Abstract
Diabetic kidney disease (DKD) is a progressive proteinuric renal disorder in patients with type 1 or type 2 diabetes mellitus. It is a common cause of end-stage kidney disease worldwide, particularly in developed countries. Therapeutic targeting of the renin–angiotensin system (RAS) is the most validated clinical strategy for slowing disease progression. DKD is paradoxically a low systematic renin state with an increased intrarenal RAS activity implicated in its pathogenesis. Angiotensin II (AngII), the main peptide of RAS, is not only a vasoactive peptide but functions as a growth factor, activating interstitial fibroblasts and mesangial and tubular cells, while promoting the synthesis of extracellular matrix proteins. AngII also promotes podocyte injury through increased calcium influx and the generation of reactive oxygen species. Blockade of the RAS using either angiotensin converting enzyme inhibitors, or angiotensin receptor blockers can attenuate progressive glomerulosclerosis in animal models, and slows disease progression in humans with DKD. In this review, we summarize the role of intrarenal RAS activation in the pathogenesis and progression of DKD and the rationale for RAS inhibition in this population.
Introduction
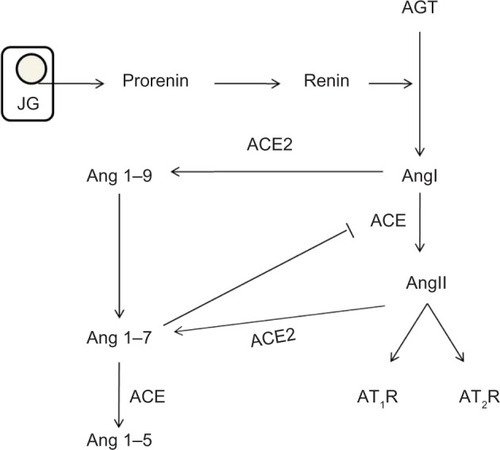
Worldwide, more than 347 million people are suffering from diabetes mellitus (DM) and its complications which are projected to be the seventh leading cause of death by 2030.Citation1 The incidence has doubled in the last two decades,Citation2–Citation4 and DM is the underlying cause of both micro- and macrovascular disorders such as diabetic kidney disease (DKD), retinopathy, coronary artery disease, and peripheral vascular disease. DKD is a progressive disease that affects patients with long-standing type 1 (insulin dependent) or type 2 (non-insulin-dependent) DM, and is functionally characterized by different degrees of albuminuria and chronic kidney disease.Citation5 Associated histological structural changes include mesangial expansion, glomerular basement membrane thickening, glomerular sclerosis, and in advanced stages, tubulointerstitial fibrosis.Citation5 In the US, it is estimated that 44% of all new end-stage renal disease (ESRD) cases are due to DKD.Citation6 Interestingly, despite the increased incidence rate of DM, recent studies have shown that the incidence of DKD has dramatically decreased with the implementation of tight glycemic control and the use of renin–angiotensin system (RAS) blockers.Citation7 The use of RAS blockers in DKD not only decreases albuminuria, but slows disease progression, which is only partially explained by their hemodynamic effects. The RAS cascade starts with the production of prorenin in the juxtaglomerular cells, which is cleaved into renin.Citation8 Renin then cleaves angiotensinogen (AGT) to form angiotensin I (AngI) which later is converted by angiotensin converting enzyme (ACE) to the octapeptide angiotensin II (AngII). AngII in turn activates both angiotensin type 1 and 2 (AT1, AT2, respectively) receptors (). AngII was previously considered to be the only active metabolite of this cascade, but evidence in the last few decades has shed light on the importance and biological role of each product including prorenin, renin, and AngI. In this review, we summarize the role of RAS in diabetic nephropathy and the mechanisms by which RAS inhibition slows disease progression.
Figure 1 RAS cascade.
Abbreviation: RAS, renin–angiotensin system.
Genetics of RAS
Host genetic variation in RAS components may predict the risk of developing DKD. It is well documented that there is an increased likelihood of developing DKD in family members who have siblings affected with the disease. Genetic studies have sought to determine variants and factors predisposing individuals to DKD.Citation9–Citation11 Some studies examined the role of the ACE gene genotype as a potential genetic risk factor and showed conflicting results. Investigators analyzed the insertion/deletion (I/D) and DD polymorphism in ACE gene in type 2 DM patients and concluded that DD polymorphisms are associated with greater risk of renal function decline, severe proteinuria, and worse outcomes.Citation12–Citation14 These findings were not seen in type 1 DM patients or in a large review of 19 studies that included type 1 and 2 patients.Citation15–Citation17 Male, and not female, patients with type 1 diabetes and the AA haplotype of the AT2 receptor gene on the X-chromosome had a lower glomerular filtration rate than those with the GT haplotype.Citation18
Hemodynamic effects of RAS on the glomerular filter
Renal autoregulation enables the kidneys to maintain a stable intraglomerular pressure and glomerular filtration rate (GFR) as the mean arterial pressure varies from 80–160 mmHg.Citation19 This is achieved through tubular–glomerular feedback (TGF) and myogenic response affecting the afferent arteriole’s tonicity, and angiotensin II (AngII) affecting the tonicity of both the afferent and the efferent arterioles.Citation20 This autoregulatory mechanism is disrupted in different diseases, including hypertension, chronic kidney disease, and diabetes (). Early in the course of DKD, an elevation in the GFR is seen, accompanied later by glomerular and renal hypertrophy along with increased intraglomerular pressure. This in turn increases the shear stress on the glomerular capillary wall resulting in progressive and sustained renal damage.Citation21
Figure 2 Hemodynamic changes in diabetic kidney disease (DKD).
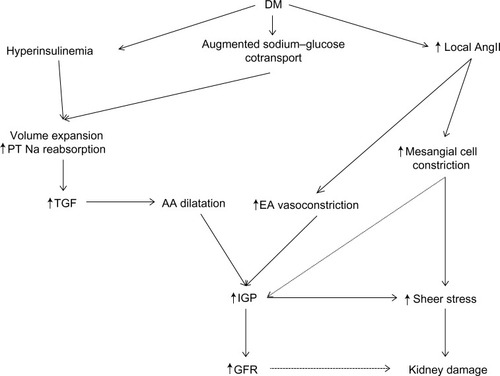
A key mechanistic factor in this hyperfiltration response in DKD is RAS mediated glomerular afferent arteriole dilatation which increases renal blood flow and intraglomerular pressure.Citation22 Other contributors to increased afferent arteriole dilatation include insulin-like growth factor I (IGF-1),Citation23 atrial natriuretic peptide,Citation24 and advanced glycation end products, the latter also influencing the micro- and macrovascular changes seen in DM.Citation25 Additionally in DM, there is an augmented sodium–glucose cotransport leading to volume expansion, along with hyperinsulinemia and local AngII stimulating sodium reabsorption in the proximal tubule.Citation26–Citation28 This increased proximal sodium reabsorption activates the tubuloglomerular feedback mechanism in the macula densa, and can then raise the GFR via dilatation of the afferent arteriole.Citation26
AngII has vasoconstrictive effects on the afferent and the efferent arterioles as well as the interlobular arteries, mediated in part by the local generation of thromboxane A2. The constrictive efficacy is greatest at the efferent arteriole due to its smaller basal diameter. This leads to a net reduced renal blood flow, increased intraglomerular pressure, and an increase in GFR.Citation29 Concurrently, AngII stimulates the release of vasodilator prostaglandins from the glomeruli and constricts glomerular mesangial cells, thereby lowering the surface area available for filtration. AngII also sensitizes the afferent arteriole to the constricting signal of tubuloglomerular feedback.Citation30–Citation33
RAS activation and its importance in DKD
The beneficial effects of RAS blockers in slowing the progression of diabetic nephropathy have been well documented,Citation34–Citation36 despite the low systemic renin state in DKD. This phenomenon is thought to be reflecting the activated intrarenal renin system or increased intrarenal sensitivity to AngII.Citation37 Likely reflecting different stages of kidney disease and different measurement methods (renin activity, protein level, RNA expression, serum potassium and bicarbonate, and immunohistochemistry and fluorescence), human clinical data measuring RAS activation have been conflicting in DKD.Citation38–Citation41 Experimental models however tend to show a consistent increase in RAS activation.Citation42–Citation46 This activation in RAS involves all factors and steps in the cascade and takes place in a paracrine fashion. summarizes the deleterious effects of RAS cascade activation in DKD.
Table 1 The deleterious effects of RAS cascade activation in DKD
Prorenin
The importance of prorenin and its biological role beyond the formation of renin was first established in the 1980s when it was found that its levels, despite the low systemic renin levels and activity, are increased in diabetic subjects, and correlate with the presence of retinopathy and nephropathy.Citation39 In DM, prorenin represents 95% of circulating renin, and the mechanism by which prorenin is implicated in the development of DKD and retinopathy in this population is under investigation.Citation47 It binds to the prorenin receptor (PRR) and, independently of AngII, triggers intracellular signaling and the activation of the mitogen-activated protein (MAP) kinases ERK1/2, leading to upregulation of TGFβ1, PAI1, collagens, fibronectin, and cyclooxygenase-2.Citation48–Citation52 This raises the possibility that high prorenin levels play a role in diabetic nephropathy by stimulating PRR and inducing profibrotic protein syntheses.Citation8 Indeed, transgenic rats overexpressing human PRR develop proteinuria, and a slowly progressive glomerulosclerosis and kidney damage independent of AngII.Citation42 Interestingly, inducible overexpression of prorenin does not result in glomerulosclerosis suggesting that prorenin may augment the fibrosis rather than causing it.Citation43,Citation53 Prorenin also participates in the generation of AngI. Prorenin binding to PRR induces a conformational change involving unfolding of the peptide from the enzymatic cleft so the cleft is now accessible to AGT and generates AngI.Citation54,Citation55
ACE
The best evidence for intrarenal renin production and activity in DM comes from spontaneous or streptozotocin (STZ)-induced diabetes animal models.Citation44,Citation56 In spite of the suppressed or normal plasma renin activity, the intrarenal Ren mRNA and protein levels, most significantly in the proximal tubule, are increased in DM.Citation44 The total renal ACE activity is significantly reduced in DM rats, with specific redistribution in diabetic kidneys.Citation56 While proximal tubule ACE activity is reduced, ACE staining intensity is enhanced in diabetic glomeruli and renal vasculature. This suggests a role for glomerular ACE in mediating nephron injury, possibly by increasing local intraglomerular AngII formation.
Renin
Early diabetes causes a significant stimulation of the proximal tubule renin mRNA expression.Citation44 Renin, independent of its enzymatic action to enhance AngII synthesis, directly increases production of transforming growth factor β (TGF-β), the fibrogenic cytokine.Citation48 Renin binds to its specific receptor on the cell surface of mesangial cells,Citation57 leading to hypertrophy,Citation58 and enhanced efficiency of angiotensinogen cleavage by renin, thereby unmasking prorenin catalytic activity.Citation57 The renin receptor was also localized in the sub-endothelium of the renal arteries suggesting that renin has a novel receptor-mediated action that could play a role in renal fibrosis.Citation59 In podocytes, high glucose is shown to cause increased AngII generation through increasing renin mRNA expression with a concomitant increase in PRR and hence augmenting the conversion from AGT to AngI.Citation60
Angiotensinogen
The high extracellular glucose in DM stimulates the synthesis of AGT in a concentration dependent manner and increases the expression of its gene (Agt) in the proximal tubule of the kidneys.Citation56,Citation59 This increased AGT protein level in the proximal tubules is due to many factors, including the effects of high extracellular glucose on Agt expression via reactive oxygen speciesCitation45 and the direct effect of glucose on its promoter. A glucose response element has been located on the Agt promoter.Citation46
Angiotensin II
As previously mentioned, in diabetic nephropathy there is an increase in the generation of the intrarenal AngII despite the systemic suppression of RAS. The deleterious effects of this rise in AngII go beyond the hemodynamic changes to involve insulin resistance, growth promotion, and tubular damage. One of the most important roles of AngII in DKD is its association with volume expansion through water and Na reabsorption. It activates the Na+–H+ antiporter in the luminal membrane through stimulation of an inhibitory G protein that decreases cyclic AMP (adenosine monophosphate) generation, minimizing the normally suppressive effect of cyclic AMP on Na+–H+ exchange.Citation61 AngII stimulates phosphatidylinositol turnover, resulting in the generation of protein kinase C.Citation61,Citation62 It also increases the secretion of aldosterone from the adrenal cortex enhancing Na+ transport in the cortical collecting tubule.Citation63 AngII inhibits proteinase activity in the proximal tubule and causes mesangial cell expansion via decreasing the activity of plasminogen activator. AngII mediated TGF-β1 upregulation and vascular endothelial growth factor release from the glomerular epithelial and mesangial cells contribute to mesangial matrix expansion.Citation64 Renal fibroblasts express AT1 receptor and respond to AngII stimuli by cell proliferation, matrix expansion, and synthesis of fibronectin, by a TGF-β-dependent mechanism.Citation65 Microinflammation of the glomeruli and tubulointerstitial regions and subsequent extracellular matrix expansion are common pathways for the progression of DKD.Citation66 AngII activates inflammatory cells by direct chemotaxis including osteopontin (OPN), RANTES and the production of other proinflammatory mediators, including MCP-1 and TGFβ. It activates protein kinase C, protein tyrosine kinases (PTK), mitogen-activating protein kinases (MAPK), extracellular signal-regulated kinase (ERK), c-Jun amino terminal kinase (JNK), p38 MAP kinase (p38 MAPK), and the activator protein-1 (AP-1). These factors are implicated and involved in proliferation, differentiation, fibrosis, and inflammation processes.Citation65 AngII has also been implicated in insulin resistance. AngII inhibits insulin-mediated GLUT4 translocation in this skeletal muscle model through a transient activation of ERK1/2 inhibiting insulin receptor substrate 1/2 (IRS-1/2) and through a direct inhibitory nitration of Akt. It induces tyrosine phosphorylation of IRS-1 by Janus kinase 2 associated with AT1 receptor stimulation which attenuates insulin-induced activation of phosphatidylinositol-3-kinase associated with IRS-1, leading to decreased insulin sensitivity.Citation67 Treatment with the angiotensin II receptor blocker (ARB) candesartan increases renal insulin receptor expression in STZ-induced diabetic rat models and the insulin resistant Zucker rats irrespective of insulin levels.Citation68
It appears that high glucose directly stimulates podocytes to increase AngII production independent of ACE activity, and increases AT1 receptor levels promoting the deleterious effects of AngII on podocytes.Citation60 AngII directly promotes podocyte injury via release of calcium from intracellular stores as well as influx from the extracellular space.Citation69 Finally, AngII activates enzyme systems like Nox4 that use NADPH oxidases as substrates for superoxide generation.Citation70 These mechanisms contribute to proteinuria and progressive renal disease.
Aldosterone
Aldosterone secretion from the adrenal cortex is mainly mediated by AngII and plasma potassium concentration. This secretion might result in a vicious cycle of local RAS stimulation causing further RAS activation and aldosterone generation.Citation71 The effects of aldosterone go beyond solute and water retention, vasoconstriction, and subsequently, increased blood pressure. In the diabetic kidney, aldosterone activity contributes to tubulointerstitial fibrosis and inflammation, key features of disease progression. Aldosterone increases extracellular matrix production through enhanced TGF-β1 and type IV collagen gene expression.Citation72 In diabetic rats, aldosterone induces glomerular and tubulointerstitial macrophage infiltration through TGFβ.Citation73 Aldosterone also stimulates ROS production through activation of NADPH oxidase in mesangial cells.Citation74 Systemically, higher circulating levels of aldosterone are associated with insulin resistance.Citation75 There is a need for large prospective trials to determine the efficacy of aldosterone inhibition in the treatment of DKD.
ACE2 and angiotensin 1–7 (Ang 1–7)
Angiotensin converting enzyme 2 (ACE2) is an enzyme related to ACE, expressed in the intrarenal endothelium and the tubular epithelial cell.Citation76 It hydrolyzes AngI to Ang 1–9, which is subsequently cleaved by ACE into Ang 1–7. ACE2 also catalyzes the conversion of AngII to Ang 1–7 (the major pathway of Ang 1–7 production). ACE2 does not produce AngII, or metabolize bradykinin (BK), and is not inhibited by ACE inhibition.Citation76,Citation77 Cardiac ischemia studies suggest that ACE2 and ACE have counterbalancing effects. Animal models with disrupted ACE2 gene showed significant cardiac abnormalities including decreased contractility and increased damage, probably mediated by the upregulation of hypoxia-induced genes. These histological findings were abrogated by concomitant ACE gene ablation.Citation78
Ang 1–7 causes intrarenal vasodilation and counterbalances the vasoconstrictor effects of AngII via stimulation of NO and prostaglandins, and synergistically acts with BK.Citation77 The effects are not limited to hemodynamics. In animal models of DKD, Ang 1–7 treatment ameliorates mesangial expansion and renal fibrosis, and reduces oxidative stress, inflammation, and lipotoxicity through blocking the activation of MAPKs by AngII and selective inhibition of glucose-stimulated protein synthesis, TGF-β1/Smad3- and VEGF-mediated pathways.Citation79,Citation80 There is a dynamic reciprocal interaction between Ang 1–7 and ACE; Ang 1–7 inhibits ACE by 30%–70%, and at the same time is degraded by it. In addition to its vasodilatory and natriuretic actions, Ang (1–7) has an antiproliferative effect on vascular smooth muscle cells.Citation77
Types of RAS blockades and their pleiotropic effects
Direct renin inhibitors (DRI), angiotensin converting enzyme inhibitors (ACEi), and angiotensin II receptor blockers (ARB) are available to treat hypertension and proteinuric kidney diseases. The use of these medications results in either decreased AngII (DRI and ACEi) or blocking its receptor AT1 (ARB). To better understand the differences in effects and actions of these medications, attention should first be paid to AngII receptor distribution. AT1 receptors are distributed throughout the kidney compartments. They are localized in the afferent and efferent arterioles, vascular smooth muscle cells, podocytes, glomerular mesangial and macula densa cells, the proximal tubule cell brush border, basolateral membranes, thick ascending limb epithelia, distal tubules, and the cortical collecting ducts.Citation81 The AT2 receptor is localized in the afferent arteriole, glomerular endothelial and mesangial cells, proximal tubule cells, and interstitial cells.Citation81,Citation82 AT1 receptor stimulation leads to renal arterial vasoconstriction, tubule epithelial sodium reabsorption, augmentation of tubulo–glomerular feedback sensitivity, and inhibition of pressure-natriuresis.Citation77 Conversely, AT2 receptor activation exerts the opposite effects with respect to cardiovascular hemodynamics and cell growth. AT2 receptor activation stimulates BK and NO production, vasodilatation, and modulates the vasoconstrictor action mediated by the AT1 receptor through endothelium-dependent vasodilatation.Citation83
DRI
AngII has a negative feedback on RAS, and during ACEi therapy, there is a rise in plasma renin activity (PRA) and concentration (PRC) as a result of decreased renal parenchymal AngII. This led to the development of DRIs for monotherapy or in combination with ACEi therapy. They have been shown to have a similar antiproteinuric and antihypertensive effects, but without significant cardiovascular benefits.Citation84,Citation85
ACEi
ACEi is probably one of the most studied and used class of antihypertensive medication. Since the late 1970s and the use of captopril, ACEi has become the cornerstone in treating hypertensive patients with proteinuria. It inhibits ACE activity by blocking the conversion from AngI to AngII, and inhibits BK degradation. Bradykinin, via its B2 receptors, stimulates NO, cGMP, prostaglandin E2, and prostacyclin. Therefore, ACEi therapy not only inhibits AngII production but also increases the production of the vasodilatory factors.Citation86 ACEi therapy also inhibits the ACE mediated degradation of Ang 1–7 augmenting the renoprotective effects of Ang 1–7. The pleiotropic effects of ACEi include anti-inflammatory and antioxidant,Citation87 antithrombotic, and profibrinolytic activities.Citation88 ACEi is also important in significantly improving arterial compliance through cytoprotection of vascular endothelium,Citation89 along with better regression of left ventricular hypertrophy compared to beta blockers.Citation90
ARB
The major clinical and physiological differences between ACEi and ARBs are related to activation of AT2 receptors. ARBs selectively block AT1 receptor without directly affecting the synthesis of AngII and subsequent AT2 activation. Some of the beneficial effects of using ARB are thought to be due to selective inhibition of AT1 and the concomitant renoprotective stimulation of AT2 receptors.
It has been proposed that ARBs might have a greater potential in preventing renal interstitial fibrosis compared to ACEi mediated by AT2 receptor proapoptotic properties.Citation91 This is supported by the fact that renal interstitial fibrosis after ureteral obstruction is enhanced in rats with either AT2 deletion, mutation, or pharmacologic blockade.Citation92,Citation93
RAS blockers and renal outcome studies
The effects and roles of RAS blockers in the progression of kidney disease are probably mostly seen due to their hemodynamic effects and blood pressure control. It is well known that blood pressure control is considered a cornerstone in the management and prevention of chronic kidney diseases.Citation94 Thus, different studies have evaluated the hypothesis whether a stricter lower blood pressure control will confer a better renal outcome, and initial societies guidelinesCitation95–Citation97 have recommended a lower blood pressure target (<130 mmHg systolic) for patients with CKD and significant proteinuria, based on the findings of post hoc analysis of major trials like MDRDCitation98–Citation100 AASK,Citation101,Citation102 and a patient level meta-analysis of eleven randomized trials.Citation103 These studies mostly included nondiabetic kidney disease patients, and thus it is hard to extrapolate their findings and assume that DKD patients will respond similarly. To address this issue, post hoc analysis of RENAALCitation104 and Irbesartan Diabetic Nephropathy Trial (IDNT)Citation105 found that higher BP targets (140–149 mmHg for RENAAL, and >145 mmHg for IDNT) resulted in higher risk of composite outcome. These studies also found that a tighter BP control (systolic <120 mmHg) resulted in increased mortality risk. It is important to mention that these studies were post hoc analyses that included a RAS blockers arm and a placebo arm, and were not designed to answer the question whether achieving a lower BP goal (<130 mmHg systolic) by increasing RAS blockers dose will have any benefit in this population. At the same time, these data were later challenged and questioned by the findings of increased risk of adverse outcomes (cardiac events, stroke, and falls) in different populations (especially elderly) and current guidelines have raised the target to 140 mmHg systolic.Citation106–Citation109
Several studies have evaluated the role of ACEi in halting DKD progression. The Captopril-Diabetes study evaluated type 1 diabetic patients with nephrotic range proteinuria and showed a significant reduction in proteinuria along with lower GFR decline rate compared to placebo.Citation110,Citation111 The ADVANCE trial compared perindopril–indapamide combination to placebo in over 11,000 patients with DM, and found a significantly lower microalbuminuria, and lower worsening macroalbuminuria incidence rate in all groups studied, including normotensive patients.Citation112,Citation113 The importance and significance of the effects of proteinuria reduction on renal outcomes was questioned since the treatment groups had better blood pressure control, which might have played a big role in preventing disease progression. Later studies showed that lowering proteinuria in patients with diabetic nephropathy (regardless of the agent used) is associated with improved outcomes and a decreased risk of ESRD.Citation114–Citation117
The IDNT and the RENAAL trial studied ARBs therapy in patients with DKD and found similar beneficiary results. ARB therapy was superior to placebo and amlodipine in decreasing proteinuria, lower rates of doubling creatinine, or the development of ESRD and mortality.Citation34,Citation118
No significant difference was found between ACEi and ARB in patients with early DKD. In the DETAIL trial, both groups (enalapril vs telmisartan) had similar 5-year GFR decline, annual changes in the GFR, blood pressure, serum creatinine, urinary albumin excretion, ESRD, cardiovascular events, and mortality.Citation119
Trials evaluating dual RAS blockades and controversies
It was previously demonstrated that ACEi/ARB combination therapy decreases proteinuria, more than monotherapy with either drug.Citation120–Citation122 These studies found no difference in adverse events in the combination group compared to monotherapy, but bore two major limitations that made their results and conclusion debatable. The sample sizes in these studies were small and patients were followed for a maximum of 16 weeks. Large scale randomized controlled trials conducted in different patient populations over a longer follow-up period failed to show any benefit on hard outcomes, including 50% drop in GFR, ESRD, and mortality.Citation123,Citation124 Patients on dual therapy had significantly higher adverse events, including hyperkalemia and hospitalization for acute kidney injury (AKI), as seen in the VA NEPHRON-D study,Citation123 and even a statistically nonsignificant trend of higher incidence of chronic dialysis, doubling in serum creatinine and death, along with a statistically significant higher incidence rate of AKI requiring dialysis, hypotension, and hyperkalemia (ONTARGET trial).Citation124 The results from studies evaluating the benefit of adding DRI (Aliskiren) to either ACEi or ARB in patients with DKD showed similar trend.Citation125,Citation126 Initially, the AVOID trial studied the combination between DRI and ARB in a group of 599 patients for 6 months and concluded that aliskiren may have renoprotective effects that are independent of its blood pressure-lowering effect in patients with hypertension and DKD, without a significant increase in adverse events.Citation125 Later, the ALTITUDE investigators evaluated 8,561 patients for a median follow-up of 32.9 months and found that the combination therapy increased the risk of composite primary endpoint (ESRD, doubling of serum creatinine, renal death, cardiovascular death, cardiac arrest, heart failure, nonfatal myocardial infarction, or nonfatal stroke), along with hyperkalemia.Citation126
As many as 40% of subjects treated with RAS blockers (ACEi or ARBs) have above normal limits levels of aldosterone (aldosterone breakthrough).Citation127,Citation128 This is thought to be due to different mechanisms that include: stimulation from tissue not inhibited by ACEi/ARBs, AngII generation via pathway not requiring ACE, or other stimuli such as high serum potassium concentrations.Citation129 Adding spironolactone (aldosterone receptor antagonist) to ACEi in DN patients with aldosterone breakthrough can decrease proteinuria.Citation128 This study, though promising and reporting no significant change in serum potassium, was limited to a small number of patients and was of short duration. Similar findings on proteinuria were noted in different small and short duration randomized controlled studies using a combination therapy of spironolactone and either ACEi or ARBs.Citation130–Citation135 Not surprisingly, all studies reported either a trend or a statistically significant higher levels of serum potassium, and greater eGFR decline in the combination therapy group. Similar findings were noted in adding eplerenone (aldosterone antagonist) to ACEi or ARBs in patients with DN and preserved renal function.Citation136
There are no long-term data regarding the beneficial effects of adding aldosterone antagonist, and the current literature suggests possible increased risk of adverse events.
Adverse events
The major adverse events in patients using RAS are hypotension, hyperkalemia, angioedema, and reduction in GFR. ACEi can result in dry cough in up to 20% of cases, while ARB is associated with cough in up to 9.9% of cases.Citation137,Citation138 Recently there is an emerging concern about the development of sprue-like enteropathy in patients using olmesartan.Citation139 Patients with bilateral renal artery stenosis, polycystic kidney disease, and advanced kidney disease may sustain a significant decrease in GFR, and in some cases AKI. The intrarenal perfusion pressure in these cases is already reduced, and patients are highly dependent on AngII to maintain a stable intraglomerular pressure and GFR. Similar but modest intrarenal hemodynamic changes are seen in elderly and DKD patients on RAS, increasing their risk of sustaining AKI in the setting of volume depletion, contrast agents, and major stressors like surgery.Citation140–Citation142
Summary and recommendations
Intrarenal activation of RAS in DKD plays a major role in disease pathophysiology and is implicated in its progression. The intrarenal generation is increased despite the systemic RAS suppression. This activation further downregulates the expression of both AT1 and AT2 receptors contributing to early diabetic nephropathy. This reduces AT2 receptor mediated beneficial counterregulatory actions. The negative impact of increased AngII on the kidneys goes beyond the regulation of intraglomerular pressure. It leads to increased insulin resistance, solute retention, stimulation of TGF-β secretion, increased synthesis, and decreased degradation of matrix proteins, all leading to matrix accumulation, kidney fibrosis, and further kidney damage. The use of ACEi and ARBs has significantly changed the incidence of ESRD and has provided renal protection through their effects on RAS, hemodynamic changes, and other pleiotropic effects that include anti-inflammatory, antioxidant, antithrombotic effects, and profibrinolytic activities. Even though a combination therapy of both ACEi and ARBs has decreased protein excretion in DKD patients, it is associated with worse renal outcomes compared to monotherapy. Studies evaluating other components of the RAS, including ACE2 and Ang 1–7 along with genetic animal models are warranted.
Studies to date have found no difference in renal outcomes between ACEi and ARBs, thus all hypertension patients with proteinuric kidney disease should be started on either ACEi or ARBs to halt disease progression. In our opinion, we believe that the evidence and the risk of adverse events overweigh the benefits of RAS blockers combination therapy. This therapy, though resulting in decreased proteinuria, carries a significant risk of adverse outcomes including hyperkalemia, increased hospitalization events, acute kidney injury, and a faster decline in renal function, and should be avoided. Another important issue we as clinicians face on a daily basis is the increase in serum potassium level in patients on RAS blockers. Little is available in the literature in regards to the use of sorbitol free sodium polystyrene sulfonate (Kayexalate), or other resins, as a secondary prevention method. A small study reports that this approach is effective and well tolerated.Citation143 We believe that all efforts to keep patients on the RAS blockers should be implemented, and other means and therapies aiming at decreasing serum potassium levels should be considered before discontinuing RAS blockers in cases of mild/non-life-threatening hyperkalemia. To date, no studies evaluating the long-term effects of such an approach are available, and the need for further study is warranted. There is also little evidence to guide decision making regarding the use of RAS blockers in predialysis advanced kidney disease patients, eg, eGFR <30 mL/min. We believe that each patient should be evaluated individually, and a risk and benefit evaluation to aid the decision should be made.
Disclosure
The authors report no conflicts of interest in this work.
References
- YacoubRLeeKHeJCThe role of SIRT1 in diabetic kidney diseaseFront Endocrinol20145166
- FoxCSPencinaMJMeigsJBVasanRSLevitzkyYSD’AgostinoRBSrTrends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart StudyCirculation2006113252914291816785337
- HuDFuPXieJIncreasing prevalence and low awareness, treatment and control of diabetes mellitus among Chinese adults: the InterASIA studyDiabetes Res Clin Pract200881225025718495287
- XuYWangLHeJPrevalence and control of diabetes in Chinese adultsJAMA2013310994895924002281
- TervaertTWMooyaartALAmannKPathologic classification of diabetic nephropathyJ Am Soc Nephrol201021455656320167701
- MauerMZinmanBGardinerRRenal and retinal effects of enalapril and losartan in type 1 diabetesN Engl J Med20093611405119571282
- BojestigMArnqvistHJHermanssonGKarlbergBELudvigssonJDeclining incidence of nephropathy in insulin-dependent diabetes mellitusN Engl J Med1994330115188259139
- NguyenGMullerDNThe biology of the (pro)renin receptorJ Am Soc Nephrol2010211182319917780
- KrolewskiASGenetics of diabetic nephropathy: evidence for major and minor gene effectsKidney Int19995541582159610201028
- TrevisanRVibertiGGenetic factors in the development of diabetic nephropathyJ Lab Clin Med199512643423497561441
- SatkoSGLangefeldCDDaeihaghPBowdenDWRichSSFreedmanBINephropathy in siblings of African Americans with overt type 2 diabetic nephropathyAm J Kidney Dis200240348949412200799
- JeffersBWEstacioRORaynoldsMVSchrierRWAngiotensin-converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus and its relationship with diabetic nephropathyKidney Int19975224734779264004
- YoshidaHKuriyamaSAtsumiYAngiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitusKidney Int19965026576648840299
- KuramotoNIizukaTItoHEffect of ACE gene on diabetic nephropathy in NIDDM patients with insulin resistanceAm J Kidney Dis199933227628110023638
- BorightAPPatersonADMireaLGenetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics StudyDiabetes20055441238124415793268
- HadjadjSTarnowLForsblomCAssociation between angiotensin-converting enzyme gene polymorphisms and diabetic nephropathy: case-control, haplotype, and family-based study in three European populationsJ Am Soc Nephrol20071841284129117376814
- KunzRBorkJPFritscheLRingelJSharmaAMAssociation between the angiotensin-converting enzyme-insertion/deletion polymorphism and diabetic nephropathy: a methodologic appraisal and systematic reviewJ Am Soc Nephrol199899165316639727374
- Pettersson-FernholmKFrojdoSFageruddJThe AT2 gene may have a gender-specific effect on kidney function and pulse pressure in type I diabetic patientsKidney Int200669101880188416598200
- PalmerBFRenal dysfunction complicating the treatment of hypertensionN Engl J Med2002347161256126112393824
- BadrKFIchikawaIPrerenal failure: a deleterious shift from renal compensation to decompensationN Engl J Med1988319106236293045546
- BakrisGLSlataperRVicknairNSadlerRACE inhibitor mediated reductions in renal size and microalbuminuria in normotensive, diabetic subjectsJ Diabetes Complications199481268167383
- AbueloJGNormotensive ischemic acute renal failureN Engl J Med2007357879780517715412
- HirschbergRBrunoriGKoppleJDGulerHPEffects of insulin-like growth factor I on renal function in normal menKidney Int19934323873978441234
- AndersonSVoraJPCurrent concepts of renal hemodynamics in diabetesJ Diabetes Complications1995943043078573753
- VlassaraHProtein glycation in the kidney: role in diabetes and agingKidney Int1996496179518048743500
- HannedoucheTPDelgadoAGGnionsaheDABoitardCLacourBGrunfeldJPRenal hemodynamics and segmental tubular reabsorption in early type 1 diabetesKidney Int1990374112611332188030
- VallonVRichterKBlantzRCThomsonSOsswaldHGlomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorptionJ Am Soc Nephrol199910122569257610589696
- YanagawaNPotential role for local luminal angiotensin II in proximal tubule sodium transportKidney Int Suppl199132S33S361881047
- DentonKMFennessyPAAlcornDAndersonWPMorphometric analysis of the actions of angiotensin II on renal arterioles and glomeruliAm J Physiol19922623 Pt 2F367F3721558155
- StahlRAParaviciniMSchollmeyerPAngiotensin II stimulation of prostaglandin E2 and 6-keto-F1 alpha formation by isolated human glomeruliKidney Int198426130346592393
- OliverJAPintoJSciaccaRRCannonPJIncreased renal secretion of norepinephrine and prostaglandin E2 during sodium depletion in the dogJ Clin Invest19806647487566999033
- VallottonMBGerber-WichtCDolciWWuthrichRPInteraction of vasopressin and angiotensin II in stimulation of prostacyclin synthesis in vascular smooth muscle cellsAm J Physiol19892575 Pt 1E617E6242512814
- IchikawiIHarrisRCAngiotensin actions in the kidney: renewed insight into the old hormoneKidney Int19914045835961745006
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- No authors listedEffects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study InvestigatorsLancet2000355920025325910675071
- ACEInhibitors in Diabetic Nephropathy Trialist GroupShould all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient dataAnn Intern Med2001134537037911242497
- PriceDAPorterLEGordonMThe paradox of the low-renin state in diabetic nephropathyJ Am Soc Nephrol199910112382239110541298
- GurleySBCoffmanTMThe renin-angiotensin system and diabetic nephropathySemin Nephrol200727214415217418683
- LuetscherJAKraemerFBWilsonDMSchwartzHCBryer-AshMIncreased plasma inactive renin in diabetes mellitus. A marker of microvascular complicationsN Engl J Med198531222141214173887168
- LaiKNLeungJCLaiKBToWYYeungVTLaiFMGene expression of the renin-angiotensin system in human kidneyJ Hypertens1998161911029533422
- TuckMLSambhiMPLevinLHyporeninemic hypoaldosteronism in diabetes mellitus. Studies of the autonomic nervous system’s control of renin releaseDiabetes1979283237241446908
- KaneshiroYIchiharaASakodaMSlowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic ratsJ Am Soc Nephrol20071861789179517494887
- PetersBGriskOBecherBDose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: absence of prorenin-induced glomerulosclerosisJ Hypertens200826110210918090546
- ZimpelmannJKumarDLevineDZEarly diabetes mellitus stimulates proximal tubule renin mRNA expression in the ratKidney Int20005862320233011115066
- HsiehTJZhangSLFilepJGTangSSIngelfingerJRChanJSHigh glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cellsEndocrinology200214382975298512130563
- ChoiKCKimNHAnMRKangDGKimSWLeeJAlterations of intrarenal renin-angiotensin and nitric oxide systems in streptozotocin-induced diabetic ratsKidney Int Suppl199760S23S279285898
- HollenbergNKDirect renin inhibition and the kidneyNat Rev Nephrol201061495519935744
- HuangYWongamornthamSKastingJRenin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanismsKidney Int200669110511316374430
- HuangYNobleNAZhangJXuCBorderWARenin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cellsKidney Int2007721455217396111
- SakodaMIchiharaAKaneshiroY(Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cellsHypertens Res200730111139114618250563
- KaneshiroYIchiharaATakemitsuTIncreased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic ratsKidney Int200670464164616807542
- FeldtSBatenburgWWMazakIProrenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptideHypertension200851368268818212269
- MercureCPrescottGLacombeMJSilversidesDWReudelhuberTLChronic increases in circulating prorenin are not associated with renal or cardiac pathologiesHypertension20095361062106919364992
- DanserAH(Pro)renin receptor and vacuolar H+-ATPaseHypertension200954221922119546373
- AdvaniAKellyDJCoxAJThe (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidneyHypertension200954226126919546380
- AndersonSJungFFIngelfingerJRRenal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlationsAm J Physiol19932654 Pt 2F477F4868238377
- HuangYBorderWANobleNAFunctional renin receptors in renal mesangial cellsCurr Hypertens Rep20079213313917442225
- NguyenGDelarueFBerrouJRondeauESraerJDSpecific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigenKidney Int1996506189719038943472
- NguyenGDelarueFBurckleCBouzhirLGillerTSraerJDPivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to reninJ Clin Invest2002109111417142712045255
- DurvasulaRVShanklandSJActivation of a local renin angiotensin system in podocytes by glucoseAm J Physiol Renal Physiol20082944F830F83918216149
- LiuFYCoganMGAngiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphateJ Clin Invest198984183912544631
- LiuFYCoganMGRole of protein kinase C in proximal bicarbonate absorption and angiotensin signalingAm J Physiol19902584 Pt 2F927F9332330986
- KiforIMooreTJFalloFPotassium-stimulated angiotensin release from superfused adrenal capsules and enzymatically dispersed cells of the zona glomerulosaEndocrinology199112928238311855477
- SinghRAlaviNSinghAKLeeheyDJRole of angiotensin II in glucose-induced inhibition of mesangial matrix degradationDiabetes199948102066207310512375
- MezzanoSARuiz-OrtegaMEgidoJAngiotensin II and renal fibrosisHypertension2001383 Pt 263563811566946
- WadaJMakinoHInflammation and the pathogenesis of diabetic nephropathyClin Sci2013124313915223075333
- VellosoLAFolliFPeregoLSaadMJThe multi-faceted cross-talk between the insulin and angiotensin II signaling systemsDiabetes Metab Res Rev20062229810716389635
- TiwariSHalagappaVKRiaziSHuXEcelbargerCAReduced expression of insulin receptors in the kidneys of insulin-resistant ratsJ Am Soc Nephrol200718102661267117855644
- GloyJHengerAFischerKGAngiotensin II modulates cellular functions of podocytesKidney Int Suppl199867S168S1709736279
- GarridoAMGriendlingKKNADPH oxidases and angiotensin II receptor signalingMol Cell Endocrinol2009302214815819059306
- HaradaEYoshimuraMYasueHAldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytesCirculation2001104213713911447075
- KangYSKoGJLeeMHEffect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic ratsNephrol Dial Transplant2009241738418682491
- HanSYKimCHKimHSSpironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic ratsJ Am Soc Nephrol20061751362137216571782
- MiyataKRahmanMShokojiTAldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cellsJ Am Soc Nephrol200516102906291216135774
- SowersJRWhaley-ConnellAEpsteinMNarrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertensionAnn Intern Med20091501177678319487712
- DonoghueMHsiehFBaronasEA novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9Circ Res2000875E1E910969042
- CareyRMSiragyHMThe intrarenal renin-angiotensin system and diabetic nephropathyTrends Endocrinol Metab200314627428112890592
- CrackowerMASaraoROuditGYAngiotensin-converting enzyme 2 is an essential regulator of heart functionNature2002417689182282812075344
- MoriJPatelVBRamprasathTAngiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicityAm J Physiol Renal Physiol20143068F812F82124553436
- ZhangKMengXLiDAngiotensin(1-7) attenuates the progression of streptozotocin-induced diabetic renal injury better than angiotensin receptor blockadeKidney Int201587235936925075768
- MiyataNParkFLiXFCowleyAWJrDistribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidneyAm J Physiol19992773 Pt 2F437F44610484527
- OzonoRWangZQMooreAFInagamiTSiragyHMCareyRMExpression of the subtype 2 angiotensin (AT2) receptor protein in rat kidneyHypertension1997305123812469369282
- ArimaSItoSAngiotensin II type 2 receptors in the kidney: evidence for endothelial-cell-mediated renal vasodilatationNephrol Dial Transplant200015444845110727534
- OhBHMitchellJHerronJRChungJKhanMKeefeDLAliskiren, an oral renin inhibitor, provides dose-dependent efficacy and sustained 24-hour blood pressure control in patients with hypertensionJ Am Coll Cardiol200749111157116317367658
- NichollsSJBakrisGLKasteleinJJEffect of aliskiren on progression of coronary disease in patients with prehypertension: the AQUARIUS randomized clinical trialJAMA2013310111135114423999933
- ErdosEGMarcicBMKinins, receptors, kininases and inhibitors – where did they lead us?Biol Chem20013821434711258670
- SironiLNobiliEGianellaAGelosaPTremoliEAnti-inflammatory properties of drugs acting on the renin-angiotensin systemDrugs Today200541960962216341292
- KothariSALeMKGandhiPJEffects of angiotensin converting enzyme inhibitors on thrombotic mediators: potential clinical implicationsJ Thromb Thrombolysis200315321722514739632
- NeutelJMEffect of the renin–angiotensin system on the vessel wall: using ACE inhibition to improve endothelial functionJ Hum Hypertens200418959960615190263
- KlingbeilAUSchneiderMMartusPMesserliFHSchmiederREA meta-analysis of the effects of treatment on left ventricular mass in essential hypertensionAm J Med20031151414612867233
- HilgersKFMannJFACE inhibitors versus AT(1) receptor antagonists in patients with chronic renal diseaseJ Am Soc Nephrol20021341100110811912272
- MaJNishimuraHFogoAKonVInagamiTIchikawaIAccelerated fibrosis and collagen deposition develop in the renal interstitium of angiotensin type 2 receptor null mutant mice during ureteral obstructionKidney Int19985349379449551401
- MorrisseyJJKlahrSEffect of AT2 receptor blockade on the pathogenesis of renal fibrosisAm J Physiol19992761 Pt 2F39F459887078
- CouserWGRiellaMCJoint International Society of Nephrology and International Federation of Kidney Foundations’ World Kidney Day Steering CommitteeWorld Kidney Day 2011: protect your kidneys, save your heartKidney Int201179548348521321555
- American Diabetes AssociationDiabetic nephropathyDiabetes Care199821S50S53
- Arauz-PachecoCParrottMARaskinPAmerican Diabetes AssociationTreatment of hypertension in adults with diabetesDiabetes Care200326Suppl 1S80S8212502624
- KDOQIKDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney diseaseAm J Kidney Dis2007492 Suppl 2S12S15417276798
- KlahrSLeveyASBeckGJThe effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of diet in Renal Disease Study GroupN Engl J Med1994330138778848114857
- PetersonJCAdlerSBurkartJMBlood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease studyAnn Intern Med1995123107547627574193
- SarnakMJGreeneTWangXThe effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease studyAnn Intern Med2005142534235115738453
- WrightJTJrBakrisGGreeneTEffect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trialJAMA2002288192421243112435255
- AppelLJWrightJTJrGreeneTIntensive blood-pressure control in hypertensive chronic kidney diseaseN Engl J Med20103631091892920818902
- JafarTHStarkPCSchmidCHProgression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysisAnn Intern Med2003139424425212965979
- BakrisGLWeirMRShanifarSEffects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL studyArch Intern Med2003163131555156512860578
- PohlMABlumenthalSCordonnierDJIndependent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitationsJ Am Soc Nephrol200516103027303716120823
- RuzickaMQuinnRRMcFarlanePCanadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for the management of blood pressure in CKDAm J Kidney Dis201463686988724725980
- TalerSJAgarwalRBakrisGLKDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKDAm J Kidney Dis201362220121323684145
- VerbekeFLindleyEVan BortelLA European Renal Best Practice (ERBP) position statement on the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for the management of blood pressure in non-dialysis-dependent chronic kidney disease: an endorsement with some caveats for real-life applicationNephrol Dial Transplant201429349049624071661
- RobertsMACommentary on the KDIGO clinical practice guideline for the management of blood pressure in chronic kidney diseaseNephrology2014191535524341660
- LewisEJHunsickerLGBainRPRohdeRDThe effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study GroupN Engl J Med199332920145614628413456
- HebertLABainRPVermeDRemission of nephrotic range proteinuria in type I diabetes. Collaborative Study GroupKidney Int1994466168816937700028
- PatelAADVANCE Collaborative GroupMacMahonSEffects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trialLancet2007370959082984017765963
- de GalanBEPerkovicVNinomiyaTLowering blood pressure reduces renal events in type 2 diabetesJ Am Soc Nephrol200920488389219225038
- AtkinsRCBrigantiEMLewisJBProteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathyAm J Kidney Dis200545228128715685505
- JafarTHStarkPCSchmidCHProteinuria as a modifiable risk factor for the progression of non-diabetic renal diseaseKidney Int20016031131114011532109
- ImaiEHanedaMChanJCReduction and residual proteinuria are therapeutic targets in type 2 diabetes with overt nephropathy: a post hoc analysis (ORIENT-proteinuria)Nephrol Dial Transplant201328102526253424013685
- BakrisGLMangrumACopleyJBVicknairNSadlerREffect of calcium channel or beta-blockade on the progression of diabetic nephropathy in African AmericansHypertension19972937447509052890
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- BarnettAHBainSCBouterPAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med2004351191952196115516696
- JacobsenPAndersenSRossingKJensenBRParvingHHDual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathyKidney Int20036351874188012675866
- MogensenCENeldamSTikkanenIRandomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) studyBMJ200032172741440144411110735
- SongJHChaSHLeeHJEffect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney diseaseNephrol Dial Transplant200621368368916330466
- FriedLFEmanueleNZhangJHCombined angiotensin inhibition for the treatment of diabetic nephropathyN Engl J Med2013369201892190324206457
- MannJFAndersonCGaoPDual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trialJ Hypertens201331241442123249829
- ParvingHHPerssonFLewisJBLewisEJHollenbergNKAVOID Study InvestigatorsAliskiren combined with losartan in type 2 diabetes and nephropathyN Engl J Med2008358232433244618525041
- ParvingHHBrennerBMMcMurrayJJCardiorenal end points in a trial of aliskiren for type 2 diabetesN Engl J Med2012367232204221323121378
- JordeUPVittorioTKatzSDColomboPCLatifFLe JemtelTHElevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failureCirculation200210691055105712196328
- SatoAHayashiKNaruseMSarutaTEffectiveness of aldosterone blockade in patients with diabetic nephropathyHypertension2003411646812511531
- YoungDBSmithMJJrJacksonTEScottREMultiplicative interaction between angiotensin II and K concentration in stimulation of aldosteroneAm J Physiol19842473 Pt 1E328E3356476112
- BianchiSBigazziRCampeseVMAntagonists of aldosterone and proteinuria in patients with CKD: an uncontrolled pilot studyAm J Kidney Dis2005461455115983956
- RossingKSchjoedtKJSmidtUMBoomsmaFParvingHHBeneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over studyDiabetes Care20052892106211216123474
- SchjoedtKJRossingKJuhlTRBeneficial impact of spironolactone in diabetic nephropathyKidney Int20056862829283616316360
- van den MeirackerAHBaggenRGPauliSSpironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal functionJ Hypertens200624112285229217053552
- MehdiUFAdams-HuetBRaskinPVegaGLTotoRDAddition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathyJ Am Soc Nephrol200920122641265019926893
- RachmaniRSlavachevskyIAmitMThe effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled studyDiabet Med200421547147515089793
- EpsteinMWilliamsGHWeinbergerMSelective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetesClin J Am Soc Nephrol20061594095117699311
- IsrailiZHHallWDCough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiologyAnn Intern Med199211732342421616218
- MatcharDBMcCroryDCOrlandoLASystematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertensionAnn Intern Med20081481162917984484
- IaniroGBibboSMontaltoMRicciRGasbarriniACammarotaGSystematic review: Sprue-like enteropathy associated with olmesartanAliment Pharmacol Ther2014401162324805127
- StirlingCHoustonJRobertsonSDiarrhoea, vomiting and ACE inhibitors: – an important cause of acute renal failureJ Hum Hypertens200317641942312764405
- YacoubRPatelNLohrJWRajagopalanSNaderNAroraPAcute kidney injury and death associated with renin angiotensin system blockade in cardiothoracic surgery: a meta-analysis of observational studiesAm J Kidney Dis20136261077108623791246
- RimMYRoHKangWCThe effect of renin-angiotensin-aldosterone system blockade on contrast-induced acute kidney injury: a propensity-matched studyAm J Kidney Dis201260457658222658321
- CherninGGal-OzABen-AssaESecondary prevention of hyperkalemia with sodium polystyrene sulfonate in cardiac and kidney patients on renin-angiotensin-aldosterone system inhibition therapyClin Cardiol2012351323622057933
