Abstract
Diabetic nephropathy is a significant cause of chronic kidney disease and end-stage renal failure globally. Much research has been conducted in both basic science and clinical therapeutics, which has enhanced understanding of the pathophysiology of diabetic nephropathy and expanded the potential therapies available. This review will examine the current concepts of diabetic nephropathy management in the context of some of the basic science and pathophysiology aspects relevant to the approaches taken in novel, investigative treatment strategies.
Introduction
Background
Diabetic nephropathy (DN) or diabetic kidney disease is a syndrome characterized by the presence of pathological quantities of urine albumin excretion, diabetic glomerular lesions, and loss of glomerular filtration rate (GFR) in diabetics. Diabetes may be classified as type 1 (autoimmune β-cell destruction and absolute insulin deficiency), type 2 (relative insulin deficiency and resistance), and other types (eg, pancreatic disease).
Epidemiology
The prevalence of diabetes is phenomenal and the projections are staggering. When one considers the morbidity, mortality, and cost of health care, the burden of the diabetes epidemic becomes apparent. Worldwide, the prevalence of diabetes was estimated at 171 million in 2000, increasing to 382 million in 2013; and is projected to reach 592 million by 2035. This represents 8%–10% of the global population, resulting in at least 548 billion dollars in health expenditure on diabetes care. Type 2 diabetes constitutes about 85%–95% of all diabetes cases.Citation1 In the US alone for 2011, 25.8 million children and adults have diabetes with another 79 million having a prediabetic state.Citation2
The diabetes epidemic has resulted in DN becoming the most frequent cause of end-stage renal disease (ESRD) in most countries. In 2009–2011, diabetes was the primary cause of ESRD in about 60% of patients in Malaysia, Mexico, and Singapore. Countries with an ESRD incidence of 40%–50% include Israel, Korea, Hong Kong, Taiwan, Philippines, Japan, the US, and New Zealand.Citation2 The incidence of ESRD due to diabetes also rises in the older age group. In 2011, the incident rates of ESRD due to diabetes in the US were 44, 266, and 584 per million for the age groups 20–44, 45–64, and 65–74 years, respectively. A similar finding was noted in the AusDiab study of 11,247 diabetic Australians.Citation3 Thus, the reason for this boom in diabetes-associated ESRD is the increasing prevalence of diabetes and the aging population.
Risk factors
Not all diabetics develop DN and in those who do, progression is variable. The main modifiable risks are hypertension, glycemic control, and dyslipidemia. Data from the Joslin Diabetes Center, Steno Diabetes Center, and AusDiab studies also strongly implicate smoking as a risk factor for DN.Citation3–Citation5 The main unmodifiable risks are age, race, and genetic profile. DN is more likely to develop in patients with a family history of DN.Citation6–Citation8 Certain racial groups are also at higher risk, such as African Americans, Mexican Americans, and Pima Indians.Citation9,Citation10 One study suggested that males had an increased risk of DN.Citation11
A meta-analysis of studies identified 24 genetic variants in 16 genes which are associated with DN. These include: ACE, ALR2, APOC1, APOE, EPO, eNOS, HSPG2, VEGF, FRMD3, CARS, UNC13B, CPVL/CHN2, and GREM1. In a subgroup of type 2 diabetic Asians, ELMO1, CCR5, and CNDP1 were also relevant.Citation12 Other meta-analyses implicated polymorphisms of ADIPOQ, PAI-1, TGFβ1, and PPARγ in the development of DN. The nature of the polymorphism varies with ethnicity.Citation13–Citation15 The complexity of genetic studies in DN is discussed in a review by Mooyaart.Citation16
Diagnosis
Stages and natural history
Incipient nephropathy is the initial presence of low but abnormal amounts of urine albumin, referred to as microalbuminuria (persistent albuminuria at level 30–299 mg/24 hours). Overt nephropathy or macroalbuminuria (persistent albuminuria at level ≥300 mg/24 hours) develops after many years in type 1 diabetes but may be present at the time of diagnosis of type 2 diabetes. Patients who progress to macroalbuminuria are more likely to develop ESRD.Citation11 The natural history depends on the type of diabetes.
In untreated type 1 diabetics, approximately 80% of patients with sustained microalbuminuria increase their albumin excretion by 10%–20% per year until overt nephropathy develops, which normally takes 10–15 years. With the development of overt nephropathy, the GFR declines at a rate of 2–20 mL/minute/year and ESRD develops in 50% within 10 years and in 75% by 20 years.Citation17 Structural changes can precede albuminuria and reduced GFR, with glomerular basement membrane thickening and mesangial expansion, can be detected as early as 2–8 years after onset of diabetes.Citation18
In type 2 diabetics, more patients have DN at the time of diagnosis of diabetes as type 2 diabetes can go unrecognized for years. The AusDiab study of diabetic Australians showed that albuminuria is common among patients with established diabetes, is present before the onset of diabetes, and becomes more prevalent with worsening glucose tolerance.Citation3 About 20%–40% of type 2 diabetics with microalbuminuria progress to overt nephropathy; and about 20% will develop ESRD after the development of overt nephropathy.Citation17,Citation19
Screening for DN
Most guidelines recommend screening with a spot urine albumin/creatinine ratio (ACR; normal >30 mg/g creatinine), from either first morning (preferred) or random specimens. An abnormal result is repeated once or twice over a few months for consistency. This is coupled with an assessment of renal function, using the Modification of Diet in Renal Disease or Chronic Kidney Disease Epidemiology Collaboration formulas for estimated GFR (eGFR) in order to stage chronic kidney disease (CKD).Citation20,Citation21 Screening begins at diagnosis of type 2 diabetes and usually 5 years after onset of type 1 diabetes. Timed collections can also be utilized and will average out diurnal variations in albumin excretion (normal >20 μg/minute).
Renal biopsy
The routine use of renal biopsy to confirm DN is much debated. Many nephrologists do not biopsy patients with classic features such as retinopathy, duration of diabetes <10 years, slow decline in GFR, gradual progression of proteinuria, and lack of active urinary sediment. Without standardized criteria, there may be significant variations in epidemiology. An Italian study of 393 type 2 diabetics highlighted this point. In centers with an unrestricted biopsy policy, the rate of finding an underlying glomerulonephritis was lower than those centers with a restricted biopsy policy (33% versus 57%). The unrestricted policy resulted in a greater proportion of patients found to have glomerulonephritis rather than diabetic glomerulosclerosis.Citation22 The prevalence of specific disease in the population can also affect the biopsy decision. In a Chinese study of 51 type 2 diabetics with >1 g/day proteinuria, one-third of patients had nondiabetic disease, predominantly IgA nephropathy.Citation23 The largest study to date looked at 620 biopsies from type 1 and 2 diabetics, with a median duration of diabetes of 10 years. Overall, 37% of patients had isolated DN, 36% had isolated nondiabetic disease, and 27% had nondiabetic disease superimposed on DN. The duration of diabetes >12 years was the best predictor for isolated DN. Interestingly, 43% of biopsies with DN demonstrated superimposed acute tubular necrosis.Citation24 Thus, a renal biopsy is useful to exclude acute tubular injury and diseases amenable to specific therapy.
Biomarkers
There are limitations in using albuminuria as a marker of DN as many patients experience GFR loss without deterioration in albuminuria and even normoalbuminuria.Citation25 In fact, histologically proven advanced diabetic glomerular lesions can develop despite normoalbuminuria.Citation26 Furthermore, low-grade albuminuria is a lesser predictor of disease progression than macroalbuminuria.Citation27 Therefore, there is interest in finding biomarkers to detect DN earlier and identify progression risk. There is also interest in urine microRNA profiling but studies are fairly preliminary.Citation28,Citation29 The most promising biomarker currently is serum TNF-α receptor levels, which may predict progression of CKD and ESRD, in type 1 and type 2 diabetics. In type 2 diabetics, the TNF-α receptor level showed prognostic value in addition to albuminuria.Citation30,Citation31 Serum uric acid is another biomarker which may also be pathogenic (discussed later). Studies of tubular biomarkers have been conflicting (). The larger studies have not shown that these biomarkers offer additional value on top of traditional prediction models. More work is needed to clarify the role of biomarkers in clinical practice.
Table 1 Tubular biomarkers
Pathogenesis
Pathology and pathophysiology
DN is characterized by structural and functional changes. In glomeruli, there is mesangial expansion, thickening of the basement membrane, and, characteristically, nodular glomerulosclerosis (Kimmelstiel–Wilson nodules). In early DN, tubular hypertrophy is present but eventually interstitial fibrosis with tubular atrophy develops, along with arteriolar hyalinosis. In advanced cases, there is an infiltrate of macrophages and T-lymphocytes. Ultrastructurally, there is podocyte loss and reduced endothelial cell fenestration.Citation32,Citation33 These characteristic pathological changes are shown in . Functionally, there is early glomerular hyperfiltration and increased albumin excretion; and with advancing nephropathy, increasing proteinuria and declining GFR. A brief description of the functional and cellular pathology is provided below. Although it is conceptually easier to describe these pathways individually, these pathways overlap and interact with one another in vivo, and enhance one another’s biophysiological effects ().
Figure 1 Characteristic histological features of diabetic nephropathy.
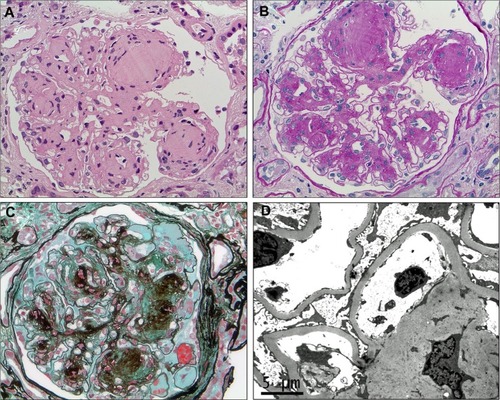
Figure 2 Overview of the pathological pathways in diabetic nephropathy.
Abbreviations: AGE, advanced glycation end-products; GBM, glomerular basement membrane; GFR, glomerular filtration rate; Mac, macrophages; Mon, monocyte; NOS, nitric oxide synthase; ROS, reactive oxygen species.
Hemodynamic factors
There is an imbalance in afferent and efferent arteriolar resistance, resulting in increased glomerular hydrostatic pressure and hyperfiltration. Activation of the renin–angiotensin system (RAS) increases angiotensin II levels, leading to efferent arteriolar vasoconstriction and production of proinflammatory and profibrotic molecules through multiple mechanisms. High angiotensin converting enzyme (ACE) levels are associated with greater albuminuria and nephropathy in diabetic mice and humans.Citation34,Citation35 Increased levels of endothelin-1 and urotensin II also contribute to vasoconstriction. Various dysregulation of nitric oxide and nitric oxide synthase has been described in DN. Nitric oxide mediates endothelium-dependent vasodilatation, and is formed from L-arginine by endothelial nitric oxide synthase. Diabetic endothelial nitric oxide synthase knockout mice develop more severe glomerular lesions and proteinuria compared to wild-type mice.Citation36
Metabolic factors
Oxidative stress and generation of reactive oxygen species (ROS) damage DNA and protein, or function as signaling amplifiers to activate cellular stress pathways such as PKC, MAPK, and NF-κB.Citation37,Citation38 Activation of the polyol pathway, with aldose reductase converting excess glucose to sorbitol, and subsequent conversion to fructose by sorbitol dehydrogenase contributes to oxidative stress by increasing the NADH/NAD+ ratio.Citation39,Citation40 A recently described novel mechanism of injury also involves endogenous fructose production with activation of fructokinase in the proximal tubule.Citation41 The formation of advanced glycation end-products (AGE) by nonenzymatic binding of glucose to proteins, lipids, and nucleic acids can lead to alteration of protein structure and function, oxidative stress, and expression of proinflammatory cytokines and growth factors.Citation42
Growth factors/cytokines
Activation of TGF-β and its downstream cytokine, CTGF, induce extracellular matrix formation and fibrosis. In kidney biopsies, glomerular expression of TGF-β1 and CTGF were higher in diabetics compared to controls, and correlated with albuminuria. PDGF expression is also increased in DN, which can modulate chemotaxis, vascular tone, and platelet aggregation. VEGF is crucial in angiogenesis but also mediates vasodilatation and leukocyte trafficking in DN.
Cell signaling and transcription factors
Increased renal gene transcription of PKC-β showed a strong relationship with glycemic control.Citation43 PKC activation has wide ranging effects, including enhancing angiotensin II actions, nitric oxide dysregulation, endothelial dysfunction, and activation of MAPK and NF-κB.Citation44,Citation45 MAPKs are intracellular kinases which integrate cell signaling into cellular responses. MAPKs activate a number of nuclear transcription factors, including NF-κB, which then regulates the gene expression of various cytokines, chemokines, and adhesion molecules. The activation of p38α isoform of the p38 MAPK pathway is most strongly associated with renal inflammation and DN.Citation46,Citation47 There may also be a role for toll-like receptors (TLR2, TLR4) and B7-1 costimulatory signaling in modulating inflammation and injury in DN.Citation48,Citation49 Finally, transcription factors bind to the promoter regions of genes and modulate transcription of messenger RNA. NF-κB has been the best studied in DN. Activation of NF-κB in both human peripheral blood mononuclear cells and kidney biopsies correlate with severity of proteinuria and glycemic control.Citation50,Citation51 A review of transcription factors in DN is provided by Sanchez and Sharma.Citation52
Inflammation
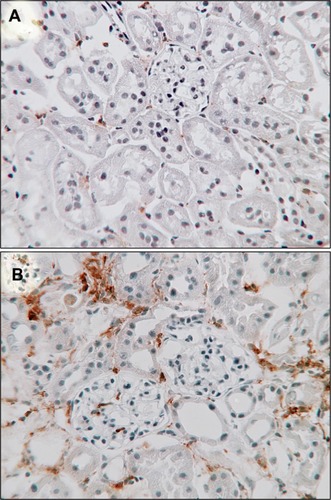
In DN, there is recruitment and activation of innate immune cells and elaboration of proinflammatory cytokines.Citation53 Macrophages and T-lymphocytes are prominent in early diabetic glomeruli while an interstitial infiltrate develops later (). Strategies impairing kidney leukocyte recruitment, proliferation, or activation have demonstrated that macrophages mediate DN.Citation54,Citation55 In humans, kidney macrophage accumulation is associated with the severity of glomerulosclerosis.Citation56 Accumulation of interstitial macrophages correlated strongly with proteinuria, interstitial fibrosis, and GFR decline.Citation57
Figure 3 Macrophages in diabetic nephropathy.
The role of lymphocytes is less clear. A higher circulating level of activated T-cells is associated with DN.Citation58 A kidney T-cell influx is common in early type 1 diabetes, and correlates with renal function and albuminuria.Citation59 However, absence of lymphocytes did not prevent fibrosis and declining renal function in experimental DN.Citation60 Recent attention has focused on the subset of regulatory T-cells (Treg), which may play a protective role in DN. Treg numbers are increased in diabetic mice.Citation60 Treg depletion in diabetic mice exacerbated albuminuria and hyperfiltration, while adoptive transfer of Treg improved DN.Citation61 In type 2 diabetics, the number of Tregs as determined by flow cytometry showed an inverse correlation with albuminuria, particularly in patients with macroalbuminuria.Citation62 Treg also demonstrated an anti-inflammatory function, which reduces the metabolic abnormalities and insulin resistance in a mouse model of type 2 diabetes.Citation63 The main proinflammatory cytokines implicated in DN are TNF-α, MCP-1, ICAM-1, IL-1, IL-6, and IL-18. These cytokines are increased in diabetic patients and show correlation with albuminuria and glomerular pathology.Citation53
Treatment
Treatment to delay DN progression involves adequate control of metabolic and hemodynamic abnormalities. In practical terms, this means adequate blood glucose lowering and control of hypertension. A description of all glucose lowering agents is beyond the scope of this review but certain agents have theoretical benefits beyond glucose lowering. Certain antihypertensives are also preferred based on studies which have demonstrated reductions in proteinuria or preservation of GFR, or both. The main pharmacological interventions described here are summarized in . Nonpharmacological approaches and alternative medicine are briefly discussed. There is also interest in novel agents, gene therapy, and stem cell treatment, which may someday find a place in the treatment armamentarium.
Table 2 Summary of pharmacological treatment of diabetic nephropathy
Glycemic control
Good glycemic control is effective in reducing diabetic microvascular complications. DCCT was a trial involving 1,365 type 1 diabetics and normoalbuminuria. After almost 10 years, patients randomized to intensive glucose control had lower incidences of microalbuminuria and macroalbuminuria.Citation64 In the UKPDS trial of 3,867 newly diagnosed type 2 diabetics, patients receiving intensive glucose treatment were less likely to develop renal failure.Citation65 In the ADVANCE trial of 11,140 type 2 diabetics, intensive therapy (mean hemoglobin A1c [HbA1c] ≤6.5%) also reduced the incidence of nephropathy compared to standard control (mean HbA1c 7.3%). Intensive glucose control reduced the risk of ESRD by 65%.Citation66 In the VADT study of 1,791 type 2 diabetics, intensive glucose control (median HbA1c 6.9%) was associated with less worsening of albuminuria and progression to macroalbuminuria but no significant difference in GFR at 6 years.Citation67 However, intensive glucose control to an HbA1c of <6% may confer excess mortality, as demonstrated in the ACCORD trial of type 2 diabetics with cardiovascular disease or cardiovascular risk factors.Citation68,Citation69 Thus, an HbA1c of <6%, particularly if associated with significant hypoglycemic episodes, should be avoided.
Certain drugs may confer beneficial effects independent of glucose lowering. PPAR-γ inhibitors such as pioglitazone and rosiglitazone have demonstrated antifibrotic and anti-inflammatory effects in the kidney of diabetic rats.Citation70–Citation72 In type 2 diabetics, the addition of rosiglitazone to metformin treatment for 32 weeks reduced albuminuria and blood pressure independent of glycemic control.Citation73 DPP-4 inhibitors (gliptins) have shown anti-inflammatory and antiapoptotic properties in DN models.Citation74 In type 2 diabetics, sitagliptin treatment for 6 months reduced albuminuria independent of HbA1c.Citation75 In a study of alogliptin in type 2 diabetics, researchers showed a reduction in oxidative stress but no change in renal function.Citation76 Lastly, SGLT-2 inhibitors such as empagliflozin may reduce hyperfiltration by their effect on tubuloglomerular feedback.Citation77 Further trial evidence is needed to determine if these agents should be preferred agents in patients with DN.
Antihypertensives
ACE inhibitors
ACE inhibitors have a strong track record in slowing disease progression in type 1 and type 2 diabetics. In the 1990s, captopril demonstrated the ability of ACE inhibitors in reducing the progression of albuminuria and decline in renal function in type 1 diabetics, independent of blood pressure lowering.Citation78–Citation80 In the Collaborative Study Group trial of 409 type 1 diabetics, captopril treatment reduced the risk of doubling of serum creatinine by 48% and reduced the composite outcome of death, dialysis, and transplantation by 50% compared to placebo.Citation80 This study also demonstrated that a sustained remission of nephrotic-range proteinuria was possible with ACE inhibitors.Citation81 This was backed up by a study which showed that patients who achieved remission (albuminuria <600 mg/day) for ≥1 year had better outcomes compared to those who did not, including slower decline in GFR and lower risk of dialysis, transplantation, or death.Citation82,Citation83
The perindopril/indapamide combination was studied in the ADVANCE trial of 11,140 type 2 diabetics. After mean follow-up of 4.3 years, perindopril/indapamide treatment reduced new onset microalbuminuria and prevented progression of microalbuminuria to overt nephropathy. However, serum creatinine and ESRD were not affected. It has also been argued that the effect on albuminuria was not independent of blood pressure, given a difference of 5.6/2.2 mmHg between the treatment groups.Citation84 Finally, the BENEDICT trial also showed that ACE inhibitor treatment could delay onset of microalbuminuria in type 2 diabetics with hypertension and baseline normoalbuminuria.Citation85
Angiotensin receptor blocker (ARB)
In the IDNT trial, 1,715 hypertensive type 2 diabetics with nephropathy were randomly assigned to receive irbesartan, amlodipine, or placebo.Citation86 Irbesartan reduced the risk of ESRD or doubling of serum creatinine by 20%–23% compared to amlodipine or placebo. In the RENAAL trial, 1,513 type 2 diabetics with nephropathy were randomly assigned to losartan or placebo, in addition to conventional antihypertensives. Losartan reduced the risk of ESRD or doubling of serum creatinine by 25%–28% compared to placebo.Citation87 These effects were also independent of blood pressure lowering. Much like the early captopril studies in type 1 diabetics, a lower residual level of albuminuria was associated with lower ESRD risk.Citation88 The ROADMAP trial of 4,447 type 2 diabetics randomized to olmesartan or placebo demonstrated that olmesartan was more effective in delaying the onset of microalbuminuria. However, the olmesartan group had a slightly lower blood pressure (mean difference 3.1/1.9 mmHg) and there appeared to be a higher rate of fatal cardiovascular events in those with preexisting coronary artery disease.Citation89
ACE inhibitor versus ARB
In the DETAIL trial, 250 type 2 diabetics with early DN were randomly assigned to enalapril or telmisartan. This trial indicated that telmisartan was not inferior to enalapril in reducing a decline in GFR over 5 years. However, there was only a relatively small proportion of patients with overt nephropathy in this study.Citation90 Given the paucity of data for ARBs in type 1 diabetics, some clinicians prefer initiating treatment with an ACE inhibitor for type 1 DN.
For primary prevention of DN, a recent meta-analysis of eight studies and 11,906 participants found that ACE inhibitors reduced the risk of new onset microalbuminuria, macroalbuminuria, or both when compared to placebo (relative risk 0.71; 95% confidence interval 0.56–0.89). However, similar benefits could not be demonstrated for ARBs.Citation91 Thus, there is no proven benefit in starting ARB treatment in normotensive, normoalbuminuric type 1 or type 2 diabetics. Neither ACE inhibitor nor ARB is currently recommended in normotensive, normoalbuminuric diabetics for primary prevention of DN.
ACE inhibitor and ARB
Earlier studies of combination ACE inhibitor and ARB reported superiority of combination therapy for lowering albuminuria and blood pressure versus either alone, in both type 1 and 2 diabetics.Citation92–Citation94 One study also showed a reduction in urinary TGF-β levels as another surrogate marker.Citation95 Despite the positive effects on these surrogate markers, the impact on preservation of GFR has not been demonstrated. The ONTARGET trial, which combined ramipril and telmisartan in patients with DN, noted no significant difference in the incidence of dialysis or doubling of serum creatinine when compared to single RAS inhibition.Citation96 In the Veterans Affairs NEPHRON-D study, the addition of lisinopril to losartan treatment did not reduce the composite endpoint of 50% reduction in eGFR, ESRD, or death.Citation97 Furthermore, combination treatment was associated with higher incidences of acute kidney injury and hyperkalemia in both these trials. Thus, the dual ACE inhibitor/ARB treatment strategy for DN has largely been abandoned.
Aldosterone antagonists
Aldosterone is the final component of the RAS cascade. Aldosterone promotes fibrosis, inflammation, and generation of ROS, along with endothelial dysfunction, cell growth, and proliferation.Citation98,Citation99 Spironolactone appears to reduce proteinuria on its own or in combination with ACE inhibitor or ARB, in both type 1 and type 2 diabetics.Citation100,Citation101 In addition to a blood pressure lowering effect, an anti-inflammatory mechanism is also likely, including reductions in MCP-1, MIF, and macrophage accumulation.Citation102 In a randomized trial of 268 type 2 diabetics, the addition of eplerenone to an ACE inhibitor reduced albuminuria.Citation103 However, the combination of aldosterone antagonists and other RAS inhibitors increases the risk of hyperkalemia and there is no long-term data on loss of renal function with combination blockade. Thus, combination of aldosterone antagonists and ACE inhibitor/ARB is unclear but, if used, careful monitoring of blood potassium is recommended along with dietary limitation of potassium intake.
Calcium channel blocker (CCB)
The addition of a non-dihydropyridine CCB to RAS inhibition may also be beneficial. Both verapamil and diltiazem have been shown to lower proteinuria in type 2 diabetics.Citation104 The effects of adding verapamil to lisinopril or trandolapril treatment were additive in reducing albuminuria and a decline in GFR.Citation105,Citation106 However, the BENEDICT-B study of verapamil in combination with trandolapril did not find an additional benefit in regression of macroalbuminuria in hypertensive type 2 diabetics independent of blood pressure lowering.Citation107 In the MARVAL study of 332 type 2 diabetics randomized to valsartan or amlodipine (a dihydropyridine CCB) for 24 weeks, valsartan was more effective than amlodipine in reducing albuminuria, including remission to normoalbuminuria.Citation108 Further evidence from the Nephros and REIN-2 studies in nondiabetic CKD suggests that dihydropyridine CCBs such as felodipine and amlodipine do not have additive value in reducing proteinuria or progression to ESRD when added to ramipril.Citation109,Citation110 Thus, the non-dihydropyridine CCBs may be considered second- or third-line agents after RAS inhibitors.
Diuretics
Similar to dietary sodium restriction, thiazide diuretics (eg, hydrochlorothiazide 50 mg) when combined with an ACE inhibitor (lisinopril 40 mg/day) reduced albuminuria in type 2 diabetics. However, the combination is associated with more frequent orthostatic symptoms.Citation82 For advanced CKD, a loop diuretic may be more appropriate. Diuretics may increase the effectiveness of ACE inhibitors and ARBs.
Blood pressure target
The current Joint National Committee (JNC 8) guidelines recommend targeting a blood pressure of <140/90 mmHg for diabetic patients, irrespective of CKD.Citation111 The 2013 European Society of Hypertension/European Society of Cardiology,Citation112 2014 Kidney Health Australia Caring for Australians with Renal Impairment,Citation113 and 2012 Kidney Disease: Improving Global Outcomes guidelines advocate a similar target. However, a lower blood pressure target is recommended by some guidelines for better control of proteinuria. The 2014 Kidney Health Australia Caring for Australians with Renal Impairment guidelines recommend a lowering of the blood pressure target from <140/90 mmHg to <130/80 mmHg in the presence of macroalbuminuria.Citation113 The 2012 Kidney Disease: Improving Global Outcomes guidelines suggest that a target of <130/80 would be more beneficial in those with micro- or macroalbuminuria. The National Kidney Foundation’s (Kidney Disease Outcomes Quality Initiative) 2007 and 2012 updated guidelines advocate blood pressure readings <130/80 mmHg in diabetics with CKD, or even lower in patients with high-level albuminuria (ACR >500 mg/g).Citation114 The Canadian Society of Nephrology continues to advocate for the lower target of <130/80 mmHg for all diabetics, regardless of CKD or albuminuria.Citation115 It is probably sufficient to say that low risk diabetics with normoalbuminuria could be treated to a target of <140/90 mmHg, while those at high risk or significant albuminuria should have a lower target of <130/80 mmHg.
Anti-lipid agents
In the Casale Monferrato study of 1,253 type 2 diabetics, apolipoprotein B and high-density lipoprotein cholesterol levels were independent risk factors for progression to overt nephropathy during 7 years follow-up.Citation19 In a large multinational case–control study of 2,535 type 2 diabetics with good control of low-density lipoprotein cholesterol, triglycerides and high-density lipoprotein cholesterol were associated with a higher risk of DN.Citation116 Data from the Joslin Diabetes Center from 439 type 1 diabetics also indicated that elevated cholesterol levels (>220 mg/dL) was associated with progression of DN.Citation117 Experimentally, statins have been shown to reduce NF-κB activation by p38 MAPK in tubular cells, AGE-mediated ROS activation, and tubular apoptosis and suppress RAS activation and aldosterone production.Citation118–Citation120
Despite the epidemiological and experimental data, there is limited data from intervention studies with regards to renal outcomes. In a study of type 2 diabetics, simvastatin reduced albuminuria and improved expression of slit diaphragm proteins compared with cholestyramine despite similar lipid reductions.Citation121 In an open-label randomized study in 104 type 2 diabetics, rosuvastatin reduced albuminuria and oxidative stress independent of lipid levels.Citation122 The Heart Protection Study noted that simvastatin treatment was associated with a lesser decline in GFR compared to placebo after an average of 4.6 years, a difference which was bigger in diabetics compared to nondiabetics.Citation123 The CARDS study of 2,838 type 2 diabetics randomized patients to atorvastatin or placebo, with a median follow-up of 3.9 years. Atorvastatin treatment improved the annual decline in eGFR, particularly in those with albuminuria.Citation124 Currently, statins are already recommended for diabetics with DN over the age of 40 years, irrespective of their baseline lipid levels. This is primarily for cardiovascular benefit rather than renal disease per se, as albuminuria has been demonstrated to be an independent risk factor for cardiovascular events and mortality.Citation125
Uric acid
Epidemiological studies demonstrate a strong link between uric acid and DN. The Joslin Diabetes Center study of 355 type 1 diabetics found that higher baseline uric acid levels was associated with early GFR loss over 4–6 years.Citation126 Data from the Coronary Artery Calcification study, which included 324 type 1 diabetics with normoalbuminuria at baseline who were followed for 6 years, showed that for every 1 mg/dL increase in uric acid levels there was an 80% increased risk of developing micro- or macroalbuminuria.Citation127 In the Steno Diabetes Center study of 263 type 1 diabetics, baseline serum uric acid at the onset of diabetes predicted development of macroalbuminuria 18 years later.Citation128
Does lowering uric acid prevent progression of DN? A post hoc analysis of RENAAL noted that uric acid lowering by losartan may have accounted for 20% of the benefit afforded by the intervention.Citation129 In diabetic mice, allopurinol attenuated albuminuria and tubulointerstitial injury, suggesting that uric acid is not just a potential marker but a therapeutic target.Citation130,Citation131 Allopurinol improves endothelial dysfunction and reduces urinary TGF-β in DN.Citation132–Citation134 The PERL study is currently enrolling type 1 diabetics into a randomized trial of allopurinol versus placebo.Citation135
Vitamin D
A low vitamin D level is common in patients with CKD. Vitamin D deficiency is linked to RAS activation and podocyte injury.Citation136,Citation137 Vitamin D may also play a role in preventing epithelial-to-mesenchymal transformation of tubular epithelial cells.Citation138 Experimentally, active vitamin D also attenuated oxidative stress by restoring Nrf2 levels, important for cellular protection against oxidative injury. This was associated with reduced NF-κB activation and lower albuminuria.Citation139
Observational data from the PRONEDI trial of type 2 diabetics with stage 2–3 CKD showed that vitamin D levels <15 ng/mL was independently a risk factor for the composite outcome of >50% increase in serum creatinine, ESRD, or death.Citation140 In the VITAL study, type 2 diabetics randomized to paricalcitol (a synthetic D2 agonist) for 24 weeks achieved significantly lower albuminuria than placebo treatment.Citation141 The upcoming VALIDATE-D study will evaluate the effect of calcitriol supplementation in patients on lisinopril to determine if there is a synergistic effect on RAS activity to lower proteinuria.Citation142 Future randomized trials will hopefully determine the usefulness of targeting the vitamin D receptor in preserving GFR in DN.
Lifestyle, diet, and alternative medicine
Although moderate-intensity aerobic physical activity is recommended for all diabetics to improve glycemic control and cardiovascular risk, the DCCT study of type 1 diabetics found no evidence that physical activity prevents DN.Citation143 Exercise may temporarily increase albumin excretion and should be avoided prior to urine collection for albumin excretion. On the other hand, the Look AHEAD study of type 2 diabetics suggested that intensive lifestyle intervention targeting weight loss may reduce progression of CKD, despite no benefit on cardiovascular outcomes.Citation144
A low protein diet is advocated by the American Diabetes Association.Citation21 A recent meta-analysis of 13 randomized controlled trials with 779 type 1 and type 2 diabetics found that a low protein diet was associated with significant improvement in GFR. However, adequate compliance was necessary for this effect on GFR. Interestingly, proteinuria was not different between low protein and regular protein patients but HbA1c decreased slightly with low protein intake (−0.26%; 95% confidence interval −0.35 to −0.18). Low protein intake was defined as 0.6–0.8 g/kg/day and regular protein intake as 1.0–1.6 g/kg/day.Citation145
Substituting soy protein for animal protein may also be beneficial in diabetics with proteinuria but studies have not been consistent.Citation146 A number of alternative medicine supplements have also been studied (). Lastly, sodium restriction to 50–70 mmol daily may enhance the action of RAS inhibitors and result in a greater reduction in albuminuria in type 2 diabetics.Citation147,Citation148 However, this degree of sodium restriction is quite difficult for most and some advocate achieving an intake of <100 mmol/day as adequate restriction.
Table 3 Diet and alternative medicine
Multifactorial risk factor reduction
The benefits of intensive multifactorial intervention in type 2 diabetics were shown in the Steno-2 trial of 160 patients with microalbuminuria. Intensive therapy included: reduced dietary fat, light/moderate exercise, smoking cessation, tight glycemic control (<6.5%), tight blood pressure control (<130/80), ACE inhibitors, and anti-lipid medications (cholesterol <4.5 mmol/L). After a mean follow-up of 7.8 years, patients receiving multifactorial intervention had significantly lower risk of overt nephropathy (hazard ratio 0.39; 95% confidence interval 0.17–0.87) than those receiving regular management.Citation149
Transplantation
Simultaneous pancreas/kidney transplantation is an effective treatment for type 1 diabetics with ESRD, with most achieving insulin independence and preventing recurrence of DN in the allograft.Citation150,Citation151 In patients with CKD after 10 years of pancreas transplantation alone, patients with sustained normoglycemia showed reductions in albuminuria and reversal of DN lesions on serial biopsy, including regression of glomerular basement membrane thickening and mesangial matrix deposition.Citation152 Some of these benefits may be offset by interstitial fibrosis and arteriolar hyalinosis due to calcineurin inhibitor (eg, cyclosporine) use. However, the same authors note that tubulointerstitial remodeling at 10 years had ameliorated some of the interstitial collagen deposition noted at 5 years, although vascular changes were not affected.Citation153
Novel agents
The diabetic milieu is a complex environment where a number of interventions may be utilized to target various pathological processes. As no single therapy completely ameliorates DN, novel strategies are needed to complement existing interventions. Some of these novel agents are described below and summarized in .
Table 4 Summary of novel agents
Renin inhibitors
Renin catalyses the rate-limiting step in the production of angiotensin II. In diabetic rats, aliskiren reduced albuminuria and glomerulosclerosis, and was more effective than perindopril in reducing interstitial fibrosis.Citation154 In type 2 diabetics after a 4-week washout of previous medications, aliskiren reduced blood pressure and albuminuria, with the effects on albuminuria persisting after withdrawal of medication.Citation155 In the AVOID trial of 599 type 2 diabetics, the combination of aliskiren 300 mg and losartan 100 mg for 6 months reduced the urine ACR independent of blood pressure.Citation156 However, the much larger ALTITUDE trial, which randomized 8,561 high-risk type 2 diabetics to aliskiren 300 mg or placebo as adjunctive to RAS inhibition, found no significant difference in renal outcomes. It is noted that the trial was terminated prematurely due to excess hyperkalemia and hypotension in the aliskiren group.Citation157 Due to the lack of good randomized controlled trial evidence supporting the use of aliskiren in combination with ACE inhibitors or ARBs, and the increased adverse effects, the combination is not recommended. From the US Food and Drug Administration perspective, the combination should be contraindicated in patients with diabetes. However, it could be considered as an alternative RAS blocker for blood pressure lowering and proteinuria reduction. More research is needed to demonstrate that aliskiren is as good as ACE inhibitors or ARBs.
Endothelin inhibitors
In diabetic rats, an ETA receptor blockade with atrasentan or avosentan reduced albuminuria and renal fibrosis.Citation158,Citation159 The ASCEND trial of 1,392 type 2 diabetics with overt nephropathy examined the effect of avosentan on time to doubling of serum creatinine, ESRD, or death. Avosentan halved proteinuria but increased fluid retention, edema, and congestive heart failure, resulting in the trial being stopped early.Citation160 Since ASCEND, two other randomized controlled trials have noted reduction in albuminuria at the cost of edema and congestive heart failure.Citation160,Citation161 The latter trial involving 1,392 type 2 diabetics was also stopped prematurely after a median follow-up of 4 months. In a randomized trial of 211 type 2 diabetics, atrasentan added to RAS inhibition for 12 weeks reduced albuminuria in association with lowering blood pressure.Citation162 Fluid overload was reported as manageable, albeit more patients discontinued treatment on the higher dose of atrasentan. The SONAR trial (NCT01858532) with atrasentan is currently in progress to evaluate renal outcomes in type 2 diabetics.
Urotensin and vasopeptidase inhibitors
Vasopeptidase inhibitors can block ACE and neutral endopeptidase. Palosuran is a competitive antagonist of the urotensin II receptor. In diabetic patients with macroalbuminuria, a 2-week course of palosuran in addition to RAS inhibitors reduced albuminuria by 24%.Citation163 The PROLONG trial is a prospective, randomized controlled crossover trial in hypertensive type 2 diabetics looking at the effects of palosuran on albuminuria and blood pressure.Citation164 This study found no significant reduction in albuminuria or blood pressure after 4 weeks of treatment. Other vasopeptidase inhibitors such as omapatrilat and ilepatril (AVE7688) have been shown to attenuate albuminuria in diabetic rats but human data are lacking.Citation165,Citation166
PKC inhibitors
Ruboxistaurin is a selective inhibitor of PKC-β. Animal studies with ruboxistaurin showed beneficial effects on reducing mesangial expansion, hyperfiltration, albuminuria, macrophage accumulation, and tubulointerstitial injury.Citation167,Citation168 Small randomized controlled studies have demonstrated that ruboxistaurin reduced urinary TGF-β excretion by >50%,Citation169 reduced albuminuria, and preserved eGFR at 1 year in type 2 diabetics.Citation170 However, when pooled data from three large studies of ruboxistaurin from diabetic retinopathy trials were analyzed (n=1,157), ruboxistaurin was no different from placebo after 3 years in reducing the rates of doubling of serum creatinine or stage 4–5 CKD.Citation171
Aldose reductase inhibitors
These inhibitors suppress sorbitol accumulation in tissues. Epalrestat reduced mesangial expansion and preserved renal function in diabetic rats.Citation172 Another inhibitor – tolrestat – prevented glomerular hypertrophy and hyperfiltration, mesangial cell hypocontractility, and albuminuria in diabetic rats.Citation173 A small study of 35 type 2 diabetics showed that epalrestat treatment for 5 years prevented progression of microalbuminuria.Citation174 A post hoc analysis of the Aldose Reductase Inhibitor–Diabetes Complications Trial concluded that progression of retinopathy/albuminuria was significantly inhibited by epalrestat.Citation175 This was a re-analysis of the original 3-year, open-label trial using a subset of patients for which data were available. On the other hand, another inhibitor – ponalrestat – did not affect urinary albumin excretion or glomerular filtration in type 1 diabetics.Citation176
Phosphodiesterase inhibitors
Cilostazol inhibits phosphodiesterase III and reduces thrombospondin-1 and TGF-β expression, attenuating hyperfiltration, albuminuria, and extracellular matrix deposition in diabetic rats.Citation177,Citation178 In humans, one study using cilostazol for 3 months in type 2 diabetics demonstrated a reduction in urinary ACR and renal production of thromboxane B2.Citation179 A small Chinese study randomized 40 type 2 diabetics to cilostazol or placebo for 6 months. Cilostazol reduced albuminuria, serum ICAM-1, and MCP-1 levels but did not affect kidney function.Citation180
Pentoxifylline is a methylxanthine-derived phosphodiesterase inhibitor that antagonizes the adenosine receptor and lowers blood viscosity. It also has anti-inflammatory and immunomodulatory properties, and lowers serum and urine TNF-α in diabetic patients with DN. In a Cochrane meta-analysis of 17 randomized trials involving 991 participants, pentoxifylline was better than placebo in reducing albuminuria and preserving serum creatinine but was equivalent to captopril. However, the studies were small and of poor methodology, with no data on ESRD or mortality.Citation181 Since the meta-analysis, other trials have examined the addition of pentoxifylline to RAS blockers and have consistently found a benefit in reducing proteinuria. Roozbeh et al enrolled 74 patients with type 2 diabetes with overt proteinuria, randomized to pentoxifylline 400 mg daily plus captopril or captopril alone. The reduction in proteinuria from baseline was greater in the pentoxifylline-treated group, associated with a modest reduction in blood pressure.Citation182 Oliaei et al enrolled 50 type 2 diabetics with proteinuria >500 mg/day despite RAS inhibition, to pentoxifylline 400 mg three times a day versus placebo. The pentoxifylline group had greater reductions in proteinuria but no difference in creatinine clearance.Citation183 Ghorbani et al enrolled 100 type 2 diabetics with proteinuria randomized to pentoxifylline 400 mg/day or placebo for 6 months. Both groups received losartan and enalapril in combination. After 6 months, pentoxifylline treatment was associated with lower proteinuria and higher creatinine clearance.Citation184 The results of the PREDIAN study are still expected.Citation185
AGE inhibitors
AGE inhibitors reduce AGE formation, enhance degradation, or break AGE crosslinks. The prototype AGE inhibitor is aminoguanidine (pimagedine), which acts by scavenging intermediates such as 3-deoxyglucosone and methyglyoxal.Citation186 Experimentally, aminoguanidine attenuates albuminuria, mesangial expansion, and collagen deposition in diabetic rats.Citation187 However, the placebo-controlled ACTION trial in 690 type 1 diabetics with overt nephropathy showed no difference in the time taken to double serum creatinine despite a reduction in proteinuria with pimagedine treatment for 2–4 years.Citation188
Pyridoxamine inhibits AGE formation and scavenges ROS and toxic carbonyls. When data from two 24-week studies were combined, pyridoxamine reduced the change from baseline creatinine in type 1 and type 2 diabetics without affecting albuminuria.Citation189 However, in a random-ized controlled trial of 317 type 2 diabetics, pyridoxamine treatment for 52 weeks did not significantly affect serum creatinine.Citation190 GLY-230 is another inhibitor of protein glycation that was studied in 21 diabetic men in a randomized trial for 14 days. GLY-230 reduced glycated albumin and albuminuria compared to baseline but not placebo.Citation191 AGE crosslink breakers, such as alegebrium, and inhibitors of the AGE receptor have shown benefit in DN models but have not been studied in humans.
Agents targeting oxidative stress
Cellular and mitochondrial ROS formation is an important contributor to the pathophysiology of DN. Targeted inhibitors of ROS generation are emerging but most have not progressed to clinical trials. Oxidative stress and inflammation in DN may also lead to a reduction in Nrf2, a nuclear transcription factor which plays a key role in antioxidant and cytoprotective mechanisms.Citation192 Bardoxolone is a potent activator of Nrf2. In the BEAM trial of 227 type 2 diabetics with eGFR 20–45 mL/minute/m2 randomized to bardoxolone (25, 75, or 150 mg daily) or placebo, bardoxolone was associated with an improvement in eGFR at 24 weeks, which was sustained to 52 weeks of treatment.Citation193 In the much larger BEACON trial of 2,185 type 2 diabetics with stage 4 CKD, patients were randomized to bardoxolone 20 mg daily or placebo. The trial was stopped after a median follow-up of 9 months due to a higher rate of cardiovascular events and increased albuminuria, with no reduction in ESRD or cardiovascular death.Citation194 Subsequently, one animal study in diabetic rats found unfavorable side effects of bardoxolone analogs, further questioning the safety profile in DN.Citation195
Glycosaminoglycans
Sulodexide is a mixture of 80% heparan sulfate and 20% dermatan sulfate. Sulodexide may reduce the enhanced heparan sulfate degradation in the glomerular basement membrane that occurs in DN. It has anti-inflammatory properties and inhibits the hyperglycemia-induced production of ROS, MCP-1, and IL-6 in endothelial cells.Citation196 It may improve endothelial dysfunction, vascular permeability, and renal hemodynamics. It may also attenuate TGF-β gene expression, extracellular matrix expansion, and inhibit HPSE-1, which plays a role in tubular epithelial-to-mesenchymal transition.Citation197
The DiNAS trial enrolled 223 type 1 and type 2 diabetics with serum creatinine ≤1.7 mg/dL in a randomized trial of sulodexide (50, 100, 200 mg/day) versus placebo for 4 months, with a further 4 months follow-up postintervention. There was a dose-dependent effect, with 200 mg/day the most effective in reducing albuminuria. RAS inhibition was not universal in this trial although post hoc analysis indicated the effect of sulodexide was additive to ACE inhibition.Citation198 The Sun-MICRO trial enrolled 1,056 type 2 diabetics with microalbuminuria in a randomized trial of sulodexide 200 mg/day versus placebo for 12 months. There was no difference between the groups in normalizing albumin excretion or reducing albuminuria by at least 50%.Citation199 The Sun-MACRO trial enrolled 1,248 type 2 diabetics with renal impairment and overt nephropathy in a randomized trial of sulodexide 200 mg/day versus placebo. The study was terminated mid-enrollment, with data on 1,029 patient-years analyzed. This showed no significant difference in doubling of serum creatinine, ESRD, or creatinine >6 mg/dL.Citation200 In both the Sun-MICRO and Sun-MACRO trials, patients were on maximum doses of RAS inhibitors. The latter studies have dampened the enthusiasm for sulodexide in DN.
Antifibrotic agents
Pirfenidone inhibits TGF-β production and TNF-α production in models of DN and non-DN kidney disease. The exact mechanism of action is unclear. In db/db mice with type 2 diabetes, pirfenidone reduced mesangial matrix expansion but did not affect albuminuria.Citation201 In a small randomized trial of 77 type 1 and 2 diabetics with established DN, pirfenidone at 1,200 mg/day for 1 year improved eGFR from baseline compared to placebo (mean intergroup difference 5.5 mL/minute/1.73 m2). Pirfenidone at the higher dose of 2,400 mg/day did not demonstrate a similar benefit and the dropout rate was high. Pirfenidone did not lower albuminuria. Larger studies are needed to validate the findings.
Gene and cell-based therapy
Gene therapy involves introducing a gene into cells to increase the production of a protein of interest. A carrier or vector such as modified adenovirus is employed to deliver the gene to the nucleus where the protein coded by the gene is produced by the cellular machinery. Gene therapy targeting TGF-β/SMAD signaling has shown promise in reducing kidney injury in diabetic models. Ka et al studied Smad7 gene therapy in the db/db mouse model of type 2 diabetes. Treatment inhibited TGF-β/SMAD and NF-κB activation, resulting in a reduction in proteinuria, macrophage infiltration, inflammation, podocyte injury, and renal fibrosis.Citation202 A similar finding was noted by Zhang et al by using gene therapy to enhance decorin expression in the streptozotocin model of type 1 diabetes. The beneficial effects were attributed to downregulation of TGF-β/SMAD signaling as decorin is a natural inhibitor of TGF-β1.Citation203 HGF gene therapy has been shown in db/db mice to enhance renal expression of SDF-1, associated with increased numbers of bone marrow-derived monocyte/macrophages with a higher proportion of M2 markers (anti-inflammatory phenotype). This was associated with a reduction in proinflammatory cytokines, reduced histological injury, and preservation of podocytes.Citation204 Kosugi et al examined soluble Flt-1 gene therapy in db/db mice. sFlt-1 is an endogenous inhibitor of VEGF and treated animals showed reduced VEGF expression in association with elevated sFlt-1 levels in the kidney. Although sFlt-1 gene therapy reduced podocyte injury and albuminuria, tubulointerstitial injury was enhanced, leading the authors to conclude that this approach would not be beneficial in DN.Citation205 Thus, there are some potential risks with gene therapy, which may be related to the inserted gene itself or the viral vector utilized but this discussion is beyond the scope of this review.
Progenitor (stem) cells are multipotent cells capable of self-renewal and differentiation into specialized cells, and are broadly categorized into embryonic stem cells and adult stem cells. Adult stem cells can be derived from bone marrow, adipose tissue, or peripheral blood. Stem cells can also be harvested from umbilical cord blood at birth. The potential benefits of stem cell treatment in DN include: 1) replacing or regenerating damaged cells, 2) modulating inflammation, 3) reducing oxidative stress, and 4) improving glycemia. There have been a number of experimental studies of stem cell treatment in DN (). Most studies have demonstrated a blood glucose lowering effect by improved pancreatic β-cell function and insulin levels, whilst some others have not. This may relate to the nature of the cells utilized or the method of delivery. Some of these studies suggest that a paracrine effect is more important as a renoprotective mechanism, rather than regeneration or replacement of injured cells. This is based on observations of low level engraftment of mesenchymal stem cells in the kidney and the production of beneficial growth factors, antifibrotic factors, and factors which protect from oxidative stress.Citation206,Citation207
Table 5 Stem cell therapy in experimental diabetic nephropathy
The main issues facing cell-based therapy include: 1) consistency of manufactured cells (phenotypic change occur with repeat passages), 2) cell delivery method (optimize tissue targeting and minimizing passive entrapment), and 3) engrafting and cell survival. Notwithstanding the limitations mentioned, both gene therapy and stem cell therapy are promising areas of research but there are currently no successful human studies to date. Further studies are also needed to confirm that mesenchymal stem cells ameliorate DN independent of its metabolic benefits.
Conclusion
DN and ESRD remains a significant problem despite best efforts to limit the impact of the disease on such end-organ damage. In such a complex milieu of diabetes where no single treatment can halt DN progression, a multifactorial approach remains the most sensible. This should include optimal glycemic control and single RAS inhibition for hypertension or albuminuria. Based on the evidence, ACE inhibitors are preferred for type 1 diabetics. Second-line antihypertensives include non-dihydropyridine CCBs and diuretics. Lipid management with a statin is prudent for cardiovascular disease even though a direct impact on renal disease has not been conclusively shown other than as part of the multifactorial risk intervention similar to the Steno-2 study (which includes aspirin). No alternative medicines or supplements have been shown to slow GFR decline although effects on albuminuria are reported by some small studies. None can be routinely recommended currently and further studies on vitamin D are awaited. Further data on uric acid management with allopurinol are also awaited. Mild salt and protein restriction may also benefit some patients but strict monitoring and compliance can be problematic.
Understanding the pathophysiology of DN has improved over the years, particularly the molecular biology aspect. Inflammation has emerged as an important theme, while treatment targets and options continue to evolve as knowledge improves. The inflammatory amplification loop mediated by macrophages may be a good candidate for inhibition to reduce DN progression. Leukocyte or monocyte/macrophage culling may not necessarily be the best long-term strategy but manipulation of the macrophage phenotype and the interaction with T-cells should be further investigated. Blocking specific cell signaling pathways involved with inflammation may be useful but can be troubled by off-target effects, which will need to be fully explored before clinical trials can proceed.
A number of potential treatment strategies have shown benefit in improving surrogate markers like albuminuria but the translation to preserving GFR and preventing ESRD has not always followed. Such is the case with dual or triple blockade of the RAS system in DN seen in recent large clinical trials. It is acknowledged that albuminuria as a surrogate marker of disease progression is flawed. Furthermore, experimental interventions which reduce histological injury and inflammation do not always reduce the level of established proteinuria. Novel biomarkers may assist in this area when more data becomes available. Despite these challenges, new strategies to complement existing treatments will nonetheless continue to be looked for.
Acknowledgments
The author thanks Dr Ian Simpson (Renal Pathologist, Department of Anatomical Pathology, Monash Health) for his contribution of the histology images and pathology descriptions. The author also thanks Mr Paul Crammer (Renal Scientist, Department of Anatomical Pathology, Monash Health) for the electron microscopy image.
Disclosure
The author reports no conflicts of interest in this work. This manuscript has not been submitted or published anywhere else.
References
- International Diabetes FederationIDF Diabetes Atlas6th edBrussels, BelgiumInternational Diabetes Federation2013 Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdfAccessed September 2, 2014
- Centers for Disease Control and PreventionNational diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United StatesAtlanta, GAUS Department of Health and Human Services2011 Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdfAccessed September 2, 2014
- TappRJShawJEZimmetPZAlbuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab)Am J Kidney Dis200444579279815492944
- ScottLJWarramJHHannaLSLaffelLMRyanLKrolewskiASA nonlinear effect of hyperglycemia and current cigarette smoking are major determinants of the onset of microalbuminuria in type 1 diabetesDiabetes200150122842284911723069
- RossingPHougaardPParvingHHRisk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational studyDiabetes Care200225585986411978681
- SatkoSGLangefeldCDDaeihaghPBowdenDWRichSSFreedmanBINephropathy in siblings of African Americans with overt type 2 diabetic nephropathyAm J Kidney Dis200240348949412200799
- PettittDJSaadMFBennettPHNelsonRGKnowlerWCFamilial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitusDiabetologia19903374384432401399
- SeaquistERGoetzFCRichSBarbosaJFamilial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathyN Engl J Med198932018116111652710189
- YoungBAMaynardCBoykoEJRacial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veteransDiabetes Care20032682392239912882868
- SmithSRSvetkeyLPDennisVWRacial differences in the incidence and progression of renal diseasesKidney Int19914058158221762285
- GallMAHougaardPBorch-JohnsenKParvingHHRisk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational studyBMJ199731470837837889080995
- MooyaartALValkEJvan EsLAGenetic associations in diabetic nephropathy: a meta-analysisDiabetologia201154354455321127830
- DellameaBSPintoLCLeitaoCBSantosKGCananiLHEndothelial nitric oxide synthase gene polymorphisms and risk of diabetic nephropathy: a systematic review and meta-analysisBMC Med Genet201415924433471
- LinZHuangGZhangJLinXAdiponectin gene polymorphisms and susceptibility to diabetic nephropathy: a meta-analysisRen Fail201436347848724344808
- ZhouTBGuoXFYinSSAssociation of peroxisome proliferator-activated receptor γ Pro12Ala gene polymorphism with type 2 diabetic nephropathy risk in Caucasian populationJ Recept Signal Transduct Res201434318018424329532
- MooyaartALGenetic associations in diabetic nephropathyClin Exp Nephrol201418219720024129556
- MolitchMEDeFronzoRAFranzMJAmerican Diabetes AssociationNephropathy in diabetesDiabetes Care200427Suppl 1S79S8314693934
- DrummondKMauerMThe early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetesDiabetes20025151580158711978659
- BrunoGMerlettiFBiggeriAProgression to overt nephropathy in type 2 diabetes: the Casale Monferrato StudyDiabetes Care20032672150215512832328
- JohnsonDWJonesGRMathewTHChronic kidney disease and measurement of albuminuria or proteinuria: a position statementMed J Aust20121974224225
- American Diabetes AssociationStandards of Medical Care in Diabetes – 2014Diabetes Care201437Suppl 1S14S8024357209
- MazzuccoGBertaniTFortunatoMDifferent patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsiesAm J Kidney Dis200239471372011920336
- MakSKGwiEChanKWClinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitusNephrol Dial Transplant19971212258825919430856
- SharmaSGBombackASRadhakrishnanJThe modern spectrum of renal biopsy findings in patients with diabetesClin J Am Soc Nephrol20138101718172423886566
- PerkinsBAFicocielloLHRoshanBWarramJHKrolewskiASIn patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuriaKidney Int2010771576419847154
- CaramoriMLFiorettoPMauerMLow glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesionsDiabetes20035241036104012663477
- PerkinsBAFicocielloLHOstranderBEMicroalbuminuria and the risk for early progressive renal function decline in type 1 diabetesJ Am Soc Nephrol20071841353136117329575
- ArgyropoulosCWangKMcClartySUrinary microRNA profiling in the nephropathy of type 1 diabetesPLoS One201381e5466223358711
- DiStefanoJKTailaMAlvarezMLEmerging roles for miRNAs in the development, diagnosis, and treatment of diabetic nephropathyCurr Diab Rep201313458259123666892
- GohdaTNiewczasMAFicocielloLHCirculating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetesJ Am Soc Nephrol201223351652422266664
- NiewczasMAGohdaTSkupienJCirculating TNF receptors 1 and 2 predict ESRD in type 2 diabetesJ Am Soc Nephrol201223350751522266663
- WeilEJLemleyKVMasonCCPodocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathyKidney Int20128291010101722718189
- ToyodaMNajafianBKimYCaramoriMLMauerMPodocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathyDiabetes20075682155216017536064
- HuangWGalloisYBoubyNGenetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouseProc Natl Acad Sci U S A20019823133301333411687636
- RudbergSRasmussenLMBangstadHJOsterbyRInfluence of insertion/deletion polymorphism in the ACE-I gene on the progression of diabetic glomerulopathy in type 1 diabetic patients with microalbuminuriaDiabetes Care200023454454810857950
- KanetsunaYTakahashiKNagataMDeficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred miceAm J Pathol200717051473148417456755
- HanedaMArakiSTogawaMSugimotoTIsonoMKikkawaRMitogen-activated protein kinase cascade is activated in glomeruli of diabetic rats and glomerular mesangial cells cultured under high glucose conditionsDiabetes19974658478539133554
- HaHLeeHBReactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucoseKidney Int Suppl200077S19S2510997686
- SrivastavaSKRamanaKVBhatnagarARole of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic optionsEndocr Rev200526338039215814847
- WilliamsonJRChangKFrangosMHyperglycemic pseudohypoxia and diabetic complicationsDiabetes19934268018138495803
- LanaspaMAIshimotoTCicerchiCEndogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathyJ Am Soc Nephrol Epub5292014
- SheetzMJKingGLMolecular understanding of hyperglycemia’s adverse effects for diabetic complicationsJAMA2002288202579258812444865
- LanghamRGKellyDJGowRMIncreased renal gene transcription of protein kinase C-beta in human diabetic nephropathy: relationship to long-term glycaemic controlDiabetologia200851466867418278479
- NohHKingGLThe role of protein kinase C activation in diabetic nephropathyKidney Int Suppl2007106S49S5317653211
- DerubertisFRCravenPAActivation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathyDiabetes1994431188262306
- SakaiNWadaTFuruichiKInvolvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathyAm J Kidney Dis2005451546515696444
- AdhikaryLChowFNikolic-PatersonDJAbnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathyDiabetologia20044771210122215232685
- FiorinaPVerganiABassiRRole of podocyte B7-1 in diabetic nephropathyJ Am Soc Nephrol20142571415142924676639
- MudaliarHPollockCKomalaMGChadbanSWuHPanchapakesanUThe role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubulesAm J Physiol Renal Physiol20133052F143F15423576640
- SchmidHBoucherotAYasudaYModular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathyDiabetes200655112993300317065335
- HofmannMASchiekoferSKanitzMInsufficient glycemic control increases nuclear factor-kappa B binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetesDiabetes Care1998218131013169702439
- SanchezAPSharmaKTranscription factors in the pathogenesis of diabetic nephropathyExpert Rev Mol Med200911e1319397838
- LimAKTeschGHInflammation in diabetic nephropathyMediators Inflamm2012201214615422969168
- ChowFYNikolic-PatersonDJMaFYOzolsERollinsBJTeschGHMonocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db miceDiabetologia200750247148017160673
- LimAKMaFYNikolic-PatersonDJThomasMCHurstLATeschGHAntibody blockade of c-fms suppresses the progression of inflammation and injury in early diabetic nephropathy in obese db/db miceDiabetologia20095281669167919466391
- FurutaTSaitoTOotakaTThe role of macrophages in diabetic glomerulosclerosisAm J Kidney Dis19932154804858488815
- NguyenDPingFMuWHillPAtkinsRCChadbanSJMacrophage accumulation in human progressive diabetic nephropathyNephrology (Carlton)200611322623116756636
- BendingJJLobo-YeoAVerganiDVibertiGCProteinuria and activated T-lymphocytes in diabetic nephropathyDiabetes19883755075113258834
- MoriyaRManivelJCMauerMJuxtaglomerular apparatus T-cell infiltration affects glomerular structure in Type 1 diabetic patientsDiabetologia2004471828814618232
- LimAKMaFYNikolic-PatersonDJKitchingARThomasMCTeschGHLymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injuryDiabetologia20105381772178220422398
- EllerKKirschAWolfAMPotential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathyDiabetes201160112954296221911743
- XuJSuHLWangJHZhangCHRole of CD4+CD25+Foxp3+ regulatory T cells in type 2 diabetic nephropathyNan Fang Yi Ke Da Xue Xue Bao2009291137139 Chinese19218134
- IlanYMaronRTukpahAMInduction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob miceProc Natl Acad Sci U S A2010107219765977020445103
- The Diabetes Control and Complications Trial Research GroupThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitusN Engl J Med1993329149779868366922
- HolmanRRPaulSKBethelMAMatthewsDRNeilHA10-year follow-up of intensive glucose control in type 2 diabetesN Engl J Med2008359151577158918784090
- PerkovicVHeerspinkHLChalmersJIntensive glucose control improves kidney outcomes in patients with type 2 diabetesKidney Int201383351752323302714
- DuckworthWAbrairaCMoritzTGlucose control and vascular complications in veterans with type 2 diabetesN Engl J Med2009360212913919092145
- GersteinHCMillerMEByingtonRPEffects of intensive glucose lowering in type 2 diabetesN Engl J Med2008358242545255918539917
- Ismail-BeigiFCravenTBanerjiMAEffect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trialLancet2010376973941943020594588
- KoGJKangYSHanSYPioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic ratsNephrol Dial Transplant20082392750276018388116
- OhgaSShikataKYozaiKThiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-kappaB activationAm J Physiol Renal Physiol20072924F1141F115017190910
- ZhangHSahaJByunJRosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathyAm J Physiol Renal Physiol20082954F1071F108118667486
- BakrisGLRuilopeLMMcMornSORosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuriaJ Hypertens200624102047205516957566
- KoderaRShikataKTakatsukaTDipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetesBiochem Biophys Res Commun2014443382883324342619
- MoriHOkadaYAraoTTanakaYSitagliptin improves albuminuria in patients with type 2 diabetes mellitusJ Diabetes Investig201453313319
- FujitaHTaniaiHMurayamaHDPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1α in type 2 diabetic patients with incipient nephropathyEndocr J201461215916624225429
- CherneyDZPerkinsBASoleymanlouNRenal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitusCirculation2014129558759724334175
- VibertiGMogensenCEGroopLCPaulsJFEuropean Microalbuminuria Captopril Study GroupEffect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuriaJAMA199427142752798295285
- The Microalbuminuria Captopril Study GroupCaptopril reduces the risk of nephropathy in IDDM patients with microalbuminuriaDiabetologia19963955875938739919
- LewisEJHunsickerLGBainRPRohdeRDThe Collaborative Study GroupThe effect of angiotensin-converting-enzyme inhibition on diabetic nephropathyN Engl J Med199332920145614628413456
- WilmerWAHebertLALewisEJRemission of nephrotic syndrome in type 1 diabetes: long-term follow-up of patients in the Captopril StudyAm J Kidney Dis199934230831410430979
- HovindPRossingPTarnowLToftHParvingJParvingHHRemission of nephrotic-range albuminuria in type 1 diabetic patientsDiabetes Care200124111972197711679467
- HovindPTarnowLRossingPCarstensenBParvingHHImproved survival in patients obtaining remission of nephrotic range albuminuria in diabetic nephropathyKidney Int20046631180118615327415
- de GalanBEZoungasSChalmersJCognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trialDiabetologia200952112328233619688336
- RuggenentiPFassiAIlievaAPPreventing microalbuminuria in type 2 diabetesN Engl J Med2004351191941195115516697
- LewisEJHunsickerLGClarkeWRRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- EijkelkampWBZhangZRemuzziGAlbuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trialJ Am Soc Nephrol20071851540154617409317
- HallerHItoSIzzoJLJrOlmesartan for the delay or prevention of microalbuminuria in type 2 diabetesN Engl J Med20113641090791721388309
- BarnettAHBainSCBouterPAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med2004351191952196115516696
- LvJPerkovicVFooteCVCraigMECraigJCStrippoliGFAntihypertensive agents for preventing diabetic kidney disease [review]Cochrane Database Syst Rev201212CD00413623235603
- JacobsenPAndersenSRossingKJensenBRParvingHHDual blockade of the renin–angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathyKidney Int20036351874188012675866
- JacobsenPAndersenSJensenBRParvingHHAdditive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathyJ Am Soc Nephrol200314499299912660333
- MogensenCENeldamSTikkanenIRandomised controlled trial of dual blockade of renin–angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) studyBMJ200032172741440144411110735
- SongJHChaSHLeeHJEffect of low-dose dual blockade of renin–angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney diseaseNephrol Dial Transplant200621368368916330466
- MannJFAndersonCGaoPDual inhibition of the renin–angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trialJ Hypertens201331241442123249829
- FriedLFEmanueleNZhangJHCombined angiotensin inhibition for the treatment of diabetic nephropathyN Engl J Med2013369201892190324206457
- WolfGRenal injury due to renin–angiotensin–aldosterone system activation of the transforming growth factor-beta pathwayKidney Int200670111914191916985515
- HuangWXuCKahngKWNobleNABorderWAHuangYAldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cellsAm J Physiol Renal Physiol20082946F1287F129518367662
- SchjoedtKJRossingKJuhlTRBeneficial impact of spironolactone in diabetic nephropathyKidney Int20056862829283616316360
- RossingKSchjoedtKJSmidtUMBoomsmaFParvingHHBeneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over studyDiabetes Care20052892106211216123474
- HanSYKimCHKimHSSpironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic ratsJ Am Soc Nephrol20061751362137216571782
- EpsteinMWilliamsGHWeinbergerMSelective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetesClin J Am Soc Nephrol20061594095117699311
- BakrisGLCopleyJBVicknairNSadlerRLeurgansSCalcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathyKidney Int1996505164116508914031
- BakrisGLBarnhillBWSadlerRTreatment of arterial hypertension in diabetic humans: importance of therapeutic selectionKidney Int19924149129191325010
- BakrisGLWeirMRDeQuattroVMcMahonFGEffects of an ACE inhibitor/calcium antagonist combination on proteinuria in diabetic nephropathyKidney Int1998544128312899767545
- RuggenentiPFassiAIlievaAEffects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trialJ Hypertens201129220721621243736
- VibertiGWheeldonNMMicroalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effectCirculation2002106667267812163426
- HerlitzHHarrisKRislerTThe effects of an ACE inhibitor and a calcium antagonist on the progression of renal disease: the Nephros StudyNephrol Dial Transplant200116112158216511682661
- RuggenentiPPernaALorigaGBlood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trialLancet2005365946393994615766995
- JamesPAOparilSCarterBL2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- ManciaGFagardRNarkiewiczK2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20133171281135723817082
- PilmoreHDograGRobertsMCardiovascular disease in patients with chronic kidney diseaseNephrology (Carlton)201419131023927055
- National Kidney FoundationKDOQI clinical practice guideline for diabetes and CKD: 2012 updateAm J Kidney Dis201260585088623067652
- RuzickaMQuinnRRMcFarlanePCanadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for the management of blood pressure in CKDAm J Kidney Dis201463686988724725980
- SacksFMHermansMPFiorettoPAssociation between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case–control study in 13 countriesCirculation20141299999100824352521
- KrolewskiASWarramJHChristliebARHypercholesterolemia – a determinant of renal function loss and deaths in IDDM patients with nephropathyKidney Int Suppl199445S1251318158881
- IshibashiYYamagishiSMatsuiTPravastatin inhibits advanced glycation end products (AGEs)-induced proximal tubular cell apoptosis and injury by reducing receptor for AGEs (RAGE) levelMetabolism20126181067107222386936
- GaoPWuXShuiHJiaRFluvastatin inhibits high glucose-induced nuclear factor kappa B activation in renal tubular epithelial cellsJ Nephrol201326228929622641573
- TobaHMitaniTTakahashiTInhibition of the renal renin-angiotensin system and renoprotection by pitavastatin in type1 diabetesClin Exp Pharmacol Physiol201037111064107020678154
- TonoloGVelussiMBroccoESimvastatin maintains steady patterns of GFR and improves AER and expression of slit diaphragm proteins in type II diabetesKidney Int200670117718616710349
- AbeMMaruyamaNOkadaKMatsumotoSMatsumotoKSomaMEffects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathyJ Atheroscler Thromb201118111018102821921413
- CollinsRArmitageJParishSSleighPPetoRHeart Protection Study Collaborative GroupMRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trialLancet200336193742005201612814710
- ColhounHMBetteridgeDJDurringtonPNEffects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS)Am J Kidney Dis200954581081919540640
- GersteinHCMannJFYiQAlbuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individualsJAMA2001286442142611466120
- FicocielloLHRosolowskyETNiewczasMAHigh-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-upDiabetes Care20103361337134320332356
- JalalDIRivardCJJohnsonRJSerum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes studyNephrol Dial Transplant20102561865186920064950
- HovindPRossingPTarnowLJohnsonRJParvingHHSerum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort studyDiabetes20095871668167119411615
- MiaoYOttenbrosSALavermanGDEffect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan TrialHypertension20115812721632472
- KimSMChoiYWSeokHYReducing serum uric acid attenuates TGF-β1-induced profibrogenic progression in type 2 diabetic nephropathyNephron Exp Nephrol20121213–4e109e12123307286
- KosugiTNakayamaTHeinigMEffect of lowering uric acid on renal disease in the type 2 diabetic db/db miceAm J Physiol Renal Physiol20092972F481F48819458127
- DoganAYarliogluesMKayaMGEffect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patientsBlood Press201120318218721133824
- ButlerRMorrisADBelchJJHillAStruthersADAllopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertensionHypertension200035374675110720589
- TalaatKMel-SheikhARThe effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney diseaseAm J Nephrol200727543544017622758
- MaahsDMCaramoriLCherneyDZUric acid lowering to prevent kidney function loss in diabetes: the Preventing Early Renal Function Loss (PERL) allopurinol studyCurr Diab Rep201313455055923649945
- ZhangZZhangYNingGDebDKKongJLiYCCombination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increaseProc Natl Acad Sci U S A200810541158961590118838678
- WangYDebDKZhangZVitamin D receptor signaling in podocytes protects against diabetic nephropathyJ Am Soc Nephrol201223121977198623123403
- XiongMGongJLiuYXiangRTanXLoss of vitamin D receptor in chronic kidney disease: a potential mechanism linking inflammation to epithelial-to-mesenchymal transitionAm J Physiol Renal Physiol20123037F1107F111522791341
- NakaiKFujiiHKonoKVitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic ratsAm J Hypertens201427458659524025724
- Fernandez-JuarezGLunoJBarrioV25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin–angiotensin systemClin J Am Soc Nephrol20138111870187624135218
- de ZeeuwDAgarwalRAmdahlMSelective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trialLancet201037697521543155121055801
- BrownJMSecinaroKWilliamsJSVaidyaAEvaluating hormonal mechanisms of vitamin D receptor agonist therapy in diabetic kidney disease: the VALIDATE-D studyBMC Endocr Disord20131313323971740
- MakuraCBNirantharakumarKGirlingAJSaravananPNarendranPEffects of physical activity on the development and progression of microvascular complications in type 1 diabetes: retrospective analysis of the DCCT studyBMC Endocr Disord20131313724083407
- WingRRBolinPBrancatiFLCardiovascular effects of intensive lifestyle intervention in type 2 diabetesN Engl J Med2013369214515423796131
- NezuUKamiyamaHKondoYSakumaMMorimotoTUedaSEffect of low-protein diet on kidney function in diabetic nephropathy: meta-analysis of randomised controlled trialsBMJ Open201335e002934
- AndersonJWBeneficial effects of soy protein consumption for renal functionAsia Pac J Clin Nutr200817Suppl 132432818296369
- KwakernaakAJKrikkenJABinnenmarsSHEffects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trialLancet Diabetes Endocrinol20142538539524795252
- HoulihanCAAllenTJBaxterALA low-sodium diet potentiates the effects of losartan in type 2 diabetesDiabetes Care200225466367111919122
- PedersenOGaedePIntensified multifactorial intervention and cardiovascular outcome in type 2 diabetes: the Steno-2 studyMetabolism2003528 Suppl 1192312939735
- BohmanSOTydenGWilczekHPrevention of kidney graft diabetic nephropathy by pancreas transplantation in manDiabetes19853433063083918902
- BilousRWMauerSMSutherlandDENajarianJSGoetzFCSteffesMWThe effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetesN Engl J Med1989321280852659996
- FiorettoPSteffesMWSutherlandDEGoetzFCMauerMReversal of lesions of diabetic nephropathy after pancreas transplantationN Engl J Med1998339269759654536
- FiorettoPSutherlandDENajafianBMauerMRemodeling of renal interstitial and tubular lesions in pancreas transplant recipientsKidney Int200669590791216518350
- KellyDJZhangYMoeGNaikGGilbertREAliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in ratsDiabetologia200750112398240417828524
- PerssonFRossingPSchjoedtKJTime course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetesKidney Int200873121419142518337712
- ParvingHHPerssonFLewisJBLewisEJHollenbergNKAliskiren combined with losartan in type 2 diabetes and nephropathyN Engl J Med2008358232433244618525041
- ParvingHHBrennerBMMcMurrayJJCardiorenal end points in a trial of aliskiren for type 2 diabetesN Engl J Med2012367232204221323121378
- SasserJMSullivanJCHobbsJLEndothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanismJ Am Soc Nephrol200718114315417167119
- GagliardiniECornaDZojaCUnlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetesAm J Physiol Renal Physiol20092975F1448F145619675181
- MannJFGreenDJamersonKAvosentan for overt diabetic nephropathyJ Am Soc Nephrol201021352753520167702
- WenzelRRLittkeTKuranoffSAvosentan reduces albumin excretion in diabetics with macroalbuminuriaJ Am Soc Nephrol200920365566419144760
- de ZeeuwDCollBAndressDThe endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathyJ Am Soc Nephrol20142551083109324722445
- SidhartaPNWagnerFDBohnemeierHPharmacodynamics and pharmacokinetics of the urotensin II receptor antagonist palosuran in macroalbuminuric, diabetic patientsClin Pharmacol Ther200680324625616952491
- VogtLChiurchiuCChadha-BorehamHEffect of the urotensin receptor antagonist palosuran in hypertensive patients with type 2 diabetic nephropathyHypertension20105551206120920231521
- SchaferSLinzWVollertHThe vasopeptidase inhibitor AVE7688 ameliorates type 2 diabetic nephropathyDiabetologia20044719810314618238
- DavisBJJohnstonCIBurrellLMRenoprotective effects of vasopeptidase inhibition in an experimental model of diabetic nephropathyDiabetologia200346796197112838387
- KoyaDHanedaMNakagawaHAmelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetesFASEB J200014343944710698958
- WuYWuGQiXProtein kinase C beta inhibitor LY333531 attenuates intercellular adhesion molecule-1 and monocyte chemotactic protein-1 expression in the kidney in diabetic ratsJ Pharmacol Sci2006101433534316891764
- GilbertREKimSATuttleKREffect of ruboxistaurin on urinary transforming growth factor-beta in patients with diabetic nephropathy and type 2 diabetesDiabetes Care200730499599617229944
- TuttleKRBakrisGLTotoRDMcGillJBHuKAndersonPWThe effect of ruboxistaurin on nephropathy in type 2 diabetesDiabetes Care200528112686269016249540
- TuttleKRMcGillJBHaneyDJLinTEAndersonPWKidney outcomes in long-term studies of ruboxistaurin for diabetic eye diseaseClin J Am Soc Nephrol20072463163617699475
- ItagakiIShimizuKKamanakaYThe effect of an aldose reductase inhibitor (epalrestat) on diabetic nephropathy in ratsDiabetes Res Clin Pract19942531471547851268
- DonnellySMZhouXPHuangJTWhitesideCIPrevention of early glomerulopathy with tolrestat in the streptozotocin-induced diabetic ratBiochem Cell Biol19967433553628883841
- IsoKTadaHKubokiKInokuchiTLong-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in type 2 diabetic patientsJ Diabetes Complications200115524124411522497
- HottaNKawamoriRFukudaMShigetaYLong-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathyDiabet Med201229121529153322507139
- RanganathanSKrempfMFerailleECharbonnelBShort term effect of an aldose reductase inhibitor on urinary albumin excretion rate (UAER) and glomerular filtration rate (GFR) in type 1 diabetic patients with incipient nephropathyDiabete Metab19931922572618339858
- WangXYanLChenWXuLZhangXThe renal protective effects of cilostazol on suppressing pathogenic thrombospondin-1 and transforming growth factor-beta expression in streptozotocin-induced diabetic ratsJ Int Med Res200937114515319215684
- TohmaTShimabukuroMOshiroYYamakawaMShimajiriYTakasuNCilostazol, a phosphodiesterase inhibitor, reduces microalbuminuria in the insulin-resistant Otsuka Long-Evans Tokushima Fatty ratMetabolism200453111405141015536593
- WatanabeJSakoYUmedaFNawataHEffects of cilostazol, a phosphodiesterase inhibitor, on urinary excretion of albumin and prostaglandins in non-insulin-dependent diabetic patientsDiabetes Res Clin Pract199322153598137717
- JiaoXMJiaoXJZhangXGCilostazol reduces microalbuminuria in type 2 diabetic nephropathyChin Med J (Engl)2013126224395439624238537
- ShanDWuHMYuanQYLiJZhouRLLiuGJPentoxifylline for diabetic kidney disease [review]Cochrane Database Syst Rev20122CD00680022336824
- RoozbehJBanihashemiMAGhezlouMCaptopril and combination therapy of captopril and pentoxifylline in reducing proteinuria in diabetic nephropathyRen Fail201032217217820199178
- OliaeiFHushmandSKhafriSBaradaranMEfficacy of pentoxifylline for reduction of proteinuria in type II diabetic patientsCaspian J Intern Med20112430931324551437
- GhorbaniAOmidvarBBeladi-MousaviSSLakEVaziriSThe effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: a double blind and randomized clinical trialNefrologia201232679079623169362
- Navarro-GonzalezJFMurosMMora-FernandezCHerreraHMenesesBGarciaJPentoxifylline for renoprotection in diabetic nephropathy: the PREDIAN study. Rationale and basal resultsJ Diabetes Complications201125531431921144773
- ThornalleyPJUse of aminoguanidine (pimagedine) to prevent the formation of advanced glycation endproductsArch Biochem Biophys20034191314014568006
- Soulis-LiparotaTCooperMPapazoglouDClarkeBJerumsGRetardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic ratDiabetes19914010132813341834497
- BoltonWKCattranDCWilliamsMERandomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathyAm J Nephrol2004241324014685005
- WilliamsMEBoltonWKKhalifahRGDegenhardtTPSchotzingerRJMcGillJBEffects of pyridoxamine in combined Phase II studies of patients with type 1 and type 2 diabetes and overt nephropathyAm J Nephrol200727660561417823506
- LewisEJGreeneTSpitalewizSPyridorin in type 2 diabetic nephropathyJ Am Soc Nephrol201223113113622034637
- KennedyLSolanoMPMeneghiniLLoMCohenMPAnti-glycation and anti-albuminuric effects of GLY-230 in human diabetesAm J Nephrol201031211011619923796
- RuizSPergolaPEZagerRAVaziriNDTargeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney diseaseKidney Int20138361029104123325084
- PergolaPERaskinPTotoRDBardoxolone methyl and kidney function in CKD with type 2 diabetesN Engl J Med2011365432733621699484
- de ZeeuwDAkizawaTAudhyaPBardoxolone methyl in type 2 diabetes and stage 4 chronic kidney diseaseN Engl J Med2013369262492250324206459
- ZojaCCornaDNavaVAnalogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effectsAm J Physiol Renal Physiol20133046F808F81923136004
- CiszewiczMPolubinskaAAntoniewiczASuminska-JasinskaKBreborowiczASulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicityTransl Res2009153311812319218094
- MasolaVZazaGGambaroGSulodexide and glycosaminoglycans in the progression of renal diseaseNephrol Dial Transplant201429Suppl 1i74i7924493873
- GambaroGKinalskaIOksaAOral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the DiNAS randomized trialJ Am Soc Nephrol20021361615162512039991
- LewisEJLewisJBGreeneTSulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trialAm J Kidney Dis201158572973621872376
- PackhamDKWolfeRReutensATSulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathyJ Am Soc Nephrol201223112313022034636
- RamachandraRaoSPZhuYRavasiTPirfenidone is renoprotective in diabetic kidney diseaseJ Am Soc Nephrol20092081765177519578007
- KaSMYehYCHuangXRKidney-targeting Smad7 gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear factor κB (NF-κB) signalling pathways, and improves diabetic nephropathy in miceDiabetologia201255250951922086159
- ZhangZWuFZhengFLiHAdenovirus-mediated decorin gene transfection has therapeutic effects in a streptozocin-induced diabetic rat modelNephron Exp Nephrol20101161e11e2120502052
- FlaquerMFranquesaMVidalAHepatocyte growth factor gene therapy enhances infiltration of macrophages and may induce kidney repair in db/db mice as a model of diabetesDiabetologia20125572059206822460762
- KosugiTNakayamaTLiQSoluble Flt-1 gene therapy ameliorates albuminuria but accelerates tubulointerstitial injury in diabetic miceAm J Physiol Renal Physiol20102983F609F61620015944
- LiDWangNZhangLMesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factorStem Cell Res Ther20134510324004644
- ZhangYYuenDAAdvaniAEarly-outgrowth bone marrow cells attenuate renal injury and dysfunction via an antioxidant effect in a mouse model of type 2 diabetesDiabetes20126182114212522596053
- SabbisettiVSWaikarSSAntoineDJBlood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetesJ Am Soc Nephrol Epub652014
- NielsenSEAndersenSZdunekDHessGParvingHHRossingPTubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathyKidney Int201179101113111821270761
- ConwayBRManoharanDJenksSMeasuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factorsKidney Int201282781281822718188
- LacquanitiADonatoVPintaudiB“Normoalbuminuric” diabetic nephropathy: tubular damage and NGALActa Diabetol201350693594223754672
- ChouKMLeeCCChenCHSunCYClinical value of NGAL, L-FABP, and albuminuria in predicting GFR decline in type 2 diabetes mellitus patientsPLoS One201381e5486323349979
- PanduruNMForsblomCSaraheimoMUrinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetesDiabetes Care20133672077208323378622
- NielsenSESugayaTHovindPBabaTParvingHHRossingPUrinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patientsDiabetes Care20103361320132420185732
- ArakiSHanedaMKoyaDPredictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathyDiabetes Care20133651248125323223350
- Kamijo-IkemoriASugayaTYasudaTClinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patientsDiabetes Care201134369169621273494
- KimSSSongSHKimIJUrinary cystatin C and tubular proteinuria predict progression of diabetic nephropathyDiabetes Care201336365666123093662
- FallahzadehMKDormaneshBSaghebMMEffect of addition of silymarin to renin–angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trialAm J Kidney Dis201260689690322770926
- KhanMISiddiqueKUAshfaqFAliWReddyHDMishraAEffect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patientsJ Nat Sci Biol Med20134233634024082728
- ParhamMAminiMAminorroayaAHeidarianEEffect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trialRev Diabet Stud20085210210918795212
- KhajehdehiPPakfetratMJavidniaKOral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled studyScand J Urol Nephrol201145536537021627399
- RossingPHansenBVNielsenFSMyrupBHolmerGParvingHHFish oil in diabetic nephropathyDiabetes Care19961911121412198908382
- LeeRHSeoMJRegerRLMultipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid miceProc Natl Acad Sci U S A200610346174381744317088535
- EzquerFEEzquerMEParrauDBCarpioDYanezAJCongetPASystemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic miceBiol Blood Marrow Transplant200814663164018489988
- EzquerFEzquerMSimonVEndovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic miceBiol Blood Marrow Transplant200915111354136519822294
- ZhouHTianHMLongYMesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in ratsChin Med J (Engl)2009122212573257919951572
- ParkJHParkJHwangSHHanHHaHDelayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injuryTransplant Proc20124441123112622564642
- ParkJHHwangIHwangSHHanHHaHHuman umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine actionDiabetes Res Clin Pract201298346547323026513
- FangYTianXBaiSAutologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines, and the p38 MAPK signaling pathwayInt J Mol Med2012301859222552764
- WangSLiYZhaoJZhangJHuangYMesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat modelBiol Blood Marrow Transplant201319453854623295166
- LvSSLiuGWangJPMesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltrationInt Immunopharmacol201317227528223791972
- ZhangYYeCWangGKidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy ratsBiomed Res Int2013201352636723762850
- ZhangLLiKLiuXRepeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in ratsStem Cells Dev201322233074308623844841
- Abdel AzizMTWassefMAAhmedHHThe role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathyDiabetol Metab Syndr2014613424606996
