephrol20031411293414569104
- LeveyASAtkinsRCoreshJChronic kidney disease as a global public health problem: approaches and initiatives – a position statement from Kidney Disease Improving Global OutcomesKidney Int200772324725917568785