Abstract
Acute renal failure associated with a fulminant, life-threatening systemic disease is rare in previously healthy young children; however, when it occurs, the most common cause is hemolytic-uremic syndrome (HUS). In most cases (90%), this abrupt and devastating illness is a result of ingestion of food or drink contaminated with pathogens that produce very potent toxins. Currently, there are no proven treatment options that can directly inactivate the toxin or effectively interfere with the cascade of destructive events triggered by the toxin once it gains access to the bloodstream and binds its receptor. However, HUS is self-limited, and effective supportive management during the acute phase is proven to be a life saver for children affected by HUS. A minority of childhood HUS cases, approximately 5%, are caused by various genetic mutations causing uncontrolled activation of the complement system. These children, who used to have a poor prognosis leading to end-stage renal disease, now have access to exciting new treatment options that can preserve kidney function and avoid disease recurrences. This review provides a summary of the current knowledge on the epidemiology, pathophysiology, and clinical presentation of childhood HUS, focusing on a practical approach to best management measures.
Introduction
Hemolytic-uremic syndrome (HUS) is one of the most common etiologies for acute kidney injury and an important cause of acquired chronic kidney disease in children.Citation1 HUS is generally classified into two main types: typical or diarrhea-associated (D+HUS) and atypical (aHUS) or diarrhea-negative HUS. In children, 90% of HUS cases are associated with a prodrome of diarrhea caused by infections with Shiga toxin-producing Escherichia coli that are able to attach to the intestinal wall; these are known as enterohemorrhagic E. coli (EHEC).Citation1 A rare form of HUS caused by streptococcus pneumoniae, usually following invasive pulmonary infection, is occasionally classified with D+HUS in the group of infectious HUS, or is included in the aHUS group as it is not preceded by diarrhea.Citation2 The majority of the remaining cases are affected by aHUS, which can be sporadic or familial. Most cases of aHUS are caused by genetic or acquired dysregulations of the complement system.Citation3 Thrombotic microangiopathy (TMA) is the common pathogenetic pathway leading to all forms of HUS as well as thrombotic thrombocytopenic purpura (TTP).Citation4 However, TTP is rare in children and is usually caused by congenital mutation in the ADMTS13 gene, whereas in adults it occurs more frequently due to abnormal autoantibodies against the ADMTS13 enzyme.Citation4 This paper will review the clinical presentation, epidemiology, and pathogenesis of HUS in children, focusing on current management options.
Clinical presentation
D+HUS
D+HUS is usually preceded by a severe form of infectious gastroenteritis that typically begins 3 days after exposure to food or water contaminated with EHEC. However, following ingestion of EHEC, incubation periods ranging from 1–12 days have been reported.Citation5,Citation6 Symptoms are nonspecific, including nausea, cramping, abdominal pain, and diarrhea that is initially watery but becomes bloody in more than 70% of cases within 2–3 days from beginning of symptoms.Citation7–Citation9 Infections with Shiga toxin-producing E. coli belonging to the EHEC family can cause extensive colonic injury with excruciating cramping pain and a severe hemorrhagic colitis; however, milder forms with minimal symptoms and non-bloody diarrhea have been described.Citation7,Citation10,Citation11 In 85%–90% of cases, the gastroenteritis is self-limited and the symptoms subside or disappear 5–8 days from start of symptoms, leading to full recovery.Citation5,Citation11–Citation14 Complications of the infectious colitis itself, including bowel perforation or massive hemorrhage, can be fatal; however, the most common and serious complication of EHEC infections is HUS, which develops in 10%–15% of affected patients 7–10 days after beginning of symptoms.Citation15
Patients that go on to develop HUS continue to be very sick; many are lethargic or irritable. Lab work is characterized by thrombocytopenia with platelet counts less than 150×109/L, hemolytic anemia with schistocytes on blood smear, and progressing azotemia. In mild cases of D+HUS in previously healthy children, the azotemia may be missed or difficult to document as serum creatinine levels can be within normal range despite a more than 50% increase from baseline.Citation16 D+HUS is a systemic disease that can affect many organs; however, some of the most serious complications relate to involvement of the central nervous system. Lethargy, which is observed in many patients on presentation, can progress to development of stupor and coma as well as seizures and strokes.Citation17 Acute kidney injury is characterized by progression to oligoanuria and severe electrolyte imbalances that require establishment of acute renal replacement therapy in 50%–70% of patients.Citation18 Despite the fulminant disease course and multi-organ involvement, D+HUS is self-limited in most cases, with eventual gradual improvement and full recovery.Citation18 Nevertheless, up to 25% of patients are left with ongoing sequelae such as chronic renal failure, hypertension, and, rarely, chronic pancreatitis and diabetes.Citation18 Central nervous system complications resolve in most cases; however, these are responsible for the majority of D+HUS-related fatalities, and permanent neurological sequelae have been reported in more than 20% of cases.Citation17
aHUS
aHUS, by definition, is not preceded by the characteristic bloody diarrhea of EHEC infection; however, since in the majority of cases it involves dysregulation of the complement system, it is frequently triggered by an infectious event including gastroenteritis with diarrhea.Citation19 Therefore, initially it may be difficult to differentiate from D+HUS. More than 60% of patients with aHUS present in childhood – the majority at less than 2 years of age, which is also common for D+HUS.Citation19,Citation20 Nevertheless, unlike aHUS, D+HUS rarely affects children younger than 1 year of age and almost never presents before 6 months of age.Citation21 The presenting symptoms are nonspecific, related to the triggering infectious event or the developing systemic TMA, resembling D+HUS symptoms.
Epidemiology
HUS is a rare disease with an incidence ranging from 0.7–8 cases/100,000 population per year with significant geographical and seasonal variability. The highest incidence of D+HUS is reported among children younger than 6 years who typically present in the late summer and autumn months.Citation8,Citation14,Citation16,Citation22–Citation25 Prevalence of D+HUS is closely related to the incidence of EHEC infections and the main reservoir for EHEC pathogens is the gastrointestinal tract of healthy cattle.Citation26 Higher incidence of D+HUS in rural areas with a developed cattle industry has been reported.Citation8,Citation23 The highest incidence of D+HUS in the world was reported in Argentina, while, in Canada, it has been observed in the province of Alberta; both geographic regions have developed beef production industries.Citation16,Citation27 Large outbreaks originating in contaminated food have been extensively publicized; one of the most well-known outbreaks in the US was caused by contaminated ground beef hamburgers served in a restaurant chain, hence the popular name for D+HUS: “hamburger disease”.Citation5 However, contrary to popular belief, most cases of D+HUS are sporadic or occur in small clusters and are not related to poorly cooked contaminated ground beef.Citation8,Citation14,Citation15,Citation22,Citation24,Citation28,Citation29 The majority of sporadic cases, as well as small and large outbreaks, are caused by other contaminated foods and beverages, such as vegetables, cider, or milk,Citation30 and transmission from person to person has also been reported.Citation5,Citation12,Citation31 Moreover, not all outbreaks of D+HUS are associated with EHEC infections; other pathogens may be responsible, the most common of which is Shigella dysenteriae type 1 (SD1).Citation32
aHUS is responsible for 10% of the total number of HUS cases, with an annual incidence that is roughly one-tenth of the incidence of D+HUS.Citation33 Up to 40% of these cases (5% of the total HUS incidence) are caused by invasive pneumococcal infections, and the rest are generally caused by complement dysregulations presenting sporadically or inherited in familial clusters.Citation33 In North America and Europe, the incidence of noninfectious aHUS has been estimated to be two per million population per year.Citation33,Citation34 Patients can present at all ages; however, the majority of new cases present in childhood, with an estimated incidence of seven per million children per year in the European Community.Citation34
Physiopathology
D+HUS
Gasser et al were the first to describe the triad of symptoms and coin the term “hemolytic-uremic syndrome” in 1955;Citation35 however, only in 1983 were Karmali et al able to identify the toxin produced by EHEC as a causative factor.Citation36,Citation37 Notwithstanding Karmali et al’s important discovery, D+HUS was reported to be associated with SD1 infectious diarrhea as early as 1975.Citation32,Citation38 The toxin isolated from stools of patients with EHEC infections and D+HUS was first named “verotoxin” due to its extremely toxic effect on Vero cells – an epithelial cell line isolated from the kidneys of an African green monkey.Citation37 Subsequently, two toxins were identified and reclassified as “Shiga-like toxins” (STX1 and STX2) due to their close similarity to the Shiga toxin produced by SD1.Citation39 The Shiga toxins are produced by pathogens in the gut and are absorbed into the blood circulation, which delivers them to various organs, including the kidneys.
The Shiga toxins are composed of 5 β-subunits that bind with high affinity to the globotriaosylceramide receptor (Gb3) and an α-subunit, which, after being internalized by cells, interferes with the ribosomal apparatus, effectively blocking protein synthesis leading to cell death.Citation39 The currently accepted mechanism leading to HUS is a TMA triggered by endothelial cell death, exposure of the sub-endothelial space, and activation of the thrombotic cascade.Citation40 Extensive microangiopathic intravascular thrombosis is responsible for the hemolysis of red cells by fragmentation, platelet consumption, and decreased glomerular perfusion leading to kidney injury.Citation40 However, in addition to a prothrombotic effect, the presence of STX in microvasculature has been shown to be vasoconstrictive, proadhesive, and proinflammatory. Recent scientific evidence suggests that the extent of endothelial cell injury observed in STX-related HUS exceeds expectations from interference with protein synthesis alone.Citation40 In vitro experiments demonstrated that bacterial lipopolysaccharide (endotoxin) acts synergistically with SD1 and EHEC toxins to enhance endothelial damage and the coagulative microangiopathy.Citation41 One of the additional emerging mechanisms for cell injury in STX-related HUS involves activation of the complement system.Citation40,Citation42 In fact, evidence involving activation of the complement system in D+HUS forms the basis for a common pathogenetic mechanism that unifies all forms of TMA, including aHUS and TTP.Citation42
Pathogens producing the STX2 toxin have been associated with more severe cases of D+HUS. E. coli O157:H7 is a pathogen that produces both forms of STX and is the most common serotype isolated from stools of D+HUS patients; however, many other serotypes have been reported to trigger D+HUS: O11:H8, Ol03-H2, O123, O26, and more.Citation23 In the most recent mega-outbreak from Germany that occurred in 2011, the responsible pathogen was E. coli 0104:H4, which previously was not known to cause large outbreaks.Citation40
aHUS
The pathogenesis of aHUS associated with invasive Streptococcus pneumoniae infections is related to the effect of the pneumococcal enzyme neuraminidase. It is postulated that by exposing the T-antigen on the surface of endothelial cells, this enzyme renders the endothelial cells vulnerable to injury by an immune response, which triggers the cascade of events leading to TMA and HUS.Citation2
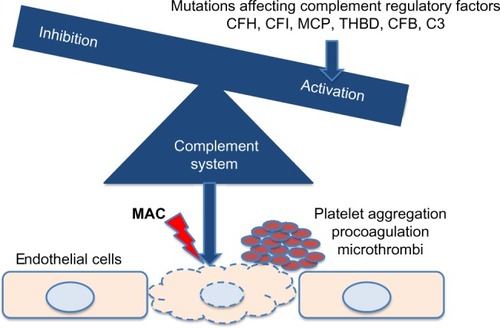
Approximately 50% of the rest of aHUS cases are caused by genetic variations of genes encoding proteins that participate in the tight control of the complement system.Citation21 As shown in , mutations in genes encoding complement factors H, I, and B, thrombomodulin, C3, and membrane cofactor protein have been reported to cause abnormal activation of the complement system.Citation3 Autoantibodies against factor H with or without associated mutations in complement factor H-related proteins have also been described.Citation3 Recently, a mutation in the gene encoding the enzyme diacylglycerol kinase ε has been shown to be responsible for aHUS in a small number of familial cases by a complement unrelated mechanism.Citation43
Figure 1 Genetic variations affecting complement factors or complement regulatory proteins cause complement activation, leading to thrombotic microangiopathy and hemolytic-uremic syndrome.
Management and prognosis
Management of EHEC infections and prevention of HUS
Gastrointestinal infection with EHEC causes one the most severe forms of gastroenteritis, associated with excruciating abdominal pain and patient discomfort; yet, in most cases, it is self-limited, resolving without specific intervention. This fact must be taken into consideration in management, and intervention that may eventually aggravate or complicate outcomes must be avoided. For example, antiperistaltic agents have been shown to increase risks for systemic complication associated with EHEC infections and should therefore be avoided.Citation44 Antibiotic treatment of EHEC infections that should also be avoided is discussed in detail in the antibiotic treatment section. Best management of patients with EHEC infections includes close monitoring for development of complications such as HUS coupled with supportive therapy. Fluid management is one of the most important components of supportive management in EHEC infections in the days before development of D+HUS. This period is characterized by diarrhea associated with nausea, vomiting, and extreme abdominal cramping pain, which are likely to lead to dehydration, intravascular volume depletion, and reduced renal perfusion. This situation can further intensify the prothrombotic environment created by STX-associated endothelial injury.Citation45,Citation46 Early volume expansion in patients affected by EHEC, implemented within the first 4 days from the onset of diarrhea, can improve renal and general outcomes of D+HUS.Citation45,Citation46 Type of fluids and way of administration should follow the general guidelines for hydration and rehydration in children with acute gastroenteritis.Citation47,Citation48
Antibiotic treatment
Antibiotic treatment of EHEC infections before and after development of D+HUS has been a subject of great interest for researchers. However, most available data were obtained from retrospective and some prospective cohort studies demonstrating conflicting results.Citation5,Citation9,Citation13,Citation49–Citation54 Prospective, blinded, and randomized studies have not been conducted, and observational studies have been affected by bias toward use of antibiotics in initially “sicker” patients. A number of meta-analyses and systematic reviews have attempted to summarize the results of available studies, reaching inconclusive results and stating that more prospective, appropriately powered studies are needed.Citation55–Citation57 Most studies found no difference or favored a negative impact of antibiotics on risk for D+HUS and outcome.Citation55,Citation56 Only one retrospective cohort analysis of a large outbreak that occurred in Japan, demonstrated benefit for using the antibiotic fosfomycin within the first 2–3 days from beginning of EHEC infection symptoms.Citation50,Citation58 The negative impact of antibiotic use on risk and outcomes of HUS is not well understood. In vitro exposure of bacteria to antibiotics induces lysis with release of toxin, which, in the gut, could potentially be more likely to be absorbed.Citation54 However, a toxin binder designed to diminish toxin absorption was not proven effective in a prospective randomized controlled trial.Citation59 Antibiotics have not been shown to directly influence interaction between toxins and their Gb3 receptor, suggesting that the antibiotic influence on risk and outcome of HUS is mediated by modulation of toxin bioavailability.Citation60–Citation62 Recently, further evidence for a possibly beneficial role of early use of antibiotics in patients with D+HUS was provided by the retrospective analysis of antibiotic use during the 2011 German mega-outbreak.Citation63 Nevertheless, based on best available evidence, the current recommendations are to avoid use of antibiotics in treatment of EHEC infections.
Shiga toxin binding and blocking agents
STX1 and STX2 are highly potent toxins in very small concentrations. Blocking STX access into the bloodstream or inactivation of blood-borne STX are major areas of interest in the search for D+HUS treatment options. SYNSORB Pk (Synsorb Biotech Inc., Calgary, AB, Canada) was a synthetic potent STX binder that was designed to bind STX in the gut, thus blocking absorption of STX; unfortunately, a large Phase III trial with this agent failed to demonstrate any benefit.Citation59
Blocking the effect of blood-borne STX with inactivating antibodies has also been attempted, and, currently, a trial with monoclonal anti-STX1 and anti-STX2 antibodies is taking place in South America.Citation64,Citation65 Nevertheless, the main challenge with STX binding and blocking agents has been timing of administration, which needs to occur before the STX effect is further amplified by a cascade of events involving the thrombotic, inflammatory, and complement systems.Citation59,Citation66
Management of D+HUS
Mortality associated with the acute phase of D+HUS has decreased over the years since first description of this syndrome, from up to 30% to less than 1% in some series.Citation18 Early recognition of symptoms followed by prompt and diligent supportive therapy is most likely responsible for this achievement. Specific supportive therapy strategies in children with D+HUS such as fluid management, renal replacement therapy and blood products transfusions, are detailed in their respective sections later in this review. A practical flowchart approach to the child at risk or presenting with HUS is shown in .
Figure 2 Practical approach to the child at risk for or presenting with HUS.
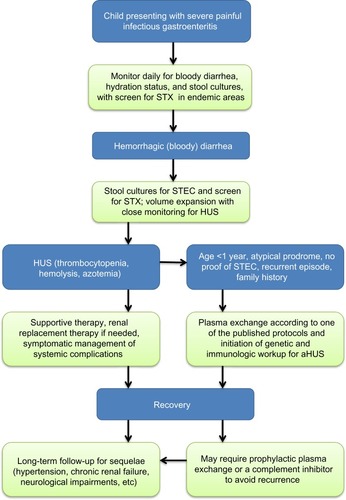
Early, effective supportive intervention depends on a high level of suspicion of D+HUS followed by diligent close monitoring for emerging HUS signs, combined with prompt and accurate diagnosis.Citation30,Citation46,Citation66 Isolation of EHEC from stool samples requires time and special culture conditions and has limited rates of success; however, new methods of screening stool samples for STX by enzyme-linked immunosorbent assay and polymerase chain reaction have greatly improved the likelihood and speed of detection.Citation67
Fluid management in D+HUS
Fluid expansion can be very beneficial and is indicated as supportive therapy for EHEC infection with gastroenteritis; however, once D+HUS develops and oligoanuria is identified, careful fluid management, including restriction, should be considered to avoid fluid overload. Fluid overload that is present at the time of initiation of urgent renal replacement therapy in critically ill children with multisystem involvement has been shown to be a bad prognostic factor.Citation68,Citation69 Among other benefits, close monitoring during the EHEC infection is important to facilitate early detection of evolving HUS and allow timely necessary adjustments to fluid management. As HUS and acute kidney injury evolve, hypertension frequently develops along with fluid and salt retention. In this situation, the general approach to management of acute kidney injury in children should be applied.Citation70–Citation72 Careful attention should be given to the type of fluids administered, aiming to minimize salt load, while total administered volume should not exceed the total of all losses such as urine output, insensible losses, ongoing diarrhea, etc. Volume required for adequate nutrition should be given priority and be included in the total fluid balance. Judicious use of loop diuretics can help manage hypertension as well as maintain adequate urine output, which can facilitate fluid management and nutrition and improve outcomes.Citation73 Additional use of antihypertensive agents may be needed to control hypertension.
Renal replacement therapy
Renal replacement therapy is required in 50%–70% of cases in the acute phase of HUS.Citation18 There are no specific indications or benefit for early initiation of acute dialysis in D+HUS.Citation66 General indications for acute renal replacement therapy in the management of acute kidney injury should be applied. Oligoanuria, severe azotemia, electrolyte abnormalities, acidosis, and need for nutritional support are among the common indications for acute dialysis.Citation30 There is no clear benefit for a specific type of renal replacement therapy; however, peritoneal dialysis has been extensively and successfully used in children with D+HUS.Citation59 Insertion of a peritoneal dialysis catheter can usually be safely performed in thrombocytopenic patients without the need for platelet transfusion, and trained staff can safely perform peritoneal dialysis on general pediatric wards.Citation74 Complications and technical failure are rare despite the severe intestinal injury caused by the EHEC infection, which is not a contraindication for peritoneal dialysis and does not interfere with the effectiveness of this modality.Citation75 Hemodialysis or continuous veno-venous hemodiafiltration are also very efficient, but require appropriate venous access and admission to an intensive care unit. Nevertheless, in patients with aHUS or in whom aHUS is suspected, hemodialysis or veno-venous hemodiafiltration may be preferred modalities since the venous access can also be used for administration of plasma exchange, which is generally indicated in these patients. Finally, despite the fact that there is no proven benefit for early renal replacement therapy, efforts should be made to start dialysis before development of fluid overload, which may be associated with a worse prognosis in a critically ill child.Citation68,Citation69
Transfusions
Platelet consumption and hemolysis are integral consequences of the TMA in HUS. The thrombocytopenia in these cases can be extreme, yet platelet transfusions are not indicated unless there is evidence of active bleeding. Transfused platelets can enhance the thrombotic events occurring in various microvasculature beds of affected patients.Citation30 Severe anemia secondary to hemolysis, on the other hand, can further destabilize patients by negative effects on the cardiovascular and respiratory systems. Erythrocyte transfusions are considered an important component of the supportive therapy in HUS, and have been reported to be required in 80% of patients.Citation7,Citation75 Use of erythropoietin early in the course of HUS has been proposed as a possible measure toward reducing the need for erythrocyte transfusions.Citation76
Treatment of aHUS – plasma exchange (PE)
Current evidence does not support use of fresh frozen plasma infusions or PE in patients with D+HUS. In contrast, PE should be instituted without delay in patients with non-pneumococcal aHUS.Citation21 Initial management of aHUS is frequently similar to D+HUS due to the infectious prodrome that typically triggers the first episode; however, once aHUS is suspected, PE treatment should be offered, given its proven efficacy in aHUS.Citation21
In a majority of non-pneumococcal aHUS cases, abnormal complement activation is responsible for the TMA, which can be efficiently controlled with daily or even twice-daily PE.Citation21,Citation77 Therefore, when aHUS is suspected, PE should be instituted immediately, as the diagnostic workup may be quite lengthy.Citation21
Complement inhibitors
Currently, the single commercially available complement inhibitor is eculizumab, a recombinant, humanized monoclonal antibody against complement component C5. This antibody effectively blocks cleavage of complement factor C5 and the formation of the complement membrane attack complex C5b-9.Citation78 Clinical trials have shown eculizumab to be more efficient than plasma therapy in prevention and treatment of aHUS episodes caused by dysregulations of the complement system.Citation78 A favorable outcome after use of eculizumab in three patients with severe D+HUS and central nervous system involvement was described in a case report.Citation79 However, a retrospective review of the extensive uncontrolled use of eculizumab, during the last large outbreak of D+HUS in Germany, did not demonstrate a benefit for patients who were treated with this agent, compared to matched patients who did not receive eculizumab.Citation63 Despite recent reports involving the complement system in the pathogenesis of D+HUS, current evidence is not sufficient to support the use of eculizumab for patients with D+HUS.Citation40,Citation42
Prognosis and long-term follow-up
The TMA underlying D+HUS is self-limited; a majority of patients with D+HUS, including children with very severe acute disease courses, experience a full recovery based on clinical and laboratory assessments.Citation16,Citation18 Nevertheless, a significant percentage are affected by long-term, mostly renal, sequelae. Unlike mortality rate, risk for long-term renal sequelae has not significantly changed over the years, remaining, on average, around 30%.Citation18,Citation80 Pneumococcal HUS is managed based on similar principles, but tends to be associated with a worse prognosis.Citation2 Children with identifiable sequelae such as hypertension, proteinuria, or impaired renal function should be treated accordingly; generally, long-term treatment with an angiotensin-converting enzyme inhibitor is indicated.Citation81,Citation82 Ideally, these patients may benefit from ongoing nephrology follow-up. However, children that seem to have made a full recovery may also benefit from long-term monitoring for development of hypertension and proteinuria, as the extent of lost nephrons during the acute phase of the disease may be significant but masked by compensation of remaining nephrons.Citation80
aHUS, particularly the familial form, has been reported to be associated with a poor prognosis; without specific treatment, patients experience relapses and ongoing disease activity leading to end-stage kidney failure in most cases.Citation77 The disease tends to recur after kidney transplantation, leading to graft loss in a majority of patients.Citation77 Nevertheless, recent advances in understanding the pathophysiology of aHUS have opened the way to new treatment protocols using PE and complement inhibitors, which are likely to significantly improve outcomes in these patients.Citation83,Citation84
Summary
HUS is a rare but very serious complication of infections with toxin-producing pathogens or genetic mutations leading to a TMA. The vast majority of cases in childhood, 90%, are caused by EHEC/Shiga toxin-producing E. coli infections. Currently, no specific treatment modalities have been shown to influence outcomes of D+HUS. Comprehensive supportive nutritional and hemodynamic management coupled with peritoneal dialysis or hemodialysis when indicated are associated with best outcomes and minimal mortality. Prevention of EHEC infections continues to be the best and most efficient strategy against D+HUS. In patients suspected of aHUS, PE should be instituted without delay and, once diagnosis is confirmed, treatment with a complement inhibitor should be considered.
Disclosure
The author reports no conflicts of interest in this work.
References
- SieglerRLThe hemolytic uremic syndromePediatr Clin North Am1995426150515298614598
- BrandtJWongCMihmSInvasive pneumococcal disease and hemolytic uremic syndromePediatrics20021102 Pt 137137612165593
- GeerdinkLMWestraDvan WijkJAAtypical hemolytic uremic syndrome in children: complement mutations and clinical characteristicsPediatr Nephrol20122781283129122410797
- BarbourTJohnsonSCohneySHughesPThrombotic microangiopathy and associated renal disordersNephrol Dial Transplant20122772673268522802583
- BellBPGoldoftMGriffinPMA multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experienceJAMA199427217134913537933395
- RileyLWRemisRSHelgersonSDHemorrhagic colitis associated with a rare Escherichia coli serotypeN Engl J Med1983308126816856338386
- BrandtJRFouserLSWatkinsSLEscherichia coli O 157:H7-associated hemolytic-uremic syndrome after ingestion of contaminated hamburgersJ Pediatr199412545195267931869
- MacDonaldIAGouldIMCurnowJEpidemiology of infection due to Escherichia coli O157: a 3-year prospective studyEpidemiol Infect199611632792848666071
- SlutskerLRiesAAGreeneKDWellsJGHutwagnerLGriffinPMEscherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic featuresAnn Intern Med199712675055139092315
- MiceliSJureMAde SaabOAA clinical and bacteriological study of children suffering from haemolytic uraemic syndrome in Tucuman, ArgentinaJpn J Infect Dis1999522333710816611
- RodrigueDCMastEEGreeneKDA university outbreak of Escherichia coli O157:H7 infections associated with roast beef and an unusually benign clinical courseJ Infect Dis19951724112211257561194
- CarterAOBorczykAACarlsonJAA severe outbreak of Escherichia coli O157:H7– associated hemorrhagic colitis in a nursing homeN Engl J Med198731724149615003317047
- OstroffSMKobayashiJMLewisJHInfections with Escherichia coli O157:H7 in Washington State. The first year of statewide disease surveillanceJAMA198926233553592661870
- PaiCHAhmedNLiorHJohnsonWMSimsHVWoodsDEEpidemiology of sporadic diarrhea due to verocytotoxin-producing Escherichia coli: a two-year prospective studyJ Infect Dis19881575105410573283256
- RowePCOrrbineELiorHRisk of hemolytic uremic syndrome after sporadic Escherichia coli O157:H7 infection: results of a Canadian collaborative study. Investigators of the Canadian Pediatric Kidney Disease Research CenterJ Pediatr199813257777829602185
- GrisaruSMidgleyJPHamiwkaLAWadeAWSamuelSMDiarrhea-associated hemolytic uremic syndrome in southern Alberta: a long-term single-centre experiencePaediatr Child Health201116633734022654544
- NathansonSKwonTElmalehMAcute neurological involvement in diarrhea-associated hemolytic uremic syndromeClin J Am Soc Nephrol2010571218122820498239
- GargAXSuriRSBarrowmanNLong-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regressionJAMA2003290101360137012966129
- NorisMCaprioliJBresinERelative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotypeClin J Am Soc Nephrol20105101844185920595690
- SalvadoriMBertoniEUpdate on hemolytic uremic syndrome: diagnostic and therapeutic recommendationsWorld J Nephrol201323567624255888
- AricetaGBesbasNJohnsonSEuropean Paediatric Study Group for HUSGuideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndromePediatr Nephrol200924468769618800230
- CummingsKCMohle-BoetaniJCWernerSBVugiaDJPopulation-based trends in pediatric hemolytic uremic syndrome in California, 1994–1999: substantial underreporting and public health implicationsAm J Epidemiol20021551094194811994234
- GriffinPMTauxeRVThe epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndromeEpidemiol Rev19911360981765120
- TarrPIHickmanROHemolytic uremic syndrome epidemiology: a population-based study in King County, Washington, 1971 to 1980Pediatrics198780141453601516
- WatersJRSharpJCDevVJInfection caused by Escherichia coli O157:H7 in Alberta, Canada, and in Scotland: a five-year review, 1987–1991Clin Infect Dis19941958348437893866
- VerweyenHMKarchHBrandisMZimmerhacklLBEnterohemorrhagic Escherichia coli infections: following transmission routesPediatr Nephrol2000141738310654337
- LopezELDiazMGrinsteinSHemolytic uremic syndrome and diarrhea in Argentine children: the role of Shiga-like toxinsJ Infect Dis198916034694752668430
- BonnetRSouweineBGauthierGNon-O157:H7 Stx2-producing Escherichia coli strains associated with sporadic cases of hemolytic-uremic syndrome in adultsJ Clin Microbiol1998366177717809620420
- KeeneWESazieEKokJAn outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meatJAMA199727715122912319103348
- TarrPIGordonCAChandlerWLShiga-toxin-producing Escherichia coli and haemolytic uraemic syndromeLancet200536594641073108615781103
- BelongiaEAOsterholmMTSolerJTAmmendDABraunJEMacDonaldKLTransmission of Escherichia coli O157:H7 infection in Minnesota child day-care facilitiesJAMA199326978838888426447
- ButlerTHaemolytic uraemic syndrome during shigellosisTrans R Soc Trop Med Hyg2012106739539922579556
- ConstantinescuARBitzanMWeissLSNon-enteropathic hemolytic uremic syndrome: causes and short-term courseAm J Kidney Dis200443697698215168377
- TaylorCMMachinSWigmoreSJGoodshipTHworking party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation SocietyClinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United KingdomBr J Haematol20101481374719821824
- GasserCGautierESteckASiebenmannREOechslinRHemolytic-uremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemiaSchweiz Med Wochenschr19558538–39905909 German13274004
- KarmaliMAPetricMLimCFlemingPCSteeleBTEscherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitisLancet198328362129913006139632
- KarmaliMASteeleBTPetricMLimCSporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stoolsLancet1983183256196206131302
- RahamanMMJamiulAlamAKIslamMRGreenoughWB3rdShiga bacillus dysentery associated with marked leukocytosis and erythrocyte fragmentationJohns Hopkins Med J1975136265701090770
- KarmaliMAInfection by verocytotoxin-producing Escherichia coliClin Microbiol Rev19892115382644022
- Petruzziello-PellegriniTNMarsdenPAShiga toxin-associated hemolytic uremic syndrome: advances in pathogenesis and therapeuticsCurr Opin Nephrol Hypertens201221443344022660553
- LouiseCBObrigTGShiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of shiga toxin and lipopolysaccharide (endotoxin) on human vascular endothelial cells in vitroInfect Immun1992604153615431548077
- NorisMMesciaFRemuzziGSTEC-HUS, atypical HUS and TTP are all diseases of complement activationNat Rev Nephrol201281162263322986360
- LemaireMFrémeaux-BacchiVSchaeferFRecessive mutations in DGKE cause atypical hemolytic-uremic syndromeNat Genet201345553153623542698
- NelsonJMGriffinPMJonesTFSmithKEScallanEAntimicrobial and antimotility agent use in persons with shiga toxin-producing Escherichia coli O157 infection in FoodNet SitesClin Infect Dis20115291130113221467017
- AkeJAJelacicSCiolMARelative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansionPediatrics20051156e673e68015930195
- HickeyCABeattieTJCowiesonJEarly volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndromeArch Pediatr Adolesc Med20111651088488921784993
- KingCKGlassRBreseeJSDugganCCenters for Disease Control and PreventionManaging acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapyMMWR Recomm Rep200352RR-1611614627948
- GuarinoAAlbanoFAshkenaziSEuropean Society for Paediatric Gastroenterology, Hepatology, and NutritionEuropean Society for Paediatric Infectious DiseasesEuropean Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in EuropeJ Pediatr Gastroenterol Nutr200846Suppl 2S81S12218460974
- DundasSToddWTNeillMATarrPIUsing antibiotics in suspected haemolytic-uraemic syndrome: antibiotics should not be used in Escherichia coli O157:H7 infectionBMJ200533075011209 author reply 120915905265
- IkedaKIdaOKimotoKTakatorigeTNakanishiNTataraKEffect of early fosfomycin treatment on prevention of hemolytic uremic syndrome accompanying Escherichia coli O157:H7 infectionClin Nephrol199952635736210604643
- PaviaATNicholsCRGreenDPHemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiologic observationsJ Pediatr199011645445512181098
- ProulxFTurgeonJPDelageGLafleurLChicoineLRandomized, controlled trial of antibiotic therapy for Escherichia coli O157:H7 enteritisJ Pediatr199212122993031640303
- SmithKEWilkerPRReiterPLHedicanEBBenderJBHedbergCWAntibiotic treatment of Escherichia coli O157 infection and the risk of hemolytic uremic syndrome, MinnesotaPediatr Infect Dis J2012311374121892124
- WongCSJelacicSHabeebRLWatkinsSLTarrPIThe risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infectionsN Engl J Med2000342261930193610874060
- PanosGZBetsiGIFalagasMESystematic review: are antibiotics detrimental or beneficial for the treatment of patients with Escherichia coli O157:H7 infection?Aliment Pharmacol Ther200624573174216918877
- SafdarNSaidAGangnonREMakiDGRisk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: a meta-analysisJAMA20022888996100112190370
- ThomasDEElliottEJInterventions for preventing diarrhea-associated hemolytic uremic syndrome: systematic reviewBMC Public Health20131379924007265
- IkedaKIdaOKimotoKPredictors for the development of haemolytic uraemic syndrome with Escherichia coli O157:H7 infections: with focus on the day of illnessEpidemiol Infect200012434334910982057
- TrachtmanHCnaanAChristenEInvestigators of the HUS-SYNSORB Pk Multicenter Clinical TrialEffect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trialJAMA2003290101337134412966125
- GrifKDierichMPKarchHAllerbergerFStrain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agentsEur J Clin Microbiol Infect Dis199817117617669923515
- KimmittPTHarwoodCRBarerMRInduction of type 2 Shiga toxin synthesis in Escherichia coli O157 by 4-quinolonesLancet199935391641588158910334263
- WalterspielJNAshkenaziSMorrowALClearyTGEffect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin IInfection199220125291563808
- MenneJNitschkeMStingeleRValidation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control studyBMJ2012345e456522815429
- BitzanMPooleRMehranMSafety and pharmacokinetics of chimeric anti-Shiga toxin 1 and anti-Shiga toxin 2 monoclonal antibodies in healthy volunteersAntimicrob Agents Chemother20095373081308719414580
- TaylorCMBitzanMReymondDShigatec: a Phase II study assessing monoclonal antibodies against Shiga toxin 1 and 2 in Shiga toxin-producing E.coli-infected childrenPediatr Nephrol201126915951596
- BitzanMTreatment options for HUS secondary to Escherichia coli O157:H7Kidney Int Suppl2009112S62S6619180140
- MeadPSGriffinPMEscherichia coli O157:H7Lancet19983529135120712129777854
- GoldsteinSLSomersMJBaumMAPediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapyKidney Int200567265365815673313
- SutherlandSMZappitelliMAlexanderSRFluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registryAm J Kidney Dis201055231632520042260
- AndreoliSPAcute kidney injury in childrenPediatr Nephrol200924225326319083019
- AndreoliSPManagement of acute kidney injury in children: a guide for pediatriciansPaediatr Drugs200810637939018998748
- BunchmanTETreatment of acute kidney injury in children: from conservative management to renal replacement therapyNat Clin Pract Nephrol20084951051418728634
- RousseauEBlaisNO’ReganSDecreased necessity for dialysis with loop diuretic therapy in hemolytic uremic syndromeClin Nephrol199034122252387099
- GrisaruSMorgunovMASamuelSMAcute renal replacement therapy in children with diarrhea-associated hemolytic uremic syndrome: a single center 16 years of experienceInt J Nephrol2011201193053921716936
- TarrPINeillMAAllenJSiccardiCJWatkinsSLHickmanROThe increasing incidence of the hemolytic-uremic syndrome in King County, Washington: lack of evidence for ascertainment biasAm J Epidemiol198912935825862916551
- PapeLAhlenstielTKreuzerMEarly erythropoietin reduced the need for red blood cell transfusion in childhood hemolytic uremic syndrome: a randomized prospective pilot trialPediatr Nephrol20092451061106419085014
- NorisMRemuzziGAtypical hemolytic-uremic syndromeN Engl J Med2009361171676168719846853
- ZuberJFakhouriFRoumeninaLTLoiratCFrémeaux-Bacchi V; French Study Group for aHUS/C3GUse of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathiesNat Rev Nephrol201281164365723026949
- LapeyraqueALMalinaMFremeaux-BacchiVEculizumab in severe Shiga-toxin-associated HUSN Engl J Med2011364262561256321612462
- RosalesAHoferJZimmerhacklLBNeed for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelaeClin Infect Dis201254101413142122412065
- CalettiMGBalestracciAMissoniMVezzaniCAdditive antiproteinuric effect of enalapril and losartan in children with hemolytic uremic syndromePediatr Nephrol201328574575023250713
- CalettiMGMissoniMVezzaniCEffect of diet, enalapril, or losartan in post-diarrheal hemolytic uremic syndrome nephropathyPediatr Nephrol20112681247125421533629
- FakhouriFFremeaux-BacchiVLoiratCAtypical hemolytic uremic syndrome: from the rediscovery of complement to targeted therapyEur J Intern Med201324649249523756030
- NorisMRemuzziGManaging and preventing atypical hemolytic uremic syndrome recurrence after kidney transplantationCurr Opin Nephrol Hypertens201322670471224076560
