Abstract
Disseminated intravascular coagulation (DIC) is the most frequent coagulation disorder in patients with prostate cancer. However, renal involvement in DIC associated with prostate cancer has rarely been documented. Herein, we present a case of metastatic prostate cancer presenting with acute renal failure (RF) triggered by DIC. An 80 year old man with metastatic prostate cancer was treated with antihormone therapy at an outpatient clinic. He was admitted to our hospital because of severe dyspnea and progressive RF. A hemorrhagic tendency was not clinically evident. Laboratory tests exhibited a significant coagulation disorder, suggestive of DIC. Despite treatment, his RF and dyspnea worsened, and he eventually passed away. An autopsy study revealed hypertensive nephrosclerosis superimposed by fibrin rich thrombi formation involving glomerular capillaries and arterioles characteristic of DIC. Additionally, focal segmental glomerulosclerosis was identified, which was presumably secondary to the glomerular endothelial and/or podocyte injury augmented by DIC. Those findings showed that glomerular injury, which was induced and subsequently exacerbated by DIC associated with prostate cancer, highly contributed to the progression of RF in our case. A differential diagnosis of DIC should be considered when a patient with prostate cancer presents with renal dysfunction.
Introduction
Disseminated intravascular coagulation (DIC) is the most frequent coagulation disorder in patients with prostate cancer.Citation1 DIC occasionally occurs as a presenting sign of metastatic prostate cancer.Citation2,Citation3 Clinical symptoms of DIC associated with prostate cancer range from a subclinical marker of disease to overt bleeding after trauma or surgical procedures.Citation3,Citation4
Despite its frequent occurrence, little is known about the clinical presentation of DIC in prostate cancer. Specifically, renal involvement in DIC as a presenting sign has not been reported in the literature. In the present article, we describe an autopsy case of metastatic prostate cancer presenting with progressive renal dysfunction, which was augmented by DIC. We have described this case with emphasis on the renal histopathology characteristic of DIC, and include a brief review of the literature. This unusual case presentation demonstrates that DIC should be considered when prostate cancer patients present with renal dysfunction due to unknown etiology and therefore a comprehensive work up for DIC should be performed even in the absence of apparent hemorrhagic tendency.
Case presentation
An 80-year-old Japanese man was found to have increased serum prostate specific antigen levels of 231.5 ng/mL (normal: <1.0 ng/mL) on a routine medical checkup. He had a long-standing history of hypertension and underwent a needle biopsy of the prostate gland that showed Gleason 4 + 5 = 9 adenocarcinoma. A computed tomography scan revealed that the tumor had invaded the bladder, and multiple metastatic lesions were found in the lumbar regions. He was started on a luteinizing hormone releasing hormone agonist (leuprolide) and androgen antagonist (flutamide), and was followed at an outpatient clinic. His serum creatinine levels were within a normal range (0.8–1.4 mg/dL) at this time.
The patient’s prostate specific antigen level increased to 1530 ng/mL 4 months later. A computed tomography scan revealed an increasing number of metastatic lesions in the lumbar bones. At this time, he complained of dyspnea that was probably due to pulmonary edema. His serum creatinine was 2.1 mg/dL. After 1 month, his dyspnea worsened as the renal failure progressed with a creatinine level of 2.89 mg/dL. A urine test showed strong presence of protein (+++) and occult blood (++). His serum total protein was 5.8 g/dL (normal: 6.0–8.3 g/dL), and his albumin was 2.3 g/dL (normal: 3.5–5.5 g/dL). He was admitted to the intensive care unit for further investigation and treatment.
Laboratory evaluation performed on the first day of intensive care unit admission revealed the following results: hemoglobin level, 10.2 g/dL; platelet count, 60 × 103/μL (normal: 150–400 × 103/μL); and total leukocyte count, 18.2 × 103/mm3 (normal: 4.0–11 × 103/mm3). His prothrombin time was 16.3 seconds (normal: 11.5–15.5 seconds), and his prothrombin time and international normalized ratio was 1.8 (normal: 1–1.25). The activated partial prothrombin time was 38.5 seconds (normal: 25.2–36 seconds); the serum fibrinogen level, 168 mg/dL (normal: 170–410 mg/dL); and D-dimer level, 28.8 μg/mL (normal: <1.0 μg/mL). His fibrin degradation product level was 93.9 μg/mL (normal: <10 μg/mL).
Although a bleeding tendency was not clinically evident, he was diagnosed with DIC based on the laboratory data. Treatment with antithrombin-III and blood transfusion was initiated. On the second day at the intensive care unit, he had oliguria associated with a serum creatinine level of 5.19 mg/dL, for which hemodialysis was started. His serum total protein was 5.1 g/dL, and his albumin level was 1.9 g/dL. The dyspnea progressively worsened despite treatment. His serum creatinine ranged from 4.5–5.0 mg/dL. Chest X-ray revealed pulmonary infiltrates in both lungs, which was suggestive of alveolar hemorrhage. On day 14, he experienced cardiac arrest and expired. An autopsy was conducted after obtaining permission from family members to elucidate the mechanisms of renal failure (RF).
Histopathology
Gross examination of the kidney revealed an irregular and roughly granular cortical surface, and there was marked congestive changes mainly involving the medulla. Microscopically, global glomerulosclerosis was noted in approximately 40% of all glomeruli. The arcuate and interlobular arteriolar walls showed moderate to severe thickening due to intimal fibrosis. These chronic glomerular and vascular alterations are reflective of hypertensive nephrosclerosis related to the patient’s long standing hypertension. The vast majority of the remaining glomeruli showed numerous thrombi formation mainly involving peripheral glomerular capillaries and afferent arterioles, reflective of severe coagulation disorder (). Some glomerular capillaries were distended by thrombi with features of thrombotic microangiopathic injury. A phosphotungstic acid hematoxylin stain identified dark blue fibrin strands constituting the thrombi (). CD41 immunostaining showed the presence of sparsely scattered platelets within the thrombi (). We believe that the formation of these thrombi under the condition of reduced nephrons due to nephrosclerosis highly contributed to progressive RF in this case.
Figure 1 Renal pathology of DIC in association with metastatic prostate cancer. The peripheral glomerular capillaries (A) and afferent arteriole (B, arrows) were distended by thrombi formation (periodic acid methenamine silver stain). Fibrin strands revealed by phosphotungstic acid hematoxylin stain constituting the thrombi (C). The presence of sparsely scattered platelets within the thrombi was detected by immunostaining with an anti-CD41 antibody (D). Focal segmental sclerosis of the glomeruli (E and F; periodic acid methenamine silver stain). Segmental sclerosis of capillary architecture associated with attachments to the Bowman’s capsule with prominence of overlying epithelial cells (E, arrows). Segmental sclerosis was also noted at the urinary pole with capillary lumina obliterated by infiltration of mononuclear leukocytes and foam cells (F, arrowhead).
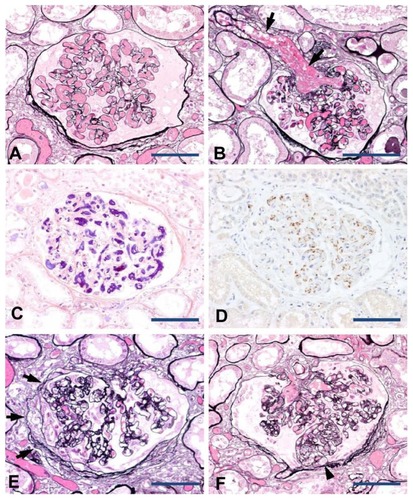
In addition, focal segmental glomerulosclerosis (FSGS) was identified in 1% of nonglobally sclerotic glomeruli. The FSGS was characterized by segmental sclerosis of capillary architecture associated with attachment to Bowman’s capsule, accompanied by the prominence of overlying epithelial cells (). Another glomerulus exhibited the segmental obliteration of a few capillaries by infiltration of mononuclear leukocytes and foam cells, and attachment to Bowman’s capsules near the urinary pole (). Active lesions or thrombi formation were not evident in the interlobular or arcuate arteries.
In the lung, multiple foci of intra-alveolar hemorrhage in the background of diffuse edema with occasional thrombin formation were observed in the small to medium sized arteries. Adenocarcinoma was identified in the prostate with direct invasion into the bladder and multiple metastatic lesions involving the thoracic and lumbar vertebrae.
Discussion
DIC has been one of the most common complications of prostate cancer since the first report in 1953.Citation5 Clinical signs of DIC are noted in only 0.4%–1.65% of patients with prostate cancer, including a bleeding tendency.Citation6 In these patients, DIC can be triggered by surgical procedures like prostate biopsy causing hemorrhage in multiple sites, such as the skin, genitourinary organs, gastrointestinal tracts and the brain.Citation3,Citation7,Citation8 To our knowledge, the occurrence rate of RF associated with DIC, as seen in our case, has not been previously documented. The incidence of DIC depends on the tumor stage, and it is enhanced in metastatic hormone refractory disease.Citation8,Citation9 Hyman et al recently reported that more than half of the patients with prostate cancer who develop DIC had high grade disease (Gleason scores of eight, nine, or ten).Citation8 In our case, the patient had prostate cancer with a Gleason score of nine and metastatic hormone refractory disease, consistent with previous reports.
DIC represents the result of a widespread activation of the coagulation pathway.Citation10 DIC proceeds from the simultaneous occurrence of systemic fibrin formation resulting from an increased generation of thrombi, impaired physiological anticoagulation mechanism, and inadequate fibrinolysis. The combination of increased formation and impaired removal of fibrin results in thrombotic occlusion of small and medium sized vessels. In cancer patients, tumor cells express a variety of procoagulant molecules including tissue factor and cancer procoagulant, which activate the host’s hemostatic system.Citation11,Citation12 Tumor cells also express fibrinolytic inhibitors such as plasminogen activator type 1, creating the hypofibrinolytic state that is characteristic of DIC.Citation13 Proinflammatory cytokines, such as tumor necrosis factor α and interleukin-6, are also involved in DIC development.Citation14,Citation15 Unfortunately, the underlying mechanism of DIC in prostate cancer has not been fully investigated and further studies are still needed. We expect that elucidation of the detailed mechanisms would enable us to invent new therapies and drugs to prevent the development of coagulation disorders in prostate cancer patients.
In our case, the most prominent histological finding was numerous thrombi within the glomerular capillary lumina and afferent arterioles. Furthermore, many afferent arterioles were occluded by thrombi, resulting in the collapse of the glomerular tufts, which is often called bloodless glomeruli. These vascular lesions are very similar to those of thrombocytopenic thrombotic purpura (TTP)/hemolytic uremic syndrome (HUS), which also occurs in prostate cancer.Citation16–Citation19 Despite the histological resemblance, major difference exists in the vascular lesions between DIC and TTP/HUS. In DIC, thrombi are fibrin rich, whereas TTP/HUS presents with platelet-rich thrombi.Citation20–Citation22 Clinically, TTP/HUS patients have normal platelet counts and prothrombin and D-dimer levels. This is in contrast to DIC, in which coagulation disorders are evident.Citation23 In our case, the DIC diagnosis was established on the basis of abnormal laboratory data, such as low platelet count, prolonged prothrombin, and increased D-dimer and fibrin degradation product levels. This diagnosis was confirmed histologically by the presence of fibrin rich thrombi revealed by the phosphotungstic acid hematoxylin stain. Additionally, TTP/HUS can be reversible when properly treated whereas DIC is refractory to treatment and has a poor prognosis, which is consistent with the present case.
Another remarkable renal finding in our case was FSGS. FSGS is a pattern of glomerular injury that occurs as a primary form or as secondary to many conditions.Citation24 The cause of primary FSGS is usually unknown, and patients often present with significant nephrotic syndrome, which was not identified in our case. In contrast, secondary forms of FSGS may be associated with a variety of conditions, including chronic hypertension, pyelonephritis, morbid obesity, and renal transplantation.Citation25–Citation28 A possible mechanism of secondary FSGS is a reduced number of nephrons, which increases glomerular pressure within remnant glomeruli and results in glomerular podocyte and/or endothelial injury.Citation25 In our case, the patient appeared to have a reduced number of nephrons due to hypertensive nephrosclerosis before the onset of DIC. We therefore speculate that the occurrence of DIC to the kidney with reduced nephrons may have augmented podocyte and/or endothelial injury of glomeruli, resulting in FSGS.
In summary, we have reported an autopsied patient who died of DIC associated with metastatic prostate cancer. This case was unique because acute RF was the initial presenting sign of DIC. We described clinicopathological aspects of DIC in prostate cancer and renal pathology of DIC along with a brief review of the literature. We propose that a differential diagnosis of DIC should be considered when a patient with prostate cancer presents with renal dysfunction and that complete physical and laboratory tests, including coagulation study, should be performed to detect a cause of renal dysfunction even in the absence of hemorrhagic tendency.
Acknowledgments
We express our appreciation to Mr Takenori Fujii for excellent technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- SmithJASolowayMSYoungMJComplications of advanced prostate cancerUrology199954Suppl 6A81410606278
- DuranITannockIFDisseminated intravascular coagulation as the presenting sign of metastatic prostate cancerJ Gen Intern Med20062111C6C817026724
- NavarroMRuizIMartínGCruzJJPatient with disseminated intravascular coagulation as the first manifestation of adenocarcinoma of the prostate. Risks of prostatic biopsyProstate Cancer Prostatic Dis20069219019116331297
- LeongCMcKenzieMRCouplandDBGascoyneRDDisseminated intravascular coagulation in a patient with metastatic prostate cancer: fatal outcome following strontium-89 therapyJ Nucl Med19943510166216647931669
- TagnonHJWhitmoreWFJrSchulmanPKravitzSCThe significance of fibrinolysis occurring in patients with metastatic cancer of the prostateCancer195361636713009653
- RuffionAManelAValignatCLopezJGPerrin-FayolleOPerrinPSuccessful use of Samarium 153 for emergency treatment of disseminated intravascular coagulation due to metastatic hormone refractory prostate cancerJ Urol20001643 Pt 178210953152
- Al-OtaibiMFAl-TaweelWBin-SalehSHerbaMAprikianAGDisseminated intravascular coagulation following transrectal ultrasound guided prostate biopsyJ Urol2004171134614665920
- HymanDMSoffGAKampelLJDisseminated intravascular coagulation with excessive fibrinolysis in prostate cancer: a case series and review of the literatureOncology201181211912521986538
- KampelLJChallenging problems in advanced malignancy: Case 2. Disseminated intravascular coagulation in metastatic hormone-refractory prostate cancerJ Clin Oncol200321163170317112915609
- LeviMde JongeEvan der PollTten CateHAdvances in the understanding of the pathogenetic pathways of disseminated intravascular coagulation result in more insight in the clinical picture and better management strategiesSemin Thromb Hemost200127656957511740680
- ContrinoJHairGKreutzerDLRicklesFRIn situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast diseaseNat Med1996222092158574967
- RicklesFRBrennerBTissue factor and cancerSemin Thromb Hemost200834214314518645918
- McMahonBKwaanHCThe plasminogen activator system and cancerPathophysiol Haemost Thromb2008363–418419419176991
- NakashimaJTachibanaMUenoMBabaSTazakiHTumor necrosis factor and coagulopathy in patients with prostate cancerCancer Res19955521488148857585524
- WadaHTanigawaMWakitaYIncreased plasma level of interleukin-6 in disseminated intravascular coagulationBlood Coagul Fibrinolysis1993445835908218855
- Morel-MarogerLKanferASolezKSraerJDRichetGPrognostic importance of vascular lesions in acute renal failure with microangiopathic hemolytic anemia (hemolytic-uremic syndrome): clinicopathologic study in 20 adultsKidney Int1979155548558480787
- MüllerNJPestalozziBCHemolytic uremic syndrome in prostatic carcinomaOncology19985521741769499193
- BiersSMSullivanMERobertsISNobleJGThrombotic microangiopathy in advanced prostatic carcinomaUrology200463238038214972503
- CaramelloVDovioACaraciPBerrutiAAngeliAThrombotic thrombocytopenic purpura in advanced prostate cancer: case report and published work reviewInt J Urol200714215015217302573
- AsadaYSumiyoshiAHayashiTSuzumiyaJKaketaniKImmunohistochemistry of vascular lesion in thrombotic thrombocytopenic purpura, with special reference to factor VIII related antigenThromb Res19853854694792861671
- KawasakiHHayashiKAwaiMDisseminated intravascular coagulation (DIC). Immunohistochemical study of fibrin-related materials (FRMs) in renal tissuesActa Pathol Jpn198737177842437764
- BurkeAPMontEKolodgieFVirmaniRThrombotic thrombocytopenic purpura causing rapid unexpected death: value of CD61 immunohistochemical staining in diagnosisCardiovasc Pathol200514315015515914300
- ParkYAWaldrumMRMarquesMBPlatelet count and prothrombin time help distinguish thrombotic thrombocytopenic purpura-hemolytic uremic syndrome from disseminated intravascular coagulation in adultsAm J Clin Pathol2010133346046520154285
- D’AgatiVDKaskelFJFalkRJFocal segmental glomerulosclerosisN Engl J Med2011365252398241122187987
- RennkeHGKleinPSPathogenesis and significance of nonprimary focal and segmental glomerulosclerosisAm J Kidney Dis19891364434562658558
- CotranRSNephrology Forum. Glomerulosclerosis in reflux nephropathyKidney Int19822135285347087289
- WarnkeRAKempsonRLThe nephrotic syndrome in massive obesity: a study by light, immunofluorescence, and electron microscopyArch Pathol Lab Med19781028431438580886
- CosioFGFrankelWLPelletierRPPesaventoTEHenryMLFergusonRMFocal segmental glomerulosclerosis in renal allografts with chronic nephropathy: implications for graft survivalAm J Kidney Dis199934473173810516356