Abstract
Renal artery stenosis (RAS) is a frequently encountered problem in clinical practice. The disease encompasses a broad spectrum of pathophysiologies and is associated with three major clinical syndromes: ischemic nephropathy, hypertension, and destabilizing cardiac syndromes. The two most common etiologies are fibromuscular dysplasia and atherosclerotic renal artery disease with atherosclerotic disease accounting for the vast majority of cases. Atherosclerotic renovascular disease has considerable overlap with atherosclerotic disease elsewhere and is associated with a poor prognosis. A wide range of diagnostic modalities and treatment approaches for RAS are available to clinicians, and with the advent of endovascular interventions, selecting the best course for a given patient has only grown more challenging. Several clinical trials have demonstrated some benefit with revascularization but not to the extent that many had hoped for or expected. Furthermore, much of the existing data is only marginally useful given significant flaws in study design and inherent bias. There remains a need for further identification of subgroups and appropriate indications in hopes of maximizing outcomes and avoiding unnecessary procedures in patients who would not benefit from treatment. In recent decades, the study of RAS has expanded and evolved rapidly. In this review, we will attempt to summarize the amassed body of literature with a focus on the epidemiology of RAS including prevalence, overlap with other atherosclerotic disease, and prognosis. We will also outline existing diagnostic and treatment approaches available to clinicians as well as summarize the findings of several major clinical trials. Finally, we will offer our perspective on future directions in the field.
Introduction
Renal artery stenosis (RAS) is general term that refers to any vascular lesion causing narrowing of the renal artery thereby impairing blood flow to the kidney. This disease encompasses a broad range of pathophysiologies, the two most common being fibromuscular dysplasia (FMD) and atherosclerotic renal artery disease.Citation1 This review will primarily focus on the epidemiology and treatment of atherosclerotic renal artery stenosis (ARAS), which accounts for the vast majority of cases. RAS is associated with three major clinical syndromes: ischemic nephropathy, hypertension, and destabilizing cardiac syndromes. However, a diagnosis of RAS may also result from an incidental finding in an otherwise asymptomatic patient. A diagnosis of ARAS is associated with a poor prognosis and often with atherosclerotic disease in other vascular beds. A wide range of diagnostic modalities and treatment approaches for RAS are available to clinicians, and with the advent of endovascular interventions, selecting the best course for a given patient has only grown more challenging. Several clinical trials have demonstrated some benefit with revascularization but not to the extent that many had hoped for or expected.Citation2,Citation3 Furthermore, much of the existing data is only marginally useful given significant flaws in study design and inherent bias. There remains a need for further identification of subgroups and appropriate indications in hopes of maximizing outcomes and avoiding unnecessary procedures in patients who would not benefit from treatment. In recent decades, the study of RAS has expanded and evolved rapidly. In this review, we will attempt to summarize the amassed body of literature with a focus on the epidemiology of RAS including prevalence, overlap with other atherosclerotic disease, and prognosis. We will also outline existing diagnostic and treatment approaches available to clinicians as well as summarize the findings of several major clinical trials. Finally, we will offer our perspective on future directions in the field.
Nonatherosclerotic renal artery disease
Nonatherosclerotic renal artery disease accounts for a wide range of etiologies. This list includes FMD, aneurysms, congenital or traumatic arteriovenous fistulas, vasculitis, neurofibromatosis, trauma, embolization, congenital bands, post-radiative therapy, and dissection. FMD is by far the most common of the nonatherosclerotic causes, accounting for 10% of all RAS.Citation1 FMD predominately affects premenopausal woman, typically ranging from 15 to 50 years of age and has been associated with a history of smoking and hypertension.Citation4 Unlike atherosclerotic renovascular disease, FMD is largely a disease of the young and healthy with few cardiovascular risk factors. Histologically, FMD may involve the intima, media, and adventitia; however, 90% involve the media.Citation1 On angiography, FMD has classically been described as having a “beads-on-a-string” appearance due to contrast filling of consecutive aneurysms along the renal artery. In patients with FMD, the distal two-thirds of the renal artery is the most commonly affected location; however, the disease may also involve carotid and vertebral arteries.Citation5 Though it may resemble vasculitis, FMD is noninflammatory, and the cause remains unknown. It is thought that a genetic component plays a role.Citation4 FMD is generally associated with a good prognosis and usually does not progress to complete occlusion.
Atherosclerotic renal artery disease
Atherosclerotic disease is the most common disease to affect the renal arteries, making up 90% of all renovascular lesions.Citation1 Atherosclerotic RAS (ARAS) typically involves the proximal third of the renal artery including the perirenal aorta and ostium.Citation5 ARAS is associated with renovascular hypertension, but more consistently, ARAS presents with nephropathy.Citation6 Unlike FMD, patients with ARAS are commonly elderly and have multiple cardiovascular risk factors. In these patients, atherosclerosis is generally systemic and not limited to the renal artery, a concept to be discussed in depth in a following section. More often than not, ARAS is a progressive disease characterized by worsening stenosis and eventual occlusion with wider implications in regards to both organ function and patient prognosis.
Pathophysiology
RAS is a common cause of secondary hypertension. Though likely an oversimplification of a more complex pathophysiology, the mechanism leading to the development of renovascular hypertension is typically classified as either renin-dependent or primarily a result of volume overload. This theory dates back to the 1930s, when Goldblatt et al performed a series of studies examining the impact of unilateral and bilateral RAS (BRAS) on blood pressure.Citation7 By clamping renal arteries in dogs, Goldblatt et al demonstrated a systemic pressor effect. They postulated that this effect was due to a substance produced by the kidneys causing vasoconstriction. The substance was ultimately isolated and identified as the proteolytic enzyme now known as renin. Renin is an early effector in the larger renin angiotensin aldosterone neurohormonal cascade. When ischemia occurs downstream of a stenotic renal artery, renin is released from juxtaglomerular cells. Renin then cleaves angiotensinogen to form angiotensin I, which must be further processed to angiotensin II by angiotensin-converting enzyme (ACE) produced in the lung endothelium and vasculature. Angiotensin II is the active enzyme and has multiple downstream effects. Angiotensin-II-mediated vasoconstriction causes hypertension, leading to pressure diuresis of the unaffected kidney. Glomerular filtration rate (GFR) is increased via vasoconstriction of the efferent arteriole. Antidiuretic hormone is released from the posterior pituitary gland, causing water conservation, further contributing to pressure diuresis. Release of aldosterone from the adrenal glands enhances exchange of sodium in the nephron-promoting volume retention. In addition, angiotensin increases sympathetic tone. The mechanism behind BRAS or unilateral RAS with a solitary kidney is due to extracellular fluid overload secondary to decreased diuresis rather than a renin-mediated mechanism. It is important to distinguish between renin-dependent and renin-independent patients as the initiation of an angiotensin II inhibitor may induce acute renal failure in a renin-dependent system.
Ischemic nephropathy can be defined as an obstruction causing decreased perfusion leading to renal ischemia and subsequent excretory dysfunction. The cause of ischemic nephropathy has not been fully elucidated. However, several interrelated mechanisms have been proposed explaining how a hemodynamically significant lesion ultimately results in interstitial fibrosis.Citation8 By one pathway, recurrent local ischemia causes tubulointerstitial injury and microvascular damage. By another, global hypoperfusion of the kidney leads to altered endothelial and epithelial factors as well as activation of the renin-angiotensin aldosterone system and subsequent vasoconstriction. Both pathways are thought to contribute to oxidative injury, increased production of fibrogenetic cytokines, and inflammation, ultimately leading to atrophy and fibrosis.
RAS may either cause or exacerbate cardiac destabilizing syndromes, including unstable angina (UA) and congestive heart failure characterized by flash pulmonary edema. RAS precipitates these conditions through three general mechanisms: volume overload, peripheral arterial vasoconstriction, and direct effects of angiotensin on the myocardium. Flash pulmonary edema can be described as a specific presentation of acute decompensated heart failure characterized by rapid fluid accumulation within the lungs. Flash pulmonary edema can occur secondary to several conditions, all of which result from an acute increase in end diastolic left ventricular pressure. Flash pulmonary edema does occur in unilateral RAS, but tends to occur more often in patients with BRAS.Citation9 In BRAS, the mechanism can be explained by impaired natriuresis and thus, a propensity for volume overload. This phenomenon was first reported by Pickering et al in 1988 in a case series of eleven hypertensive patients with BRAS and recurrent pulmonary edema.Citation10 UA is defined as pain due to cardiac ischemia that is new in onset or is increasing in frequency or intensity. The prototypical cause of UA is atherosclerosis and plaque rupture. RAS, however, may contribute to UA via an acute angiotensin-mediated increase in afterload. Increased left ventricular workload leads to increased oxygen demand, resulting in myocardial ischemia.
Prevalence
Historically, it has been difficult to determine the true prevalence of RAS in the general population. Prior to newer, noninvasive diagnostic techniques, most studies were performed postmortem or were selective of subpopulations undergoing angiography. For example, several decades ago, a large series of 14,152 patients who underwent angiography found insignificant disease (<50% stenosis) in one or more vessels in 5.1% of patients and significant stenosis in 6.3% of patients.Citation11 A more recent population-based study utilizing a noninvasive screening technique found similar results. The authors found that 6.8% of both Black and White elderly patients enrolled in the Cardiovascular Health Study had ≥60% stenosis or occlusion as determined by renal duplex sonography.Citation12 There was no correlation between RAS and ethnicity while disease was independently associated with age, hyperlipidemia, and hypertension. It is well established that the prevalence of RAS is increased in elderly patients, particularly in those with additional comorbid conditions such as diabetes, aortoiliac occlusive disease, coronary artery disease (CAD), or hypertension.Citation1,Citation13
Overlap of RAS with other atherosclerotic disease
It has long been recognized that patients with known atherosclerotic vascular disease in one arterial bed are likely to have or develop disease in another. The risk of concurrent ARAS ranges from 26% to 50% with a diagnosis of arterial atherosclerotic disease elsewhere in the arterial vasculature.Citation14 Specifically, the overlap of RAS with CAD, peripheral arterial disease (PAD), and carotid arterial disease has been well described. There also appears to be a relationship between the severity of RAS and the incidence of atherosclerotic disease. In a study by Wollenweber et al, ~31% of patients with mild atherosclerotic narrowing (<50% occlusion) of a renal artery had symptomatic arterial disease in the coronary, cerebrovascular, or peripheral vascular circulation.Citation15 This proportion was increased to 49% in patients with moderate to severe stenosis (>50%).
In patients with CAD, the risk of RAS (>50% stenosis) ranges from 22% to 89%.Citation14 The prevalence of RAS tends to increase with the number of coronary vessels involved. In fact, the presence of significant CAD with greater than two vessel involvement was found to be an independent predictor of RAS with a sensitivity of 0.84 and specificity of 0.77.Citation16 A history of percutaneous coronary intervention (PCI) is quite prevalent among patients with significant RAS, ~39%.Citation17 Not surprisingly, clinically overt CAD is more common among patients with RAS than without. In a study of patients with unsuspected RAS, 58% were shown to have clinical CAD (documented myocardial infarction, positive PCI, history of coronary artery bypass grafting, electrocardiogram change, or angina) compared with 39% of patients without RAS (P=0.002).Citation18
The overlap of PAD with RAS is significant. In a group of patients who underwent angiography as a part a routine evaluation for a known vascular pathology, greater than 50% RAS was found in 38% of patients with abdominal aortic aneurysm, 33% with aorto-occlusive disease, and 39% with lower-extremity occlusive disease.Citation20 A prospective study of patients with RAS documented by ultrasonic duplex scanning demonstrated a similarly high prevalence of PAD as assessed by ankle/brachial systolic pressure ratio.Citation21 For those with high-grade RAS, the prevalence of severe PAD was 73% versus 25% with RAS of less than 60%. Another study observed a trend for patients with increasing degrees of renal artery disease to have increasing degrees of lower extremity arterial disease.Citation22
Carotid artery lesions are more common and more severe in patients with renovascular hypertension. It has been shown that 40% to 46% of patients with significant RAS have moderate to severe occlusion of the carotid circulation.Citation14 A case-control study of patients without history or symptoms of cerebrovascular disease (CVD) compared patients with renovascular hypertension to those with essential hypertension (EH).Citation23 Almost twice as many patients with renovascular hypertension had carotid arterial stenosis as compared to those with EH. In the same study, it was found that the plaques in patients with renovascular hypertension were more heavily calcified than those with EH. Just as with PAD, increasing severity of RAS correlates with an increasing prevalence of CAD. From mild RAS to severe RAS, the prevalence increases from 7% to 28%, a striking four-fold difference.Citation22 There is also an association of RAS with clinical CVD. This relationship was examined in a series of autopsies of patients with clinical evidence of stroke, who died between 1980 and 1997.Citation24 The authors identified significant atherosclerotic RAS (>75%) in 10.4% of patients. Furthermore, patients with carotid artery stenosis were more than four times as likely to have RAS than patients without carotid artery stenosis (24.4% versus 5.9%, P=0.0001). Conversely, 67.7% of patients with RAS had carotid artery stenosis. Others have found that a history of CVD is an independent predictor of the presence of RAS.Citation17 In a large series of over 14,000 patients, a history of CVD was elicited in 9.2% without RAS versus 20.2% with RAS (odd’s ratio: 2.3, 95% confidence interval [CI]: 2.0–2.7, P=0.000001).Citation17
Prognosis
ARAS is a progressive disease in regards to both lesion and kidney function, conferring a poor prognosis to affected patients. Caps et al followed the natural progression of RAS in 295 renal arteries in 170 patients with serial duplex scans.Citation25 Stratified by initial degree of stenosis, the 3-year cumulative incidence of renal artery disease progression was 18% in normal arteries, 29% of arteries with <60% stenosis, and 49% of those with ≥60% stenosis at baseline. Total occlusion occurred in only nine (6.3%) of the arteries with ≥60% stenosis. The authors concluded that while renal artery disease progression occurs frequently, progression to total renal artery occlusion does not. An alternative study observed progression to total occlusion in 39% of patients with ≥75% stenosis on renal arteriography.Citation26 Unfortunately, complete occlusion occurs even in patients medically treated with adequate blood pressure control. In the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial, progression to complete occlusion occurred in 16% of patients treated medically.Citation27 At baseline, several conditions have been shown to be significantly associated with renal artery disease progression, including severity of disease and comorbid conditions including diabetes and hypertension. Specifically, a stepwise Cox proportional hazards model included four baseline factors that were significantly associated with the risk of renal artery disease progression.Citation25 These included systolic blood pressure ≥160 mmHg (relative risk [RR] =2.1; 95% CI: 1.2–3.5), diabetes mellitus (RR =2.0; 95% CI: 1.2–3.3), and high-grade stenosis (>60% or occlusion) in either artery, ipsilateral (RR =1.9; 95% CI: 1.2–3.0) or contralateral (RR =1.7; 95% CI: 1.0–2.8).
Atherosclerotic RAS is associated with decreased kidney function, renal atrophy, and ultimately, renal failure. In a series of 204 kidneys with ARAS in 122 subjects followed by duplex scans for an average of 33 months, the 2-year cumulative incidence of renal atrophy was 5.5% in normal renal arteries, 11.7% with <60% stenosis, and 20.8% with 60% stenosis (P=0.009, log rank test).Citation28 The authors also identified several baseline factors associated with a high risk of renal atrophy including hypertension, severe stenosis, and low renal cortical blood flow velocity. The occurrence of renal atrophy was well-correlated with increases in serum creatinine concentrations. This decline in organ function was also observed in a prospective study of patients with ARAS who were treated medically.Citation29 Over the average follow-up period of 28 weeks, 46% had increased serum creatinine, 25% to 50% had a decline in GFR, and 37% had a decrease in kidney size by more than 10%. It is not surprising then, that in a population of patients requiring dialysis, 12% likely developed end stage renal disease (ESRD) secondary to ARAS.Citation30 To make matters worse, ESRD due to ARAS is associated with worse survival rates as compared to other etiologies. The median survival rates in ESRD secondary to various etiologies was 25 months, 55 months, and 133 months for renovascular disease, malignant hypertension, and polycystic kidney disease, respectively.Citation31 Increased mortality associated with ARAS has been observed even in the absence of ESRD with 2-year survival rates of 96%, 74%, and 47% for unilateral RAS, BRAS, and RAS affecting a solitary functioning kidney, respectively.Citation32 It is evident that severity of disease also predicts survival. Four-year adjusted survival for patients with 50%, 75%, and ≥95% stenosis is 70%, 68%, and a dismal 48%, respectively. Furthermore, bilateral disease has been associated with a 4-year survival of 47% as compared with 59% for patients with unilateral disease (P<0.001).Citation19
Diagnosis
There is a wide array of diagnostic modalities available for the identification of atherosclerotic RAS. According to the American College of Cardiology/American Heart Association (ACC/AHA) Clinical Practice Guidelines, duplex ultrasonography, computed tomographic angiography (CTA), and magnetic resonance angiography (MRA) all receive a class I indication (level B evidence) as a screening test to establish the diagnosis of RAS.Citation33 When the clinical index of suspicion is high and the results of noninvasive tests are inconclusive, catheter angiography is then recommended for screening as well. Each of these diagnostic techniques comes with its own unique set of advantages and disadvantages, providing clinicians with a number of approaches for any given patient. Receiving a Class III recommendation is captopril renography (also known as captopril scintigraphy), an older diagnostic modality widely used in the past.Citation33 However, due to significant limitations and a sensitivity and specificity inferior to modern diagnostic techniques, this modality has fallen out of favor. Current guidelines state that captopril renal scintigraphy is not recommended as a screening test to diagnose RAS (level C evidence).Citation33
Duplex ultrasonography combines direct visualization of renal arteries (B-mode imaging) with Doppler velocity measurements of blood flow. Information provided by this technique includes location and degree of stenosis, measurement of kidney size, visualization of adjacent processes (obstruction, masses, AAA, etc), and assessment of intrinsic small vessel renovascular disease. In addition, new software has allowed for visualization of the entire renal artery including the distal portion; however, ability to visualize accessory renal arteries remains limited. Duplex boasts a number of advantages. This noninvasive test is not affected by medications that the patient may be taking, the level of renal function, whether the disease is unilateral or bilateral, or if it affects a solitary functioning kidney. It is the least expensive imaging modality and does not require the use of intravenous contrast unlike CTA or MRA. Duplex is particularly useful for follow up after implantation of metallic stents.Citation5 However, duplex is not without limitations. The test is time-consuming with prolonged examination times (~45 to 60 minutes). It can be technically challenging in obese patients or in the presence of abdominal gas, and there is also significant dependence on the operator. Notably, Hansen et al demonstrated a 10% to 20% rate of failure due to the operator’s inexperience or the presence of obesity or bowel gas.Citation34 To maximize results, an experienced technician should perform the test on a fasting patient in the morning.Citation32,Citation34 Detection of stenosis by ultrasound is determined by the measurement of a high peak systolic velocity (>180 or >200 cm/second) or by the renal aortic ratio, which is calculated by dividing the peak systolic velocity of the renal artery by the peak systolic velocity of the adjacent aorta.Citation35 A normal ratio is <3.5. The sensitivity and specificity of a renal aortic ratio >3.5 as compared to contrast arteriography has been estimated at 84% and 97%, respectively.Citation35 This translates to a positive predictive value of 94% for the detection of stenosis greater than 60%. A larger study by Olin et al, observed a higher value of 98% for both sensitivity and specificity.Citation36
CTA is a form of diagnostic imaging that utilizes computer software to reconstruct an image from X-rays projected from several directions targeted at the same vessel of interest. Multidetector computed tomography (MDCT) boasts increased speed of image acquisition as well as higher spatial resolution. Initially, the image output is a series of overlapping axial images. Through post-processing of volumetric data, a three-dimensional angiographic representation is rendered.Citation32 In addition to identifying stenotic lesions, CTA is useful for the visualization of adjacent anatomic structures. CTA has several advantages as compared to MRA, including higher spatial resolution and less artifact due to implanted metal stents; although vessel wall calcification can create potential difficulties in estimating the degree of stenosis. Compared to duplex, CTA is less operator-dependent. The requirement for injection of nephrotoxic iodinated contrast (100 to 150 cc) is a significant drawback, especially in the screening of ARAS, a population with increased prevalence of azotemia. While the time required for image acquisition is short, prolonged post-procedure processing time may be considered a limitation. CTA has proven to be a useful reliable test in the identification of RAS. For single-slice CTA, the sensitivity and the specificity for the detection of stenoses range between 88%–100% and 92%–98%, respectively.Citation37,Citation38 For multidetector computed tomography, the sensitivity and specificity ranges from 86%–93% and 90%–100%, respectively.Citation39,Citation40
MRA is a method of imaging that utilizes the property of nuclear magnetic resonance. The technique allows for the visualization of the target vessel structure without the need for ionizing radiation. Intravascular contrast is required to enhance the imaging of blood vessels; however, gadolinium contrast is less nephrotoxic than the ionized contrast used for CTA. Therefore, MRA can be performed in patients with some degree of renal insufficiency (though, not in patients with severe renal insufficiency or dialysis dependence due to the risk of nephrogenic systemic fibrosis with gadolinium contrast in those with a GFR ≤30 mL/minute/1.73 m2), congestive heart failure, and dye allergy. Like CTA, MRA is less operator-dependent as compared to ultrasonography. MRA also contributes additional information not provided by other techniques. For example, MRA allows for the visualization of surrounding anatomical structures including accessory renal arteries that are otherwise not seen on ultrasonography. Furthermore, renal perfusion may be assessed, and GFR can be determined via calculation of gadolinium clearance. Unfortunately, MRA is the most expensive modality. In addition to cost, MRA has several other limitations. MRA cannot be used for patients with pacemakers or other metallic objects. Accordingly, MRA cannot be used to evaluate in-stent restenosis. While the aorta and proximal portion of the renal artery do not significantly move with respiration, persistent craniocaudal motion of the distal segment limits imaging in this portion of the renal artery.Citation41 While ARAS is typically a disease of the proximal renal artery, stenosis due to FMD is often distally located and accuracy with MRA may be limited.Citation32 The sensitivity and specificity for the identification of RAS have been shown to be greater than 90% by several studies.Citation42–Citation44 According to a meta-analysis of 25 studies published between 1985 to 2001, the sensitivity and specificity of non-enhanced MRA were 94% (95% CI: 90%–97%) and 85% (95% CI: 82%–87%), respectively.Citation45 For gadolinium-enhanced MRA, sensitivity was 97% (95% CI: 93%–98%) and specificity was 93% (95% CI: 91%–95%). Thus, specificity and positive predictive value were significantly better for gadolinium-enhanced MRA (P<0.001).
While arterial angiography remains the gold standard for the diagnosis of RAS, it is typically used only after a positive noninvasive screening test. Angiography is also recommended in cases in which RAS is highly suspected and definitive noninvasive imaging cannot be obtained or in cases in which peripheral access is already obtained such as for imaging of the coronary arteries or abdominal aorta.Citation33 Although more invasive than other techniques, the risk of complications is low. These risks include access-related complications, embolization, contrast-related allergic reactions, and contrast-induced nephropathy. The risk of contrast-induced nephropathy is <3% in patients without diabetes or chronic kidney disease; though caution is warranted in patients with either of these conditions as the risk is increased dramatically.Citation33
Treatment
Medical therapy is a central pillar in the approach to treatment of RAS. Major goals include glycemic control optimization, cholesterol reduction, smoking cessation, blood pressure reduction, and primary prevention with aspirin, if indicated. According to ACC/AHA guidelines, ACE inhibitors, angiotensin receptor blockers, calcium channel blockers, and beta-blockers all receive a class I indication for the treatment of hypertension associated with RAS.Citation33 ACE inhibitors/angiotensin receptor blockers are a mainstay of most hypertensive regimens and have been shown to be 86% to 92% effective.Citation1 A limitation of modulation of the renin–angiotensin system is the potential to induce acute renal failure in patients with RAS to solitary functioning kidneys, severe bilateral stenoses, or advanced chronic kidney disease. While management of hypertension is a primary goal in the treatment of RAS, care should be taken to avoid reducing perfusion pressure so low as to induce ischemic nephropathy in pressure-dependent kidneys. Another major drawback of medical therapy is the apparent inability to prevent progression of existing stenosis, which has been the primary impetus for the development of more permanent and decisive solutions such as surgical and percutaneous revascularization.
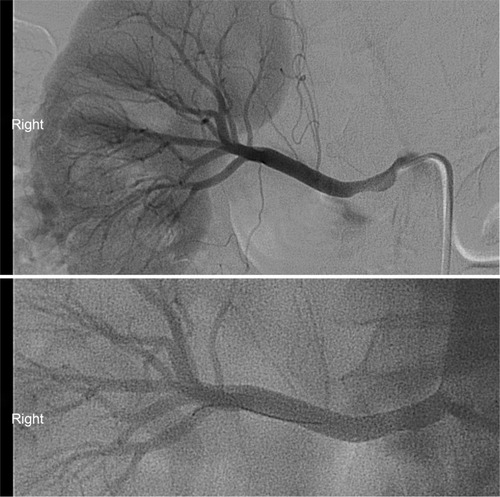
Revascularization of the renal artery can be accomplished surgically or endovascularly. Bypass grafts, aortorenal or nonanatomic, and the more technically challenging aortorenal endarterectomy make up the various surgical approaches to revascularization. These techniques are effective treatment options and are comparable to balloon angioplasty; however, major complications associated with surgery have been reported to be twice as common as compared to the endovascular approach.Citation46 For this reason, angioplasty has largely replaced surgical therapy as a first-line treatment. To assess the efficacy of stent placement, angioplasty with stent placement was compared to angioplasty alone in a randomized prospective trial of patients with ostial ARAS.Citation47 The results favored angioplasty with stent implantation in regards to rates of primary patency, restenosis, and need for intervention at 6 months. Currently, clinical guidelines endorse stent implantation in ostial lesions in patients with an established indication for revascularization (class I recommendation, level of evidence: B).Citation33 An example of a severely stenotic renal artery before and after successful angioplasty and stenting is shown in .
Endovascular interventions have been used, with limited success, to treat the three clinical syndromes associated with RAS: ischemic nephropathy, hypertension, and destabilizing cardiac syndromes. The treatment of asymptomatic RAS is not well established in an asymptomatic unilateral RAS in a viable kidney; though, it may be considered in a BRAS or unilateral RAS of a solitary viable kidney, a Class IIb indication (level of evidence: C). Specific instances of severe hypertension receive a Class IIa indication for revascularization. Guidelines state that percutaneous revascularization is reasonable for patients with RAS and accelerated hypertension, resistant hypertension, malignant hypertension, hypertension with an unexplained unilateral small kidney, and hypertension with intolerance to medication (level of evidence: B). Revascularization for the preservation of renal function also receives a Class IIa recommendation. Intervention can be considered for patients with RAS and progressive chronic kidney disease with BRAS or a RAS to a solitary functioning kidney (level of evidence: B). Intervention may also be considered for patients with chronic renal insufficiency with unilateral RAS, a Class IIb indication (level of evidence: C). RAS with UA is a class IIa indication. Currently, the only class I indication for catheter-based intervention is for patients with hemodynamically significant RAS and recurrent, unexplained congestive heart failure or flash pulmonary edema (level of evidence: B). Current guidelines endorse few indications for endovascular intervention in the treatment of clinical syndromes related to RAS due to a lack supporting clinical evidence.Citation33
Clinical trials
Several major clinical trials have largely shaped our current understanding of the effectiveness of percutaneous intervention as compared to conservative treatment. Among the first large clinical trials was the DRASTIC trial.Citation27 In this study, 106 patients with RAS (≥50%) and resistant hypertension were randomized to renal angioplasty versus medical therapy. At 3 months, blood pressures were similar in the two groups, 169±28 and 99±12 mmHg in the angioplasty group and 176±31 and 101±14 mmHg in the drug therapy group (P=0.25 for systolic blood pressure, P=0.36 diastolic blood pressure). At 12 months, there were still no significant differences in blood pressures, diastolic or systolic. The authors concluded that in the treatment of patients with hypertension and RAS, angioplasty has little advantage over antihypertensive drug therapy. As is the theme with the existing body of clinical data, this trial has significant limitations. First, a sample size of 106 patients is less than ideal and possibly insufficient to see the true effect of invasive intervention. Notably, this trial examined the effectiveness of angioplasty alone, which has been shown to be inferior to angioplasty with stenting.Citation46 Therefore, the therapy evaluated by this trial does not reflect current standard practice. Furthermore, RAS was defined as greater than 50%, indicating that many non-hemodynamically significant lesions were treated. Finally, 22 out of the 55 patients originally randomized to medical therapy crossed over to the angioplasty arm because of persistent hypertension despite treatment with three or more drugs or because of deterioration of renal function. These patients were still analyzed as intention-to-treat (ITT). While the lack of benefit observed in this trial may be real, multiple flaws inherent in the study design prevent any convincing conclusions from being drawn.
Another major clinical trial is known as the STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery (STAR) trial.Citation48 STAR was a multicenter randomized study of 140 patients with RAS. Inclusion criteria included renal impairment (GFR <80 mL/minute) and well-controlled blood pressure (<140/90 mmHg) on a stable medication regimen for 1 month prior to enrollment. Patients were randomized to renal artery stenting and medical therapy or medical therapy alone. The primary outcome was a >20% decrease in creatinine clearance during 2 years of follow-up. Ten of 64 patients (16%) in the stent placement group and 16 patients (22%) in the medication group reached the primary endpoint (hazard ratio: 0.73; 95% CI: 0.33–1.61). Several serious complications occurred in the stent group including two procedure-related deaths (3%), one late death secondary to an infected hematoma, and one patient required dialysis secondary to cholesterol embolism. There was no difference between the two groups in regards to other secondary endpoints: changes in blood pressure, incidence of refractory or malignant hypertension and pulmonary edema, cardiovascular morbidity and mortality, and total mortality. The authors concluded stent placement with medical treatment had no clear effect on progression of impaired renal function but led to a small number of significant procedure-related complications. They state that their findings favor a conservative approach to patients with ARAS, focused on cardiovascular risk factor management. Unfortunately, STAR represents yet another trial plagued with bias and design flaws. Of the 140 patients, 33% had only mild RAS (50%–70%). Furthermore, 12 of 64 patients (19%) in the stenting arm had RAS <50% and did not receive a stent but were still analyzed in ITT. An additional six patients in the stent arm did not receive a stent (one received balloon angioplasty, one died before placement, two declined the stent, and two technical failures) but all were analyzed in ITT. It is also worth noting that >50% had unilateral disease. This is important because bilateral disease is usually required to observe changes in creatinine clearance, which was the primary endpoint.
The third and largest clinical trial to date is known as Angioplasty and Stenting for Renal Artery Lesions (ASTRAL), a multicenter, prospective randomized study of 806 patients with uncontrolled or refractory hypertension or unexplained renal dysfunction with concomitant imaging to suggest RAS.Citation49 The primary outcome was change in renal function measured by mean slope of the reciprocal of a serum creatinine curve. Secondary outcomes were change in blood pressure, time to renal and major cardiovascular events, and mortality. Over a 5-year period, the rate of progression of renal impairment was −0.07 × 10−3 L/μmol per year in the revascularization group and −0.13 × 10−3 L/μmol per year in the medical therapy group. This was a difference favoring revascularization of 0.06 × 10−3 L/μmol per year (95% CI: −0.002 to 0.13; P=0.06). Over the same time period, the mean serum creatinine level was 1.6 μmol/L lower in the revascularization group than in the medical therapy group (95% CI: −8.4 to 5.2 [0.02 mg per deciliter; 95% CI: −0.10 to 0.06]). In regards to secondary endpoints, there was no significant difference in systolic blood pressure, while the decrease in diastolic blood pressure was smaller in the revascularization group. Similar rates of renal and major cardiovascular events were seen in both groups with hazard ratios of 0.97 (95% CI: 0.67–1.40; P=0.88), and 0.94 (95% CI: 0.75–1.19; P=0.61), respectively. Several serious complications associated with revascularization were observed in 23 patients including two deaths, and three amputations of toes or limb. From these results, the author’s concluded that there was no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease. Selection bias is of particular concern in this trial. For patients to be enrolled, the treating physician had to determine that the patient was reasonable to be randomized, meaning it was uncertain if the subject would benefit from revascularization. If an individual was thought to need revascularization within 6 months, they were excluded. Ultimately, only 83% randomized to stenting actually underwent the procedure. Interestingly, one-quarter of patients had normal renal function at baseline and 41% had <70% stenosis, but renal function was the primary outcome. It is concerning that recruitment took 7 years, and 42% of all sites only randomized between one to five patients while 61% randomized nine or fewer patients. Furthermore, the major adverse event rate at 24 hours was 9%, while the usual accepted rate is 2%.
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial is a multicenter, randomized trial sponsored by the National Heart, Lung, and Blood Institute with results published in early 2014 in the New England Journal of Medicine.Citation50 CORAL was designed to compare optimum medical therapy alone to stenting with optimum medical therapy and is thought to have corrected for flaws inherent in previous trials of its kind. The primary outcome is a composite endpoint of major cardiovascular and renal adverse events. To be included in the trial, patients were required to have atherosclerotic renal stenosis of ≥60% with a systolic pressure gradient of 20 mmHg or stenosis ≥80% with no gradient necessary and systolic hypertension of ≥155 mmHg on at least two antihypertensive medications. After a median follow-up period of 43 months, the authors found no significant difference between the two groups in regards to the composite endpoint, any of the individual components of the composite endpoints, or all-cause mortality.
In summary, the major trials published to date have been unable to prove a clinical benefit of endovascular revascularization for the treatment of RAS. However, significant design flaws and selection bias plague many of these studies, thus, limiting the usefulness of available data. While a truly definitive answer remains elusive, the most recent data from CORAL is more convincing than that of previous studies, thus favoring medical therapy alone over stenting for the treatment of ARAS. Ongoing and future studies are likely to provide additional constructive data, further informing clinical decision-making.
Need for clinical predictors
Despite a lack supporting data, marginal benefits seen in some studies may be more pronounced if appropriate subgroups were to be identified. Presumably, such patients would consistently benefit from revascularization. Several clinical predictors of successful outcomes following revascularization have been proposed.
Rocha-Singh et al followed 150 consecutive hypertensive patients with 180 renal artery lesions (≥75% stenosis) after undergoing renal artery stenting.Citation51 Multivariate logistic regression analysis was used to identify clinical variables related to improved blood pressure control at a mean follow-up of 13 months. Only BRAS or mean arterial pressure >110 predicted blood pressure improvement. While brain natriuretic peptide (BNP) is already a well-known marker of congestive heart failure and major cardiovascular events, it may also play a role in predicting outcomes following RAS. Though initially released from the myocardium in response to myocyte stretch, BNP’s primary physiological site of action is in the kidney. Interestingly, angiotensin II has been implicated in inducing the production of BNP.Citation52 In a study of 27 patients with refractory hypertension and significant RAS, BNP was measured before and after renal artery stent placement.Citation53 The authors observed an elevated BNP level in patients with significant RAS, and results indicated that a baseline BNP >80 pg/mL appears to predict blood pressure response after successful stent revascularization.
Elevated pulse pressure (PP) has been used as a predictor of increased cardiovascular events, and evidence suggests that PP may help to predict outcomes following RAS revascularization as well. A retrospective study at the Loyola University Medical Center found that a low PP was associated with improvements and stabilizations in renal function as well as improvements and stabilizations in blood pressure.Citation54 The authors concluded that a wide PP may reflect more advanced vascular stiffness and renal disease, distinguishing patients less likely to benefit from revascularization.
Poor treatment response following revascularization might be explained by irreversible microvascular disease downstream of stenotic lesions secondary to longstanding hypertension. Hypertension induced glomerulosclerosis and nephrosclerosis may increase the resistance to flow through both affected and unaffected kidneys. An increase in resistance could, therefore, serve as a marker of structural alterations of the renal microvasculature. Clinically, increased intrarenal resistance can be observed by less diastolic flow and a more resistant waveform. The resistive index quantifies this observation with the formula [1 − (end-diastolic velocity ÷ maximal systolic velocity)] × 100. In 2001, Radermacher et al tested the hypothesis in 131 patients who underwent renal artery stent implantation.Citation55 It was concluded that a resistive index value ≥80 reliably identifies patients in whom invasive intervention will not improve renal function, blood pressure, or kidney survival. A study published 2 years later observed results to the contrary.Citation56 One hundred seventy-six patients with severe (≥70%) ostial ARAS were divided into three strata of resistive indices, <0.7, 0.7–0.8, and >0.8. All groups underwent renal artery stenting and all groups had similar reductions in blood pressure at discharge and similar improvements in renal function at 6-month follow-up.
The measurement of fractional flow reserve (FFR) is a technique commonly used in the evaluation of coronary artery lesions. FFR measures pressure differences across specific arterial lesions obtained during maximum hyperemia and is a marker of the severity of vascular disease. More recently, the technique was adapted to study renal arteries.Citation57 Using papaverine (endothelium independent vasodilator) to induce hyperemia of the renal vasculature of 13 patients, investigators found no correlation between blood pressure gradient, hyperemic pressure gradient, or FFR with angiographic stenosis. The best correlation was found between hyperemic pressure gradient and FFR (r=0.94). The poor correlation between angiographic stenosis and hemodynamic parameters implies that angiography alone provides insufficient diagnostic information.
Renal frame count (RFC) is a method of assessing and quantifying perfusion of the kidneys in the setting of RAS. By utilizing the frame counter on standard angiographic images, one can count the number of frames required for dye to pass through the renal parenchyma and reach a distally defined point. It has been hypothesized that following revascularization, improvement in two variables of renal perfusion, RFC and renal blush grade, would correlate with improvement in renovascular hypertension.Citation58 This hypothesis was tested in a series of 24 patients with unilateral RAS who underwent revascularization. The authors found that the RFC and renal blush grade were in fact impaired in RAS and improved following renal artery stenting. Specifically, an improvement in RFC >4 was associated with lower blood pressures. Though not statistically significant, they also found that a baseline RFC ≥25 was 75% predictive of response to renal stent therapy. An optimal baseline RFC predictive of clinical response may be better defined by a larger study.
Characterization of renal artery plaques through intravascular ultrasound with virtual histology (VH-IUVS) has also shown promise in identifying a population potentially responsive to intervention. In a series of 25 patients who underwent VH-IUVS prior to renal artery revascularization, investigators found that a larger necrotic core was significantly associated with deterioration in estimated GFR following intervention.Citation59 Percentage of necrotic core has also been linked to a lack of improvement in RFC following RAS stenting. A similar series utilized VH-IUVS to characterize the stenotic arterial segment prior to revascularization, while RFC was measured before and after intervention.Citation60 The results demonstrated an association between increased necrotic core and lack of improvement in RFC following revascularization.
Cardiac destabilizing syndromes
While improvements in hypertension and renal function following revascularization of RAS remain elusive, it is important to remember the clear benefits seen in the treatment of cardiac destabilizing syndromes. As previously mentioned, flash pulmonary edema in the setting of hemodynamically significant RAS has earned the only Class I indication for revascularization according to current guidelines (ACC/AHA).Citation33 In the case of bilateral stenosis, the goal of stent placement is the restoration of renal blood flow, thus promoting the return of natriuresis. In addition, patients whose kidney function is no longer renin-dependent may be started on ACE inhibitors, thus improving long-term survival in the context of heart failure. In unilateral stenosis, reperfusion and reversal of renal ischemia removes the stimulus for angiotensin release, preventing acute increases in left ventricular afterload. This logic appears to be supported by convincing clinical data demonstrating benefits of revascularization. Koshla et al analyzed prospective registry data of patients with refractory hypertension and hemodynamically significant RAS presenting with UA (20 patients) or congestive heart failure (28 patients).Citation61 All patients underwent renal artery stent implantation with or without coronary intervention. Results indicated a benefit for revascularization, independent of having received PCI. New York Heart Association classification was improved in congestive heart failure patients and Canadian Cardiovascular Society angina classification was improved in patients with UA. Both effects were sustained in 73% of patients at a mean follow-up of 8.4 months. Bloch et alCitation9 reported similar results from a retrospective analysis of 90 patients (41% BRAS, 12% unilateral RAS) with pulmonary edema. Following revascularization, more than three-quarters of patients with BRAS and one-third of patients with unilateral RAS remained free of pulmonary edema. In addition, investigators have demonstrated reduction in hospitalizations due to heart failure, improvement in New York Heart Association functional classification, and increased utilization of ACE inhibitors following RAS revascularization.Citation62
Conclusion
RAS is a prevalent disease commonly encountered in association with other atherosclerotic vascular disease. RAS is associated with poor prognosis in terms of lesion progression, decline of renal function, and overall mortality. The detection of RAS can be accomplished via several effective diagnostic modalities, the choice of which can be tailored to the needs of individual patients. Once identified, the next step in management of RAS is unclear. Renal artery stenting is a therapy that has shown great promise, but has, thus far, not lived up to its expected and much hoped for potential. More outcome studies are needed to justify routine renal artery stenting for hypertension or chronic kidney disease. Better clinical predictors are needed to help with patient selection in hopes of identifying subgroups that might more consistently benefit from renal artery revascularization. Finally, it is important to recognize the well-established benefits of percutaneous intervention seen in the treatment of cardiac destabilizing syndromes.
Disclosure
The authors report no conflicts of interest in this work.
References
- SafianRDTextorSCRenal-artery stenosisN Engl J Med2001344643144211172181
- BlumUKrummeBFlügelPTreatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplastyN Engl J Med19973364594659017938
- BurketMWCooperCJKennedyDJRenal artery angioplasty and stent placement: predictors of a favorable outcomeAm Heart J2000139647110618564
- SlovutDPOlinJWFibromuscular dysplasiaN Engl J Med2004350181862187115115832
- DieterRSchmidtWPacanowskiJJaffMRRenovascular hypertensionExpert Rev Cardiovasc Ther20053341342215889969
- GottamNNanjundappaADieterRRenal artery stenosis: pathophysiology and treatmentExpert Rev Cardiovasc Ther20097111413142019900024
- GoldblattHLynchJHanzelRFSummervilleWWStudies on experimental hypertension I: the production of persistent elevation of systolic blood pressure by means of renal ischemiaJ Exp Med193459334737919870251
- TextorSCIschemic nephropathy: where are we now?J Am Soc Nephrol20041581974198215284283
- BlochMJTrostDWPickeringTGSosTAAugustPPrevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placementAm J Hypertens1999121 Pt 11710075377
- PickeringTGHermanLDevereuxRBRecurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisationLancet1988286105515522900930
- CrowleyJSantosRPeterRProgression of renal artery stenosis in patients undergoing cardiac catheterizationAm Heart J199813659139189812088
- HansenKJEdwardsMSCravenTEPrevalence of renovascular disease in the elderly: a population-based studyJ Vasc Surg200236344345112218965
- ConlonPJO’RiordanEKalraPANew insights into the epidemiologic and clinical manifestations of atherosclerotic renovascular diseaseAm J Kidney Dis200035457358710739776
- SinghMMorshedi-MeibodiASteenLDieterRSOverlap of atherosclerotic diseaseDieterRSDieterRIIIDieterRJrPeripheral Arterial DiseaseNew YorkMcGraw-Hill Professional2009177184
- WollenweberJShepsSDavisGClinical course of atherosclerotic renovascular diseaseAm J Cardiol196821160715634745
- Weber-MzellDKotankoPSchumacherMKleinWSkrabalaFCoronary anatomy predicts presence or absence of renal artery stenosis: a prospective study in patients undergoing cardiac catheterization for suspected coronary artery diseaseEur Heart J200223211684169112398826
- RihalCSTextorSCBreenJFIncidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiographyMayo Clin Proc200277430931611936924
- ValentineRClagettGMillerGMyersSMartinJChervuAThe coronary risk of unsuspected renal artery stenosisJ Vasc Surg1993183433439 discussion 439–4408377237
- ConlonPJLittleMAPieperKMarkDBSeverity of renal vascular disease predicts mortality in patients undergoing coronary angiographyKidney Int20016041490149711576364
- OlinJWMeliaMYoungJRGraorRARisiusBPrevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhereAm J Med1990881N46N51N
- LouieJIsaacsonJZierlerRBergelinRStrandnessDPrevalence of carotid and lower extremity arterial disease in patients with renal artery stenosisAm J Hypertens1994754364398060577
- ZierlerRBergelinRPolissarNCarotid and lower extremity arterial disease in patients with renal artery atherosclerosisArch Intern Med199815877617679554682
- RossiGRossiAZaninLExcess prevalence of extracranial carotid artery lesions in renovascular hypertensionAm J Hypertens1992518151736936
- KurodaSNishidaNUzuTPrevalence of renal artery stenosis in autopsy patients with strokeStroke2000311616510625716
- CapsMTPerissinottoCZierlerREProspective study of atherosclerotic disease progression in the renal arteryCirculation19989825286628729860789
- SchreiberMJPohlMANovickACThe natural history of atherosclerotic and fibrous renal artery diseaseUrol Clin North Am19841133833926464247
- van JaarsveldBCKrijnenPPietermanHThe effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study GroupN Engl J Med2000342141007101410749962
- CapsMTZierlerREPolissarNLRisk of atrophy in kidneys with atherosclerotic renal artery stenosisKidney Int19985337357429507221
- DeanRHKiefferRWSmithBMRenovascular hypertension: anatomic and renal function changes during drug therapyArch Surg198111611140814157305653
- MaillouxLUNapolitanoBBellucciAGVernaceMWilkesBMMosseyRTRenal vascular disease causing end-stage renal disease, incidence, clinical correlates, and outcomes: a 20-year clinical experienceAm J Kidney Dis19942446226297942820
- MaillouxLUBellucciAGMosseyRTPredictors of survival in patients undergoing dialysisAm J Med19888458558623364444
- GuttormsenBGimelliGRenal artery diseaseDieterRSDieterRIIIDieterRJrPeripheral Arterial DiseaseNew YorkMcGraw-Hill Professional2009619638
- HirschATHaskalZJHertzerNRAmerican Association for Vascular SurgerySociety for Vascular SurgerySociety for Cardiovascular Angiography and InterventionsSociety for Vascular Medicine and BiologySociety of Interventional RadiologyACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial DiseaseAmerican Association of Cardiovascular and Pulmonary RehabilitationNational Heart, Lung, and Blood InstituteSociety for Vascular NursingTransAtlantic Inter-Society ConsensusVascular Disease FoundationACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease FoundationCirculation200611311e463e65416549646
- HansenKJTribbleRWReavisSWRenal duplex sonography: evaluation of clinical utilityJ Vasc Surg19901232272362204735
- TaylorDCKettlerMDMonetaGLDuplex ultrasound scanning in the diagnosis of renal artery stenosis: a prospective evaluationJ Vasc Surg1988723633693276934
- OlinJWPiedmonteMRYoungJRDeAnnaSGrubbMChildsMBThe utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosisAnn Intern Med1995122118338387741367
- WittenbergGKennWTschammlerASandstedeJHahnDSpiral CT angiography of renal arteries: comparison with angiographyEur Radiol19999354655110087131
- GalanskiMProkopMChavanASchaeferCJandeleitKOlbrichtC[Accuracy of CT angiography in the diagnosis of renal artery stenosis]Rofo19941616519525 German7803775
- HahnUKönigCWMillerSMultidetector CT Angiography – is it a valuable screening tool to detect significant renal artery stenosis?Rofo20011731210861092 German11740668
- WillmannJKWildermuthSPfammatterTAortoiliac and renal arteries: prospective intraindividual comparison of contrast-enhanced three-dimensional MR angiography and multi-detector row CT angiographyRadiology2003226379881112601190
- VasbinderGBMakiJHNijenhuisRJMotion of the distal renal artery during three-dimensional contrast-enhanced breath-hold MRAJ Magn Reson Imaging200216668569612451582
- SnidowJJJohnsonMSHarrisVJThree-dimensional gadolinium-enhanced MR angiography for aortoiliac inflow assessment plus renal artery screening in a single breath holdRadiology199619837257328628861
- RieumontMJKaufmanJAGellerSCEvaluation of renal artery stenosis with dynamic gadolinium-enhanced MR angiographyAJR Am J Roentgenol1997169139449207498
- FainSBKingBFBreenJFKrugerDGRiedererSJHigh- spatial-resolution contrast-enhanced MR angiography of the renal arteries: a prospective comparison with digital subtraction angiographyRadiology2001218248149011161166
- TanKTvan BeekEJBrownPWGvan DeldenOMTijssenJRamsayLEMagnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysisClin Radiol200257761762412096862
- WeibullHBergqvistDBergentzSEJonssonKHulthénLManhemPPercutaneous transluminal renal angioplasty versus surgical reconstruction of atherosclerotic renal artery stenosis: a prospective randomized studyJ Vasc Surg1993185841850 discussion 850–8528230572
- Van de venPJGKaateeRBeurtlerJJArterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trialLancet199935391492822869929021
- BaxLWoittiezAJKouwenbergHJStent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trialAnn Intern Med200915012840841W15019414832
- WheatleyKIvesNGrayRASTRAL InvestigatorsRevascularization versus medical therapy for renal-artery stenosisN Engl J Med2009361201953196219907042
- CooperCJMurphyTPCutlipDECORAL InvestigatorsStenting and medical therapy for atherosclerotic renal-artery stenosisN Engl J Med20143701132224245566
- Rocha-SinghKJMishkelGJKatholiREClinical predictors of improved long-term blood pressure control after successful stenting of hypertensive patients with obstructive renal artery atherosclerosisCatheter Cardiovasc Interv199947216717210376497
- WieseSBreyerTDraguAGene expression of brain natriuretic peptide in isolated atrial and ventricular myocardium: influence of angiotensin II and diastolic fiber lengthCirculation2000102253074307911120697
- SilvaJAChanAWWhiteCJElevated brain natriuretic peptide predicts blood pressure response after stent revascularization in patients with renal artery stenosisCirculation2005111332833315655135
- DieterRSDarkiANanjundappaAUsefulness of Wide pulse pressure as a predictor of poor outcome after renal artery angioplasty and stentingAm J Cardiol2009104573273419699353
- RadermacherJChavanABleckJUse of Doppler ultra-sonography to predict the outcome of therapy for renal-artery stenosisN Engl J Med2001344641041711172177
- ZellerTMüllerCFrankUStent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosisCatheter Cardiovasc Interv200358451051512652503
- SubramanianRWhiteCJRosenfieldKRenal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenosesCatheter Cardiovasc Interv200564448048615789382
- MitchellJASubramanianRWhiteCJPredicting blood pressure improvement in hypertensive patients after renal artery stent placement: renal fractional flow reserveCatheter Cardiovasc Interv200769568568917351955
- TakumiTMathewVBarsnessGWThe association between renal atherosclerotic plaque characteristics and renal function before and after renal artery interventionMayo Clin Proc201186121165117222134935
- PrasadAIlapakurtiMHuPRenal artery plaque composition is associated with changes in renal frame count following renal artery stentingJ Invasive Cardiol201123622723121646647
- KhoslaSWhiteCJCollinsTJJenkinsJSShawDRameeSREffects of Renal Artery Stent Implantation in Patients with Renovascular Hypertension Presenting with Unstable Angina or Congestive Heart FailureAm J Cardiol19978033633669264441
- GrayBHOlinJWChildsMBSullivanTMBacharachJMClinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failureVasc Med20027427527912710843