Abstract
Background:
Hypertensive patients usually have a blunted nocturnal decrease, or even increase, in blood pressure during sleep. There is also a tendency for increased occurrence of cardiovascular events between 6 and 12 am due to increased morning blood pressure surge (MBPS). Co-occurrence of metabolic syndrome (MetS) and hypertension is also a common problem. Hyperuricemia might trigger the development of hypertension, chronic renal failure, and insulin resistance. In this study, we aimed to determine whether there is a relationship between hyperuricemia, MetS, nocturnal blood pressure changes, and MBPS.
Method:
A total of 81 newly diagnosed hypertensive MetS patients were included in this study. Ambulatory blood pressure monitoring of patients was done and patients’ height, weight, and waist and hip circumferences were recorded. Fasting blood glucose (FBG), lipid profile, creatinine, potassium, uric acid, hematocrit levels were studied.
Results:
Non-dipper (ie, those whose blood pressure did not drop overnight) patients had higher waist–hip ratios (WHR) (P = 0.003), uric acid (P = 0.0001), FBG (P = 0.001), total and low-density lipoprotein cholesterol levels (P = 0.0001). Risk analysis revealed that hyperuricemia was a risk factor for non-dipping pattern (P < 0.0001, odds ratio = 8.1, 95% confidence interval = 1.9–33.7). Patients in the highest quadrant for uric acid levels had higher FBG (P = 0.001), low-density lipoprotein cholesterol (P = 0.017), WHR (P = 0.01), MBPS (P = 0.003), and night diastolic blood pressure compared with lowest quadrant patients (P = 0.013). Uric acid levels were also positively correlated with night ambulatory blood pressure (ABP) (r = 0.268, P =0.05), night diastolic blood pressure (r =0.3, P =0.05), and MBPS (r =0.3, P = 0.05).
Conclusion:
Evaluation of hypertensive patients should also include an assessment of uric acid level and anthropometric measurements such as abdominal obesity. Hyperuricemia seems to be closely related to undesired blood pressure patterns and this may signal to the clinician that an appropriate therapeutic approach is required.
Keywords:
Introduction
The incidence of hypertension (HT) increases with age and nearly half of the population aged 60–69 years and three-quarters of those aged over 70 years are hypertensive. Ischemic heart disease and stroke risk increase when systolic blood pressure (SBP) rises above 115 mmHg and diastolic blood pressure (DBP) rises above 75 mmHg.Citation1 Healthy, normotensive individuals have a 10%–20% nocturnal decrease in blood pressure (BP) during the night. This decrease is under the influence of psychosocial, behavioral, and neurohumoral (sympathetic nervous system, renin–angiotensin system) factors.Citation2–Citation4 Hypertensive patients usually have a blunted nocturnal decrease, or even increase, in BP during sleep. This has been reported to increase the risk for cardiovascular diseases. It is well known that there is a tendency for increased occurrence of cardiovascular events between 6 and 9 am, just after waking from sleep.Citation5,Citation6 This is usually a result of increased morning blood pressure surge (MBPS) in hypertensive individuals and 24-hour ambulatory blood pressure monitoring (ABPM) is the gold standard to evaluate these nocturnal changes and MBPS in hypertensive patients.
Metabolic syndrome (MetS) is another cardiovascular risk factor that is very common in hypertensive patients. In fact, presence of HT is one of the criteria for diagnosis of MetS according to the Adult Treatment Panel III.Citation7 Presence of three of the following five conditions is needed for diagnosing a patient as MetS: abdominal obesity (waist circumference ≥ 102 cm in males and ≥88 cm in females), HT (SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg), fasting blood glucose (FBG) ≥110 mg/dL, triglycerides (TGs) ≥150 mg/dL, and high-density lipoprotein (HDL) <40 mg/dL in males and <50 mg/dL in females. Insulin resistance (IR) is considered the main underlying problem in MetS. However, genetic factors, hyperuricemia, nutritional behaviors, and lifestyle have also been speculated to have effects.Citation8,Citation9 Further, although hyperuricemia is underestimated by some clinical practitioners,Citation10,Citation11 some reports link presence of hyperuricemia with development of HT, chronic renal failure, IR, diabetes mellitus (DM), and endothelial dysfunction (ED). In this study, we aimed to determine whether there is a relationship between hyperuricemia, MetS, nocturnal BP changes, MBPS, and abdominal obesity. Therefore, we suggest decreasing uric acid levels is a target for treatment of HT, chronic renal failure, IR, DM, and ED patients.
Patients and methods
A total of 81 newly diagnosed hypertensive patients (61 female; age [mean ± standard deviation (SD)] 58.4 ± 9.4 years) were included. None of the patients had any history of HT, hyperlipidemia, DM, gouty arthritis, cardiovascular disease, or stroke, and none was taking any medications for any of these clinical conditions. Patients with an office BP measurement ≥ 140/90 mmHg were diagnosed as hypertensive according to seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure criteriaCitation12 and were evaluated for inclusion. Individuals who also fulfilled two further Adult Treatment Panel III criteria were included in the study and ABPM was undertaken on these participants. An Oscar™ 2 Ambulatory Blood Pressure Monitor (SunTech Medical®, Raleigh, USA ABD) was used for the ABPM. BP measurements were done at 15-minute periods between 7 am and 11 pm and at 30-minute periods between 11 pm and 7 am. Patients were asked to record their sleep and waking times and these records were taken under consideration when making necessary calculations. In case of insufficient measurements or technical problems, the ABPM was repeated.
The resultant ABPM reports were evaluated to define dipper versus (vs)non-dipper status (ie, whether patients’ BP did or did not, respectively, decrease during the night). Individuals with a decrease in night/sleep time mean SBP > 10% compared with morning mean SBP were considered dipper patients and all others were considered non-dipper patients.Citation13 MBPS was calculated by subtracting the mean SBP value of the hour that the lowest SBP was measured during sleep from the second hour SBP following waking.Citation14 Office, daytime, night, and 24-hour mean SBP and DBP values were also recorded. Office BP measurements were done by the same person, following the guidance of the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, on at least two separate occasions on different days.
Anthropometric measurements included height, weight, waist and hip circumference measurements. All measurements were done by the same person. Waist circumference measurements were acquired while patients were standing up and breathing slowly, between the upper end of the iliac crest and the lower end of the lowest rib. Hip measurements were also done while patients were standing, from the widest point of the hip.Citation15 Body mass index and a waist–hip ratio (WHR) were calculated and recorded for each patient.
Blood glucose, lipid profile (total cholesterol [Tcol], HDL cholesterol, low-density lipoprotein [LDL] cholesterol, TG), creatinine, potassium, uric acid, hematocrit and thyroid-stimulating hormone levels were studied from an 8-hour fasting blood sample using standard laboratory techniques. The hyperuricemia threshold was accepted as 6 mg/dL.
Statistical analyses were performed with SPSS software (v 11.0, IBM, Armonk, NY, USA). All numerical data presented here are expressed as the mean ± SD. Data normality was analyzed by Kolmogorov–Smirnov test. All numerical variables with normal distribution are expressed here as mean ± SD, while variables with skew distribution are expressed as median, interquartile range. Categorical variables are given as percentages and were compared using the Chi-square test. Normally distributed numeric variables were compared with independent-samples Student’s t-test, and skew distributed numeric variables were compared using the Mann–Whitney U test. A P value < 0.05 was considered statistically significant.
Results
Demographic and anthropometric characteristics and laboratory and ABPM data for the study group are summarized in . Non-dipper patients had higher WHR (0.92 ± 0.09 vs 0.87 ± 0.06, P = 0.003), uric acid (5.0 ± 1.3 vs 3.7 ± 0.9, P = 0.0001), FBG (106.1 ± 14.5 vs 94.6 ± 13.9, P = 0.001), Tcol and LDL cholesterol levels (237.4 ± 41.6 vs 184.3 ± 34.3 and 153, 45.5 vs 97, 35, respectively, P = 0.0001, ). Risk analysis revealed that hyperuricemia (uric acid > 6 mg/dL) was a risk factor for non-dipping pattern (P < 0.0001, odds ratio =8.1, 95% confidence interval =1.9–33.7).
Table 1 Demographic and clinical characteristics of study group (N = 81)
Table 2 Comparison of dipper and non-dipper patients
Further analysis revealed that patients in the highest quadrant for uric acid levels had higher FBG (108.3 ± 13.9 vs 91.6 ± 16.7, P = 0.001), LDL cholesterol (148, 68 vs 123, 57 P = 0.017) WHR (0.92 ± 0.09 vs 0.85 ± 0.06, P = 0.01), MBPS (44.3 ± 9.8 vs 35.6 ± 7.2, P = 0.003), and night DBP (67.5 ± 8.5 vs 61.4 ± 4.9, P = 0.013, ) compared with those in the lowest quadrant. Uric acid levels were also positively correlated with night ambulatory blood pressure (ABP) (r = 0.268, P < 0.05), night DBP (r = 0.3, P < 0.05), and MBPS (r = 0.3, P < 0.05, ).
Figure 1 Blood pressure – uric acid relationship.
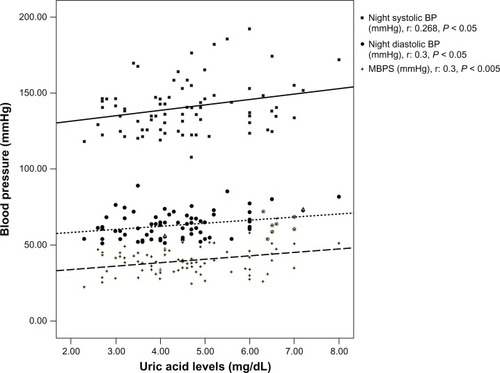
Table 3 Comparison of patients in lowest and highest uric acid quadrants
Discussion
HT is not only a significant cardiovascular risk factor by itself but also usually accompanies other cardiovascular risk factors such as abdominal obesity, DM, and hyperlipidemia.Citation16 Occurrence of these pathological conditions together is called “metabolic syndrome” and is associated with a strong likelihood of suffering from cardiovascular diseases.Citation7 All these pathologies are thought to occur as a result of the common pathophysiological pathway, IR.Citation7
Genetic, environmental, and nutritional factors seem to interfere with the presence and degree of IR. Increased abdominal fat may lead to increased resistance to insulin, resulting in hyperinsulinemia and diabetic tendencies. Increased fat tissue and various other nutritional factors are speculated to increase IR and incidence of related complications. One such factor is increased fructose intake, which leads to hyperuricemia by increasing insulin resistance and abdominal fat tissue, a condition classically associated with gouty arthritis but usually not considered a significant problem if there are no arthritic symptoms.Citation10
However, in this study, we found that hyperuricemia is closely associated with HT, diabetic tendencies, and abdominal obesity. Subjects with a non-dipping BP pattern had higher uric acid levels, WHR, FBG, and Tcol and LDL cholesterol levels compared with their dipper counterparts (). Risk analysis revealed that increased uric acid levels were a significant risk factor for non-dipper BP pattern (P < 0.0001, odds ratio =8.1, 95% confidence interval = 1.9–33.7). Also, patients in the highest quadrant for uric acid levels had higher FBG, LDL cholesterol, WHR, MBPS, and night DBP compared with those in the lowest quadrant (). All these results signify the coexistence of hyperuricemia with well-known cardiovascular risk factors such as DM, hyperlipidemia, obesity, and HT, but it is unknown whether hyperuricemia is the reason for or the result of this.
In a study by Wasada et al of 160 subjects, patients with uric acid levels in the highest quadrant had the highest body mass index and FBG as well as a hyperlipidemic profile.Citation17 Similarly, Lin et al reported that patients with MetS have a tendency to hyperuricemia and that uric acid levels are correlated with insulin levels, TGs, the homeostatic model assessment of insulin resistance, both SBP and DBP, and abdominal obesity.Citation18 Korpachev et al analyzed a group of 90 patients and reported that uric acid was related to abdominal obesity and IR.Citation19 Further, some large-scale epidemiological studies have reported an association between hyperuricemia, MetS, and abdominal obesity.Citation20–Citation22
Hyperuricemia may be a secondary result of decreased renal uric acid clearance in some patients.Citation20–Citation22 However, some studies have reported increased uric acid production in patients with MetS who have normal renal function. For example, See et al analyzed data on 28,745 subjects and reported that hyperuricemia is only weakly associated with renal function but is strongly associated with MetS with or without a low glomerular filtration rate.Citation23 The authors also reported that the main cause of hyperuricemia was increased production rather than decreased clearance of uric acid. Another possible explanation for this elevation might be increased renal tubular re-absorption due to hyperinsulinemia.Citation24 Supporting this theory, we found a close association between the MetS components, FBG, WHR, and uric acid levels in a group of patients with normal renal function.
Another important finding of our study is the close association of hyperuricemia with non-dipping pattern and increased MBPS. Non-dipping patients are known to have a higher degree of cardiovascular risk compared with those who dip. It is well known that there is a tendency for increased occurrence of myocardial infarction, sudden death, and hemorrhagic or ischemic stroke 6 and 9 am, just after waking from sleep.Citation5,Citation6 This is thought to be secondary to increased MBPS in hypertensive individuals. Increased vascular resistance secondary to increased sympathetic and renin–angiotensin system activities have also been speculated to be the main underlying reason for increased MBPS.Citation25 Similarly, increased cortisol levels (as a result of circadian rhythm) may also increase vascular resistance and cause a hypertensive attack.Citation26 Moreover, patients with MetS might be more prone to increased MBPS simply because of their IR. Hyperinsulinemia increases sympathetic activity and intra-vascular volume as well as causing ED and angiotensin II receptor upregulation.Citation27–Citation30
Thus, at this stage, we think that uric acid might closely interact with every component of MetS. We found that MBPS was clearly related to the degree of abdominal obesity and hyperuricemia, while we also found a relationship between uric acid levels, WHR, and FBG. Patients with increased uric acid levels also had higher MBPS. Supportive of our findings is a recent analysis of the Framingham Heart Study by Sundström et al, who similarly reported that serum uric acid level was an independent predictor of HT.Citation31 Zoppini et al, Kim et al, and Holme et al also reported that increased uric acid levels are independently associated with increased cardiovascular mortality, stroke, and congestive heart failure in three different epidemiological studies.Citation32–Citation34 We think that increased uric acid levels may induce IR and abdominal obesity, two conditions that increase uric acid levels with a positive feedback mechanism. All three of these conditions increase BP separately or in additive manner through some common mechanisms. As such, we feel that evaluation of hypertensive patients should also include assessment of uric acid levels and anthropometric measurements, as abdominal obesity and hyperuricemia seem to be closely related with undesired BP patterns, and these assessments may alert the clinician to any problems needing treatment.
Author contributions
Emre Tutal is the main author of this article. Derun Taner Ertugrul, Burak Sayin, Avsin Ibis, Siren Sezer, and Nurhan Özdemir assisted in collecting study data and writing the article.
Disclosure
The authors declare no conflicts of interest in this work.
References
- LewingtonSClarkeRQizilbashNPetoRCollinsRProspective Studies CollaborationAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236093491903191312493255
- KarioKSchwartzJEGerinWRobayoNMaceoEPickeringTGPsychological and physical stress-induced cardiovascular reactivity and diurnal blood pressure variation in women with different work shiftsHypertens Res200225454355112358139
- KarioKJamesGDMarionRAhmedMPickeringTGThe influence of work- and home-related stress on the levels and diurnal variation of ambulatory blood pressure and neurohumoral factors in employed womenHypertens Res200225449950612358133
- KawasakiTCuginiPUezonoKCircadian variations of total renin, active renin, plasma renin activity and plasma aldosterone in clinically healthy young subjectsHorm Metab Res199022126366392076861
- MullerJEToflerGHStonePHCircadian variation and triggers of onset of acute cardiovascular diseaseCirculation19897947337432647318
- MarlerJRPriceTRClarkGLMorning increase in onset of ischemic strokeStroke19892044734762648651
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in AdultsExecutive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)JAMA2001285192486249711368702
- KatsikiNPapanasNFonsecaVAMaltezosEMikhailidisDPUric acid and diabetes: is there a link?Curr Pharm Des Epub December 26, 2012.
- CohenEKrauseIFraserAGoldbergEGartyMHyperuricemia and metabolic syndrome: lessons from a large cohort from IsraelIsr Med Assoc J2012141167668023240372
- BorgesRLRibeiroABZanellaMTBatistaMCUric acid as a factor in the metabolic syndromeCurr Hypertens Rep201012211311920424936
- JalalDIChoncholMChenWTargherGUric acid as a target of therapy in CKDAm J Kidney Dis201361113414623058478
- ChobanianAVBakrisGLBlackHRJoint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureNational Heart, Lung, and Blood InstituteNational High Blood Pressure Education Program Coordinating CommitteeSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension20034261206125214656957
- WhiteWBLaroccaGMImproving the utility of the nocturnal hypertension definition by using absolute sleep blood pressure rather than the “dipping” proportionAm J Cardiol200392211439144114675581
- KarioKPickeringTGUmedaYMorning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective studyCirculation2003107101401140612642361
- SnijderMBZimmetPZVisserMDekkerJMSeidellJCShawJEIndependent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab StudyInt J Obes Relat Metab Disord200428340240914724659
- KaplanNMKaplan’s Clinical Hypertension8th edPhiladelphia, PALippincott Williams & Wilkins2002
- WasadaTKatsumoriKSaekiAIwataniM[Hyperuricemia and insulin resistance]Nippon Rinsho1996541232933296 Japanese.8976108
- LinJDChiouWKChangHYLiuFHWengHFSerum uric acid and leptin levels in metabolic syndrome: a quandary over the role of uric acidMetabolism200756675175617512306
- KorpachevVVHurinaNMKorpachevaTIShuprovychAAMosendzIO[Peculiarities of uric acid balance disorders in patients with type 2 diabetes and metabolic syndrome.]Fiziol Zh2009553133140 Ukrainian.19526867
- SchmidtMIDuncanBBWatsonRLSharrettARBrancatiFLHeissGA metabolic syndrome in whites and African-Americans. The Atherosclerosis Risk in Communities baseline studyDiabetes Care19961954144188732701
- YooTWSungKCShinHSRelationship between serum uric acid concentration and insulin resistance and metabolic syndromeCirc J200569892893316041161
- OnatAUyarelHHergençGSerum uric acid is a determinant of metabolic syndrome in a population-based studyAm J Hypertens200619101055106217027827
- SeeLCKuoCFChuangFHSerum uric acid is independently associated with metabolic syndrome in subjects with and without a low estimated glomerular filtration rateJ Rheumatol20093681691169819531754
- Quiñones GalvanANataliABaldiSEffect of insulin on uric acid excretion in humansAm J Physiol19952681 Pt 1E1E57840165
- KarioKPickeringTGHoshideSMorning blood pressure surge and hypertensive cerebrovascular disease: role of the alpha adrenergic sympathetic nervous systemAm J Hypertens200417866867515288883
- OttoMESvatikovaABarrettoRBEarly morning attenuation of endothelial function in healthy humansCirculation2004109212507251015136499
- HallJELouisKDahl Memorial Lecture. Renal and cardiovascular mechanisms of hypertension in obesityHypertension19942333813948125566
- ReavenGMLithellHLandsbergLHypertension and associated metabolic abnormalities – the role of insulin resistance and the sympathoadrenal systemN Engl J Med199633463743818538710
- RocchiniAPKatchVKveselisDInsulin and renal sodium retention in obese adolescentsHypertension19891443673742676858
- SteinbergHOChakerHLeamingRJohnsonABrechtelGBaronADObesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistanceJ Clin Invest19969711260126108647954
- SundströmJSullivanLD’AgostinoRBLevyDKannelWBVasanRSRelations of serum uric acid to longitudinal blood pressure tracking and hypertension incidenceHypertension2005454283315569852
- ZoppiniGTargherGNegriCElevated serum uric acid concentrations independently predict cardiovascular mortality in type 2 diabetic patientsDiabetes Care20093291716172019542211
- KimSYGuevaraJPKimKMChoiHKHeitjanDFAlbertDAHyperuricemia and risk of stroke: a systematic review and meta-analysisArthritis Rheum200961788589219565556
- HolmeIAastveitAHHammarNJungnerIWalldiusGUric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS)J Intern Med2009266655857019563390