Abstract
Aim
The objective of the study reported here was to describe dose equivalence and hemoglobin (Hb) stability in a cohort of unselected hemodialysis patients who were switched simultaneously from epoetin alfa to darbepoetin alfa.
Methods
This was a multicenter, observational, retrospective study in patients aged ≥18 years who switched from intravenous (IV) epoetin alfa to IV darbepoetin alfa in October 2007 (Month 0) and continued on hemodialysis for at least 24 months. The dose was adjusted to maintain Hb within 1.0 g/dL of baseline.
Results
We included 125 patients (59.7% male, mean [standard deviation (SD)] age 70.4 [13.4] years). No significant changes were observed in Hb levels (mean [SD] 11.9 [1.3] g/dL, 12.0 [1.5], 12.0 [1.5], and 12.0 [1.7] at Months −12, 0, 12 and 24, respectively, P=0.409). After conversion, the erythropoiesis-stimulating agent (ESA) dose decreased significantly (P<0.0001), with an annual mean of 174.7 (88.7) international units (IU)/kg/week for epoetin versus 95.7 (43.4) (first year) and 91.4 (42.7) IU/kg/week (second year) for darbepoetin (65% and 64% reduction, respectively). The ESA resistance index decreased from 15.1 (8.5) IU/kg/week/g/dL with epoetin to 8.1 (3.9) (first year) and 7.9 (4.0) (second year) with darbepoetin (P<0.0001). The conversion rate was 354:1 in patients requiring high (>200 IU/kg/week) doses of epoetin and 291:1 in patients requiring low doses.
Conclusion
In patients on hemodialysis receiving ESAs, conversion from epoetin alfa to darbepoetin alfa was associated with an approximate and persistent reduction of 65% of the required dose. To maintain Hb stability, a conversion rate of 300:1 seems to be appropriate for most patients receiving low doses of epoetin alfa (≤200 IU/kg/week), while 350:1 would be better for patients receiving higher doses.
Introduction
Anemia is the complication that has the greatest impact on perceived quality of life in patients with chronic kidney disease on hemodialysis. Therapy with erythropoiesis-stimulating agents (ESAs) significantly reduces the need for transfusions, hospital admissions, and overall mortality.Citation1–Citation4
“Epoetin alfa” is a recombinant erythropoietin with the same amino acid sequence as human erythropoietin (EPO).Citation5 Subcutaneous (SC) administration has a higher therapeutic effect than intravenous (IV) administration, because the effect of epoetin alfa on erythropoiesis depends more on peak levels than on through levels.Citation5,Citation6 However, it is almost exclusively used intravenously in hemodialysis patients to avoid the production of anti-EPO antibodies.Citation2
In comparison to epoetin alfa, darbepoetin alfa contains an increased amount of sialic acid-containing carbohydrate chains.Citation7–Citation9 It shows a much longer elimination half-life than epoetin alfa, with prolonged erythropoietic effect.Citation10–Citation15 Another peculiarity is the equivalence of IV and SC dosing requirements.Citation1,Citation10,Citation16 The initially recommended conversion factor was 200 international units (IU) of epoetin alfa per 1 μg of darbepoetin alfa.Citation12 However, several studies have found that, after switching from epoetin alfa to darbepoetin alfa, a mean dose reduction of between 17% and 39% is required to maintain stable hemoglobin (Hb) levels.Citation3,Citation17–Citation23 Further, there is wide inter-patient variability in the conversion rate.Citation23,Citation24 Despite the fact that label instructions remain unchanged, all these observations have resulted in nephrologists using a conversion rate of 250:1 in clinical practice.Citation20–Citation23
Due to a decision of the Central Purchasing Center of the Basque Health Service, Spain, the type of ESA was changed simultaneously for all hemodialysis patients in the area. The present study aimed to assess the effect on Hb levels and dose requirements of switching from epoetin alfa to darbepoetin alfa in a cohort of unselected hemodialysis patients.
Materials and methods
We performed an observational, retrospective study of ten dialysis units in the Basque Country, Spain, with data collected from March to May 2011. The main inclusion criteria were patients aged ≥18 years who switched from epoetin alfa to darbepoetin alfa in October 2007 (index date) who had been on hemodialysis for at least 15 months before the index date and remained on hemodialysis for at least 24 months after switching. Only patients with active neoplasia or bleeding were excluded. The study was conducted in accordance with the Helsinki Declaration and the guidelines for Good Clinical Practice. The study protocol was approved by the ethics committee in each participating center. Institutional review board/ethics committee approval was obtained for experimental investigation on human subjects.
The date of switch was considered the index date (Month 0). The conversion rate was left to the discretion of each nephrologist. Both ESAs were administered intravenously using pre-filled syringes. The administration frequency of epoetin alfa was 2–3 times/week and darbepoetin alfa was administered once or twice weekly (two times/week for doses >80 μg/week). Hb was measured monthly, and the ESA dose was adjusted to maintain Hb within 1.0 g/dL of the baseline value. All patients received IV iron 100 mg/month, except if ferritin levels exceeded 600 mg/dL. If ferritin levels decreased to below 200 mg/dL, the dose of IV iron was increased to 100 mg/week. The target for ferritin was 300–500 mg/dL.
The retrospective follow-up period was 36 months: −12 and +24 months relative to the index date. We collected data on Hb, ESA dose, iron dose, weight, bleeding, transfusion of red blood cells, quality of hemodialysis water, serum ferritin, transferrin saturation index (TSI), and C-reactive protein (CRP). Aluminum levels were monitored twice a year. Charlson index was collected annually. Only adverse reactions leading to the discontinuation of darbepoetin alfa were collected.
Statistical analysis
All ESA doses were converted to IU/kg/week using the 200:1 conversion rate. The erythropoiesis-stimulating agent resistance index (ERI) was calculated by dividing the ESA dose by the Hb level.Citation22
Changes from baseline at post-baseline visits were evaluated using paired t-test, Wilcoxon signed-rank test, or McNemar’s test as appropriate. Changes in continuous variables over time were evaluated using repeated-measures analysis of variance. Univariate and multivariate linear regression models were used to investigate the influence of several variables on the ERI. Statistical analyses were performed using SAS software (v 9.2; SAS Institute, Cary, NC, USA).
Results
Patients
There were 242 prevalent hemodialysis patients at the index date. Of these, 125 were included in the study (67 were excluded due to kidney transplant, 17 due to change to peritoneal dialysis, three due to active neoplasia or bleeding, and 30 due to death).Citation25
Patient characteristics are summarized in . The main comorbidities were hypertension (82.4%), peripheral vascular disease (21.6%), diabetes with end organ damage (20.8%), cerebrovascular disease (17.6%), chronic pulmonary disease (13.6%), arrhythmia (12.8%), coronary disease (12.8%), myocardial infarction (10.4%), and congestive heart failure (10.4%).
Table 1 Population characteristics at the time of switch from epoetin alfa to darbepoetin alfa
The mean dry weight, Charlson index, and TSI levels remained unchanged after conversion. Ferritin and CRP levels showed a moderate but significant increase over time. The bacterial content and aluminum levels in the water for hemodialysis decreased ().
Table 2 Changes in patient characteristics and hemodialysis water over time
Hb levels over time
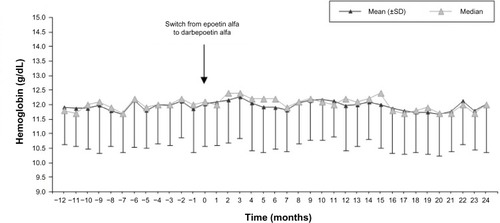
No significant changes in mean and median monthly values of Hb were observed through the follow-up period (mean [SD] 11.9 [1.3] g/dL at Month −12, 12.0 [1.5] at Month 0, 12.0 [1.5] at Month 12, 12.0 [1.7] at Month 24, P=0.409; ). Only a slight, nonsignificant increase in mean Hb levels was observed within the first 3 months after the switch (12.3 [1.4] g/dL at Month 3), which quickly returned to prior levels (12.1 [1.6] g/dL at Month 4, 11.9 [1.4] g/dL at Month 6) after minor dose adjustments during the first 2 months of darbepoetin alfa ().
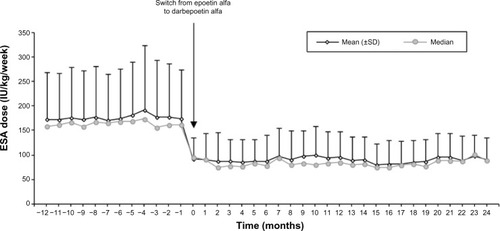
Figure 2 Changes in erythropoiesis-stimulating agent (ESA) dose over time.
Abbreviation: SD, standard deviation.
Patients were on target levels (10–12 g/dL) for a mean (SD) of 4 (3) months with epoetin alfa and 8 (5) months with darbepoetin alfa, which represents 42.6% (25.2) and 44.2% (21.0) of the time, respectively (P=0.616). The coefficient of variation (SD/mean*100) of the Hb levels did not change between the two periods: 11.4% (95% confidence interval [CI] 10.8%–12.0%) with epoetin alfa; 11.9% (95% CI 11.4%–12.4%) with darbepoetin alfa.
No significant changes over time were observed in the number of bleedings or transfusions (data not shown). The percentage of patients suffering a bleeding episode or requiring a blood transfusion was <1% in all months.
ESA doses over time
The ESA dose with epoetin alfa did not show significant variation in the year prior to the switch (annual mean of 174.7 [88.7] IU/kg/week [54.1 (29.8) μg/week]; and ). After conversion, the ESA dose decreased abruptly and significantly (P<0.0001), and, from then on, remained unchanged. Between Months 0 and 12, the mean dose was 95.7 (43.4) IU/kg/week (23.3 [12.5] μg/week). A slight but nonsignificant additional decrease was observed during the second year (mean 91.4 [42.7] IU/kg/week; ). The conversion rate at baseline for the overall group was 332:1.
Table 3 Absolute and relative changes in erythropoiesis-stimulating agent (ESA) dose and erythropoiesis-stimulating agent resistance index (ERI)
Resistance index
The ERI decreased from an annual mean of 15.1 (8.5) IU/kg/week/g/dL with epoetin alfa to 8.1 (3.9) during the first year and 7.9 (4.0) during the second year with darbepoetin alfa ( and ).
Figure 3 Changes in erythropoiesis-stimulating agent (ESA) resistance index over time.
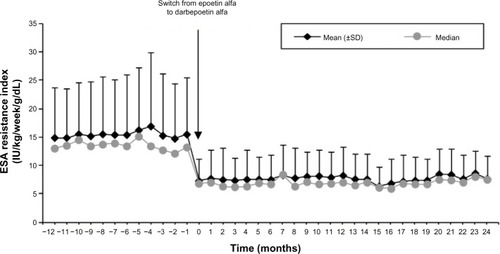
No significant relationship with the ERI was observed for iron dose, quality of hemodialysis water, or CRP levels (data not shown). The Charlson index was the only variable significantly associated with ERI (P=0.007), independently from the type of ESA used.
Results according to baseline ESA dose
There were significant differences in the reduction of ESA dose between subgroups defined by their baseline dose: patients requiring high doses had a greater reduction than patients on medium or low doses (). The resulting conversion rate was 291:1 for patients on low doses of epoetin alfa (≤200 IU/kg/week) and 354:1 for patients requiring doses >200 IU/kg/week.
Safety
There were no discontinuations of darbepoetin alfa due to adverse reactions during the follow-up period.
Discussion
The present study analyzed the switch from epoetin alfa to darbepoetin alfa in hemodialysis patients. After the switch, the mean ESA dose decreased by 65%, and this reduction was maintained during the subsequent 2 years, without noticeable changes in Hb levels. Thus, an approximately double efficiency of darbepoetin alfa treatment was observed. Further, the median values of ESA dose and ERI were much closer to the mean values after the switch. These findings support that there are more “resistant” patients with epoetin alfa,Citation17 in whom the conversion rate would be even higher than 350:1.
Our results are consistent with those from randomized and controlled trials;Citation3,Citation26 however, the dose reduction was higher in our study, which could be related to the higher proportion of individuals requiring high ESA doses. In prior studies, there was a gradual reduction in the dose of ESA in the first year after conversion due to progressive dose adjustments in response to Hb levels above targets. In our experience, using a conversion rate of about 330:1, we achieved the early stabilization of Hb and dose of darbepoetin alfa.
The quality of water for hemodialysis improved during the study, from baseline values fulfilling requirements of “pure water” to final values close to those for “ultrapure water”.Citation27,Citation28 A good hemodialysis water quality can decrease the ERI.Citation29–Citation31 However, the bacterial and aluminum contents with epoetin alfa were already very low relative to the maximum levels allowed for pure water (<100 CFU/mL and <10 μg/L, respectively).Citation27,Citation28 Also, we did not observe any trend toward improvement in inflammatory parameters (CRP and ferritin levels).
In our population, the conversion rate was higher in patients with relative erythropoietin resistance. These results differ from an Italian study that found the same rate irrespective of the previous dosage of epoetin alfa.Citation32 However, these results are similar to those reported by Bock et al: from a conversion factor of around 200:1 in patients using lower doses (<5,000 IU/week) to more than 300:1 at doses of >10,000 IU/week.Citation23 In that study, only 49% of patients used IV epoetin alfa. An explanation for the differences between the conversion factors at different doses was proposed by Nissenson,Citation17 who suggested that epoetin loses some efficacy at high doses. This effect would be related to the use of epoetin in weekly or less frequent schedules.Citation33
Our study design did not allow for the detailed analysis of within-subject variability. The monthly stability of Hb levels was comparable, and achieved a mean of 8 months during the 2 years of treatment with darbepoetin alfa. This also raises the possibility of reducing the frequency of Hb checks. Hb stability was accompanied by an increased ESA dose stability.
Regarding between-subject variability, we did not find any difference in the coefficient of variation of Hb levels between epoetin alfa and darbepoetin alfa, in line with prior publications that suggest that this type of variability is due to intrinsic factors associated with the disease and its comorbidities.Citation34
The main limitation of the study is the fact that data were retrospectively collected. We excluded from the present analysis around 50% of all prevalent hemodialysis patients.Citation25 Although there is a possible bias due to the inclusion of survivors, the exclusion of 30% of the more stable patients due to transplantation should compensate for this. Thus, we think that our cohort can be regarded as representative of hemodialysis patients receiving ESAs.
Conclusion
In patients on hemodialysis receiving ESAs, conversion from epoetin alfa to darbepoetin alfa was associated with an approximate and persistent 65% reduction of the required dose. To maintain Hb stability, a conversion rate of 300:1 seems to be appropriate for most patients receiving low doses of epoetin alfa (≤200 IU/kg/week), while 350:1 would be better for patients on higher doses.
Acknowledgments
Writing assistance was provided by Dr Neus Valveny from Trial Form Support. The authors wish to acknowledge Amgen SA for the financial support of this study.
Disclosure
The conclusions, interpretations, and opinions expressed herein are those of the authors. This study was financially supported by Amgen. The authors report no other conflicts of interest in this work.
References
- MacdougallICOptimizing the use of erythropoietic agents – pharmacokinetic and pharmacodynamic considerationsNephrol Dial Transplant200217Suppl 5667012091611
- LocatelliFAljamaPBárányPEuropean Best Practice Guidelines Working GroupRevised European best practice guidelines for the management of anaemia in patients with chronic renal failureNephrol Dial Transplant200419Suppl 2ii1ii4715206425
- NissensonARSwanSKLindbergJSRandomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patientsAm J Kidney Dis200240111011812087568
- FishbaneSThe role of erythropoiesis-stimulating agents in the treatment of anemiaAm J Manag Care201016Suppl IssuesS67S7320297874
- McGowanTVaccaroNMBeaverJSMassarellaJWolfsonMPharmacokinetic and pharmacodynamic profiles of extended dosing of epoetin alfa in anemic patients who have chronic kidney disease and are not on dialysisClin J Am Soc Nephrol2008341006101418417741
- PergolaPEGartenbergGFuMWolfsonMRaoSBowersPA randomized controlled study of weekly and biweekly dosing of epoetin alfa in CKD Patients with anemiaClin J Am Soc Nephrol20094111731174019808215
- DordalMSWangFFGoldwasserEThe role of carbohydrate in erythropoietin actionEndocrinology19851166229322993996313
- EgrieJCBrowneJKDevelopment and characterization of novel erythropoiesis stimulating protein (NESP)Nephrol Dial Transplant200116Suppl 331311402085
- JoyMSDarbepoetin alfa: a novel erythropoiesis-stimulating proteinAnn Pharmacother2002367–81183119212086553
- MacdougallICGraySJElstonOPharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patientsJ Am Soc Nephrol199910112392239510541299
- MacdougallICDarbepoetin alfa: a new therapeutic agent for renal anemiaKidney Int Suppl200280556111982814
- AljamaPBommerJCanaudBNESP Usage Guidelines GroupPractical guidelines for the use of NESP in treating renal anaemiaNephrol Dial Transplant200116Suppl 3222811402087
- HudsonJQSameriRMDarbepoetin alfa, a new therapy for the management of anemia of chronic kidney diseasePharmacotherapy2002229 Pt 2141S149S12222584
- LocatelliFOlivaresJWalkerREuropean/Australian NESP 980202 Study GroupNovel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiencyKidney Int200160274174711473657
- IbbotsonTGoaKLDarbepoetin alfaDrugs2001611420972104 discussion 2105–210611735636
- MacdougallICRobertsDEColesGAWilliamsJDClinical pharmacokinetics of epoetin (recombinant human erythropoietin)Clin Pharmacokinet1991202991132029809
- NissensonARDosing darbepoetin alfaAm J Kidney Dis200240487212324929
- BarnettALCrémieuxPYDose conversion from epoetin alfa to darbepoetin alfa for patients with chronic kidney disease receiving hemodialysisPharmacotherapy2003235690693 discussion 693–69412741447
- DerayGOnce-weekly erythropoietic therapy: is there a difference between the available preparations?Nephrol Dial Transplant200318112455245614551393
- JacobsCFreiDPerkinsACResults of the European Survey on Anaemia Management 2003 (ESAM 2003): current status of anaemia management in dialysis patients, factors affecting epoetin dosage and changes in anaemia management over the last 5 yearsNephrol Dial Transplant200520Suppl 3iii3iii2415824128
- LocatelliFCanaudBGiacardyFMartin-MaloABakerNWilsonJTreatment of anaemia in dialysis patients with unit dosing of darbepoetin alfa at a reduced dose frequency relative to recombinant human erythropoietin (rHuEpo)Nephrol Dial Transplant200318236236912543893
- MolinaMGarcía HernándezMANavarroMJDe GraciaMCOrtuñoTEstudio comparativo sobre el tratamiento de la anemia renal en el paciente en hemodialisis: cambio de via de administracion de epoetina alfa frente a conversion a darbepoetina [Change of EPO treatment from subcutaneous epoetin to intravenous epoetin or darbepoetin alpha]Nefrologia2004246564571 Spanish15683029
- BockHAHirt-MinkowskiPBrünisholzMKeuschGReySvon AlbertiniBSwiss EFIXNES trial investigatorsDarbepoetin alpha in lower-than-equimolar doses maintains haemoglobin levels in stable haemodialysis patients converting from epoetin alpha/betaNephrol Dial Transplant200823130130817890745
- MolinaMNavarroMJde GraciaCAlvarezGde AlarconRGarciaMAChange in darbepoetin alfa administration schedule affects erythropoiesis-stimulating agent resistance in patients with chronic kidney disease receiving hemodialysisRen Fail200830877878318791951
- Consejería de Sanidad del Gobierno vascoUnidad de información sobre pacientes renales de la CAPV 2011, UNIPAR, CAPV 2011 [Health Service of the Basque Country Government. Information unit about renal patients from the Basque Country Authonomous Community 2011, UNIPAR, CAPV 2011]Publisher Servicio Central de Publicaciones del Gobierno VascoVitoria-Gasteiz, Spain2012 Available from: http://www.osakidetza.euskadi.net/r85-pkpubl02/es/contenidos/informacion/osk_publicaciones/es_publi/memorias.htmlAccessed June 3, 2014
- MolinaMGarcía HernándezMANavarroMJPérez SilvaFCachoMDe GraciaMCTratamiento de la anemia renal con administracion una vez cada dos semanas de darbepoetina alfa en pacientes con insuficiencia renal cronica predialisis previamente tratados con epoetina alfa [Treatment of renal anemia with darbepoetin alfa administered once every other week in predialysis patients with chronic kidney disease and previously treated with epoetin alfa]Nefrologia20042415459 Spanish15083958
- EBPG Working GroupEuropean best practice guidelines for haemodialysis (Part 1). Section IV.1 Water treatment systemNephrol Dial Transplant200217Suppl 74562
- Pérez GarcíaRGonzález ParraECeballosFSpanish Society of NephrologyGuías de gestión de calidad del líquido de diálisis (LD) [Guidelines for quality management of dialysis solutions]Nefrologia200424Suppl 2142 Spanish15083969
- SchifflHLangSMFischerRUltrapure dialysis fluid slows loss of residual renal function in new dialysis patientsNephrol Dial Transplant200217101814181812270990
- FuruyaRKumagaiHTakahashiMSanoKHishidaAUltrapure dialysate reduces plasma levels of beta2-microglobulin and pentosidine in hemodialysis patientsBlood Purif200523431131615980621
- MolinaMNavarroMJPalaciosMEImportancia del liquido de dialisis ultrapuro en la respuesta al tratamiento de la anemia renal con darbepoetina en el paciente en hemodialisis [Importance of ultrapure dialysis liquid in response to the treatment of renal anaemia with darbepoetin in patients receiving haemodialysis]Nefrologia2007272196201 Spanish17564565
- IcardiASaccoPSalvatoreFRomanoULong-term intravenous epoetin-alpha/darbepoetin-alpha ratio in iron-replete hemodialysis patientsJ Nephrol2007201737917347977
- ElliottSPhamEMacdougallICErythropoietins: a common mechanism of actionExp Hematol200836121573158418922615
- Pérez-RuixoJJCucala-RamosMGarcía-GonzaloEDel Val RomeroBValvenyNBetween subjects variability in haemoglobin and dose are not associated with the erythropoiesis-stimulating agent used to treat anaemia in dialysis: a meta-analysisBr J Clin Pharmacol2013751152522803621