Abstract
Background:
We investigated the effects of aliskiren in terms of its inhibition of the renin–angiotensin–aldosterone system (RAAS) as well as that on blood pressure (BP), and renal and cardiac protection in elderly chronic kidney disease (CKD) patients with hypertension.
Methods:
Nineteen elderly CKD patients (nine males, ten females, aged 74.6 ± 5.8 years) were assigned to receive 150 mg/day of aliskiren added to existing antihypertensives for 6 months. Changes in plasma renin activity (PRA), angiotensin I (Ang I), angiotensin II (Ang II), aldosterone (Ald), BP, estimated glomerular filtration rate (eGFR), urine albumin/creatinine ratio (UACR), left ventricular ejection fraction (LVEF), interventricular septum thickness (IVST), left ventricular posterior wall thickness (LVPWT), and plasma brain natriuretic peptide (BNP) levels were evaluated.
Results:
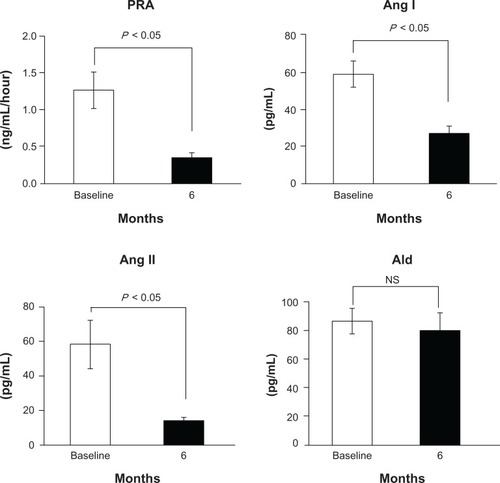
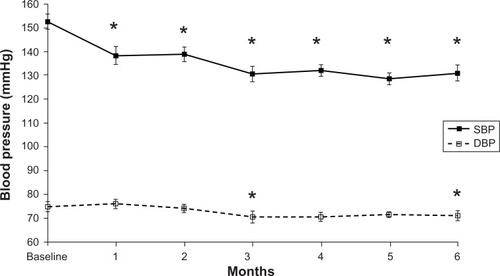
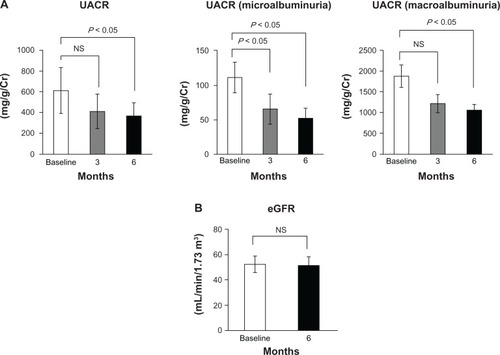
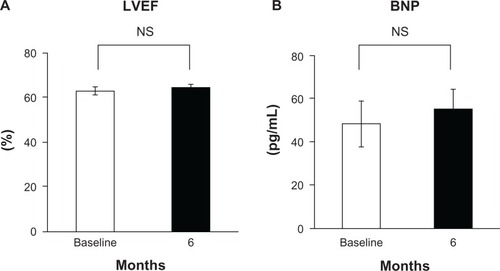
Aliskiren suppressed the RAAS as follows: PRA 1.3 ± 1.0 to 0.3 ± 0.3 ng/mL/hour, P < 0.05; Ang I 59.5 ± 32.1 to 26.0 ± 17.3 pg/mL, P < 0.05; Ang II 58.4 ± 62.1 to 14.3 ± 9.0 pg/mL, P < 0.05; and Ald 86.1 ± 38.3 to 80.1 ± 52.6 pg/mL, not significant (NS). Aliskiren reduced BP (153.6/77.2 ± 14.9/10.4 to 130.9/72.2 ± 15.6/9.9 mmHg, P < 0.05). It also reduced UACR (747.1 ± 1121.4 to 409.6 ± 636.8 mg/g, P < 0.05), whereas it did not change eGFR (52.1 ± 29.2 to 51.2 ± 29.3 mL/min/1.73 m2, NS), LVEF (66.8 ± 7.9 to 66.5% ± 6.8%, NS), IVST (10.1 ± 1.8 to 9.9 ± 1.8 mm, NS), LVPWT (10.0 ± 1.6 mm to 10.0 ± 1.4 mm, NS), or BNP (48.2 ± 46.0 to 54.9 ± 41.1 pg/mL, NS).
Conclusion:
Aliskiren was effective for BP control and reduced UACR while maintaining eGFR and heart function in elderly CKD patients with hypertension.
Introduction
Elderly patients with chronic kidney disease (CKD) have been increasing in association with the increase in the population of elderly people globally.Citation1,Citation2 CKD is a major factor that contributes to the morbidity and mortality of elderly people.Citation3 Furthermore, CKD increases the risk of cardiovascular mortality and morbidity.Citation4 The renin–angiotensin–aldosterone system (RAAS) plays pivotal roles in the pathogenesis of CKD as well as hypertension and cardiovascular disease.Citation5,Citation6 Indeed, several studies have reported that the blockade of the RAAS by angiotensin I–converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) showed beneficial effects on hypertension, CKD, and cardiovascular disease in CKD patients, including the elderly.Citation6–Citation10 Aliskiren suppresses the RAAS by directly inhibiting plasma renin activity (PRA).Citation11 Recently, evidence has accumulated suggesting that aliskiren is effective for blood pressure (BP) control and inhibits the progression of CKD and cardiovascular disease in patients with hypertension and CKD;Citation12–Citation16 however, its effects on elderly CKD patients have not been fully elucidated because most clinical trials have excluded the elderly and few studies have targeted elderly CKD patients. In the present study, we assessed the efficacy of aliskiren on BP control, renoprotection, and cardioprotection in elderly CKD patients with hypertension.
Methods
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Jichi Medical University. Written informed consent was obtained from all patients.
Patients
Between February 2011 and December 2011, 23 elderly patients (over 65 years of age) who had CKD and hypertension were enrolled in this study. CKD was defined as the estimated glomerular filtration rate (eGFR) being > 15 and ≤60 mL/minute/1.73 m2 and/or the urine albumin/creatinine ratio (UACR) being >30 mg/g. Hypertension was defined by clinical systolic BP (SBP) ≥140 mmHg and diastolic BP (DBP) ≥90 mmHg. Exclusion criteria were as follows: type I diabetes mellitus or type II diabetes mellitus with poor glucose control (glycosylated hemoglobin [HbA1c] >9% at the start of the observation period), hyperpotassemia (>5.5 mEq/mL), history of stroke, coronary heart disease, severe arrhythmia, or any medical or surgical condition that may have affected the pharmacokinetics of the study drug. The patients who had decreased eGFR (<60 mL/minute/1.73 m2) with diabetic mellitus and who had been receiving ACEIs or ARBs were suspended in this study in accordance with the results of the ALTITUDE study, which showed that adverse effects such as stroke, renal impairment, hyperkalemia, and hypotension were observed in patients who received aliskiren added to ACEIs or ARBs among high-risk patients with diabetes and renal impairment than in patients who received a placebo.
Study protocol
This was a 28-week, multicenter study consisting of a 4-week observation period to fix any drugs, including existing antihypertensives, and a 24-week treatment period with aliskiren. After the 4-week observation period, all eligible patients entered the 24-week treatment period, during which they received aliskiren at 150 mg orally in the morning once daily. BP was measured at a clinic when they consulted primary care physicians. The reported BP was the average of three measurements. eGFR, plasma potassium, plasma BNP, plasma PRA, plasma angiotensin I (Ang I), plasma angiotensin II (Ang II), and plasma aldosterone (Ald) levels were measured at baseline and week 24 in the treatment period. UACR was measured at baseline and at week 12 and week 24 in the treatment period. Left ventricular ejection fraction (LVEF), interventricular septum thickness (IVST), and left ventricular posterior wall thickness (LVPWT) were measured by echocardiogram at baseline and week 24 in the treatment period. Standard laboratory tests were performed in the observation period and at baseline, and weeks 12 and 24 in the treatment period.
Laboratory methods
eGFR was calculated using a modified version of the Modification of Diet in Renal Disease formula of the Japanese Society of Nephrology, as follows: eGFR (mL/minute/1.73 m2) =194 ×age−0.287 × serum creatinine−1.094 (multiplied by 0.739 for females).Citation17 Microalbuminuria was defined as UACR of >30 mg/g and <300 mg/g. Macroalbuminuria was defined as UACR > 300 mg/g.
Plasma BNP, PRA and Ald levels were determined by radioimmunoassay. Plasma Ang I and Ang II levels were measured by double-antibody radioimmunoassay; the details of these methods have been described elsewhere.Citation18,Citation19 PRA, Ang I, Ang II and other blood-chemistry parameters were determined by a clinical chemistry laboratory (SRL, Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Mann–Whitney test or repeated-measures analysis of variance was used to compare continuous data. Differences with a P-value < 0.05 were considered significant.
Results
Twenty-three patients entered the treatment period after the 1-month observation period (). Nineteen patients completed the study. One discontinued the study owing to the development of a hemodynamically unstable condition associated with severe viral infection with herpes zoster. One discontinued the study owing to dizziness. The dizziness was resolved after aliskiren withdrawal. One discontinued the study owing to symptomatic hypotension. The symptomatic hypotension recovered to the basal level after aliskiren withdrawal. One discontinued the study and suspended aliskiren treatment; he was excluded from this study because he had decreased eGFR (<60 mL/minute/1.73 m2) and diabetes mellitus with treatment of an ARB, in accordance with the results of the ALTITUDE study. However, he did not have any side effects upon the addition of aliskiren. Increased serum potassium was not observed in any patients. shows the characteristics of the study patients. The study patients were nine males and ten females, and their average age was 74.6 ± 5.8 years (range 65.0–84.7 years). Their average eGFR was 52.1 ± 29.2 mL/minute/1.73 m2 and average UACR was 747.1 ± 1121.4 mg/g. Nine patients had microalbuminuria (UACR: 111.3 ± 79.8 mg/g) and seven patients had macroalbuminuria (UACR: 1877.9 ± 1182.6 mg/g). All patients had been taking antihypertensive drugs before the treatment period, including calcium antagonists (18 patients), AECIs (two patients), ARBs (16 patients), β-blockers (one patient), α-blockers (seven patients), αβ-blockers (two patients), and diuretics (four patients). Details of antihypertensive drugs in each patient are shown in . None of the patients had their BP well controlled, despite the use of antihypertensives prior to aliskiren treatment.
Figure 1 Patient flow chart.
Table 1 Patient baseline characteristics (n = 19)
Table 2 Patient clinical characteristics before aliskiren treatment
Blockade of RAAS by aliskiren
The blockade effect of RAAS by aliskiren in the patients is shown in . PRA decreased from 1.3 ±1.0 ng/mL/hour at baseline to 0.3 ± 0.3 ng/mL/hour at week 24 (P < 0.05). Ang I decreased from 59.5 ± 32.1 pg/mL at baseline to 26.0 ± 17.3 pg/mL at week 24 (P < 0.05). Ang II decreased from 58.4 ± 62.1 pg/mL at baseline to 14.3 ± 9.0 pg/mL at week 24 (P < 0.05). Aldosterone (Ald) decreased from 86.1 ± 38.3 pg/mL at baseline to 80.1 ± 52.6 pg/mL at week 24 (not significant).
Effect of aliskiren on BP
SBP (±SD) decreased from 153.6 ± 14.9 mmHg at baseline to 130.9 ± 15.6 mmHg at week 24 (P < 0.05) (). DBP (±SD) also decreased from 77.2 ± 10.4 mmHg at baseline to 72.2 ± 9.9 mmHg at week 24 (P < 0.05) ().
Effect of aliskiren on UACR and eGFR
UACR (all patients: n =19) decreased from 747.1 ± 1121.4 mg/g at baseline to 480.5 ± 791.2 mg/g at week 12 (P < 0.05), followed by a further decrease to 409.6 ± 636.8 mg/g at week 24 (P < 0.05) (). In the subanalysis of the microalbuminuria and macroalbuminuria groups, microalbuminuria (n =9) decreased from 111.3 ± 79.8 mg/g to 65.6 ± 79.5 mg/g at week 12 (P < 0.05), followed by a further decrease to 53.2 ± 52.3 mg/g at week 24 (P < 0.05), and macroalbuminuria (n =7) also decreased from 1878.0 ± 1182.6 mg/g to 1214.1 ± 935.3 (not significant), followed by a further decrease to 1039.7 ± 692.0 at week 24 (P < 0.05) (). The eGFR did not significantly change during the treatment period (52.1 ± 29.2 mL/minute/1.73 m2 at baseline vs 51.2 ± 29.3 mL/minute/1.73 m2 at week 24) ().
Effect of aliskiren on heart function and plasma BNP level
LVEF did not change during the treatment period (66.8% ± 7.9% at baseline vs 66.5% ± 6.8% at week 24) (). IVST and LVPWT did not change in the treatment period (IVST, 10.1 ± 1.8 mm at baseline vs 9.9 ± 1.8 mm at week 248; LVPWT, 10.0 ± 1.6 mm at baseline vs 10.0 ± 1.4 mm at week 24) either. Also, plasma BNP level did not change during the treatment period (48.2 ± 46.0 pg/mL at baseline vs 54.9 ± 41.1 pg/mL at week 24) ().
Discussion
The results of this study show that aliskiren suppressed RAAS and significantly decreased BP and UACR, whereas it did not change eGFR, LVEF, IVST, LVPWT, and BNP levels in elderly CKD patients with hypertension. Among RAAS components, PRA, Ang I, and Ang II were significantly decreased by aliskiren in elderly CKD patients in this study; however, aldosterone was not decreased. These results may suggest the possibility that aliskiren cannot overcome aldosterone breakthrough in the same way as ACEIs and ARBs in elderly CKD patients can.
Hypertension has a high impact on the decline of renal function and the development of cardiovascular disease in elderly patients.Citation20–Citation22 The subanalysis of Systolic Hypertension in the Elderly Program showed that SBP is a strong, independent predictor of a decline in renal function among elderly patients.Citation21 Okada et al also reported that SBP is a significant predictor of decline in renal function and the development of end-stage renal failure in elderly CKD patients.Citation22 The Hypertension in the Very Elderly Trial reported that cardiovascular events were significantly less common in the decreased BP group (143.5/77.9 mmHg) with antihypertensives than in the placebo group (158.5/84.0 mmHg) among elderly patients.Citation20 In this study, the enrolled patients’ BP was not controlled well, despite antihypertensives prior to aliskiren treatment. The dose of each antihypertensive may not have been enough to control their BP.
In addition, CKD itself is a major risk factor for cardiovascular disease. Several studies have reported that CKD increases the risk of cardiovascular disease in elderly hypertensive patients.Citation4,Citation23 Although the stage of CKD is defined by GFR, albuminuria is also an independent risk factor for the development of end-stage renal disease and cardiovascular disease in CKD patients.Citation24–Citation26 The risk of renal events was increased substantially more by microalbuminuria, even in the same GFR patients.Citation24 The risk of cardiovascular disease was also increased by microalbuminuria, and it was further increased with decreased GFR in CKD patients.Citation26 Macroalbuminuria is also a risk factor for accelerated decline of renal function.Citation25 These lines of evidence suggested that decreased BP and albuminuria could inhibit the progress of CKD and the development of cardiovascular disease in elderly CKD patients. In the present study, aliskiren decreased BP, microalbuminuria, and macroalbuminuria, while it did not change eGFR, LVEF, VST, LVPWT, or plasma BNP levels in elderly CKD patients. These results suggest that aliskiren has beneficial effects on BP control and renoprotection, which may lead to inhibition of the development of cardiovascular disease in elderly CKD patients. The effects of aliskiren for albuminuria, eGFR, LVEF, VST, LVPWT, and plasma BNP did not change between early stage CKD (stages 1 and 2) and advanced-stage CKD (stages 3–5) (data not shown). It should be noted that renal replacement therapies, while paying attention to the risk of a decrease of residual renal function, could be alternative therapies to manage hypertension in the stage 5 CKD patients in this study.
The albuminuria decreased in accordance with BP reduction in this study. These results suggested aliskiren may decrease albuminuria, mainly by a BP-lowering effect in the elderly CKD patients in this study. Although not statistically significant, plasma BNP levels tended to increase from baseline to week 24. Since BNP reduction has consistently been associated with an improved outcome in heart failure,Citation27,Citation28 aliskiren may not have beneficial effects for heart function in elderly CKD patients with hypertension. Further large-scale and long-term studies will need to investigate the effects and mechanism of renoprotection and cardioprotection of aliskiren in elderly CKD patients.
Several clinical studies have demonstrated that aliskiren is effective for hypertension by monotherapy and combination therapy with other antihypertensives such as ACEIs, ARBs, and calcium antagonists in elderly hypertensive patients;Citation16,Citation29 however, recently the ALTITUDE study investigating the effects of aliskiren added to ACEIs or ARBs in patients at high risk for cardiovascular disease and with diabetes and renal impairment was suspended because more adverse effects such as stroke, renal impairment, hyperkalemia, and hypotension were observed in patients who received aliskiren than in patients who received a placebo. In accordance with the results of that study, one patient who had decreased eGFR (<60 mL/minute/1.73 m2) with diabetes mellitus treated with an ARB were suspended in this study at a time when he did not have any adverse events upon the addition of aliskiren. Although many patients in the present study who received aliskiren had been taking ACEIs (2/23 patients) or ARBs (16/23 patients), no patients developed any serious adverse events. The patients in the present study maintained their cardiac function and had no complication of diabetes mellitus. These conditions of the patients may have affected the fact that serious side effects of aliskiren with ARBs or ACEIs were not observed in elderly CKD patients in the present study. The further studies investigating the effects and safety of aliskiren, including its combination with ACEIs and ARBs, will be required in elderly CKD patients.
There are several limitations in this study. First, it was a single-arm trial without a control group. Second, the number of patients was small and treatment period was short. Therefore, the results may not reflect outcomes shown in previous large-scale trials such as the ALTITUDE study. Further large-scale, double-blind, long-term clinical trials will need to investigate the effects of aliskiren in elderly CKD patients.
In conclusion, aliskiren reduced BP and UACR while maintaining eGFR, LVEF, and plasma BNP levels in elderly CKD patients with hypertension, which may inhibit the development of cardiovascular disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- ImaiEHorioMIsekiKPrevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficientClin Exp Nephrol200711215616317593516
- CoreshJSelvinEStevensLAPrevalence of chronic kidney disease in the United StatesJAMA2007298172038204717986697
- CoreshJAstorBCGreeneTEknoyanGLeveyASPrevalence of chronic kidney disease and decreased kidney function in the adult US population: third National Health and Nutrition Examination SurveyAm J Kidney Dis200341111212500213
- RitzEMinor renal dysfunction: an emerging independent cardiovascular risk factorHeart200389996396412922986
- FerrarioCMStrawnWBRole of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular diseaseAm J Cardiol200698112112816784934
- RusterCWolfGRenin-angiotensin-aldosterone system and progression of renal diseaseJ Am Soc Nephrol200617112985299117035613
- RuggenentiPPernaARemuzziGACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in NephropathyJ Am Soc Nephrol200112122832283711729254
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- BerlTHunsickerLGLewisJBCardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathyAnn Intern Med2003138754254912667024
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- NussbergerJWuerznerGJensenCBrunnerHRAngiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalaprilHypertension2002391E1E811799102
- ItoSNakuraNLe BretonSKeefeDEfficacy and safety of aliskiren in Japanese hypertensive patients with renal dysfunctionHypertens Res2010331626619927154
- PerssonFRossingPSchjoedtKJTime course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetesKidney Int200873121419142518337712
- MorishitaYHanawaSChindaJIimuraOTsunematsuSKusanoEEffects of aliskiren on blood pressure and the predictive biomarkers for cardiovascular disease in hemodialysis-dependent chronic kidney disease patients with hypertensionHypertens Res201134330831321124333
- ParvingHHPerssonFLewisJBLewisEJHollenbergNKAliskiren combined with losartan in type 2 diabetes and nephropathyN Engl J Med2008358232433244618525041
- McMurrayJJPittBLatiniREffects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failureCirc Heart Fail200811172419808266
- MatsuoSImaiEHorioMRevised equations for estimated GFR from serum creatinine in JapanAm J Kidney Dis200953698299219339088
- RodbardDStatistical quality control and routine data processing for radioimmunoassays and immunoradiometric assaysClin Chem19742010125512704370388
- EmanuelRLCainJPWilliamsGHDouble antibody radioimmunoassay of renin activity and angiotensin II in human peripheral plasmaJ Lab Clin Med19738146326404348744
- BeckettNSPetersRFletcherAETreatment of hypertension in patients 80 years of age or olderN Engl J Med2008358181887189818378519
- YoungJHKlagMJMuntnerPWhyteJLPahorMCoreshJBlood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP)J Am Soc Nephrol200213112776278212397049
- OkadaTNakaoTMatsumotoHPrognostic significance of home blood pressure control on renal and cardiovascular outcomes in elderly patients with chronic kidney diseaseHypertens Res200932121123112919816503
- HenryRMKostensePJBosGMild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn StudyKidney Int20026241402140712234312
- IshaniAGranditsGAGrimmRHAssociation of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trialJ Am Soc Nephrol20061751444145216611715
- HalbesmaNKuikenDSBrantsmaAHMacroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screeningJ Am Soc Nephrol20061792582259016899519
- TonelliMJosePCurhanGSacksFBraunwaldEPfefferMProteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trialBMJ20063327555142616714328
- LatiniRMassonSAnandIThe comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFTEur Heart J200425429229914984917
- JourdainPJondeauGFunckFPlasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter StudyJ Am Coll Cardiol200749161733173917448376
- SchmiederREPhilippTGuerediagaJLong-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12-month randomized, double-blind comparator trial with hydrochlorothiazideCirculation2009119341742519139391