Abstract
Chronic kidney disease (CKD) is a complex debilitating condition affecting more than 70 million people worldwide. With the increased prevalence in risk factors such as diabetes, hypertension, and cardiovascular disease in an aging population, CKD prevalence is also expected to increase. Increased awareness and understanding of the overall CKD burden by health care teams (patients, clinicians, and payers) is warranted so that overall care and treatment management may improve. This review of the burden of CKD summarizes available evidence of the clinical, humanistic, and economic burden of CKD and the current unmet need for new treatments and serves as a resource on the overall burden. Across countries, CKD prevalence varies considerably and is dependent upon patient characteristics. The prevalence of risk factors including diabetes, hypertension, cardiovascular disease, and congestive heart failure is noticeably higher in patients with lower estimated glomerular filtration rates (eGFRs) and results in highly complex CKD patient populations. As CKD severity worsens, there is a subsequent decline in patient health-related quality of life and an increased use of health care resources as well as burgeoning costs. With current treatment, nearly half of patients progress to unfavorable renal and cardiovascular outcomes. Although curative treatment that will arrest kidney deterioration is desired, innovative agents under investigation for CKD to slow kidney deterioration, such as atrasentan, bardoxolone methyl, and spherical carbon adsorbent, may offer patients healthier and more productive lives.
Introduction
Chronic kidney disease (CKD) is a debilitating disease affecting approximately 7% of all people aged 30 years and older, which translates to more than 70 million people in developed countries worldwide.Citation1 This number is likely to be much higher given the unknown prevalence in underdeveloped countries. The increased prevalence of diabetes, hypertension, and obesity and an aging population will only perpetuate the rise of CKD.Citation2–Citation5 Patients have been, and continue to be, diagnosed with CKD later in the disease cycle, and therefore have to be prepared for life on dialysis or to undergo kidney transplant. However, with better screening, early management, and innovative pharmacologic therapies, the disease progression may be delayed and patients with CKD may enjoy healthier and more productive lives.
The objective of this targeted literature review is to present the clinical, humanistic, and economic burden of CKD and the current corresponding unmet treatment need. We searched the PubMed database via the National Library of Medicine Gateway and conducted supportive desktop research (eg, ClinicalTrials.gov). Search categories included “chronic kidney disease,” “epidemiology,” “disease classification,” “progression,” “patient-reported outcomes,” “economics,” and “treatment.” The language and date limits applied to the search were English only and 1980 to February 2012, respectively. Original research, key reviews, current guidelines, and drug-specific reports/press releases were selected for inclusion. Findings are presented qualitatively.
The complex clinical nature of CKD is characterized and presented, including a description of the foundational interrelated factors of disease and progression that underlie the true burden and unmet medical needs of CKD. Further, current treatment options are reviewed to outline the existing unmet treatment need. Future treatments under development to address these key unmet needs are also presented.
Increasing prevalence and global burden of CKD
The trend for increased prevalence of CKD in the USA and select countries, irrespective of the calculation, implies persistent and rapid growth worldwide. Reported prevalence estimates across countries range broadly from approximately 2.0% to 44%.Citation1–Citation5 The broad range in prevalence exemplifies the differences in patient populations and unmet clinical, humanistic, and economic needs across the globe. In the USA, the fastest increase in prevalence is occurring among those aged 65 years and older. Across three databases, the Kidney Early Evaluation Program, National Health and Nutrition Examination Survey, and Medicare, prevalence in the elderly population (aged ≥ 65 years) was approximately 44%, with the highest representation observed in those aged 80 years and older.Citation4 Across countries (Australia, Canada, China, Iceland, Italy, Japan, Mexico, Netherlands, Norway, Singapore, Spain, Switzerland, Thailand, and USA), estimates were similarly high in the elderly and ranged from 23.4% to 44%.Citation1,Citation4
Prevalence estimates for several modifiable risk factors affecting initiation and/or progression of disease have also increased. National Health and Nutrition Examination Survey 2001–2008 data report that diabetes, hypertension, cardiovascular disease, and congestive heart failure are more prevalent in patients with estimated glomerular filtration rates (eGFRs) <60 mL/min/1.73 m2; additionally, the prevalence of hypertension is twofold and the prevalence of cardiovascular disease is fivefold greater compared with those with eGFR > 60 mL/min/1.73 m2.Citation5 As the prevalence of diabetes, hypertension, and other risk factors rise, so does the severity of CKD. For example, the frequency of diabetes increased more than five times by CKD stage and eGFR category. Diabetes occurred in an estimated 7% of patients in stage 1/2 (eGFR > 60 mL/min/1.73 m2), 18% of patients in early stage 3 (eGFR 45 mL/min/1.73 m2 to <60 mL/min/1.73 m2), 27% of patients in late stage 3 (eGFR 30 mL/min/1.73 m2 to <45 mL/min/1.73 m2), and 40% of patients in stages 4 and 5 (eGFR < 30 mL/min/1.73 m2).Citation5
Given the increase in CKD prevalence and associated risk factors, more global epidemiological research is needed to better characterize the international burden of CKD. With improved patient-level definitions of CKD, large-scale epidemiological studies may present more adequately representative populations with CKD across countries.
Clinical burden
Complexity of disease
Attention to traditional measures of kidney function (eg, eGFR) is no longer adequate to optimally manage and care for patients with CKD. With the increase in CKD patients diagnosed with diabetes, hypertension, and obesity, consideration must also be given to these and other preexisting, emerging risk factors and comorbid illnesses. Patients with significantly increased risk, with or without confirmed CKD, may require more aggressive management to avoid the consequences of accelerated disease progression.
Disease classification and staging
The diagnosis, treatment, and management of CKD depend on classification and staging of the disease as set forth by international, country-specific, and other clinical guidelines.Citation6–Citation13 As evidenced by a variety of definitions and staging systems in the scientific literature, identification of optimal patient care strategies and interpretation of data are complicated. To date, the most frequently cited and used CKD staging system is that developed by the National Kidney Foundation, Dialysis Outcomes Quality InitiativeCitation12,Citation14,Citation15 ().
Table 1 Classification of chronic kidney disease
Although measurement of eGFR is considered the gold standard for diagnosing and evaluating progression of CKD, there is a movement among clinicians and researchers to improve clinical guidelines specifically related to diagnosis, classification, and staging. The following frequently reported criticisms of current guidelines have prompted the discussion for modification of current clinical guidelines.Citation16,Citation17
A diagnosis based on current eGFR estimation formulas is imprecise.
There is an absence of risk-stratification across patients; those at high risk for disease progression are not identified, and stage 3 is too broad (eg, stage 3 should be stratified into 3a [eGFR 45–59 mL/min/1.73 m2] and 3b [eGFR 30–44 mL/min/1.73 m2]).
Patient variability (age, sex, race, ethnicity) is not considered in current methods of evaluation (eg, eGFR and proteinuria estimation, at risk for progression, prognosis).
In an effort to collaborate and provide a foundational set of international CKD guidelines that address these criticisms, Kidney Disease Improving Global Outcomes (KDIGO) formed a dedicated workgroup. Publication of the KDIGO “Clinical practice guideline on CKD classification and management” is anticipated in 2012. The proposed guideline structure and associated discussion points are as follows:Citation17
Guideline 1: Definition and stages of CKD. Stages modified and enriched to include different degrees of proteinuria; splitting stage 3 into 3a and 3b; define differences between kidney damage and disease.
Guideline 2: Identification and evaluation of CKD. eGFR, high-risk population evaluation, appropriateness and frequency of testing.
Guideline 3: Estimation of glomerular filtration rate (GFR). New equations that address “within-individual” biological variability, age, and ethnicity.
Guideline 4: Estimation of proteinuria. Regional variability in methods; change lexicon from microalbuminuria to albuminuria mild, moderate, or severe.
Guideline 5: Definition of progression of CKD. Definition of change in eGFR, acute versus chronic change, duration and stability of change, prognostic indicators, frequency of testing.
Because of the complex nature of CKD, emphasis on well-defined disease classification and staging is paramount for optimal patient care. The evidence presented here elucidates several clinical unmet needs: for example, well-defined guidelines for use by primary care physicians, internists, and nephrologists; early identification, prevention, and management of patients at risk for CKD (pre-CKD); “on-time” referral to a nephrologist when patients with early CKD are progressing to intermediate or advanced stages of CKD; optimal management for patients with confirmed CKD and those at risk for accelerated progression (eg, complex cases with multiple comorbidities); and educational programs and tools developed for primary care physicians, internists, and nephrologists to address a host of topics (eg, clinical guidelines, population-specific treatment, and management).
Risk factors and comorbid illness
The intertwined nature of risk factors and comorbid illnesses complicates the characterization of CKD. These terms are often used interchangeably due to the continuous and progressive nature of this disease. Some patients may have risk factors for developing CKD, while others may have risk factors contributing to the progression of CKD. Some comorbid illnesses are risk factors for both the initiation and the progression of disease (eg, diabetes and cardiovascular disease). However, some risk factors are simply nonmodifiable patient characteristics (eg, ethnicity, sex). A host of preexisting and traditional progression factors has been reported, with emerging risk factors and biomarkers also identified.Citation18 presents common risk categories and associated factors.
Table 2 Common chronic kidney disease (CKD) risk categories and risk factors
As noted, some conditions fit across categories (eg, preexisting and advancing cardiovascular disease) and the presence of multiple risk factors and/or comorbid illnesses leads to progression of CKD and increased mortality.Citation19 Risk factors with overwhelming impacts on patients and health care systems include diabetes,Citation20–Citation25 anemia,Citation20,Citation22–Citation31 hypertension,Citation20–Citation23,Citation25,Citation27,Citation31 and hyperlipidemia.Citation4,Citation20,Citation32–Citation35 Patients with multiple risk factors and/or comorbidities bear the greatest burden.Citation22,Citation36,Citation37 Importantly, several of the debilitating risk factors and comorbidities are modifiable, and disease progression may be delayed with active patient–clinician collaboration and appropriate treatment. More attention to and early active management of modifiable risk factors and comorbidities are necessary to thwart rapid disease progression.
Disease progression
Stated simply, in CKD, “disease progression” means deterioration of kidney function. However, the underlying pathophysiology of progression is intricate. It has been postulated that any loss of functional renal mass, irrespective of cause, leads to glomerular hyperfiltration with an increased single-nephron glomerular filtration rate (GFR) and, subsequently, the loss of the ability of the remaining nephrons to autoregulate.Citation14,Citation38,Citation39 Renal injury progresses, with glomerular and tubular hypertrophy, sclerosis, and interstitial fibrosis. Proteinuria, decline in GFR, hypertension, kidney failure, and death from uremia are classic clinical features of the renal injury characteristic of progressive CKD.Citation14
Few studies with stage-to-stage CKD progression models have been published and each varies in terms of design and population of interest. Common prognostic variables across these models include demographic and laboratory variables such as age, sex, and eGFR, with average kidney function loss, measured by eGFR, between 2 and 8 mL/min each year.Citation40–Citation45 Tangri and colleaguesCitation44 found that the addition of fewer standard variables such as diabetes, hypertension status, blood pressure, and body weight demonstrated no improvement in model performance. However, the authors reported that these variables are clearly important for the diagnosis and management of CKD. The high prevalence of these conditions and imprecision with respect to measuring disease severity may have affected performance of these variables in this modeling study.Citation44 Further research is needed to accurately predict progression of CKD from those susceptible to and at risk for CKD to those actively progressing through the stages of CKD. Of note, two ongoing studies, the Chronic Renal Insufficiency Cohort studyCitation46 and the CKD Prognosis Consortium meta-analyses,Citation16 are actively engaged in research to better understand kidney function and risk factors of CKD progression in diverse and complex patient populations.
Humanistic burden
Patient perspective is an important component of CKD-related care. In the case of any chronic disease without a cure, patient perspective can be the best source for understanding the illness experience, treatment expectations and experience, and unmet needs with current treatments. Given the few studies identified with measures of CKD burden from the patient’s perspective, opportunities for this type of research are vast.
The most burdensome conditions commonly reported by CKD patients across identified studies were cognitive impairment, dementia, sleep disturbance, pain, and emotional and physical dysfunction. Of these, physical dysfunction was the most pervasive and debilitating.Citation47–Citation54 Instruments used to measure patient perspective were the Medical Outcomes Study Short Form – 36, the Kidney Disease Quality of Life Short Form – 36 (KDQOL-36), Health Utilities Index 3, and a time trade-off approach.Citation47,Citation48,Citation50,Citation53,Citation54 Perception of general health as measured by the Medical Outcomes Study Short Form – 36 was low across all eGFR groups defined by Chin and colleagues.Citation47 With an increase in illness severity and a decrease in eGFR, mental health component scores were similarly low across groups, whereas the physical component scores were reduced significantly with reduced eGFR, particularly in those with an eGFR < 45 mL/min/1.73 m2 (stage 3) and 30 mL/min/1.73 m2 (stage 4).Citation47,Citation53 When compared with the general population, patients with CKD scored lower on six of eight subscales – physical function, role limitation – physical, general health, vitality, role limitation – emotional, and the physical component score.Citation50 Mean scores on KDQOL-36 components, Health Utilities Index 3, and time trade-off suggested considerable loss of function and well-being in patients with CKD compared with the general population. Decline in eGFR was also monotonically associated with a decline in patient-reported health as measured by the Burden of Kidney Disease (BKD) and the Effects of Kidney Disease subscales (EKD) of the KDQOL-36 (CKD-specific measures; higher score indicates better health). The scores were highest (BKD: 85.9; EKD: 92.4) in patients with eGFR > 60 mL/min/1.73 m2 (stage 1–2). With subsequent decreases in eGFR, perceived health decreased. For those with stage 3 CKD (30 mL/min/1.73 m2 ≤ eGFR < 60 mL/min/1.73 m2), BKD was 85.4 and EKD was 91.6, with continued reduction to a BKD of 74.9 and EKD of 87.5 for those with stage 4 (15 mL/min/1.73 m2 ≤ eGFR < 30 mL/min/1.73 m2), and BKD of 63.4 and EKD of 80.4 for those with stage 5 (eGFR < 15 mL/min/1.73 m2). The lowest perceived health (BKD: 38.6; EKD: 62.7) was observed in patients on dialysis.Citation48 Modifiable risk factors associated with lower quality of life were less education, less exercise, depression, history of cardiovascular disease, lower income, and unemployment.Citation47,Citation48,Citation53 and highlight the decline in health-related quality of life with the progression of CKD.
Figure 1 Health-related quality of life and progression of chronic kidney disease (CKD) by stage.
Adapted from Clin J Am Soc Nephrol. Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Copyright. 2009.Citation51
Abbreviation: KDQOL-36, Kidney Disease Quality of Life – 36.
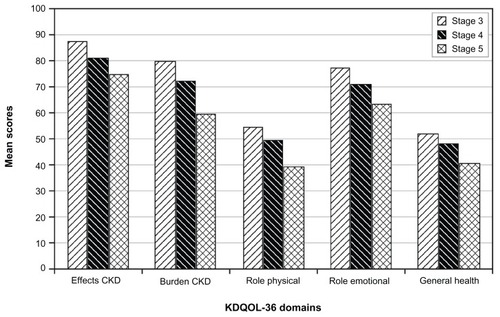
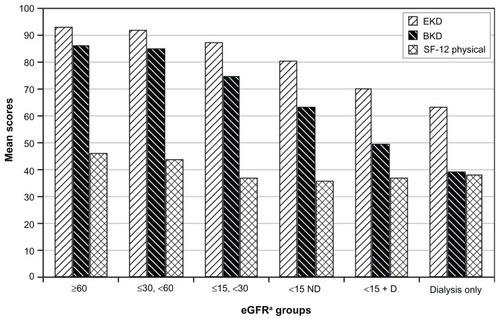
Figure 2 Health-related quality of life (HRQOL), quality of life (QOL), and progression of chronic kidney disease by glomerular filtration rate.
Abbreviations: BKD, Burden of Kidney Disease, subscale of Kidney Disease Quality of Life; D, dialysis; CKD, chronic kidney disease; EKD, Effects of Kidney Disease; subscale of eGFR, estimated glomerular filtration; KDQOL; SF-12 Physical, Medical Outcomes Study Short Form – 12, physical component; ND, no dialysis.
Economic burden
CKD is associated with significant economic burden. Disease progression, increased disease severity, and deterioration of health increase resource utilization and escalate costs. Across identified studies, in the 12 to 24 months before dialysis initiation, substantial increases in costs due to hospitalization were reported.Citation20,Citation21,Citation23,Citation25,Citation32,Citation36,Citation55–Citation58 A study in a Medicare-claims cohort of patients with CKD reported the annual mean number of days hospitalized (9.51) and mean number of physician visits (10.28).Citation59 The mean annual number of physician visits increased monotonically by CKD stage, with 4.43 visits in early stage CKD (stages 1 and 2 combined) and 6.53 visits in late-stage CKD (stages 3 and 4 combined).Citation55 Available cost data worldwide were from the USA and Germany. Irrespective of country and cost measure used (eg, total health care costs per patient, in-hospital costs, total medical payments) in each study, the identified costs were consistently high and increased with each CKD stage. Across CKD stages, total health care costs per patient ranged from US$1183 to $35,292 (per month) in the USA; annual and in-hospital costs were €3581 and €2926 to €9687, respectively, in Germany.Citation20,Citation25,Citation33,Citation60,Citation61 By stage, cost data were available from select studies ().
Table 3 Increased cost by chronic kidney disease (CKD) stage
In reported studies, the impact of CKD on employers is also significant. Of the studies identified, most were conducted in an older working population with CKD. This was not unexpected given that most patients with CKD are elderly. As the general working population increasingly includes individuals older than 65 years, the working population with CKD may also increase, thereby placing a profound and growing burden on employers.
All identified studies that evaluated an employer population with CKD were conducted in the USA.Citation29,Citation37,Citation62,Citation63 In a matched case-control study, employees with CKD were older; more likely to be male; and more likely to have hypertension, diabetes, and other chronic comorbid conditions than controls.Citation37 Annual costs attributable to CKD ranged from US$1187 for stage 3 to US$21,826 for stage 5.Citation37 Upon evaluation of an employer-sponsor population health improvement program, workdays missed exceeded 10 hours per week for employees with CKD.Citation64 Unadjusted costs are presented in .
Anemia-related morbidity is of significant concern in the workplace. Overall burden and/or costs for patients with CKD and anemia are significantly higher than for those with CKD and no anemia.Citation24,Citation26 Treatment for predialysis employees with anemia resulted in improved work productivity by 91.5%, reduced absenteeism by 52.3 days per year, and reduced health care costs by approximately US$4417 per patient per year.Citation63 A similar study reported incremental direct and indirect cost savings with anemia treatment in employees with CKD and anemia compared with those whose anemia was untreated.Citation29
Current treatment options
Over the past few decades, few new treatment options have been made available for CKD patients. Without curative treatment, the primary aims remain to slow the progression of CKD and subsequent loss of kidney function and cardiovascular disease. Current objectives to address these outcomes include control of hypertension, dyslipidemia, proteinuria, hyperglycemia, anemia, and bone mineral disorders.Citation12 Given the complex nature of CKD and the interrelated risk factors, comorbidities, and complications represented in these patients, a comprehensive and collaborative treatment strategy of nonpharmacological (lifestyle management) and pharmacological management is recommended.
Lifestyle management
Key components of lifestyle management are those supporting the primary treatment aims of CKD. Obesity, smoking, sedentary lifestyle, high cholesterol, and hypertension increase the risk for adverse outcomes in patients with CKD.Citation12,Citation42,Citation65–Citation70 Other important components of lifestyle management are mental health and social support. Although understudied, an important pilot study in late CKD was identified. Cabness et alCitation71 proposed evaluation of psychological measures linked to biophysiological measures in patients with CKD. As has been found with other chronic and debilitating diseases, mental health and social support may be of significant value to patients with CKD.Citation72
Current renoprotective drug therapy
Current renoprotective drug therapy includes treatment with angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), statins, insulins, and insulin sensitizers, depending on patient need. All of these therapies reduce proteinuria. In addition to their primary use, antihypertensive agents and those for hyperglycemia also demonstrate a slowed decline in or improvement of GFR. Supporting studies for each class are summarized in current Kidney Disease Outcomes Quality Initiative guidelines.Citation73
Hypertension
Optimum control of hypertension is paramount in the management of CKD. In multiple clinical trials, ACEIs or ARBs slowed progression of diabetic and nondiabetic kidney disease between 16% and 56%.Citation74–Citation79 The landmark trial by the Collaborative Study Group demonstrated the effectiveness of ACEIs in slowing progression of diabetes and CKD in patients with type 1 diabetes and macroalbuminuria, regardless of presence or absence of hypertension.Citation77 In patients with type 2 diabetes and overt nephropathy, ARBs were more effective than conventional therapy in the progression of nephropathy, despite similar blood pressure control.Citation74,Citation77,Citation80 In patients with nondiabetic CKD, ACEIs slowed disease progression, and the benefits were greater in patients with higher levels of proteinuria.Citation75,Citation78,Citation79,Citation81 As evidenced by the African American Study of Kidney Disease trial, setting blood pressure targets reduced proteinuria and slowed progression in African Americans with hypertensive CKD.Citation82 Special attention and further research are needed to optimally manage hypertension in other at-risk and high-risk populations.
Dyslipidemia
Dyslipidemia is a cardiovascular risk factor for CKD. It is associated with decreased renal function in the general population and in patients with CKD. Statins (3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors) are recommended for treatment of dyslipidemia in patients with CKD.Citation73 Results from the landmark Study of the Heart and Renal Protection (SHARP) trial support statin therapy to reduce cardiovascular events in a varied group of patients with advancing CKD. Although improvement in renal outcomes was not found, a 17% risk reduction in major atherosclerotic events was observed in CKD patients treated with ezetimibe and simvastatin compared with placebo, despite lower compliance (ezetimibe/simvastatin: 71%; placebo: 9%).Citation83 With improved compliance, statin therapy may result in significantly greater cardiovascular benefits than observed in Study of Heart and Renal Protection (SHARP).Citation83
Hyperglycemic control for diabetic nephropathy
Hyperglycemia, the defining feature of diabetes, is a fundamental cause of kidney damage. In patients with diabetes, the target HbA1c (glycated hemoglobin) is less than 7%. Intensive glycemic control prevents the development and progression of albuminuria.Citation84–Citation87 The Diabetes Control and Complications Trial (DCCT) demonstrated a 54% reduction in risk of albuminuria in patients with type 1 diabetes with intensive antihyperglycemic therapy.Citation84 The follow-up study to DCCT, Epidemiology of Diabetes Interventions and Complications (EDIC), demonstrated persistent beneficial effects on albumin excretion and reduced incidence of hypertension up to 8 years after the DCCT study was completed. Long-term benefits of intensive treatment were clearly demonstrated in the EDIC study.Citation85 No data evaluating the renoprotective effects of tight glycemic control in patients with established nephropathy were identified.
Although current renoprotective pharmacotherapies are the mainstay of CKD treatment, they are only partially effective; 20% to 40% of patients progress to unfavorable renal outcomes in spite of therapy.Citation88 None of the current agents target all pathological mechanisms in CKD. Nor do they adequately reduce eGFR decline or significantly delay or stop progression to ultimately reverse disease or thwart adverse renal endpoints. Therefore, continued research to find new agents with new mechanisms of action may lead to more effective therapy for patients with CKD ().
Table 4 Current renoprotective drug therapy
Emerging renoprotective drug therapy
A variety of new renoprotective agents targeting primary destructive pathological mechanisms of CKD are in development.Citation89 Those identified via ClinicalTrials.gov include AST-120 (spherical carbon adsorbent), atrasentan, bardoxolone methyl, CTP-499, pentoxifylline, and VTP-27999. To date, AST-120 and bardoxolone methyl have advanced to Phase III clinical trials.Citation90–Citation92 Brief descriptions of agents with ongoing Phase II and Phase III trials follow.
Atrasentan (ABT-627)
Atrasentan is a highly selective endothelin-A receptor antagonist that blocks the effect of endothelin-1, a protein that constricts blood vessels, raises blood pressure, and affects kidney function. Phase II data with atrasentan showed reduction in albuminuria.Citation90 A Phase IIb study is recruiting patients with type 2 diabetes and nephropathy currently treated with the maximum tolerated dose of a renin–angiotensin system inhibitor. Atrasentan (dose not reported) is an oral agent with once-daily administration.Citation93 Data availability was not reported.
Bardoxolone methyl (RTA-402)
Bardoxolone methyl is an antioxidant inflammation modulator – a potent inducer of the transcription factor NrF2, an important biological target that controls the production of many of the body’s detoxification enzymes. This agent activates the NrF2 pathway aiming to decrease oxidative stress and inflammation, which contributes to kidney decline. Phase IIb data suggest the potential to prevent patients from progressing to later-stage disease and dialysis; reversal of disease is also suggested. A Phase III clinical trial, Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes is currently recruiting patients with CKD in type 2 diabetes. Bardoxolone methyl 20 mg is an oral agent with once-daily administration. Trial results are expected in June 2013.Citation90,Citation91
Spherical carbon adsorbent (AST-120)
AST-120 is a spherical carbon adsorbent that acts locally to remove uremic toxins and precursors in the gastrointestinal tract, thereby preventing saturation in the blood stream and nephrotoxicity. It has been approved and marketed in Japan, South Korea, and the Philippines for prolonging time to initiation of dialysis therapy and improving uremic symptoms in patients with chronic renal failure.Citation94 Two Phase III outcome clinical trials have been completed and data are forthcoming:Citation92,Citation95 Evaluating Prevention of Progression in Chronic Kidney Disease and Evaluating Prevention of Progression in Chronic Kidney Disease Including Assessment of Quality of Life. The trials evaluated AST-120 added to standard-of-care therapy for prevention of progression in moderate to severe CKD. The dosage studied in clinical trials was 9 g/day, divided into three doses per day. Clinical endpoints studied include initiation of dialysis, kidney transplant, or doubling of serum creatinine.
Other agents
Other promising agents planned for entry into Phase II trials include CTP-499, a first-in-class candidate from the deuterium platform, with anti-inflammatory, anti-fibrotic, and antioxidant properties,Citation96 and VTP-27999, a potent selective renin inhibitor;Citation97 Phase II studies are not yet recruiting. Aliskiren was a promising agent for high-risk patients with diabetes and renal impairment until an interim review by the data-monitoring committee concluded that patients were unlikely to benefit from treatment added on top of standard antihypertensives. In addition, treatment with aliskiren was associated with more adverse events than other treatments. Upon recommendation by the data-monitoring committee, the trial was terminated in December 2011.Citation98
Emergent drug therapies with anticipated renoprotective effects are presented in . These agents provide new therapeutic options for patients with CKD. Several agents have new or unique mechanisms of action and better outcomes may be expected. For agents that have advanced to Phase III trials, positive efficacy and safety outcomes are anticipated.
Table 5 Key emergent renoprotective drug therapy
Conclusion
The overarching, primary unmet need for new treatment for CKD is evident. Curative treatment or the ability to arrest deterioration of the kidney in CKD is not currently possible. However, several related unmet needs provide opportunity for action. This review has summarized the considerable clinical, humanistic, and economic CKD burden; resulting unmet need; and promising new pharmacological agents that target multiple pathological mechanisms or mechanisms marginally affected by traditional therapies.
Because CKD is prevalent and growing globally, the paucity of studies across disciplines on this topic is a cause for concern. In currently available research, broad study populations characteristic of patients with CKD are not adequately represented, nor are countries. Prospective epidemiological studies are needed to adequately characterize patient populations, particularly in countries with the most rapid increase in CKD rates. This research would support disease classification and staging efforts.
Patients are key decision makers in the health care process. Qualitative research with a focus on the patient perspective has been scant. Patients can be the best source for researchers and clinicians to understand the patient’s illness experience, level of health literacy, treatment expectations and experience, and unmet needs with current treatments. Placing more emphasis on the patient perspective could improve and benefit overall care.
Although the majority of papers identified were in the field of health economics, more research is needed to better understand costs (direct and indirect) in different patient populations (eg, different ethnic groups, patients of working age, and patients with multiple comorbidities), in different settings (eg, employer, managed care, Medicare, Medicaid, and national plans) across countries.
As reported, several sponsor agencies worldwide have developed guidelines for the treatment of CKD. In 2012, KDIGO is expected to publish new international guidelines on this topic. Anticipated improved classification and staging may support earlier recognition of kidney dysfunction in the primary care setting, better referral to nephrologists, and targeted treatment plans by stage of CKD.
Continued basic and clinical trial research is needed to improve the understanding of pathological mechanisms associated with CKD initiation and progression, as well as the effects of treatment in various patient populations worldwide. Better understanding of the mechanisms may lead to the development of drugs that will stop the deterioration of the kidneys, reverse disease, or effect a cure.
CKD is a prevalent, complex, and growing condition worldwide. With the aging of the population and the increase of risk factors associated with initiation and progression of disease in many nations, a significant burden is placed on patients, families, employers, health care systems, and society as a whole. With action, the unmet needs identified in this review can be addressed. The ultimate goal is to discover a curative treatment or one that will arrest deterioration of the kidney. This will offer patients healthier and more productive lives and, eventually, decrease overall system costs so that scarce resources can be allocated elsewhere.
Authors’ contributions
LB and SH performed the review and wrote the first draft. VS and BL reviewed all drafts. CCM and LB wrote the final draft. All authors contributed to the conception and design, and read and approved the final manuscript.
Acknowledgments
We would like to thank Bonnie Kase for her scientific review and Kate Lothman for her high-quality editorial contributions.
Disclosure
Mitsubishi Tanabe Pharma Corporation provided financial support for this research. VS and BL are employees of Mitsubishi Tanabe Pharma America. LB, SH, and CCM have acted as research consultants to Mitsubishi Tanabe Pharma America. The authors declare no other competing interests with respect to this article.
References
- ZhangQLRothenbacherDPrevalence of chronic kidney disease in population-based studies: systematic reviewBMC Public Health2008811718405348
- CollinsAJFigure 12.1CKD and the Public Health Agenda for Chronic Diseases2 Available from: http://www.worldkidneyday.org/page/prevalence-of-diseaseAccessed February 17, 2012
- CoreshJSelvinEStevensLAPrevalence of chronic kidney disease in the United StatesJAMA2007298172038204717986697
- StevensLALiSWangCPrevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP)Am J Kidney Dis2010553 Suppl 2S23S3320172445
- US Renal Data System (USRDS)Costs of CKD2011 USRDS Annual Data Report Volume One: Atlas of Chronic Kidney Disease in the United StatesBethesda, MDNational Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases2011 Available from: http://www.usrds.org/2011/pdf/v1_ch06_11.pdfAccessed February 17, 2012
- Nephrology Pharmacy Associates, IncBailieGRJohnsonCAMasonNASt PeterWLChronic Kidney Disease 2006: A Guide to Select NKF-KDOQI Guidelines and RecommendationsNew York, NYNational Kidney Foundation Available from: http://www.kidney.org/professionals/kls/pdf/Pharmacist_CPG.pdfAccessed February 17, 2012
- Kidney Disease Outcomes Quality Initiative (KDOQI)KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney DiseaseAm J Kidney Dis2007492 Suppl 2S12S15417276798
- LevinAHemmelgarnBCulletonBCanadian Society of NephrologyGuidelines for the management of chronic kidney diseaseCMAJ2008179111154116219015566
- MacGregorMSTaalMWRenal Association Clinical Practice Guideline on detection, monitoring and management of patients with CKDNephron Clin Pract2011118Suppl 1c71c10021555905
- National Collaborating Centre for Chronic ConditionsChronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary careClinical guideline no. 73 [updated Sep 2008; cited February 17, 2012]. Available from: http://www.guideline.gov/content.aspx?id=14330Accessed February 17, 2012
- National Institute for Health and Clinical ExcellenceEarly identification and management of chronic kidney disease in adults in primary and secondary careClinical guideline no. 73 [updated March 30, 2010; cited February 17, 2012]. Available from: http://guidance.nice.org.uk/CG73/Guidance/pdf/EnglishAccessed February 17, 2012
- National Kidney FoundationK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratificationAm J Kidney Dis2002392 Suppl 1S1S26611904577
- Scottish Intercollegiate Guidelines Network (SIGN)Diagnosis and management of chronic kidney disease: a national guideline [updated 2008; cited February 17, 2012]. Available from: http://www.sign.ac.uk/pdf/sign103.pdfAccessed February 17, 2012
- LeveyASAtkinsRCoreshJChronic kidney disease as a global public health problem: approaches and initiatives – a position statement from Kidney Disease Improving Global OutcomesKidney Int200772324725917568785
- HallanSIOrthSRThe conundrum of chronic kidney disease classification and end-stage renal risk prediction in the elderly – what is the right approach?Nephron Clin Pract20101164c307c31620664285
- LeveyASde JongPECoreshJThe definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference reportKidney Int2011801172821150873
- Kidney Disease Improving Global Outcomes (KDIGO)Guideline scopeKDIGO guideline for CKD classification and management [web page on the Internet] Available from: http://www.kdigo.org/clinical_practice_guidelines/CKD.phpAccessed February 17, 2012
- KronenbergFEmerging risk factors and markers of chronic kidney disease progressionNat Rev Nephrol200951267768919935815
- KeithDSNicholsGAGullionCMBrownJBSmithDHLongitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organizationArch Intern Med2004164665966315037495
- BaumeisterSEBögerCAKrämerBKEffect of chronic kidney disease and comorbid conditions on health care costs: A 10-year observational study in a general populationAm J Nephrol201031322222920068286
- KubackiMCarterCHerreraADWangJLopezJMPiechCTHealth plan retention and pharmacy costs of newly diagnosed patients with chronic kidney disease in a managed care populationAmerican Health and Drug Benefits200927283290
- LalibertéFBookhartBKVekemanFDirect all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: a managed care perspectiveJ Manag Care Pharm200915431232219422271
- LondonRSolisAGoldbergGAWadeSChanWWExamination of resource use and clinical interventions associated with chronic kidney disease in a managed care populationJ Manag Care Pharm20039324825514613468
- NissensonARCollinsAJHurleyJPetersenHPereiraBJSteinbergEPOpportunities for improving the care of patients with chronic renal insufficiency: current practice patternsJ Am Soc Nephrol20011281713172011461944
- RobbinsJDKimJJZdonGChanWWJonesJResource use and patient care associated with chronic kidney disease in a managed care settingJ Manag Care Pharm20039323824714613467
- ErshlerWBChenKReyesEBDuboisREconomic burden of patients with anemia in selected diseasesValue Health20058662963816283863
- KhanSAmediaCAJrEconomic burden of chronic kidney diseaseJ Eval Clin Pract200814342243418373575
- LefebvrePDuhMSButeauSBookhartBModySHMedical costs of untreated anemia in elderly patients with predialysis chronic kidney diseaseJ Am Soc Nephrol200617123497350217082245
- MoyneurEBookhartBKModySHFournierAAMallettDDuhMSThe economic impact of pre-dialysis epoetin alpha on health care and work loss costs in chronic kidney disease: an employer’s perspectiveDis Manag2008111495818279115
- van NootenFEGreenJBrownRFinkelsteinFOWishJBurden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States: review of the literatureJ Med Econ201013224125620438399
- WishJSchulmanKLawANassarGHealthcare expenditure and resource utilization in patients with anaemia and chronic kidney disease: a retrospective claims database analysisKidney Blood Press Res200932211011819372704
- KhanSSKazmiWHAbichandaniRTighiouartHPereiraBJKauszATHealth care utilization among patients with chronic kidney diseaseKidney Int200262122923612081582
- MeyerABunzemeierHHausbergMImpact of different stages of chronic kidney disease on in-hospital costs in patients with coronary heart diseaseNephrol Dial Transplant20082361955196018083761
- SchumockGTAndressDLMarxSESterzRJoyceATKalantar-ZadehKAssociation of secondary hyperparathyroidism with CKD progression, health care costs and survival in diabetic predialysis CKD patientsNephron Clin Pract20091131c54c6119590235
- ThorpMLEastmanLSmithDHJohnsonESManaging the burden of chronic kidney diseaseDis Manag20069211512116620197
- SmithDHGullionCMNicholsGKeithDSBrownJBCost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO populationJ Am Soc Nephrol20041551300130615100370
- TaylorTNSalinitriFDSatterhwaiteDWMedical care spending associated with chronic kidney disease by stage of disease [abstract PUK9]Value Health2011143A75
- BrennerBMGoldszerRCHostetterTHGlomerular response to renal injuryContrib Nephrol19823348666749423
- HostetterTHOlsonJLRennkeHGVenkatachalamMABrennerBMHyperfiltration in remnant nephrons: a potentially adverse response to renal ablationAm J Physiol19812411F85F937246778
- BarbourSJErLDjurdjevOKarimMLevinADifferences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patientsNephrol Dial Transplant201025113663367220368302
- DjamaliAKendziorskiCBrazyPCBeckerBNDisease progression and outcomes in chronic kidney disease and renal transplantationKidney Int20036451800180714531814
- KuklaAAdullaMPascualJCKD stage-to-stage progression in native and transplant kidney diseaseNephrol Dial Transplant200823269370017881429
- OrlandoLABelascoEJPatelUDMatcharDBThe chronic kidney disease model: a general purpose model of disease progression and treatmentBMC Med Inform Decis Mak2011114121679455
- TangriNStevensLAGriffithJA predictive model for progression of chronic kidney disease to kidney failureJAMA2011305151553155921482743
- TrivediHSPangMMCampbellASaabPSlowing the progression of chronic renal failure: economic benefits and patients’ perspectivesAm J Kidney Dis200239472172911920337
- FeldmanHIAppelLJChertowGMChronic Renal Insufficiency Cohort (CRIC) Study InvestigatorsThe Chronic Renal Insufficiency Cohort (CRIC) Study: Design and MethodsJ Am Soc Nephrol2003147 Suppl 2S148S15312819321
- ChinHJSongYRLeeJJModerately decreased renal function negatively affects the health-related quality of life among the elderly Korean population: a population-based studyNephrol Dial Transplant20082392810281718372390
- GorodetskayaIZeniosSMcCullochCEHealth-related quality of life and estimates of utility in chronic kidney diseaseKidney Int20056862801280816316356
- KrishnanAVKiernanMCNeurological complications of chronic kidney diseaseNat Rev Neurol200951054255119724248
- MolstedSPrescottLHeafJEidemakIAssessment and clinical aspects of health-related quality of life in dialysis patients and patients with chronic kidney diseaseNephron Clin Pract20071061c24c3317409766
- MujaisSKStoryKBrouilletteJHealth-related quality of life in CKD Patients: correlates and evolution over timeClin J Am Soc Nephrol2009481293130119643926
- NulsenRSYaqoobMMMahonAStoby-FieldsMKellyMVaragunamMPrevalence of cognitive impairment in patients attending pre-dialysis clinicJ Ren Care200834312112618786078
- PorterAFischerMJBrooksDQuality of life and psychosocial factors in African Americans with hypertensive chronic kidney diseaseTransl Res2012159141122153804
- RichardsonMMSaris-BaglamaRNAnatchkovaMDPatient experience of chronic kidney disease (CKD): results of a focus group study [abstract 175]Am J Kidney Dis2007494B68
- AlexanderMBradburyBDKewalramaniRBarlevAMohantySAGlobeDChronic kidney disease and US healthcare resource utilization in a nationally representative sampleAm J Nephrol200929547348219039210
- MixTCSt PeterWLEbbenJHospitalization during advancing chronic kidney diseaseAm J Kidney Dis200342597298114582041
- St PeterWLKhanSSEbbenJPPereiraBJCollinsAJChronic kidney disease: the distribution of health care dollarsKidney Int200466131332115200439
- VekemanFYameogoNDLefebvrePBaileyRAMcKenzieRSPiechCTHealthcare costs associated with nephrology care in pre-dialysis chronic kidney disease patientsJ Med Econ201013467368021050062
- SchneiderKMO’DonnellBEDeanDPrevalence of multiple chronic conditions in the United States’ Medicare populationHealth Qual Life Outcomes200978219737412
- AmediaCAJrManaging chronic kidney diseaseManag Care2003 Spec No812 discussion 17–2012751138
- USRDSCosts of chronic kidney disease2010 Annual Data Report Volume One: Atlas of Chronic Kidney Disease in the United States [cited February 17, 2012]Bethesda, MDNational Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases2010 Available from: http://www.usrds.org/2010/pdf/V1_09.pdfAccessed February 17, 2012
- SullivanSEmployer challenges with the chronic kidney disease populationJ Manag Care Pharm2007139 Suppl DS19S2118177215
- PapatheofanisFBookhartBKMuserEPiechCTAn examination of productivity and resource utilization associated with epoetin alfa treatment in employees with predialysis chronic kidney diseaseJ Occup Environ Med200850558458918469628
- HarshmanRNaimACarterJRichersonGNairKVEarly detection, screening, and management of chronic kidney disease among actively employed – an integrated population health management approach [abstract PUK20]Value Health2011143A78
- De CosmoSLamacchiaORauseoACigarette smoking is associated with low glomerular filtration rate in male patients with type 2 diabetesDiabetes Care200629112467247017065686
- KramerHTuttleKRLeeheyDObesity management in adults with CKDAm J Kidney Dis200953115116519101399
- MafraDGuebre-EgziabherFFouqueDBody mass index, muscle and fat in chronic kidney disease: questions about survivalNephrol Dial Transplant20082382461246618390566
- NavaneethanSDYehnertHMoustarahFSchreiberMJSchauerPRBeddhuSWeight loss interventions in chronic kidney disease: a systematic review and meta-analysisClin J Am Soc Nephrol20094101565157419808241
- PoortmansJROuchinskyMGlomerular filtration rate and albumin excretion after maximal exercise in aging sedentary and active menJ Gerontol A Biol Sci Med Sci200661111181118517167160
- TuttleKRSunwoldDKramerHCan comprehensive lifestyle change alter the course of chronic kidney disease?Semin Nephrol200929551252319751897
- CabnessJMillerCFlowersKPromoting resilience in ESRD: evaluation of a group cognitive-behavioral intervention for patients on hemodialysisJ Nephrol Social Work2006253036
- HothKFChristensenAJEhlersSLRaichleKALawtonWJA longitudinal examination of social support, agreeableness and depressive symptoms in chronic kidney diseaseJ Behav Med2007301697617219057
- Kidney Disease Outcomes Quality Initiative (K/DOQI) GroupK/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney diseaseAm J Kidney Dis200341:4Suppl 3IIVS191
- BrennerBMCooperMEde ZeeuwDRENAAL Study InvestigatorsEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathyThe GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia)Lancet19973499069185718639217756
- LewisEJHunsickerLGBainRPRohdeRDThe effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study GroupN Engl J Med19933292014561462 Erratum in: N Engl J Med. 1993;330(2):1528413456
- LewisEJHunsickerLGClarkeWRCollaborative Study GroupRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- MaschioGAlbertiDJaninGEffect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study GroupN Engl J Med1996334159399458596594
- RuggenentiPPernaAGherardiGRenoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuriaLancet1999354917635936410437863
- ParvingHHAndersenARSmidtUMSvendsenPAEarly aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathyLancet198318335117511796133986
- JafarTHStarkPCSchmidCHAIPRD Study GroupAngiotensin-converting enzyme inhibition and progression of renal disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal diseaseKidney Int20016031131114011532109
- AppelLJWrightJTJrGreeneTAfrican American Study of Kidney Disease and Hypertension Collaborative Research GroupLong-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African AmericansArch Intern Med2008168883283918443258
- BaigentCLandrayMJReithCSHARP InvestigatorsThe effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trialLancet201137797842181219221663949
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitusThe Diabetes Control and Complications Trial Research GroupN Engl J Med1993329149779868366922
- Writing Team for the Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications Research GroupSustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) studyJAMA2003290162159216714570951
- JenkinsAJLyonsTJZhengDDCCT/EDIC Research GroupLipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathyKidney Int200364381782812911531
- LevinSRCoburnJWAbrairaCEffect of intensive glycemic control on microalbuminuria in type 2 diabetes. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Feasibility Trial InvestigatorsDiabetes Care200023101478148511023140
- HirschSAn update on proteinuric chronic kidney disease: the dual-goal approachCleve Clin J Med2008751070571318939386
- VilayurEHarrisDCEmerging therapies for chronic kidney disease: what is their role?Nat Rev Nephrol20095737538319455178
- AbbottPositive results from phase 2 study of atrasentan for treatment of diabetic kidney disease published in the Journal of the American Society of Nephrology [press release]Abbott Park, ILAbbott2011 [Mar 4]. Available from: http://www.abbott.com/news-media/press-releases/2011Mar04.htmAccessed February 17, 2012
- Reata PharmaceuticalsBardoxolone methyl [web page on the Internet]Irving, TXReata Pharmaceuticalsnd Available from: http://www.reatapharma.com/pip_rta402.aspAccessed February 17, 2012
- Mitsubishi Tanabe Pharma CorporationA study of AST-120 for evaluating prevention of progression in chronic kidney disease (EPPIC-1)ClinicalTrialsgov [website on the Internet]Bethseda, MDUS National Library of Medicine2007 [updated October 1, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00500682?term=ast-120&rank=7. NLM identifier: NCT00500682Accessed October 1, 2012
- AbbottA prospective, double-blind, placebo-controlled, multicenter study to evaluate efficacy and safety of atrasentan, including thoracic bioimpedance, in type 2 diabetic subjects with nephropathyClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2011 [updated August 11, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT01399580?term=atrasentan+and+kidney+disease&rank=3. NLM identifier: NCT01399580Accessed October 1, 2012
- KurehaBusiness Report 2010TokyoKureha2010 Available from: http://www.kureha.co.jp/en/ir/pdf/br2010.pdfAccessed February 17, 2012
- Mitsubishi Tanabe Pharma CorporationA study of AST-120 for evaluating prevention of progression in chronic kidney disease including assessment of quality of life (EPPIC-2)ClinicalTrials.gov [website on the Internet]Bethseda, MDUS National Library of Medicine2007 [updated October 1, 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00501046?term=AST-120&rank=8. NLM identifier: NCT00501046Accessed October 1, 2012
- Concert PharmaceuticalsCTP-499 – first-in-class treatment candidate for diabetic nephropathyTechnology [web page on the Internet]Lexington, MAConcert Pharmaceuticalsnd Available from: http://www.concertpharma.com/research/index.html#CTP499Accessed February 17, 2012
- Vitae PharmaceuticalsVTP-27999 programDirect inhibition of renin offers potentially better renal protection than current therapies for the 27 million people worldwide with chronic kidney disease (CKD) [web page on the Internet]Fort Washington, PAVitae Pharmaceuticalsnd Available from: http://www.vitaepharma.com/view.cfm/56/Chronic-Kidney-Disease-ReninAccessed February 17, 2012
- NovartisNovartis announces termination of ALTITUDE study with Rasilez®/Tekturna® in high-risk patients with diabetes and renal impairment [media release]BaselNovartis2011 [Dec 20]. Available from: http://www.novartis.com/newsroom/media-releases/en/2011/1572562.shtmlAccessed February 17, 2012
- TaalMWBrennerBMPredicting initiation and progression of chronic kidney disease: Developing renal risk scoresKidney Int200670101694170516969387
- LeveyASEckardtKUTsukamotoYDefinition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO)Kidney Int20056762089210015882252