Abstract
Purpose
The pathological manifestations in the kidneys in systemic lupus erythematosus (SLE) are commonly known as lupus nephritis. We have studied the pathological changes in renal biopsies from 59 cases of clinically diagnosed SLE obtained over a 15-year period from a racially diverse population in the Sydney metropolitan area. Our aim was to see if there was any regional variation in the morphological changes.
Methods
Renal biopsy changes were assessed by routine light, immunofluorescence, and electron microscopy. We used the modified 1974 World Health Organization classification of lupus nephritis to classify cases into six classes. Disease severity was assessed by age, sex, and across racial groups, including Caucasian, Asian, Middle Eastern, Mediterranean, Indian subcontinental, South American, and Pacific Islander.
Results
Our analysis showed that cases of lupus nephritis contributed 5.4% of our total renal biopsies examined over a 15-year period. The overall incidence of biopsy-proven cases was 0.49 per 100,000 per year. The ages of our patients ranged from 10 to 79 years, with most below 50 years of age. A female to male ratio was determined to be 4.4:1. There was no relationship to ethnicity, nor was there a relationship between any of these parameters and the class or severity of disease.
Conclusion
Renal biopsy with multimodal morphological and immunohistochemical analysis remains the gold standard for diagnosis and determination of the level of disease in lupus nephritis. Based on this approach we have identified an incidence rate for southwest Sydney that is slightly higher but comparable to that found in a similar study from the United Kingdom. We also found that there was no relationship between sex, race, or age and severity of disease.
Systematic lupus erythematosus (SLE) is a well-known systemic autoimmune disease that can affect all organs, including skin, joints, liver, the central nervous system, heart, serous membranes, and kidneys. There are major and minor clinical manifestations of SLE, with many of the minor ones subtle and elusive. Among the major manifestations are arthritis, discoid and molar skin rash, photosensitivity, serositis, neuromuscular, serological, and renal symptoms. The diagnosis is usually supported by positive serological tests that may include anti-phospholipid antibody, lupus anticoagulant, antiDNA antibody, anti-Smith antibody, and antinuclear antibody tests.Citation1 The kidneys are frequently found to be affected by SLE on microscopic examination of renal biopsies. However, the extent of involvement may vary between individuals, and sometimes the changes do not reflect the severity of the clinical manifestations.
Our department is located in the main metropolitan area of Sydney and serves a large population in the southwest region of over 800,000 people. The patients that make up this population comprise many ethnic groups. In this paper we assess the renal biopsy findings of 59 cases obtained over the last 15 years that were diagnosed clinically with SLE. Our aim was to investigate if there were any significant morphological differences in the renal biopsies from this heterogeneous population of patients.
Material and methods
Renal biopsies were performed by nephrologists using ultrasound guidance and were then sent to the Department of Anatomical Pathology for analysis. Processing was routine with all cases examined by light microscopy, direct immunofluorescence microscopy (IF), or by immunohistochemistry as well as by electron microscopy (EM) according to the method of Yong and Warren.Citation2 Briefly, renal biopsies were received fresh in the laboratory, usually within 30 minutes of harvesting, and were examined under a dissecting microscope and tissue samples divided for light microscopy, IF, or EM. Samples for IF were snap frozen with liquid nitrogen, then sectioned at a thickness of 4μ. They were then air dried and treated with fluorescein isothiocyanate-labeled antibodies to immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), kappa light chain (KLC), lambda light chain (LLC), complement component 1q (C1q), complement component 3c (C3c) and fibrinogen (FIB) (Dako, Glostrup, Denmark). The tissue sample processed for light microscopy was stained with hematoxylin and eosin, Masson’s trichrome, periodic acid-Schiff, methenamine silver and congo red. In some cases the sample selected for immunofluorescence did not contain glomeruli. In such instances paraffin sections were stained by standard automated immunohistochemical methods. The samples of renal tissue selected for EM were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, processed, and stained using routine methods. Samples were examined with a Morgagni 268D transmission EM (FEI, Eindhoven, The Netherlands) at 80 kV
Clinical information
Clinical information included in this publication was obtained from the written information on the request form and from discussions with the treating clinician or a member of the team when the cases were reviewed at routine multidisciplinary renal biopsy meetings. There was no comprehensive search of the medical records to include all clinical and laboratory test results on all patients. It was not our aim to carry out a comprehensive clinicopathological correlation on each patient included in this study but to look at the renal biopsy changes and their relationship to the broad clinical presentations.
Classification of lupus nephritis
All patients had either a confirmed clinical diagnosis or were suspected of having SLE, and all had been fully assessed clinically with laboratory tests supporting the diagnosis of SLE. Renal biopsies were performed to determine whether the kidneys were affected by disease and if so, to determine the class of lupus nephritis morphologically so that appropriate treatment could be prescribed. Repeat renal biopsies were performed on some patients to determine progress or treatment response, if there was relapse of the disease, or if there was other pathology.
There have been at least two modifications of the classification of lupus nephritis from the widely accepted original World Health Organization (WHO) classification of 1974.Citation3–Citation5 Although we acknowledge that the modified 1974 broad classification of lupus nephritis is no longer commonly used, for the purpose of this study we used this system without further subclassifications.Citation6,Citation7 We have been using this modified original classification for 15 years with our renal biopsy cases being broadly divided into six classes of lupus nephritis ().
Table 1 Classes of lupus nephritis
Indications for renal biopsy
Not all patients with SLE are subjected to renal biopsies. The renal biopsies reported here were from patients that had attended renal clinics in the South Western Sydney Local Health District (SWSLHD) between the years 1997 to 2012. These biopsies were carried out after full clinical assessment by experienced nephrologists. Generally, their approach was conservative, and therefore it can be assumed that renal biopsies were performed on selected patients based on established clinical criteria. All patients had been either diagnosed clinically with SLE or had other symptoms suggestive of SLE.
Results
The total number of renal biopsies performed in our laboratory over the 15-year period was 1412. Of these, 73 were from 61 patients who had been clinically diagnosed with SLE or had other symptoms suggestive of SLE, and these comprised 5.4% of total biopsies received. Seven of these patients had two biopsies during this period, one patient had three biopsies, and one patient had four biopsies. Two patients were found not to have SLE. Therefore, the overall incidence calculated from the 59 remaining biopsy-confirmed lupus nephritis cases from our population of 800,000 over the 15-year period was 0.49 per 100,000 per year. Cases of SLE not biopsied were not included in our analysis. The clinical indications for renal biopsy as supplied to us on the pathology request form are summarized in .
Table 2 Clinical indications for renal biopsy
The SSWLHD comprises a population of diverse ethnic backgrounds. The ethnicity of our patients included Asian, Caucasian, Middle Eastern, Indian subcontinental, Pacific Islander, Mediterranean, and South American (). No difference between racial groups or relationship between severity of disease and racial grouping was established.
Table 3 Distribution of cases within classes of lupus nephritis based on race
Clinical information summary data for all patients and the distribution of cases within the six classes based on final diagnosis are shown in . The gender ratio was 81% female and 19% male patients, giving an overall female to male ratio of 4.4:1. Patients’ ages ranged from 10 years to 79 years with the majority of cases below the age of 50. Analysis of the raw data showed that approximately 68%) of SLE cases biopsied presented with proteinuria (nephrotic and non-nephrotic), 24% with nephrotic syndrome, 31%) with hematuria, 29% with both hematuria and proteinuria, and 22% with increasing creatinine. Three patients had clinically documented hypertension. Class V patients clinically had nephrotic syndrome; and hematuria was more common in patients with Class III and Class IV lupus nephritis.
Table 4 Patient clinical information based on final diagnosis
Correlation of classes with clinical presentation and immunofluorescence staining
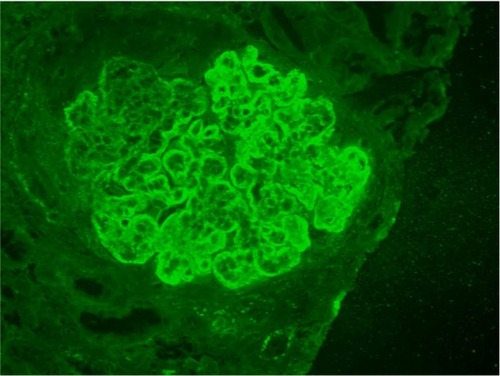
Summary pathology data is shown in . Findings of immunoglobulin deposition, complement deposition, hypertensive changes, inflammatory infiltrate, tubular casts, and the presence of active versus chronic disease are shown for each class of lupus nephritis. Generally, patients with non-nephrotic and nephrotic range proteinuria tended to have Class I, Class II, or Class V lupus nephritis, and those with hematuria or severe renal impairment had Classes III and IV lupus nephritis. There were no cases that were strictly classified as Class VI lupus nephritis. Results for immunofluorescence staining showed Classes II to V were positive for all classes of tested immunoglobulins, light chains, complement C1q and C3c, and in many cases fibrinogen (). The intensity of fluorescence varied for different proteins tested. Those cases where immunofluorescence testing was incomplete because of technical reasons or were not able to be tested were noted. These cases, however, showed deposits or thickening in the glomerular capillary basement membrane or mesangial matrix either by histological special stains or EM.
Table 5 Case pathology findings for classes of lupus nephritis
Morphological features of lupus nephritis classes
There were essentially no light microscopic abnormalities in Class I lupus nephritis (). There were negative IF findings of various classes of immunoglobulins and complement in the mesangium. EM sometimes showed granular deposits in the mesangium, and in rare instances trace deposits that were hardly discernable by EM rare found in capillary loops ().
Figure 1 Class I lupus nephritis. Mesangium and capillary loops appear normal in hematoxylin and eosin-stained section (A), Masson’s trichrome (B), periodic acid-Schiff (C) and methenamine silver (D). Transmission electron microscopy also shows no abnormality in the capillary loops (E) or mesangium (F).
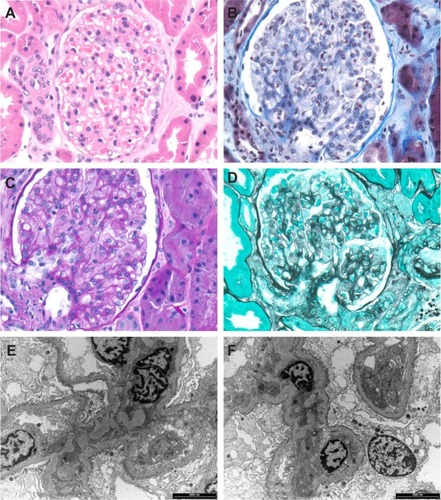
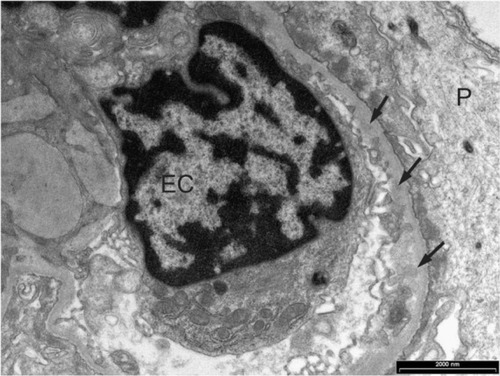
Figure 2 Glomerular capillary loop with granular trace deposits (arrows) in the subendothelium of the capillary basement membrane in a case with Class I lupus nephritis.
Abbreviations: EC, endothelial cell; P, podocyte.
Class II lupus nephritis was unremarkable by light microscopy or showed only mild focal or diffuse mesangial proliferative changes of the glomerular tuft with positive IF and EM changes, usually in the mesangium ( and ). Generally, there were no obvious capillary basement membrane changes or deposits present by light microscopy, but small deposits in the capillary loops were occasionally seen by EM ().
Figure 3 Class MB lupus nephritis.
Abbreviation: IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; KLC, kappa light chain; LLC, lambda light chain; C1q, complement 1q; C3c; complement 3c; Fib, fibrinogen.
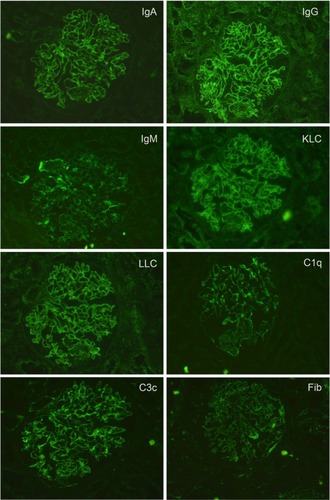
Figure 4 Class II lupus nephritis showing mesangial hypercellularity (asterisks).
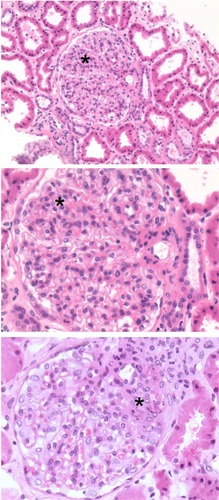
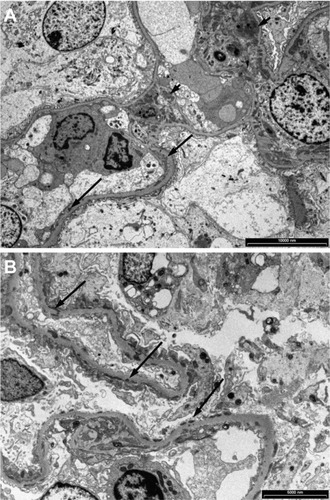
Figure 5 Granular electron dense deposits in Class MB lupus nephritis. (A) in the mesangium (short arrows) and (B) in the capillary basement membrane loops (long arrows).
Class III lupus nephritis was essentially a focal and segmental proliferative glomerulonephritis by light microscopy, with usually less than 50% of glomeruli involved. There were established abnormalities by IF and EM (–). Overlap changes were found in both Class III and Class IV lupus nephritis; the differences were that in Class IV the changes were more diffuse, or global, in glomerular tufts and more pronounced or established. Sometimes the clear distinction and separation between Class III and Class IV was difficult. However, from a treatment perspective the separation between Class III and Class IV may be unimportant as the treatment is the same.
Figure 6 Class III lupus nephritis showing a case with typical spectrum and pattern of positive immunofluorescence staining.
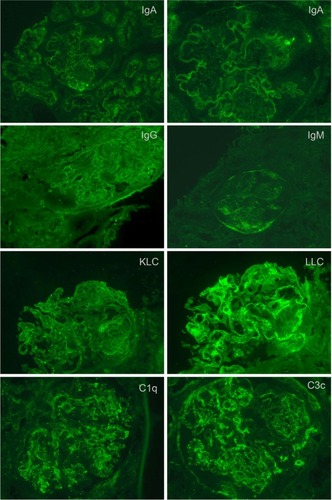
Figure 7 Electron microscopy showing (A) large fine granular deposits in the mesangial region (arrows), (B) prominent fine granular subendothelial deposits (arrows) and some intramembranous deposits (arrow heads) in a case of Class III lupus nephritis.
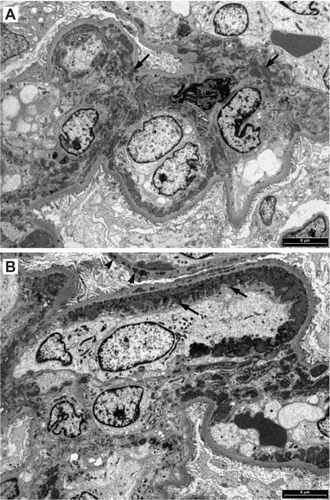
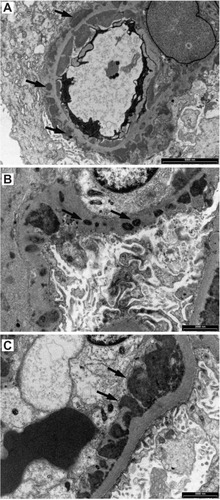
Figure 8 When tissue for immunofluorescence microscopy is not available, special histological stains and conventional immunohistochemical staining visualized with diaminobenzidine (DAB) can reveal glomerular deposits in Class III to Class VI. (A) Hematoxylin and eosin showing some crowding of nuclei in a segmental mesangial proliferative lesion; (B) Masson’s trichrome stain showing segmental mesangial proliferation and thickened capillary loops; (C) deposits of IgM (arrows); (D) deposits of IgG (arrows); (E) mesangial deposits of C3c (arrows); (F) deposits of C3c in capillary loops.
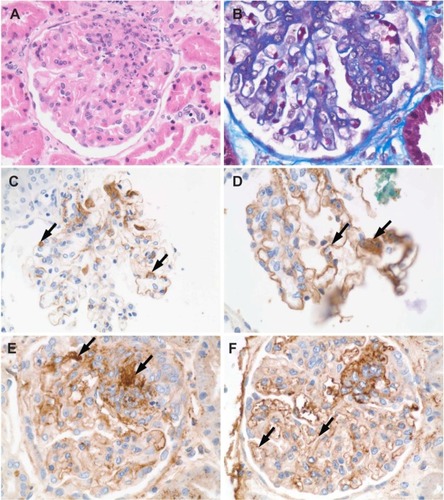
Usually more than 50% of glomeruli were globally affected In Class IV lupus nephritis. Class IV lupus nephritis also had the well-recognized “stigmata” of lupus nephritis, such as “wire loop” lesions, necrotizing lesions, and hemotoxylin or hemotoxiphil bodies (). In severe Class IV lupus nephritis, cellular crescents and vascular thrombi were a feature. In both Class IV and Class V lupus nephritis, immune deposits could be seen in the trichrome stains as fuschinophilic deposits (). By IF () and EM (), deposits were classically seen in all glomerular structural compartments, including the subepithelium, within the basement membranes, subendothelium, and mesangium.
Figure 9 Class IV lupus nephritis. (A) Mesangial hypercellularity (asterisk) and deposits (arrows) associated with the capillary loops seen in a hematoxylin and eosin-stained section. (B) Foci of necrosis (asterisk), capillary loop deposits (arrow), and vascular thrombi (arrow head) are also seen. (C) Fuchsinophilic deposits (arrows) appear clearer in a Masson’s trichrome-stained section. (D) Hematoxylin bodies (arrows) appearing as darkly stained nuclei that blend into the background.
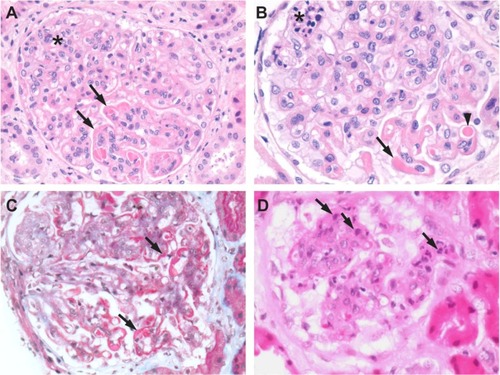
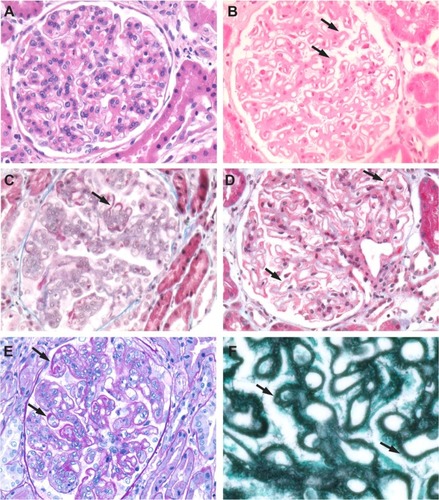
Figure 10 Class V lupus nephritis. Histological special stains are useful for further delineating the extent of glomerular deposits. (A) H&E-stained section of a Class V case showing mesangial hypercellularity. (B) H&E-stained section from a Class V case showing heavy deposits in the mesangium and capillary loops (arrows). (C) Fuchsinophilic deposits (arrows) on capillary loops seen in a Masson’s trichrome-stained section. (D) Deposits (arrows) in a Masson’s trichrome-stained section. (E) Deposits (arrows) seen in a PAS-stained section. (F) Basement membrane “spikes” in capillary loop deposits (arrows) seen in a methenamine silver-stained section.
Discussion
In our study cohort, SLE patients comprised 5.4% of the total number of patients subjected to renal biopsy over a 15-year period. All these patients had clinical manifestations of SLE or other symptoms suggestive of SLE. The renal biopsy was performed to determine the severity of disease and to determine the class of lupus nephritis in order to assist with the determination of medical treatment options. Like others, we have demonstrated that primary and repeat renal biopsies can provide valuable information on the class type, severity, progression, and evolution of the disease process that cannot be obtained by any other clinical or laboratory tests. Our biopsies were examined using several different test modalities, including special histochemistry, IF, and transmission EM.
We found that the clinical indications for performing a renal biopsy included proteinuria, especially in the nephrotic range, hematuria with active urinary sediment, new clinically diagnosed cases of SLE, SLE patients with deteriorating renal function, deterioration of clinical features, suspected progression or reactivation of disease from unknown cause, or noncompliance with medication. Only in one or two instances in our cases was a biopsy performed because of a suspicion of transformation from one class to another. The most frequent indication for biopsy was with new spontaneous cases of SLE where the biopsy was performed to confirm renal involvement.
The more recent reclassification of lupus nephritis35 from its very simplistic form of 1974 to a more detailed and complicated form with subclassifications has added complexity but has not provided much more information to significantly alter basic treatment. In the old classification, the morphological features of necrosis, degree of inflammation, and other characteristics were described and therefore taken into treatment consideration.
It is generally accepted among renal pathologists that renal biopsy diagnosis should be based on all available clinical information, laboratory test results, and morphological changes. Analysis of our 59 cases showed that clinical information provided in the request form by clinicians was minimal or rudimentary. In 82% of the cases, no clinical information was provided other than the patient had SLE, and in 21% of the cases, no clinical information was provided. The remainder of the SLE patients had proteinuria and or hematuria or increasing blood creatinine levels.
The overall incidence of lupus nephritis found in our cohort of 0.49 per 100,000 individuals per year was slightly higher but comparable to a similar study of biopsy-proven lupus nephritis in the United Kingdom that found an overall annual incidence rate of 0.40 per 100,000 per year.Citation8 Most of our SLE patients were female and were below the age of 50 years. Asian-Australian patients were the most numerous in our study, followed by those of Caucasian ethnicity. There was no association of race, sex, or age to any class of lupus nephritis, which is in agreement with other groups who have found no race variance among whites, blacks, or other races.Citation6 Furthermore, there was no relationship between the distribution of histological severity and any racial group, which is in agreement with the findings of another Australian single-center study.Citation9 Generally, the severity of symptoms was related to the class of lupus nephritis, as others have found.Citation7 Classes I and II have milder symptoms of proteinuria and or hematuria, often non-nephrotic range proteinuria, or microscopic hematuria. Class V lupus nephritis cases tend to have nephrotic range proteinuria. Class III and Class IV lupus nephritis patients have more severe proteinuria and hematuria as well as renal insufficiency. However, the occasional patient may have an inconsistent correlation between severity of disease and morphological renal biopsy changes. Although the transformation of one class of lupus to another may occurCitation10 and may be a common phenomenon in patients with lupus flare,Citation11 we only observed transformation in two patients. One of our patients transformed to Class IV from Class III and another transformed from Class III to Class IV The two patients who transformed from one class to another had presented with nephrotic range proteinuria and worsening renal insufficiency in spite of ongoing treatment. We also note that transformation of one type of glomerulonephritis to another form also may occur in non-lupus nephritis.Citation12
Assessing “activity” and “chronicity” from renal biopsy changes requires careful and diligent examination. Differentiation of active lesions, such as cellular proliferation of the glomerular tufts, necrosis, cellular crescents, hyalinosis, and vasculitis, from more established chronic lesions, such as glomerulosclerosis, tubular atrophy, and interstitial fibrosis, is important as active lesions require more aggressive treatment and are potentially reversible whereas chronic lesions are not reversible. Others have attempted to quantify activity and provide an “activity index.”Citation13 There was good correlation between activity and severity of disease in our cases. Active disease was found in all classes of lupus nephritis, whereas chronic disease was found in Classes IIB to V and mainly in Classes III and IV However, there was no correlation of disease activity by race, sex, or age. In patients of either sex and of varying ages, disease activity was related to the class of lupus nephritis seen in the renal biopsy.
SLE patients can also develop all forms of glomerulonephritis in addition to lupus nephritis.Citation14 In our series there were no patients who had been diagnosed with an additional form of glomerulonephritis. Non-lupus nephritis such as thin membrane disease or drug-induced tubulointerstitial nephritis may also be seen in SLE patients. We have on many occasions encountered dual pathology in non-SLE patients. We have diagnosed focal and segmental glomerulosclerosis and IgA nephropathy in diabetic patients with diabetic glomerulosclerosis. It is important to be aware that SLE patients may have non-lupus nephritis for treatment and prognostic purposes.Citation14
Vasculitis and vascular lesions in renal biopsies have been reported in lupus nephritis,Citation15 but the frequency varies. In our experience vasculitis is relatively rare and we did not see a single instance in our 59 cases. We have seen protein thrombi in glomerular capillaries in some Class III and Class IV lupus nephritis, but such pathology is rare. We have seen vasculitic lesions, such as hyaline change, endothelial swelling, and thickening in small arteries and arterioles. The literature also describes “hematoxylin bodies” or “hematoxyphil bodies” as common or important diagnostic features of lupus nephritis, and their appearance is thought to signify active and more aggressive disease.Citation16 In our experience hematoxylin bodies were only infrequently seen. “Nuclear dust” in necrotizing lesions and in active cellular crescents was more commonly encountered, frequently in association with fibrin deposits.
Complement and fibrinogen were seen in various compartments of the glomeruli as IF deposits of immunoglobulins. It was common to find what is colloquially known as “full-house” deposits, meaning that in most cases of lupus nephritis, especially in Classes III and IV, all classes of immunoglobulins and complements were seen. In our experience deposits of immunoglobulins and C3c were frequently found in tubular basement membranes and sometimes in the interstitium. These were detectable by both IF and EM. Tubulointerstitial lesions are now being recognized as playing a role in the pathogenesisCitation17 of the disease and may be severe in Class IV disease.Citation18 It is therefore important to include in the pathology report positive IF findings in the tubulointerstitium.
In our study we did not attempt to make a comprehensive correlation between the clinical features and pathological findings. The cases in our study were not related to specific infection or drugs. We are aware there is an association with bacterial or viral infection or ingestion of drugs in less than 10% of cases of lupus nephritis. Drugs, including hydralazine, chlorpromazine, isoniazid, and quinine, have been known to cause or at least exacerbate lupus nephritis.Citation19 Bacterial and viral infections, including human immunodeficiency virus, have been reported to be associated with lupus nephritis.Citation20
The long-term course of lupus nephritis is unpredictable. Some patients affected by Class III or Class IV lupus make a complete recovery after treatment, while a small group of patients progress rapidly to end-stage renal disease (ESRD) and require dialysis or transplantation.Citation21 It seems the progression and long-term outcome of lupus nephritis in individual patients is unpredictable. Recent advances in treatment may have a significant impact on the outcome of lupus nephritis.Citation22,Citation23 The pathogenesis of lupus nephritis is comprehensively discussed in many monographs and text books of renal pathology, and yet the exact mechanism is still unknown.Citation24 It seems that there is a genetic or familial basis to a number of cases that has been estimated to be about 12% in one series.Citation25
Conclusion
From our perspective we can conclude that cases of lupus nephritis comprised 5.4% of our renal biopsy cases. Our morphological findings support the general notion that in the majority of cases immunoglobulin deposits and the immune complex play a role in renal lesions. We, like others, found that Classes III and IV lupus nephritis are associated with more severe disease and morphologically active renal lesions. Renal biopsies remain the gold standard in providing vital information for treatment. We share the view with others that renal biopsy is essential and must be done in cases of lupus nephritis if clinically indicated.Citation26
Acknowledgments
We thank Xiaojuan Wu for providing excellent immunohistochemistry; Thi Nguyen for special histochemistry; Jasmina Mitic, Manasi Joshi, and Carlyn Evangelista for assistance with electron microscopy; and Chantai Campbell for typing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- HochbergMCUpdating the American College of Rheumatology revised criteria for the Classification of Systemic Lupus ErythematosusArthritis Rheum199740917259324032
- YongJLCWarrenBAA practical approach to the diagnosis of renal disease by biopsyPathology19942643703967892034
- ChurgJBernsteinJGlassockRJRenal Diseases: Classification and Atlas of Glomerular Diseases2nd ed.New YorkIgaku-Shoin Medical Publishers1995
- WeeningJJD’AgatiYSchwartzMMThe classification of glomerulonephritis in systemic lupus erythematosus revisitedKidney Int200465252153014717922
- McCluskeyRTLupus NephritisKidney Pathology Decennial 1966–1975SommersSCNew YorkAppleton-Century-Crofts197543589
- KorbetSMSchwartzMMEvansJLewisEJSevere lupus nephritis: racial differences in presentation and outcomeJ Am Soc Nephrol200718124425417167111
- LeakerBFairleyKFDowlingJKincaid-SmithRLupus nephritis: clinical and pathological correlationQJM1987622381631793659257
- PatelMClarkeAMBruceINSymmonsDPMThe prevalence and incidence of biopsy-proven lupus nephritis in the UKArthritis and Rheum20065492963296916947632
- OngCNichollsKBeckerGEthnicity and lupus nephritis—an Australian single centre studyIntern Med J2010 [Epub ahead of print.]
- GinzlerEMNicastriADChenCKProgression of mesangial and focal to diffuse lupus nephritisN Engl J Med197429146936964604972
- LuJTarnLSLaiFMRepeat renal biopsy in lupus nephritis: a change in histological pattern is commonAm J Nephrol201134322022521791918
- ElderGPerlSYongJLCFletcherJMackieJProgression from Goodpasture’s disease to membranous glomerulonephritisPathology19952732332368532389
- AustinHA3rdBoumpasDTVaughanEMBalowJEPredicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic dataKidney Int19944525445508164443
- HertigADrozDLesavrePGriinfeldJPRieuPSLE and idiopathic nephrotic syndrome: coincidence ornot?Am J Kidney Dis20024061179118412460036
- BanfiGBertaniTBoeriYRenal vascular lesions as a marker of poor prognosis in patients with lupus nephritis. Gruppo Italiano per lo studio della Nefrite Lupica (GISNEL)Am J Kidney Dis19911822402481867181
- SilvaFThe Nephropathies of Systemic Lupus ErythematosusNew YorkChurchill-Livingstone1983
- GiannakakisKFaraggianaTHistopathology of lupus nephritisClin Rev Allergy Immunol201140317018020514528
- YuFWuLHTanYTubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society systemKidney Int201077982082920182417
- SolingerAMDrug-related lupus. Clinical and etiologic considerationsRheum Dis Clin North Am19881411872022840692
- D’AgatiYAppelGBHIV infection and the kidneyJ Am Soc Nephrol1997811381529013459
- WorrallJGSnaithMLBatchelorJRIsenbergDASLE: A Rheuma-tological view. Analysis of the clinical features, serology and immunogenics of 100 SLE patients during long term follow upQJM1990742753193302385739
- ZimmermanRRadhakrishnanJValeriAAppelGAdvances in treatment of lupus nephritisAnnul Rev Med200152637811160768
- StoneJHEnd-stage renal disease in lupus: Disease activity, dialysis and the outcome of transplantationLupus1998796546599884106
- DattaSKMannyNAndrzejewskiCAndré-SchwartzJSchwartzRSGenetic studies of autoimmunity and retrovirus expression in crosses of New Zealand Black mice I—Xenotropic virusJ Exp Med19781473 854871204726
- BuckmanKJMooreSKEbbinAJCoxMBDuboisELFamilial systemic lupus erythematosusArch Intern Med19781381116741676718317
- OrtegaLMSchultzDRLenzOPardoYContrerasGNReview: Lupus nephritis: pathologic features, epidemiology and guide to therapeutic decisionsLupus201019555757420089610