Abstract
Background
In nephrotic syndrome, the combination of furosemide and albumin infusion is a standard regimen to treat systemic edema. The efficacy of synthetic human atrial natriuretic peptide (hANP) for nephrotic syndrome to ameliorate the systemic edema and retain renal functions has not been fully demonstrated.
Trial design
We conducted a prospective, randomized, controlled, open-label clinical trial. Patients were randomly assigned by a stratified biased coin design.
Methods
A total of 12 patients with nephrotic syndrome between the ages of 20 to 79 years were enrolled and randomly assigned to either the conventional (CON) group treated with furosemide and albumin, and hANP group, in which carperitide was administered in addition to the conventional therapies. The primary end points were: (1) the differences in serum creatinine levels, and (2) the reduction of total dosage of furosemide and albumin by the treatments of hANP. Secondary end points were body weight, systolic blood pressure, heart rate, serum protein, albumin, and urinary protein excretion.
Results
A total of 13 patients were enrolled, and one patient was excluded due to severe pneumonia. In both hANP (n = 7) and CON (n = 5) groups, body weight was reduced after 2-week treatments. Serum creatinine levels at follow-up significantly increased compared with baseline. The increase in serum creatinine levels (Δ serum creatinine) was smaller in the hANP group compared with the CON group (P = 0.31). The serum uric acid, serum urea nitrogen, and urinary protein excretion were reduced in the hANP group, and increased in the CON group, though these differences were not statistically significant. The usage of hANP significantly reduced the total dosage of furosemide (P < 0.05) during the treatment periods. No adverse effects were observed.
Conclusions
The concomitant use of synthetic hANP with conventional therapies is beneficial for reducing the dosage of loop diuretics, and the elevation of serum creatinine and uric acid may be avoided.
Introduction
Atrial natriuretic peptide (ANP) was discovered in 1992, and it was found that the exogenous infusion of ANP decreases systemic blood pressure, while increasing glomerular filtration rate (GFR), filtration fraction, and salt and water excretion.Citation1 Synthetic human atrial natriuretic peptide (hANP), carperitide, has been used for the treatment of heart failure in Japan. The administration of hANP exerted beneficial effects in experimental and clinical acute renal failure.Citation2,Citation3 Although brain natriuretic peptide (BNP) demonstrated qualitatively similar physiological effects with potent blood pressure-lowering effects, the effects of BNP on renal function in patients with heart failure remained controversial. One study demonstrated that low-dose synthetic human BNP, nesiritide, improves renal function in heart failure patients following myocardial infarction,Citation4 whereas another reported that nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine.Citation5
In nephrotic syndrome, the combination of furosemide and albumin infusion is commonly used to relieve systemic edema and the symptoms of congestive heart failure. We often encounter the cases where the administration of furosemide worsens renal function and induces abnormalities of electrolytes. The previous case report demonstrated the efficacy of hANP in ameliorating systemic edema while maintaining renal function in a patient with nephrotic syndrome.Citation6 This finding prompted us to examine the effectiveness of hANP on systemic edema in nephrotic syndrome and compare the adverse effects with conventional therapy.
Subjects and methods
Study design, setting, and participants
We conducted the prospective, randomized, controlled, open-label clinical trial at Okayama University Hospital. Patients were eligible and included in the study if they presented with nephrotic syndrome complicated with congestive heart failure, massive pleural effusion, ascites, and peripheral edema. The diagnosis of nephrotic syndrome was based on urinary protein excretion of more than 3.5 g/day, serum total protein level less than 60 g/L, or serum albumin level less than 30 g/L. Subjects with a serum creatinine level of more than 305 μmol/L, with systemic hypotension (systolic blood pressure < 100 mmHg), with cardiogenic shock, and with ischemic heart disease within one month before their entry were excluded. The diagnosis of diabetic nephropathy among subjects was made on the basis of their having a history of diabetes for at least more than 10 years, while also presenting with diabetic retinopathy. The use of antihypertensive agents such as angiotensin II receptor blockers, angiotensin converting enzyme inhibitors, β or α blockers, or aldosterone blockers was permitted, and could be administered concurrently for maintaining participants’ blood pressure under 125/75 mmHg. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the Institutional Review Board of Okayama University Hospital (#050305). All patients provided written informed consent to participate in this study.
Study protocol and outcome measures
A total of 13 patients ranging in age from 20 to 79 years old were enrolled in the study between January 2007 and October 2010. Participants were randomly assigned by way of stratified biased coin design, to either the synthetic human atrial natriuretic peptide (hANP, n = 7) or the conventional treatment (CON, n = 6) groups. Participants were treated for 2 weeks (). Incidentally, more diabetic patients were assigned to the hANP group. One patient with diabetic nephropathy in the CON group was excluded due to the onset of severe pneumonia during the intervention. In the CON group, furosemide at maximum dosage of 200 mg/day was orally or intravenously administered during the course of the study, and daily doses were adjusted to maintain participants’ urine volume more than 2000 mL/day. When serum albumin level was less than 20 g/L, 50 mL of 25% albumin per week was supplemented. In the hANP group, synthetic hANP, carperitide, was continuously administered concurrently with the conventional therapies. The starting dose of synthetic hANP was 0.01 μg/kg/min and the infusion rate was gradually increased up to 0.2 μg/kg/min. To maintain participants’ blood pressure at more than 100 mmHg, the daily dosage of antihypertensive agents was reduced or discontinued.
Table 1 Comparison of clinical characteristics of synthetic human atrial natriuretic peptide treatment (hANP) and conventional treatment (CON) groups at baseline
The primary end points of this study were: (1) the preservation of renal function evaluated by the changes in serum creatinine level; and (2) the reduction of total dosage of furosemide and albumin by the treatments of hANP, (ie, carperitide). Secondary end points were participants’ body weight, systolic blood pressure, heart rate, serum protein, albumin, and urinary protein excretion. In a preliminary evaluation, the standard deviation of the total dosage of furosemide in the patients with nephrotic syndrome was normally distributed with a standard deviation of 250 mg. If the true difference in means between the experimental and control conditions is 500 mg, we will need to study five experimental subjects and five control subjects to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) of 0.8. The Type I error probability associated with testing this null hypothesis is 0.05.
Statistical analysis
All data are shown as the means ± SEM (standard error of the mean) unless otherwise noted. Differences between paired variables were analyzed by nonparametric tests, ie, Wilcoxon and Mann–Whitney tests. Statistical analysis was conducted with PASW Statistics 18 (SPSS Inc, Chicago, IL). P values of less than 0.05 were considered statistically significant.
Results
A total of 13 patients were randomly assigned to the hANP (n = 7) and CON (n = 6) groups and treated throughout the course of the study. One patient in the CON group developed a severe infection and was excluded from the study (). During the study period, any adverse effects, such as hypotension and deterioration of renal function, were not observed. At baseline, there were no significant differences in various clinical parameters such as blood pressure, serum total protein, and urinary protein excretion (). However, it is important to note that more patients with diabetic nephropathy were assigned to the hANP group (n = 6) than to the CON group (n = 2). Across both groups, body weight was reduced among all participants after a 2-week treatment regimen, although this change was not statistically significant (p=0.063 in hANP and p=0.223 in CON groups). In both hANP and CON groups, serum creatinine levels at follow-up significantly increased when compared with baseline ().
Table 2 Clinical parameters at baseline and follow-up in synthetic human atrial natriuretic peptide treatment (hANP) and conventional treatment (CON) groups
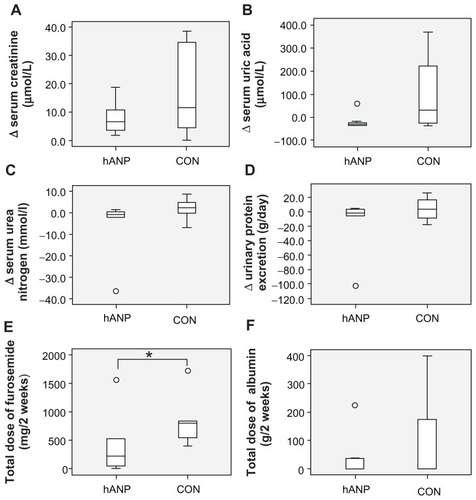
The increase in serum creatinine levels (Δ serum creatinine) tended to be smaller in the hANP group (3.95 μmol/L; range 0.9–18.6) compared with CON group (11.5 μmol/L; range 0–38.9) (P = 0.31) (). The serum uric acid, serum urea nitrogen, and urinary protein excretion were reduced in hANP group, while the CON group exhibited an increase in these levels; however, these differences were not statistically significant (). The administration of hANP significantly reduced the total dosage of furosemide (220 mg [range 0–1560] vs 800 mg [range 400–1720]; P < 0.05) (). The usage of hANP also reduced the total volume of infused albumin, but this reduction was also not statistically significant (0 g [range 0–225] vs 0 g [range 0–400]; P = 0.80) ().
Discussion
The majority of patients with nephrotic syndrome experience severe edema due to primary renal sodium retention where the tubular sodium reabsorption, mainly in the distal tubule, is enhanced and predominates over the mechanisms involved in regulating extrarenal volume mechanisms.Citation7 In addition to the inability of the renal distal tubule to excrete salt, vascular hyperpermeability also plays a role in the pathophysiology of nephrotic edema.Citation8 Two extremes of volume status, hypervolemia and hypovolemia, may be found in patients with nephrotic syndrome; however, hypovolemia is predominately due to consequences of conventional therapies.Citation9 Renal sodium retention should normally be counterbalanced by enhanced secretion of sodium in the inner medullary collecting duct, primarily mediated by the release of ANP. This regulatory pathway is curtailed in patients and rats with nephrotic syndrome by enhanced catabolism of cyclic GMP following phosphodiesterase activation,Citation10 or by the impairment of subsequent ANP signaling pathways such as cyclic GMP-dependent protein kinases.Citation11 The line of conceptual changes of pathogenesis associated with nephrotic syndrome prompted us to assume that the administration of hANP for nephrotic syndrome is beneficial for increasing sodium excretion and relieving generalized edema.
In the current study, synthetic hANP relieved systemic edema in patients with nephrotic syndrome without any of the possible adverse effects that may have impacted renal function, including elevation of serum creatinine, urea nitrogen, and uric acid when compared with conventional therapies (ie, furosemide and albumin). ANP was reported to play an important role in albumin-induced natriuresis in patients with nephritic syndrome;Citation12 the use of hANP in conjunction with furosemide and albumin has synergistic effects on the sodium excretion. We can speculate that the excessive reduction of blood pressure associated with the administration of synthetic ANP or BNP may lead to the deterioration of renal function. In previous reports, the low dose of nesiritide 0.0075 μg/kg/min improved renal function,Citation13 whereas the intermediate dose 0.01 μg/kg/min had a converse effect on renal function.Citation5,Citation14 In our study, we maintained participants’ systolic blood pressure at more than 100 mmHg by adjusting the dosage of carperitide from 0.01 to 0.20 μg/kg/min. BNP exerted more potent hypotensive actions when compared with ANP, and the study also suggested that the deterioration of renal function may be due to the excessive reduction of systemic blood pressure.
ANP has been classified as a renoprotective peptide, since it also inhibits the renin–angiotensin system (RAS). By inhibiting RAS, ANP may cause vasodilatation, suppression of sympathetic tone, and cell growth arrest. Through these biological mechanisms, the administration of hANP exerted beneficial effects on experimental and clinical acute renal failure.Citation2,Citation3 Contrary to these reports, ANP was also reported to increase urinary albumin excretion in normoalbuminuric patients with type 1 and type 2 diabetes.Citation15,Citation16 In patients with nephrotic syndrome, plasma ANP levels were significantly higher, and the elevated ANP enhanced urinary protein excretion. This is not due to modulation of GFR or filtration fraction, but rather is most probably attributable to an increase in glomerular permeability.Citation17 Thus, we were concerned that carperitide might increase urinary protein excretion in the patients with nephrotic syndrome; however, the administration of carperitide did not increase urinary protein excretion levels. In the current study, three of seven patients in the hANP group were introduced into hemodialysis therapies during a 4-year follow-up period, whereas two of five patients in the CON group were introduced into hemodialysis.
There were some limitations in the current study. First, this study was an open-label trial, thus it is possible that the results may have been influenced by unconscious bias of the investigators, who were unblinded and aware of who received which intervention. Second, the randomization protocol did not completely balance the ratio between diabetic and nondiabetic kidney diseases, which may affect the outcome of the study. Third, the current investigation was a pilot study that aimed to demonstrate the clinical usefulness of hANP in nephrotic syndrome, and the sample population may have been too small to reach statistically significant effects. Most notably, a larger sample population may have yielded significant results across the various primary and secondary end points, with the exception of the reduction in dosage of furosemide. Another criticism is that the cost of synthetic hANP is much higher than furosemide; however, the reduction of albumin infusion may compensate for the medical cost. At this stage, we conclude that concomitant use of synthetic hANP with conventional therapy is beneficial for reducing the dosage of loop diuretics. Future research is required to demonstrate that the concomitant use of synthetic hANP with conventional therapy is beneficial for improving intractable generalized edema among patients with nephrotic syndrome, while curtailing such adverse effects as elevation in serum creatinine and uric acid levels.
Disclosure
The authors declare no conflict of interest in this work.
References
- OhyamaYMiyamotoKSaitoYMinaminoNKangawaKMatsuoHCloning and characterization of two forms of C-type natriuretic peptide receptor in rat brainBiochem Biophys Res Commun199218327437491347995
- AllgrenRLMarburyTCRahmanSNAnaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study GroupN Engl J Med1997336128288349062091
- NakamotoMShapiroJIShanleyPFChanLSchrierRWIn vitro and in vivo protective effect of atriopeptin III on ischemic acute renal failureJ Clin Invest19878036987052957391
- ChenHHMartinFLGibbonsRJLow-dose nesiritide in human anterior myocardial infarction suppresses aldosterone and preserves ventricular function and structure: a proof of concept studyHeart200995161315131919447837
- WangDJDowlingTCMeadowsDNesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinineCirculation2004110121620162515337695
- KikumotoYWadaJMakinoHThe application of synthetic hANP in diabetic nephropathy with nephrotic syndromeDiabetes Care200629117217316373924
- SchrierRWFassettRGA critique of the overfill hypothesis of sodium and water retention in the nephrotic syndromeKidney Int1998535111111179573524
- RostokerGBeharALagrueGVascular hyperpermeability in nephrotic edemaNephron200085319420010867533
- Vande WalleJGDonckerwolckeRAPathogenesis of edema formation in the nephrotic syndromePediatr Nephrol200116328329311322379
- DeschenesGFerailleEDoucetAMechanisms of oedema in nephrotic syndrome: old theories and new ideasNephrol Dial Transplant200318345445612584259
- PericoNRemuzziGEdema of the nephrotic syndrome: the role of the atrial peptide systemAm J Kidney Dis19932233553668372830
- TulassayTRascherWLangRESeyberthHWScharerKAtrial natriuretic peptide and other vasoactive hormones in nephrotic syndromeKidney Int1987316139113952956451
- ZhaoQWuTGLinYLiBLuoJYWangLXLow-dose nesiritide improves renal function in heart failure patients following acute myocardial infarctionHeart Vessels20102529710320339970
- EjazAAMartinTDJohnsonRJProphylactic nesiritide does not prevent dialysis or all-cause mortality in patients undergoing high-risk cardiac surgeryJ Thorac Cardiovasc Surg2009138495996419660420
- McKennaKSmithDMooreKGlenAKessonCMThompsonCJEnhanced albuminuric response to atrial natriuretic peptide in normoalbuminuric patients with Type 1 diabetes mellitus – a pilot studyDiabet Med200017646346810975216
- MooreKBMcKennaKOsmanMTormeyWPMcDonaldDThompsonCJAtrial natriuretic peptide increases urinary albumin excretion in people with normoalbuminuric type-2 diabetesIr J Med Sci20071762677317476567
- PlumJMirzaianYGrabenseeBAtrial natriuretic peptide, sodium retention, and proteinuria in nephrotic syndromeNephrol Dial Transplant1996116103410428671965