Abstract
Introduction
Secondary hyperparathyroidism develops in nearly all patients with end-stage renal disease. Parathyroidectomy is often performed when medical therapy fails. The most common postoperative complication, hungry bone syndrome (HBS), requires early recognition and treatment.
Materials and methods
A total of 84 patients who underwent parathyroidectomy because of secondary hyperparathyroidism were investigated. Detailed analysis of laboratory parameters (calcium, phosphate, parathyroid hormone, hemoglobin, and urea levels) and baseline characteristics (age at time of surgery, duration of renal replacement therapy, and medication) was performed to detect preoperative predictors for the development of HBS.
Results
Average overall follow-up of the cohort was 4.7 years. Within this time frame, 13 of 84 patients had to undergo a second surgery because of recurrent disease, and HBS occurred in 51.2%. Only decreased preoperative calcium levels and younger age at time of surgery were significant predictors of HBS. Minimal levels of calcium were detected 3 weeks after surgery. Preoperative vitamin D therapy could not prevent HBS and could not shorten the duration of intravenous calcium supplementation.
Conclusion
HBS is a very common complication after parathyroidectomy. Younger patients and patients with low preoperative calcium levels were at higher risk for the development of HBS. Remarkably, preoperative vitamin D therapy could not prevent HBS and had no impact on the length of intravenous calcium supplementation. Intensive monitoring of calcium levels must be performed for at least 3 weeks after surgery.
Introduction
In patients with chronic kidney disease (CKD), secondary hyperparathyroidism (sHPT) is a very common complication; sHPT will ultimately develop in approximately all patients with end-stage renal disease (ESRD).Citation1 Three major factors influence stimulation of the parathyroid gland in patients with chronic renal failure: diminished 1,25-dihydroxyvitamin D levels, hypocalcemia, and hyperphosphatemia. Therefore, medical management of sHPT in patients with CKD includes the use of phosphate binders, active vitamin D analogs, and/or calcimimetics.Citation2,Citation3 Management of sHPT is based on recommendations from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative for the evaluation and management of CKD with mineral and bone disorder.Citation4 The number of patients requiring parathyroidectomy because of severe sHPT ranges from 2.6%–40% in the end-stage renal disease population.Citation5–Citation9 In patients with severe hyperparathyroidism and medical treatment failure, parathyroidectomy is the safest treatment option. Efficacy rates are favorable in the hands of an experienced surgeon.Citation10,Citation11
Hungry bone syndrome (HBS) occurs in up to 95% of patients and is the most common complication presenting immediately after parathyroidectomy.Citation1,Citation5,Citation11,Citation12 HBS is characterized by a precipitous postoperative decrease in the plasma concentrations of calcium and phosphorus causing tetany, seizures, cardiac arrhythmias, muscle cramps, weakness, and headache.Citation5,Citation13 Preoperative risk factors for the development of HBS are controversial in the current literature.Citation1,Citation5,Citation14
In everyday clinical practice, it would be helpful to predict which subpopulation of patients undergoing parathyroidectomy because of severe sHPT might be at high risk for HBS. Therefore, we analyzed data from a large cohort of patients who underwent parathyroidectomy because of severe sHPT to detect preoperative predictors for the development of HBS.
Materials and methods
Patients
Our present study included 84 patients who underwent parathyroidectomy because of sHPT between January 1, 1995, and April 30, 2005, in our hospital.
Data collection included patient sex; age at the time of surgery; and operative-, laboratory-, medication-, and dialysis-related data.
The following laboratory parameters were evaluated in all patients from the medical charts preoperative and, for multiple time points, postoperatively: hemoglobin, urea, serum creatinine, calcium, phosphate, and intact parathormone.
In addition, we collected detailed information about medication. Daily doses of vitamin D, phosphate-binding drugs (sevelamer hydrochloride, calcium acetate, and algeldrate), and antihypertensive drugs were obtained preoperatively and postoperatively.
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee of the University of Tuebingen.
Diagnosis
All patients with the diagnosis of sHPT were receiving renal replacement therapy (hemodialysis or peritoneal dialysis) or had a functioning transplant. The diagnosis of sHPT was made according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative clinical practice guidelines for bone metabolism and disease in CKD.Citation4
Surgical procedure
Parathyroidectomy was performed in patients with symptomatic sHPT that was refractory to medical therapy. All patients underwent bilateral neck exploration with an attempt to identify all parathyroid glands. When four or more glands could be detected at the time of surgery, total parathyroidectomy with or without forearm autotransplantation or subtotal parathyroidectomy was performed at the discretion of the surgeon. If fewer than four glands were detected, all other identifiable parathyroid glands were removed. In this setting, thymectomy was not performed routinely.
HBS
HBS is defined as the requirement of intravenous calcium supplementation to control serum calcium levels postoperatively after parathyroidectomy, despite optimization of supportive therapy (for example, vitamin D, oral calcium supplementation). When intravenous calcium supplementation was administered to the patient, duration of supplementation was documented.
Statistical analysis
Continuous data were expressed as mean ± standard deviation and percentages. Median with interquartile range was used where distribution was not normal. We processed all data using the software program GraphPadPrism version 5 (GraphPad Software, Inc, La Jolla, CA, USA). We made comparisons between different groups using Fisher’s exact test. The level of significance was defined as P < 0.05; a high level of significance was defined as P < 0.01, and a highly significant level was defined as P < 0.001.
Results
Demographics, baseline characteristics, and surgical procedures
Between January 1995 and April 2005, a total of 84 patients who underwent parathyroidectomy because of sHPT were identified in our nephrology and surgery department. During work-up of these patients, sufficient data were given in all patients.
There were 42 female and 42 male patients with a median age of 50.9 years (age range, 20–75 years). There was no predominant diagnosis concerning the underlying disease leading to chronic renal failure in these patients. Total parathyroidectomy was performed in 78 (92.9%) of 84 patients with sHPT. In 74 of these patients, autotransplantation was performed. Subtotal parathyroidectomy was performed in six patients (7.1%). At the time of surgery, 55 patients were receiving hemodialysis, 23 were receiving peritoneal dialysis, and six had a functioning transplant. Time from renal replacement therapy to surgery was 5.5 years (range, 1 month to 15 years), and 42 patients received vitamin D preoperatively. In our study, median follow-up time was 4.7 years after parathyroidectomy; baseline characteristics of the study population are shown in .
Table 1 Clinical data of study population
Postoperative course
Parathyroid hormone (PTH) levels decreased dramatically after surgery (136.1 ± 81.7 pmol/L preoperatively to 4.8 ± 7.4 pmol/L within 1 week after surgery; P < 0.0001) (). Within the first 3 years after surgery, PTH levels were significantly lower compared with preoperative PTH levels (1 year: 50.0 ± 73.2 pmol/L; 2 years: 100.8 ± 191.4 pmol/L; 3 years: 98.1 ± 142 pmol/L; all P < 0.05). Remarkably PTH levels were higher 4 years after surgery compared with preoperative PTH levels (164.0 ± 304.6 pmol/L at 4 years after surgery; P > 0.05).
Figure 1 Change in serum calcium levels after parathyroidectomy.
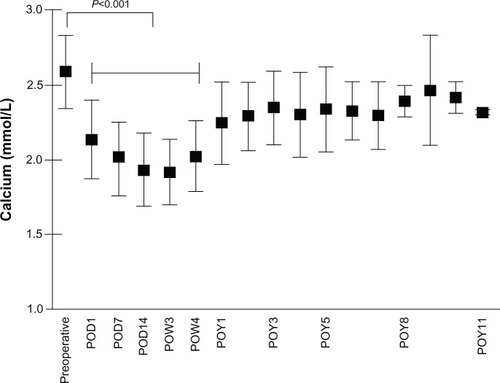
Analogous with the course of PTH levels, serum calcium levels decreased postoperatively from 2.6 ± 0.24 mmol/L to 2.1 ± 0.26 mmol/L (P < 0.0001) on postoperative day 1. All patients undergoing surgical treatment of sHPT received a high-calcium dialysis bath on the first postoperative day. Minimal calcium levels were found 3 weeks after surgery at 1.92 ± 0.22 mmol/L ().
Figure 2 Change in PTH levels after parathyroidectomy.
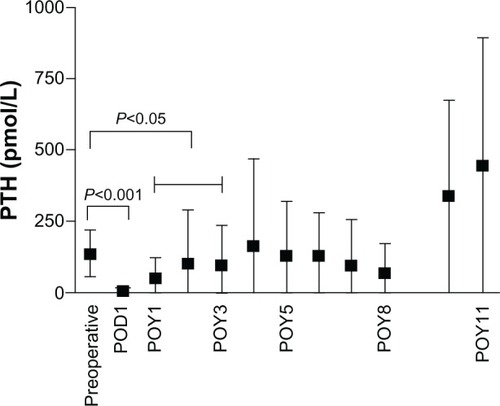
Regarding phosphate levels, there was a decrease after surgery compared with preoperative phosphate levels from 1.9 ± 0.59 mmol/L to 1.34 ± 0.44 mmol/L (P < 0.0001), but at 1 year after parathyroidectomy, phosphate levels had already reached levels higher than baseline (1.95 ± 0.62 mmol/L). Within the next few years after surgery, phosphate levels remained stable.
The number of patients requiring vitamin D increased after surgery from 42 patients to 78 patients. Analogically, daily doses of vitamin D increased from 1.09 ± 0.82 μg/day to 2.42 ± 1.51 μg/day (P < 0.01). Regarding long-term follow-up, the required doses of vitamin D decreased significantly at 1 to 6 years after surgery compared with preoperative doses (all P < 0.05). Within 4 years after surgery, the number of patients requiring phosphate-binding drugs was reduced. In addition, the doses of sevelamer, calcium acetate, and algeldrate decreased within 4 years after surgery (P < 0.05, P > 0.05, and P > 0.05, respectively). Concerning the use of antihypertensive drugs, no differences could be detected between preoperative and postoperative doses of angiotensin-converting enzyme inhibitors (P=0.96), angiotensin 2 receptor antagonists (P=0.29), beta-blockers (P=0.90), calcium antagonists (P=0.86), and diuretics (P=0.99).
Average overall follow-up in the entire group was 4.7 years. Within this time frame, 13 of 84 patients had to undergo a second surgery because of recurrent HPT. In all of these patients, total parathyroidectomy was done initially (eleven patients with autotransplantation, two patients without autotransplantation). In six patients, recurrent graft-HPT was identified as the origin of recurrent HPT, whereas in another six patients, a second neck exploration was necessary. In one patient, both recurrent graft-HPT and adenoma of the residual parathyroid gland could be detected.
HBS
HBS was defined as the requirement of intravenous calcium supplementation to control serum calcium levels postoperatively after parathyroidectomy, despite optimization of supportive therapy (for example, vitamin D, oral calcium supplementation, dialysis calcium). Overall, HBS developed in 43 (51.2%) of 84 patients. There were 20 female and 23 male patients.
In the patients who went on to have HBS, 26 patients were treated with vitamin D preoperatively, whereas 17 patients received no vitamin D preoperatively. In the group of patients who did not experience HBS, 19 patients received vitamin D, and the remaining 20 patients were not treated with vitamin D.
Within the group of patients who experienced HBS after surgery, the duration of intravenous calcium supplementation was independent of whether the patients received vitamin D before surgery (intravenous calcium supplementation, 7.8 ± 9.0 days versus 6.8 ± 7.3 days; P=0.07). In addition, no differences could be detected between serum calcium levels in the HBS group who received vitamin D therapy before surgery compared with the HBS group who did not receive vitamin D therapy on postoperative days 1 (P=0.9), 7 (P=0.54), and 14 (P=0.93).
During work-up, several preoperative variables including calcium, phosphate, PTH, hemoglobin, and urea levels were investigated regarding prediction of the development of HBS. Furthermore, age at the time of surgery and duration of renal replacement therapy were analyzed as possible risk factors. It is interesting to note that only decreased preoperative calcium levels and younger age at the time of surgery were significant predictors of HBS (P=0.04 and P=0.03, respectively). No other preoperative variables were significant predictors of HBS ().
Table 2 Clinical data of patients with and without HBS
Discussion
In our present study, we analyzed data from 84 patients who underwent parathyroidectomy because of refractory sHPT in a referral center in Germany. Preoperative parameters were investigated to predict which subpopulation of patients might be at high risk for the development of HBS during short-term follow-up.
Our study population consisted of 84 patients (42 women and 42 men). The mean age at the time of initiation of renal replacement therapy was 45.9 years, and time from renal replacement therapy to surgery was 5.5 years. There was no predominance of underlying kidney disease, but interestingly, only two of 84 patients had diabetic nephropathy. These baseline characteristics of our study population are in line with the results of Malberti et al,Citation7 who found an admission age between 18 and 54 years, duration of renal replacement therapy of 66 ± 43 months, and nondiabetic nephropathy as underlying renal disease.
Concerning the long postoperative period, the recurrence rates of 15% during a long follow-up of 4.2 years after parathyroidectomy are in line with previous reports.Citation11,Citation15,Citation16 It is noteworthy that, and stays in contrast to another study,Citation14 total parathyroidectomy with autotransplantation was performed in 78 of 84 patients in our study, and no recurrent disease was seen in the group of patients who underwent subtotal parathyroidectomy as the first surgical procedure. Up to now, no randomized trials have compared these three techniques (total parathyroidectomy with or without autotransplantation and subtotal parathyroidectomy), but most surgeons have performed subtotal parathyroidectomy or total parathyroidectomy with autoimplantation. The “procedure of choice” for the treatment of sHPT is different among surgeons.Citation17,Citation18 Nevertheless, both procedures seem to be extremely successful in controlling the hyperparathyroidism in short-term and long-term follow-up.Citation19
After surgery, a rapid shift of calcium from the bloodstream to bone occurs, which is caused by abrupt removal of elevated PTH levels.Citation20,Citation21 The symptoms of hypocalcemia range from mild symptoms, including weakness, to severe symptomatic hypocalcemia with seizures and cardiac arrhythmias.Citation5,Citation13 Postoperative hypocalcemia is a potentially life-threatening event and results in a prolonged hospital stay, including admission to intermediate or intensive care units.Citation12 As expected, PTH levels decreased significantly postoperatively in our study population. Analogous with the course of PTH levels and in line with recent studies,Citation12,Citation22 serum calcium levels had decreased significantly after surgery. Nevertheless, it is noteworthy that minimal levels of calcium were detected 3 weeks after surgery, whereas a previous study stated minimal calcium levels 9 days after surgery.Citation12 These findings underline the importance of intensive “long-term” monitoring after parathyroidectomy.
Medical management of hypocalcemia yields an increase in costs for the medical health care system. Therefore, we investigated the incidence of HBS and performed an analysis to predict which patients are at high risk for the development of HBS after parathyroidectomy. The incidence of HBS in our study (51.2%) was above the range quoted in some reportsCitation11,Citation14 but was equal to the results from Viaene et al.Citation12 Comparisons among the different studies seem to be impossible because of varying definitions, as well as different presurgical and postsurgical protocols including medications and investigated parameters.Citation1,Citation2,Citation11,Citation12,Citation14 We defined HBS as the requirement for intravenous calcium supplementation to control serum calcium levels postoperatively.
In a first step, we attempted to detect preoperative parameters, which are associated with an increased risk for the development of HBS. Parameters included calcium, phosphate, PTH, hemoglobin, and urea levels. In addition, age at the time of surgery and duration of renal replacement therapy were analyzed as predictors for HBS. Interestingly, only lower preoperative calcium levels and younger age at the time of surgery were significant predictors of HBS. Age as a risk factor for the development of HBS still remains controversial; younger age at the time of surgery as a risk factor for HBS has been reported previously,Citation5,Citation12,Citation14 but other studies have stated that older patients are at higher risk for the development of HBS.Citation20,Citation25 Our data suggest that younger patients should be monitored more closely; however, explanations as to why younger patients are at a higher risk for HBS are still missing. On the other hand, in patients with primary hyperparathyroidism, advanced age has been associated with postoperative hypocalcemia.Citation20,Citation23,Citation24 These studies suggested that vitamin D deficiency and poorer nutritional intake in older patients promote a higher risk for the development of HBS. At this time point, it still remains unclear why younger age constitutes a risk factor for the development of HBS. It could be hypothesized that the increases in bone formation and osteoblasts after parathyroidectomy are more pronounced in younger versus older patients. Additional larger studies need to be conducted to support this finding.
Analogous with the study by Goldfarb et al,Citation14 and in contrast to previous studies,Citation12,Citation25 neither phosphate, PTH, hemoglobin, or urea levels preoperatively, nor the duration of renal replacement therapy were significant predictors of HBS in our study. In addition, we could identify lower preoperative calcium levels as a significant predictor of HBS, which was recently reported in a study from Torer et al,Citation1 including 36 patients who were receiving hemodialysis.
Furthermore, we investigated the role of preoperative vitamin D therapy on postoperative serum calcium levels, the duration of intravenous vitamin D supplementation, and the incidence of HBS. Regarding the risk for HBS, no differences were observed between patients who received vitamin D preoperatively compared with patients who were not treated with vitamin D before surgery. No differences could be detected between serum calcium levels in the HBS group with vitamin D therapy before surgery compared with the HBS group without vitamin D, and preoperative vitamin D therapy showed no differences on the duration of intravenous calcium supplementation. Our data provide no explanation for the recommendation of preoperative use of vitamin D to avoid HBS.Citation21
We also investigated whether there was a decrease in prescriptions of the most common antihypertensive drugs (angiotensin-converting enzyme inhibitors, angiotensin 2 receptor antagonists, beta-blockers, calcium antagonists, and diuretics) after parathyroidectomy. We could not detect statistically significant differences between preoperative and postoperative doses during long-term follow-up in our patient cohort. Some case series,Citation26 as well as data from Kestenbaum et alCitation27 have reported significant decreases in blood pressure in patients after parathyroidectomy.
It is noteworthy that none of the included patients in our study was treated with calcimimetics before surgery. Whether the use of calcimimetics before surgery had affected the rates of HBS needs to be elucidated.
Study limitations
We acknowledge that our study had several limitations, which are mainly related to its retrospective nature. The major strengths of this study were the number of included patients, the detailed analysis of preoperative medications, and the investigation of many risk factors within a single patient cohort.
Conclusion
In conclusion, parathyroidectomy can be performed in patients with severe HPT with an acceptable outcome when medical therapy has failed. Postoperative hypocalcemia is a very common complication after parathyroidectomy. Younger patients and patients with low preoperative calcium levels are at higher risk for the development of HBS. It is noteworthy that minimal levels of calcium were detected 3 weeks after surgery, which underlines the importance of intensive “long-term” monitoring of patients up to 3 weeks after surgery. Preoperative vitamin D therapy could not prevent HBS and had no impact on the length of intravenous calcium supplementation.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorerNTorunDTorerNPredictors of early postoperative hypocalcemia in hemodialysis patients with secondary hyperparathyroidismTransplant Proc20094193642364619917359
- ChengSPLiuCLChenHHLeeJJLiuTPYangTLProlonged hospital stay after parathyroidectomy for secondary hyperparathyroidismWorld J Surg2009331727918953601
- GoodmanWGRecent developments in the management of secondary hyperparathyroidismKidney Int20015931187120111231381
- National Kidney FoundationK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratificationAm J Kidney Dis2002392 Suppl 1S1S26611904577
- MittendorfEAMerlinoJIMcHenryCRPost-parathyroidectomy hypocalcemia: incidence, risk factors, and managementAm Surg2004702114119 discussion 119–12015011912
- FoleyRNLiSLiuJGilbertsonDTChenSCCollinsAJThe fall and rise of parathyroidectomy in US hemodialysis patients, 1992 to 2002J Am Soc Nephrol200516121021815563573
- MalbertiFMarcelliDConteFLimidoASpottiDLocatelliFParathyroidectomy in patients on renal replacement therapy: an epidemiologic studyJ Am Soc Nephrol20011261242124811373348
- CunninghamJDaneseMOlsonKKlassenPChertowGMEffects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidismKidney Int20056841793180016164656
- KestenbaumBSeligerSLGillenDLParathyroidectomy rates among United States dialysis patients: 1990–1999Kidney Int200465128228814675061
- LocatelliFCannata-AndíaJBDrüekeTBManagement of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasis on the control of hyperphosphataemiaNephrol Dial Transplant200217572373111981055
- JofréRLópezGóméz JMMenárguezJParathyroidectomy: whom and when?Kidney Int Suppl200385S97S10012753276
- ViaeneLEvenepoelPBammensBClaesKKuypersDVanrenterghemYCalcium requirements after parathyroidectomy in patients with refractory secondary hyperparathyroidismNephron Clin Pract20081102c80c8518758187
- StricklandPLRecabarenJAre preoperative serum calcium, parathyroid hormone, and adenoma weight predictive of postoperative hypocalcemia?Am Surg200268121080108212516813
- GoldfarbMGondekSSLimSMFarraJCNoseVLewJIPostoperative hungry bone syndrome in patients with secondary hyperparathyroidism of renal originWorld J Surg20123661314131922399154
- ShihMLDuhQYHsiehCBTotal parathyroidectomy without autotransplantation for secondary hyperparathyroidismWorld J Surg200933224825418958522
- GasparriGCamandonaMAbbonaGCSecondary and tertiary hyperparathyroidism: causes of recurrent disease after 446 parathyroidectomiesAnn Surg20012331656911141227
- CattanPHalimiBAïdanKReoperation for secondary uremic hyperparathyroidism: are technical difficulties influenced by initial surgical procedure?Surgery2000127556256510819065
- RothmundMWagnerPKScharkCSubtotal parathyroidectomy versus total parathyroidectomy and autotransplantation in secondary hyperparathyroidism: a randomized trialWorld J Surg19911567457501767541
- GagnéERUreñaPLeite-SilvaSShort- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patientsJ Am Soc Nephrol199234100810171450363
- BrasierARNussbaumSRHungry bone syndrome: clinical and biochemical predictors of its occurrence after parathyroid surgeryAm J Med19888446546603400660
- CruzDNPerazellaMABiochemical aberrations in a dialysis patient following parathyroidectomyAm J Kidney Dis19972957597629159312
- MazzaferroSChiccaSPasqualiMChanges in bone turnover after parathyroidectomy in dialysis patients: role of calcitriol administrationNephrol Dial Transplant200015687788210831645
- FareseSThe hungry bone syndrome – an updateTher Umsch2007645277280 German [with English abstract]17685087
- ErbilYBozboraAOzbeyNPredictive value of age and serum parathormone and vitamin d3 levels for postoperative hypocalcemia after total thyroidectomy for nontoxic multinodular goiterArch Surg2007142121182118718086985
- ErbilYBarbarosUTemelBThe impact of age, vitamin D(3) level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomyAm J Surg2009197443944619324110
- GoldsmithDJCovicAAVenningMCAckrillPBlood pressure reduction after parathyroidectomy for secondary hyperparathyroidism: further evidence implicating calcium homeostasis in blood pressure regulationAm J Kidney Dis19962768198258651246
- KestenbaumBAndressDLSchwartzSMSurvival following parathyroidectomy among United States dialysis patientsKidney Int20046652010201615496173