Abstract
Background
Although deep hypothermic circulatory arrest (DHCA) is a useful option to protect the central nervous system during aortic arch operations, the influence of simultaneous renal ischemia remains controversial.
Patients and Methods
This is a retrospective observational study. Sixty-three patients who underwent thoracic aortic surgery with DHCA and 24 patients who underwent cardiac surgery without DHCA were included in this study. The mean age, preoperative serum creatinine (Cr) level, preoperative estimated glomerular filtration rate (eGFR), peak serum Cr level up to 48 hrs post-operative, elevation rate of Cr compared to the preoperative serum Cr, urine volume rate up to 48 hrs post-operative and AKI staging using the KDIGO criteria were estimated for each patient. Clinical parameters for 3 months after the operation and the 3-month post-operative mortality rate were assessed. Mean values indicating kidney function or distribution of the AKI stages were compared between patients with and without DHCA. Patients with DHCA were further divided according to the duration of ischemia to compare the values for the kidney function of each group, distribution of AKI stages and mortality.
Results
The parameters indicating AKI of the patients with DHCA were significantly more severe than those without DHCA. Patients who had undergone an ischemic state for more than 40 min revealed significantly higher peak serum Cr, elevation rate of serum Cr, less urine volume up to 48 hrs post-operative compared with those without DHCA. Distribution of the AKI stages was related to the duration of ischemia. The 3-month post-operative mortality of the patients with DHCA was significantly higher than those without DHCA.
Limitations
This study had limitations such as its retrospective design and small number patients, and the data will be required confirmation with other prospective studies.
Conclusion
DHCA is closely related to AKI up to 48 hrs post-operative and death during the 3 months following surgery.
Introduction
Deep hypothermic circulatory arrest (DHCA) was developed for cerebral protection during aortic arch operations.Citation1 Since the nervous system has high metabolic demands and limited energy reserves, it is vulnerable to ischemia. A few minutes of global ischemia will result in neuronal death in a normothermic brain.Citation2 DHCA clinically refers to the lowering of the nasopharyngeal temperature to below 20°C. Since oxygen consumption decreases by 50% for every 10 degree decrease in body temperature, hypothermia can reduce the metabolic rate of the brain and increase the duration of ischemia.Citation3
While DHCA provides a bloodless surgical field for the surgical treatment of aortic arch or the descending/thoracoabdominal aorta, all of the end-organs are at risk of ischemic injury.Citation4 The kidney is one of the most sensitive organs to ischemia, in addition to neuronal tissues. Recently, post-operative acute kidney injury (AKI) has been associated with increased mortality,Citation5–7 and the incidence of AKI after thoracic aortic surgery ranges from 18% to 55%.Citation8–10
Since the RIFLE criteria were first developed for diagnosing AKI, the diagnostic criteria have been validated and modified.Citation11,Citation12 While the Kidney Disease: Improving Global Outcomes (KDIGO) established the most recent criteria,Citation13 only a few studies have examined the relationships between DHCA and AKI using the criteria and most of the past studies have lacked urine volume as a marker.Citation5–10,Citation14,Citation15 We adopted the KDIGO criteria and examined both serum Cr and urine volume levels to show the influences of DHCA on kidney function.
Patients and Methods
This was a retrospective and observational study using data collected from clinical records stored at Juntendo University Shizuoka Hospital.
Patients
Sixty-three patients who underwent open proximal (root or supracoronary ascending [±aortic valve] and aortic arch) thoracic aortic surgery with DHCA via median sternotomy between 2017 and 2019 at the Juntendo University Shizuoka Hospital, Shizuoka, Japan, were included in this study. Twenty-four patients who underwent cardiac surgery without DHCA were included as controls. The study was approved by the ethical committee of the Juntendo University Shizuoka Hospital, and the need for individual consent was waived. Patients were not required to give informed consent to the study because the analysis used anonymized clinical data that were obtained after each patient agreed to treatment by written consent. We also applied an opt-out method for consent in this study by using a poster on a bulletin board and the hospital website. These procedures were in accordance with the Ethical Guidelines for Medical and Health Research involving Human Subjects by the Japanese government.Citation18
Operative Procedure
The surgical procedure has been described previously.Citation16,Citation17 The protocols for ventilator management, extubation and postextubation care, including supplemental oxygen delivery, are described in . In brief, the patient was placed on full cardiopulmonary bypass (CPB), and systemic core cooling was then initiated. When the nasopharyngeal temperature (NPT) reached 15–18°C, the pump was turned off and the patients were exsanguinated into the cardiotomy reservoir. When the CBP pump was restarted, the patient was rewarmed slowly to maintain the temperature difference between the CPB circuit arterial blood and the patient’s NPT < 10°C. After surgery, the patient was weaned from CPB according to the protocol, ie, when the patient’s rectal temperature (RT) was >36.5°C.Citation19
Table 1 Ventilation Protocol for Thoracic Aortic Surgery
Adjunctive antegrade (ACP) or retrograde (RCP) cerebral perfusion was used for protection in all circulatory arrest cases. For ACP cases, the right axillary artery was cannulated with an 8-mm Dacron side graft and ACP was performed with the base of the innominate and left common carotid arteries clamped and perfusion via the right axillary graft at a flow rate of 5–15 mL/kg/min and an inflow temperature of 12°C to a target right radial arterial line pressure of 50–70 mmHg. For RCP cases, a right-angled, long 26F superior vena cava cannula was used with an average flow rate of 150–450mL/min at 12°C retrograde via a snared superior vena cava cannula to a target central venous pressure of 25mmHg.Citation20
Data Collection
Clinical data including each patient’s age, gender, body weight and preoperative serum creatinine concentration (Cr) were obtained from the electronic medical record system in the Juntendo University Shizuoka Hospital. The peak concentration of serum Cr and urine volume (UV) up to 48 hrs post-operative were collected. To evaluate chronic damage of kidney function, serum Cr concentrations were also obtained 3 months after the operation with or without DHCA. The estimated glomerular filtration rate (eGFR) was calculated using the formula established by the Japanese Society of Nephrology for Japanese people: 194×s-Cr−1.094×age−0.287 (×0.739 if female).Citation21 Other parameters were calculated according to the following formula:
The stage of acute kidney injury (AKI) was made according to the KDIGO clinical guideline for acute kidney injury.Citation13 AKI staging using only Cr standards was applied to assess the staging without urine volume standards. We compared the clinical parameters, including age, preoperative Cr, preoperative eGFR, peak Cr, elevation rate of Cr, total UV within post-operative 48h, UV rate and distribution of the AKI stage, between patients with or without DHCA. The patients were then divided into three groups, A, B and C, according to the duration of ischemia (min). Group A patients, who had no ischemic time, served as controls. Those in groups B and C underwent ischemia for ≤39 min (median ischemic time) and >40 min, respectively. We compared the above-mentioned parameters between these groups. Finally, we compared the ΔeGFR, mean eGFR 3 months after the operation and mortality between patients with or without DHCA, in groups (A to C) and in the post-operative AKI stages.
Among patients with DHCA, we evaluated the brain transfusion method. The patients were divided into two groups: those who were treated with ACP and those with RCP. Patients undergoing similar approaches were compared between those with or without DHCA.
Statistical Analysis
Data are expressed as the mean±standard error (SE). The mean values were compared between two groups by Student t-test and between three or more groups by analysis of variance (ANOVA). Dunnett’s test was applied for post hoc analysis of the differences between control group A and the other groups. Categorical variables were compared using Chi-square test. Statistical significance was considered at P<0.05. All statistical analyses were performed by JMP 11 (SAS Institute Inc., Cary, NC) software.
For the propensity score-matched analysis, we investigated subjects with DHCA (n=63) and those without DHCA (n=24). We calculated the propensity score using multivariable logistic regression model with confounding factors of gender, age, body weight and a difficulty rank for cardiovascular trainees in Japan ().Citation22 We constructed a 1:1 (caliper distance: 0.2) matched pair to help control for selection bias. Matching was done using a semi-automated add-in package for JMP.
Table 2 Baseline Characteristics with and without DHCA and Operative Methods
Results
The Effects by DHCA on Kidney Function
The mean age of patients who underwent DHCA (n=63) was 70.4±1.2 years, while that of patients without DHCA (n=24) was 68.5±2.0 years (). The preoperative serum Cr levels of patients with and without DHCA were 0.92±0.03 mg/dL and 0.85±0.05 mg/dL, respectively (). Even preoperative eGFR, which was corrected by the patient’s age and gender, showed no significant differences (49.8±2.2 mL/min/1.73m2, 58.0±3.7mL/min/1.73m2; with DHCA and without DHCA, respectively) (). These findings suggest that the preoperative kidney function was not different between patients with and without DHCA.
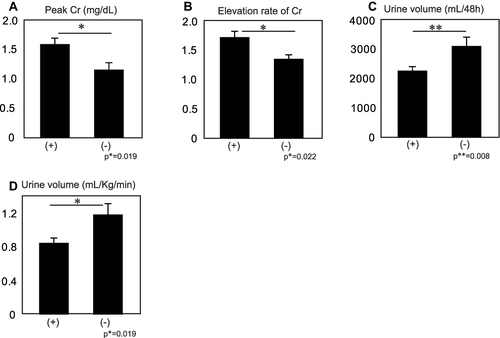
The peak serum Cr value within the 48-hrs post-operative period was significantly higher for patients with DHCA than that for patients without DHCA (1.58±0.09 mg/dL, 1.15±0.15 mg/dL, respectively; p*=0.019) (). The elevation rate of the serum Cr within the 48 hrs post-operative reveals significantly higher responses among patients with DHCA (1.71±0.03), compared with the value of patients without DHCA (1.34±0.13, p*=0.022) (). The collected urine volume within the 48-hrs post-operative of patients with DHCA was 2251.8±162.5mL while that without DHCA was 3087.3±263.4 mL (p**=0.008) (). Urine volume rate was also significantly lower in patients with DHCA compared with that of patients without DHCA (0.85±0.07, 1.19±0.11, respectively; p*=0.019) (). Distribution of the AKI stage (KDIGO) suggested that patients with DHCA suffered from more severe AKI compared with patients without DHCA (Pearson, p*=0.010) (). Between the propensity-matched patients with or without DHCA, (n=24, each group), similar results were observed compared with unmatched patients (Pearson, p*=0.041) (). Taken together, these results suggested that DHCA is a possible cause of post-operative AKI.
Table 3 AKI Stage (KDIGO): The Distribution of AKI Stage (KDIGO) Correlated with DHCA
Table 4 AKI Stage (KDIGO) Among the Propensity-Matched Patients: The Distribution of AKI Stage (KDIGO) Correlated with DHCA
Figure 1 Pre- and post-operative data for the patients with (+) (n=63) or without DHCA (-) (n=24). The data are expressed as the mean ± standard error (SE). (A) Peak serum creatinine at 48 hrs post-operative. Peak serum creatinine was significantly elevated in the patients with DHCA compared with those without (p*=0.019). (B) Elevation rate of serum creatinine at 48 hrs post-operative. The mean elevation rate of serum creatinine in the patients with DHCA was significantly higher than those without (p*=0.022). (C) Urine volume at 48 hrs post-operative. The mean 48h-collected urine volume was significantly less in the patients with DHCA than in those without (p**=0.008). (D) Urine volume rate at 48 hrs post-operative. The mean urine volume rate (mL/kg/h) is significantly less in the patients with DHCA than those without DHCA (p*=0.019).
Influence of Cerebral Perfusion Methods
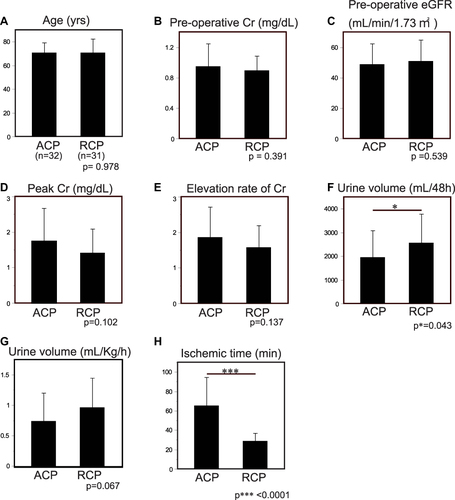
Patients with DHCA supported by ACP (n=32) and RCP (n=31) were compared with the remaining clinical subjects. Age, preoperative Cr, eGFR, peak Cr, elevation rate of Cr, urine volume and AKI stages (KDIGO) were not statistically significant between groups ( and ). Total urine volume 48 hours post-operative and ischemic time were significantly different among patients with ACP than those with RCP (p*=0.043, p***<0.0001, respectively) ().
Table 5 Distribution of AKI Stage (KDIGO) Between Patients with ACP or RCP
Figure 2 Influence of the cerebral perfusion methods. (A) Mean age of the patients who had undergone ACP or RCP. (B) Preoperative Cr levels. No significant difference was observed. (C) Preoperative eGFR. (D) Peak Cr levels during post-operative 48h. (E). Elevation rate of Cr. (F) Urine volume during post-operative 48. The patients treated with RCP showed more urine excretion than those with ACP (p*=0.043). (G) Urine excretion rate (mL/kg/min) during post-operative 48h. (H) Ischemic time during operation. Patients treated with ACP underwent significantly longer ischemia than patients with RCP. The cause is mentioned in Discussion.
Ischemic Duration and Kidney Function
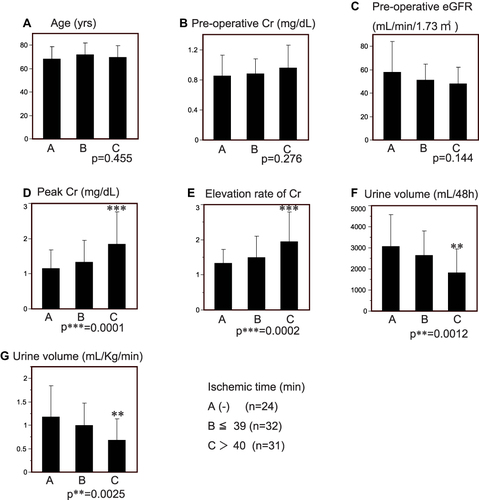
The changes of kidney function at 48-hrs post-operative were assessed according to the duration of ischemia. The mean ages of the control group A, and groups B and C were 68.5±2.1, 71.8±1.8 and 769.6±9.9 years, respectively, and there was no significant difference between those groups (). The mean preoperative serum Cr levels were not significantly different (A, B and C; 0.85±0.05, 0.88±0.03 and 0.96±0.30 mg/dL, respectively) (), as well as the preoperative mean eGFR levels (A, B and C; 58.0±3.7, 51.2±2.4 and 48.4±1.4 mL/min/1.73m2, respectively) (). The mean peak serum creatinine concentration at 48 hrs post-operative for group C (ischemic duration ≥40 min) was 1.84±0.16 mg/dL, which revealed a significant elevation compared with that of control group A (1.15±0.15 mg/dL; p***=0.0001) (). The elevation rate of serum Cr of patients in group C was also significantly higher than the control group (1.94±0.15 vs 1.34±0.13; p***=0.0002) (). A longer duration of ischemia led to a decrease in the mean collected urine volume at 48 hrs post-operative period (A, B, and C; 2087.3±256.9, 2653±204.3, and 1836±202.0mL, respectively). Group C patients showed a significant decrease in the urine volume, compared with group A patients (p**=0.0012) (). Furthermore, the urine excretion rate of patients in group C was significantly less than control group A (1.19±0.13 vs 0.89±0.08 mL/kg/min, p**=0.0025) (). Distribution of the AKI stage (KIDIGO) revealed significant differences among the groups and suggested that the longer duration of ischemia led to more severe AKI (Pearson, p**=0.0027) ().
Table 6 Ischemic Duration and AKI
Figure 3 Ischemic duration and AKI. Patients in group A did not undergo DHCA, as controls. Those belonging to groups B and C underwent ischemia for ≤39 minutes and >40 minutes, respectively. Data are expressed as the mean ± SE. (A) Age. There were no differences in the mean age between the groups. (B) Preoperative serum creatinine. No significant differences were observed. (C) Preoperative eGFR. No significant differences were observed. (D) Peak serum creatinine at 48 hrs post-operative. Patients in group C showed a significant elevation in mean serum creatinine compared with the control group A (p***=0.0001). (E). Elevation rate of serum creatinine at 48 hrs post-operative. The mean elevation rate of serum creatinine of group C was significantly higher compared with the control group A (p***=0.0002). (F) Urine volume at 48 hrs post-operative. The mean 48h-collected urine volume was significantly less in group C compared with the control group A (p**=0.0012). (G) Urine volume rate at 48 hrs post-operative. The urine volume rate in group C was significantly less than that in the control group A (p**=0.0025).
Long-Term Effects of DHCA on Kidney Function and Mortality
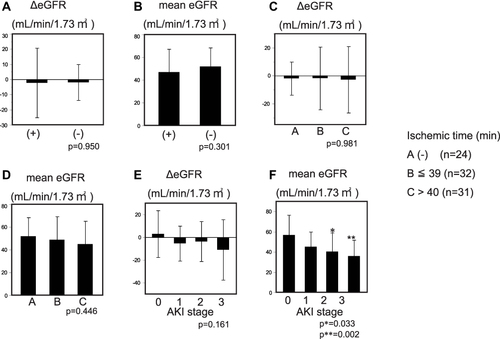
The long-term effects of DHCA on kidney function and mortality were assessed. The difference between the preoperative eGFR and that at 3 months after operation (ΔeGFR) showed that there was no difference between patients with and without DHCA (p=0.950) (). The mean eGFR of patients with DHCA at 3 months after the operation was 49.9±2.6 L/min/1.73m2, while that of patients without DHCA was 51.9±4.0 mL/min/1.73m2 (p=0.301.; ). The mortality within the 3-month post-operative period of patients with DHCA was 22.2% compared with 4.2% for those without DHCA (). Chi-square test suggested that the mortality was significantly higher for patients with DHCA compared with those without (Pearson, p*=0.046) (). The mortality between propensity-matched patients with and without DHCA showed no significant difference (Pearson, p=0.073) ().
Table 7 Long-Term Effects of DHCA on Kidney Function and Mortality the 3-Month Post-Operative Mortality
Table 8 The 3-Month Post-Operative Mortality of the Propensity-Matched Patients
Figure 4 Long-term effects of DHCA on kidney function and mortality. (A) Long-term change of eGFR between patients with or without DHCA. No significant difference was observed. (B) Mean eGFR 3 months after operation. No significant difference was observed. (C) ΔeGFR. Difference between 3-month post-operative and preoperative eGFR. No significant difference was observed for any of the groups. (D) Three-month post-operative eGFR. The mean 3-month post-operative eGFR was not significantly different between groups. (E) ΔeGFR and post-operative AKI stage. Differences between the 3-month post-operative and preoperative eGFR. The patients were divided by post-operative AKI stage (KDIGO). Patients who avoided post-operative AKI belong to Group 0. (F) Three-month post-operative eGFR and AKI stage. The mean 3-month post-operative eGFRs for patients with AKI stage 2 and 3 were significantly less than those without AKI (p*=0.033, p**=0.0022, respectively).
The ΔeGFR values for patients in groups A, B and C were −1.96±11.95, −1.78±22.47 and −2.81±23.75 mL/min/1.73m2, respectively (p=0.981) (). The mean eGFR at 3 months after the operation for groups A, B and C were 51.9±16.6, 48.8±20.3 and 44.9±20.3 mL/min/1.73m2, respectively (p=0.446) (). The 3-month post-operative mortality was not significantly different between groups (Pearson, p=0.104) ().
Table 9 Three-Month Post-Operative Mortality
We divided the patients according to the AKI stage within the post-operative 48-hrs. The ΔeGFR values for the patients without AKI (Stage 0), Stage 1, 2 and 3 (KDIGO) were 3.0±20.4, −5.3±15.3, −3.7±17.6 and −10.9±26.5 mL/min/1.73m2, respectively (p=0.161) (). The mean 3-month post-operative eGFR values for patients in Stages 0, 1, 2 and 3 group were 56.7±19.7, 45.2±14.5, 40.3±18.6 and 35.8±15.8 mL/min/1.73m2. Patients who underwent Stage 2 or 3 AKI revealed significant reductions of the eGFR 3 months after the operation compared with those patients without AKI (Stage 2 vs Stage 0; p*=0.033, Stage 3 vs Stage 0; p**=0.0022) (). No significant differences in the mortality were observed during the 3-months post-operative according to the AKI stages ().
Table 10 Three-Month Post-Operative Mortality and AKI Stage
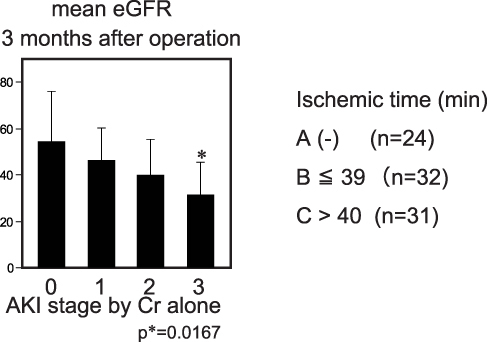
The effect of staging AKI by Cr standards alone was assessed. Distribution of AKI stage between the patients with and without DHCA was not significantly different by using both Cr and urine volume standards (Pearson, p=0.0761) (). Similar results were observed in the relationships between the distribution of AKI stage and ischemic time (Pearson, p*=0.011) (). While the late mean eGFR (3 months after operation) showed a similar tendency of reducing eGFR by progression of Stage, a significant reduction was observed in group C compared with control group A (Stage 3 vs Stage 0; p*=0.029) (). There were no significant differences between the data using Cr and urine volume standards and Cr standards alone ().
Table 11 Distribution of AKI Stage Between Patients with and without DHCA
Table 12 Distribution of AKI Stage and Ischemic Time
Table 13 Three-Months Post-Operative Mortality
Figure 5 Effect of AKI staging with Cr standards alone. The eGFR of patients with Stage 3 AKI were significantly less than patients without AKI (p*=0.0167).
Taken together these findings, it is suggested that 1) there is a close relationship between DHCA and post-operative AKI; 2) patients who have undergone ischemia for more than 40 min may show significant increases of serum Cr and reduction of urine volume, resulting in severe AKI; 3) the long-term effects of DHCA for kidney function are less apparent among the surviving patients, while post-operative mortality is greater among patients with DHCA; and 4) The severity of AKI relates the reduction of long-term kidney function, leading to advanced CKD. Since the small number of cases included and retrospective nature of our study does not allow us to generalize the results obtained, further large multicenter prospective studies are needed to validate these data.
Discussion
One of the primary goals in aortic arch surgery is to preserve cerebral function, since brain tissue has a high metabolic rate and is especially sensitive to ischemia. These limitations have been overcome through the development of two options to minimize cerebral ischemia: DHCA to suppress cerebral metabolism and ACP or RCP to supply the metabolites to the brain.Citation22 However, the risk of post-operative AKI still remains, despite the use of these options.
We adopted the diagnostic criteria for AKI established by the KDIGO, which was a modified version of the prior RIFLE and AKIN criteria. Patients who were missed for AKI, using both the RIFLE and AKIN criteria, showed poor prognoses.Citation11–13 Although, according to these criteria, patients must be evaluated based upon elevation of serum creatinine and urine excretion rate, urine excretion has been ignored in most similar reports.Citation5–10,Citation14,Citation15 Our findings showed that there was no significant difference of AKI distribution between patients with and without DHCA by using Cr standards alone ( and ). The AKI staging with serum Cr alone in our cohort tended to classify milder stages and possibly influenced the statistical underestimation ( and ).
Our control patients without DHCA did not undergo aortic arch surgery. Every patient with aortic arch disease underwent treatment with DHCA and ACP or RCP. We compared the influence of the cerebral perfusion methods (). The only significant difference between patients was ischemic time. This phenomenon was derived from our institutional protocol. The surgeons selected RCP in the ascending arch replacement and they perform ACP for partial and total arch replacement. Our protocol of DHCA required the patient’s NPT at 15–18°C; belonging to the “deep hypothermia” category (14.1–20°C).Citation17 However, the appropriate temperature remains controversial, at moderate temperatures with selective ACP selected more frequently by a meta-analysis.Citation17 All of the control patients underwent median sternotomy, CPB, mechanical ventilation and post-extubation care in the intensive care unit (ICU). All of the patients who participated in this study underwent surgery with a difficulty rank B or C by a cardiovascular surgery trainee in JapanCitation22 and the proportion of patients with rank C with and without DHCA were 60.3% and 66.7%, respectively (n.s. chi-square test). Patients without DHCA were considered suitable as the negative controls for this study.
Previous observations have suggested that DHCA is not a risk factor for AKI in thoracic aortic surgery.Citation8 While 28% of the participating patients underwent DHCA in this study, 72% among our overall population underwent DHCA. The incidence of AKI among patients with DHCA was 20.6% and 65.1% in previous observations and our study, respectively. This discrepancy might be a consequence of the age. The mean ages of patients in the former study and ours were 59 and 70 years, respectively; thus, our patients were older. The mean ages of patients with AKI were 66and 70 years in both populations. It is suggested that age distribution is critical for determining the incidence of AKI.
The duration of ischemia and AKI risk have been described previously; with studies suggesting that over 30 minutes of ischemia by DHCA was a risk factor for AKI including overall severity.Citation14,Citation23 The occurrence of AKI after partial nephrectomy remains controversial. One report has suggested that the occurrence rate depends on the duration of ischemia,Citation24 while another suggested that the human kidney is resistant against ischemia for less than 1 hour.Citation25 Under these conditions, one kidney is preserved. Surgery with DHCA burdens the kidneys with more stress. Even one report that described a favorable survival rate following operation with DHCA limited the cases to a duration of ischemia below 50 minutes.Citation26 These observations suggest that there is a critical point for severe AKI and survival at around 50 minutes of ischemia.
It has been suggested that long-term effects of DHCA do not relate to the late kidney function but rather mortality. Most of the surviving patients recover from AKI. Patients who have undergone severe AKI tend to develop and transfer to CKD. Similar results have been reported among patients who have undergone cardiac surgery.Citation26 In this cohort, patients who have undergone post-operative AKI are at higher risk of late myocardial infarction, as a post-operative risk. It has been determined that the post-operative serum Cr level affects the statistical outcome(s).Citation27 Thus, the severity of AKI correlates with post-operative cardiovascular events and mortality.Citation26 AKI has been identified as a significant risk factor of CKD.Citation28 Our results show that DHCA is not a risk for CKD but might lead to higher mortality among patients with severe AKI during the perioperative period.
Limitations
This study was a retrospective study in a single center based on medical records, and the data will be required confirmation with other prospective studies.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, Y.S. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Ethics Approval Statement
This study was approved by the Ethics Committee of the Juntendo University Hospital and carried out in accordance with Declaration of Helsinki principles (No. E21-0143).
Author Contributions
All the authors made a significant contribution to the work reported, whether that is in conception, study design, execution, acquisition data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest associated with this paper.
Acknowledgments
We are indebted to the nephrologists, cardiac surgeons, nursing and clinical engineering staff at the Juntendo University Shizuoka Hospital for their collaboration and participation in this study. We also thank to Dr Keiichi Tambara for his technical advices.
Additional information
Funding
References
- Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–1063.
- Parissis H, Hamid U, Soo A, Al-Alao B. Brief review on systematic hypothermia for the protection of central nervous system during aortic arch surgery: a double-sword tool? J Cardiothorac Surg. 2011;153:54.
- Michenfelder JD, Milde JH. The effect of profound levels of hypothermia (below 14 degrees C) on canine cerebral metabolism. J Cereb Blood Flow Metab. 1992;12:877–880. doi:10.1038/jcbfm.1992.120
- James ML, Andersen ND, Swaminathan M, et al. Predictors of electrocerebral inactivity with deep hypothermia. J Thorac Cardiovasc Surg. 2014;147:1002–1007. doi:10.1016/j.jtcvs.2013.03.022
- Takahashi T, Hasegawa T, Hirata N, et al. Impact of acute kidney injury on in-hospital outcomes in patients with DeBakey type III acute aortic dissection. Am J Cardiol. 2014;113:1904–1910. doi:10.1016/j.amjcard.2014.03.023
- Roh GU, Lee JW, Nam SB, Lee J, Choi JR, Shim YH. Incidence and risk factors of acute kidney injury after thoracic aortic surgery for acute dissection. Ann Thorac Surg. 2012;94:766–771. doi:10.1016/j.athoracsur.2012.04.057
- Tsai HS, Tsai FC, Chen YC, et al. Impact of acute kidney injury on one-year survival after surgery for aortic dissection. Ann Thorac Surg. 2012;94:1407–1412. doi:10.1016/j.athoracsur.2012.05.104
- Englberger L, Suri RM, Li Z, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi:10.1186/cc9960
- D’Onofrio A, Cruz D, Bolgan I, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010;16 Suppl 1:S32–S36. doi:10.1111/j.1751-7133.2010.00170.x
- Arnaoutakis GJ, Bihorac A, Martin TD, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007;134:1554–1560. doi:10.1016/j.jtcvs.2007.08.039
- Maccariello E, Soares M, Valente C, et al. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med. 2007;33:597–605. doi:10.1007/s00134-007-0535-0
- Ronco C, Levin A, Warnock DG, et al.; AKIN Working Group. Improving outcomes from acute kidney injury (AKI): report on an initiative. Int J Artif Organs. 2007;30:373–376. doi:10.1177/039139880703000503
- Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. doi:10.1186/cc11454
- Mori Y, Sato N, Kobayashi Y, Ochiai R. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J Anesth. 2011;25:799–804. doi:10.1007/s00540-011-1210-8
- Jang WS, Kim WH, Choi K, et al. Incidence, risk factors and clinical outcomes for acute kidney injury after aortic arch repair in paediatric patients. Eur J Cardiothorac Surg. 2014;45:e208–e214. doi:10.1093/ejcts/ezu132
- Schechter MA, Shah AA, Englum BR, et al. Prolonged postoperative respiratory support after proximal thoracic aortic surgery: is deep hypothermic circulatory arrest a risk factor? J Crit Care. 2016;31:125–129. doi:10.1016/j.jcrc.2015.10.021
- Tian DH, Wan B, Bannon PG, et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann Cardiothorac Surg. 2013;20:148–158.
- Ethical Guidelines for Medical and Health Research Involving Human Subjects. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf. Accessed September 6, 2022.
- Chong SY, Chow MY, Kang DS, Sin YK, Sim EK. Ti LK Deep hypothermic circulatory arrest in adults undergoing aortic surgery: local experience. Ann Acad Med Singapore. 2004;33:289–293.
- Ganapathi AM, Hanna JM, Schechter MA, et al. Antegrade versus retrograde cerebral perfusion for hemiarch replacement with deep hypothermic circulatory arrest: does it matter? A propensity-matched analysis. J Thorac Cardiovasc Surg. 2014;148:2896–2902. doi:10.1016/j.jtcvs.2014.04.014
- Matsuo S, Imai E, Horio M, et al.; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi:10.1053/j.ajkd.2008.12.034
- The Japanese board of cardiovascular surgery. Available from: http://cvs.umin.jp/std/result3.html. Accessed September 6, 2022. In Japanese.
- Kim WH, Park MH, Kim HJ, et al. Potentially modifiable risk factors for acute kidney injury after surgery on the thoracic aorta: a propensity score-matched case-control study. Medicine. 2015;94:e273. doi:10.1097/MD.0000000000000273
- Zhang Z, Zhao J, Dong W, et al. Acute kidney injury after partial nephrectomy: role of parenchymal mass reduction and ischemia and impact on subsequent functional recovery. Eur Urol. 2016;69:745–752. doi:10.1016/j.eururo.2015.10.023
- Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506–517. doi:10.1681/ASN.2012080786
- Damberg A, Carino D, Charilaou P, et al. Favorable late survival after aortic surgery under straight deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2017;154:1831–1839.e1. doi:10.1016/j.jtcvs.2017.08.015
- Hansen MK, Gammelager H, Jacobsen CJ, et al. Acute kidney injury and long-term risk of cardiovascular events after cardiac surgery: a population-based cohort study. acute kidney injury and long-term risk of cardiovascular events after cardiac surgery: a population-based cohort study. J Cardiothorac Vasc Anesth. 2015;29:617–625. doi:10.1053/j.jvca.2014.08.020
- He L, Wei Q, Liu J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92:1071–1083. doi:10.1016/j.kint.2017.06.030
