Abstract
Introduction
This report describes our experience using a low-dose synthetic adrenocorticotropic hormone (ACTH) analog for patients affected by nephrotic syndrome who had not responded to or had relapsed after steroid and immunosuppressive treatments.
Patients and methods
Eighteen adult nephrotic patients with an estimated glomerular filtration rate >30 mL/min were recruited. Histological pictures included ten of membranous nephropathy, three of membranous proliferative glomerulonephritis, three of minimal change, and two of focal segmental glomerular sclerosis. All patients received the synthetic ACTH analog tetracosactide 1 mg intramuscularly once a week for 12 months. Estimated glomerular filtration rate, proteinuria, serum lipids, albumin, glucose, and potassium were determined before and during the treatment.
Results
One of the 18 patients discontinued the treatment after 1 month because of severe fluid retention, and two patients were lost at follow-up. Complete remission occurred in six cases, while partial remission occurred in four cases (55.5% responder rate). With respect to baseline, after 12 months proteinuria had decreased from 7.24±0.92 to 2.03±0.65 g/day (P<0.0001), and serum albumin had increased from 2.89±0.14 to 3.66±0.18 g/dL (P<0.0001). Total and low-density lipoprotein cholesterol had decreased from 255±17 to 193±10 mg/dL (P=0.01), and from 168±18 to 114±7 mg/dL (P=0.03), respectively. No cases of severe worsening of renal function, hyperglycemia, or hypokalemia were observed, and no admissions for cardiovascular or infectious events were recorded.
Conclusion
Tetracosactide administration at the dosage of 1 mg intramuscularly per week for 12 months seems to be an acceptable alternative for nephrotic patients unresponsive or relapsing after steroid-immunosuppressive regimens. Further studies should be planned to assess the effect of this low-dose ACTH regimen also in nephrotic patients not eligible for kidney biopsy or immunosuppressive protocols.
Introduction
Steroid-immunosuppressive regimens for patients with nephrotic syndrome depend on the histological patterns and on the severity of the glomerular damage. Unfortunately, in several cases nephrotic syndrome does not reverse or frequently recurs. In addition, immunosuppressant and steroid treatments have limitations with regard to duration and dosage administration, and may expose patients to mild-to-severe side effects.Citation1–Citation6
Among possible pharmacological treatments of nephrotic syndrome, adrenocorticotropic hormone (ACTH) or the synthetic ACTH analog tetracosactide () has raised new interest, because of its noncorticosteroid mechanism of action,Citation7,Citation8 namely direct melanocortin receptor-mediated immunomodulation and podocyte protection.Citation9
ACTH was employed many years ago for the treatment of nephrotic syndrome in children and adults.Citation10–Citation12 It has been reappraised, due to its efficacy in reducing proteinuria and lipids in cases of glomerulonephritis resistant to conventional treatment with steroids and immunosuppressors. This effect has persisted for several patients many months after the discontinuation of treatment.Citation13–Citation17 Since ACTH action is mediated by a protective effect activity on podocytes, it may be favorably used in different types of glomerular disease. Namely, ACTH reduces foot-process effacement and podocyte apoptosis, and ameliorates the decline in glomerular expression of podocyte markers, including vimentin, nephrin, and podocin.Citation8
A natural, highly purified ACTH gel has been recently employed in the US, and has exhibited a good therapeutic effect on proteinuria in idiopathic nephrotic syndrome,Citation18 advanced diabetic nephropathy with nephrotic-range proteinuria,Citation19 and idiopathic membranous nephropathy.Citation20 This natural form is different from the synthetic ACTH 1–24, available outside the US, in terms of pharmacokinetics, pharmacodynamics, and physiologic actions. Melanocortin peptides, pharmacologically active molecules derived from proopiomelanocortin, exist in natural ACTH but not in the synthetic form. Similarly, the insulinotropic effect of the endogenous hormone is due to the C-terminal ACTH 1–39 which is omitted from the synthetic analog.Citation21
Synthetic ACTH 1–24 is the form available in Europe, and its standard dosage is 1 mg given intramuscularly twice a week for 1 year. This schedule was as effective as methylprednisolone plus a cytotoxic agent for 6 months in idiopathic membranous nephropathy.Citation22 In addition to nephrotic syndrome, other potential indications of this drug are neurologic diseases (acute exacerbations of multiple sclerosis and infantile myoclonic encephalopathy with hypsarrhythmia), rheumatologic diseases (acute rheumatic fever, rheumatoid arthritis, lupus erythematosus, periarteritis nodosa, psoriatic arthritis, scleroderma, rheumatoid spondylitis, and Still’s disease), dermatologic diseases (exfoliative dermatitis, dermatomyositis, and pemphigus), gastrointestinal diseases (regional enteritis and ulcerative colitis), and as adjuvant treatment to improve tolerability to chemotherapy.
Although ACTH administration is usually well tolerated, such side effects as hypokalemia, fluid retention, and hyperglycemia have been reported. This study reports our experience of a different ACTH-administration schedule, namely 1 mg once a week for 12 months with the aim of reducing side effects and increasing tolerability.
Patients and methods
Adult patients affected by nephrotic syndrome who were unresponsive or relapsing after immunosuppressive-steroid treatment were considered for the study. Patients with persisting nephrotic syndrome or proteinuria reduction less than 50% after treatment were defined as “non-responders”, whereas patients with a nephrotic syndrome recurrence following an initial partial or complete remission were considered as “relapsers”. Patients with advanced chronic kidney disease (stages IV–V), infections, diabetes, glaucoma, active cancer, severe hypertension, peptic ulcer, or pregnancy were excluded. Eighteen patients were ultimately included. The characteristics of the studied population are reported in .
Table 1 Histological diagnosis and basal and 12-month proteinuria, serum albumin, and total cholesterol
Histological diagnoses were membranous nephropathy (MN) in ten cases, membranous proliferative glomerulonephritis (MPGN) in three cases, minimal-change disease (MCD) in three cases, and focal segmental glomerular sclerosis (FSGS) in two cases.
All patients had previously received different immunosuppressive treatments such as: high-dose prednisolone, cyclophosphamide, cyclosporine, and mycophenolate mofetil. These drugs were stopped at least 3 months before the first ACTH administration (). Treatment with angiotensin-converting enzyme inhibitors (ten cases), angiotensin-receptor blockers (one patient), both angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers (five patients), and statins (15 patients) remained unmodified. All patients received ACTH (synthetic form 1–24) at a dose of 1 mg intramuscularly once a week for 12 months.
The following parameters were studied before and during the 12-month treatment period: arterial blood pressure, body weight, estimated glomerular filtration rate, 24-hour urinary protein excretion, and serum levels of glucose, glycated hemoglobin, total and low-density lipoprotein (LDL) cholesterol, potassium, and albumin. Complete remission was defined as stable or improved renal function and urine protein excretion <0.3 g/day. Partial remission was defined as stable or improved renal function together with urine protein excretion reduced by >50% or lower than 3.5 g/day.Citation23 Patients were followed up for 24–36 months after the end of treatment. All patients gave their informed consent for the study.
Statistics
Descriptive statistics are given as means ± standard error or prevalence as required. Statistical analysis was performed using analysis of variance for repeated measurements. Differences were considered statistically significant at P<0.05.
Results
One of the 18 recruited patients (5.5%) dropped out at month 2 because of furosemide-unresponsive fluid retention, while two patients were lost at follow-up after 2 months. Six cases of complete remission and four cases of partial remission occurred (55.5% of responders, namely 33.3% complete remission and 22.2% partial remission). Complete remissions occurred at months at months 2 (one case), 4 (two cases), and 12 (three cases) of treatment, with a median value of 8 months.
The outcome after the end of the 12-month tetracosactide treatment was available in 12 patients, since three patients were lost at follow-up. Total remission was maintained in two (at 36 months of follow-up) of the six patients with complete remissions; in three of them, nephrotic syndrome relapsed (patients affected by MN, FSGS, and minimal-change disease), and in one nonnephrotic proteinuria occurred (MN) (). One patient (MN) who showed partial remission at the end of tetracosactide treatment presented with nephrotic proteinuria at 36 months (). One patient (MPGN) who did not respond to tetracosactide treatment, and one who showed partial remission, were on complete remission at the 24-month follow-up ().
The results of the 15 patients who completed the 12-month study period are shown in . The reduction in proteinuria was significant from month 6 of treatment to month 12 ( and ). Serum total cholesterol decreased significantly (), together with changes in urine protein excretion at month 6 of treatment and even more at month 12 ( and ). Similarly, serum LDL cholesterol decreased significantly from month 6 to month 12 ( and ). Serum albumin increased significantly at month 12 ().
Figure 2 Urine protein excretion (g/day) at baseline and during the treatment period (means ± standard error).
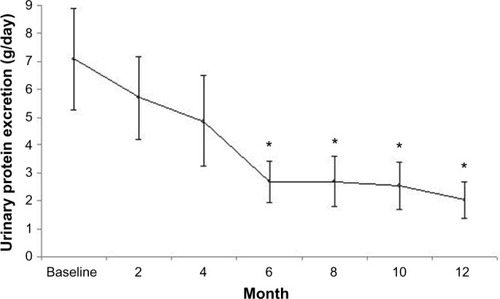
Figure 3 Total cholesterol (upper line) and low-density lipoprotein cholesterol (lower line) serum levels at baseline and during the treatment period (mean ± standard error).
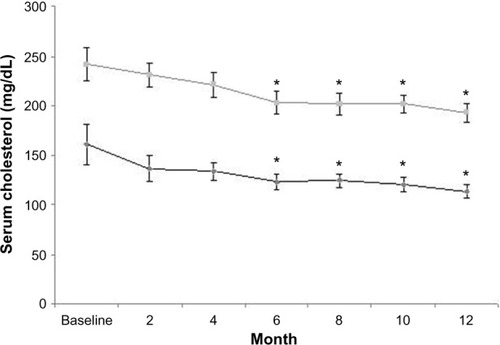
Table 2 Proteinuria, serum albumin, eGFR (MDRD), urea, serum total cholesterol, LDL cholesterol, glycemia, HbA1c, potassium, blood pressure, and body weight pretreatment and at end of treatment period (mean ± standard error) in the 15 patients who completed the study
Renal function, serum glucose, glycated hemoglobin, serum potassium, urea, blood pressure, and body weight remained stable during treatment ( and ). No cases of hypokalemia or hyperglycemia occurred. No severe fluid retention unresponsive to furosemide was reported in the 15 patients who completed the study. No admission due to infectious or cardiovascular events was recorded throughout the study period.
Discussion
Our findings show that Tetracosactide administered at a dose of 1 mg intramuscularly once a week for 1 year was effective in reducing proteinuria in patients resistant or relapsing to conventional treatment, with a 55.5% response rate (33.3% complete remission and 22.2% partial remission).
The only two recent papers that described the effect of synthetic ACTH in adult nephrotic patients are those of Berg and ArnadottirCitation15 and Ponticelli et al.Citation22 When compared to their data, the remission rate we observed is lower. However, it is noteworthy that Ponticelli et al treated only MN patients at their first-line treatment, and hence had a higher chance of favorable results with respect to resistant or relapsing nephrotic patients. Two patients stopped the ACTH therapy because of severe dizziness or fluid retention. An additional two patients developed glucose intolerance, one onychodystrophy, one patient folliculitis, and six developed a bronze color of the skin. These side effects did not cause a cessation of treatment, and they completely disappeared after the end of therapy.
Berg and ArnadottirCitation15 studied a population similar to our series, but the prevalence of unwanted side effects was not reported. From their data, it emerges that one patient had a rapid progression to end-stage renal disease within 9 months of the end of the study, one patient showed a psychiatric syndrome, and one patient was deceased 3 months after the end of ACTH therapy.
The response rate we observed was similar to Bomback et al.Citation24 Most of the remissions occurred within 6 months of therapy, and this is in keeping with Hladunewich et al.Citation20 These studies employed ACTH gel, as well as the recent investigation on FSGS patients.Citation25 In this study, side effects were reported in 21 of the 24 studied patients treated with ACTH gel. These consisted of fluid retention (five patients), mood alteration (four patients), elevated blood pressure (three patients), dyspepsia (two patients), hyperglycemia (one patient), upper respiratory tract symptoms (four patients), muscle cramps (four patients), polyuria (one patient), rash (two patients), nausea, headache, and palpitations (one patient), and worsening of renal function (one patient). However, most adverse events were mild and transient.Citation25
In our series, the treatment schedule was well tolerated: we had only one case of dropout due to fluid retention. In fact, sodium and water retention, potassium wasting, and expansion of effective circulating volume may be related to mineralocorticoid-receptor activation due to the ACTH-induced cortisol production.Citation26
The weekly dose of synthetic ACTH analog we administered was lower than that used by other authors.Citation14–Citation17,Citation22 This lower dosage can explain the negligible occurrence of side effects or complications related to Tetracosactide administration. It is important to keep in mind that the insulin-like effect of ACTH is related to the C-terminal sequence, and thus it should not occur with tetracosactide (synthetic ACTH 1–24).Citation27
In accordance with other studies,Citation14,Citation16 we observed a decrease in total and LDL cholesterol from month 6 to 12. The reduction in serum lipids occurred not only in remitting patients: this may be in agreement with some evidence suggesting that ACTH may affect serum lipids independently of changes in proteinuria.Citation16
Berg et al showed that ACTH has a pronounced lipid-lowering effect,Citation14,Citation16 mediated by modification of apolipoprotein metabolism. This change can restore glomerular expression of apolipoprotein J (clusterin), which was found to be reduced in MN.Citation28 The clusterin competes in the terminal component of complement C5b-9 for the same receptor in podocytes with megalin, identified as the target of the C5b-9 injury.Citation28
The melanocortins (ACTH and α-, β-, and γ-melanocyte-stimulating hormone) derive from the same precursor – proopiomelanocortin. The melanocortins exhibit anti- inflammatory and protective effects in vitro and in vivo in many conditions of local or systemic inflammation.Citation9,Citation29 A recent report identified gene expression of the melanocortin receptor MC1R in podocytes, glomerular endothelial cells, mesangial cells, and tubular epithelial cells. The administration of MS05, a specific MC1R agonist, in rats with passive Heymann nephritis significantly reduced proteinuria and oxidative stress, and improved podocyte morphology.Citation7
The antiproteinuric effect of tetracosactide that we observed in patients unresponsive to previous steroid therapy seems to support the potential nonsteroidogenic mechanism accounting for the ACTH 1–24 therapy.Citation30
Because of the nonsteroid-related action of ACTH, namely its favorable effects on podocyte structure and functions, it may be used in those patients for whom immunosuppressive or steroid treatment may not be free from risks, eg, in cases when renal biopsy cannot be safely performed or where the patient is at high risk of complications due to classical immunosuppressive regimens. Furthermore, there are situations where patients do not give their informed consent to procedures and/or treatments. These conditions may represent a possible indication for first-line therapy with ACTH in nephrotic patients. Future studies should address this point.
In summary, our investigation showed that a low dosage of ACTH was able to significantly reduce proteinuria and cholesterol in a cohort of nephrotic patients resistant or relapsing to immunosuppressive regimens. Although our regimen resulted in a lower rate of remissions with respect to the standard dosage, the treatment was safe and well tolerated. The preliminary results of our pilot investigation need to be confirmed by randomized controlled studies. In addition, because ACTH mechanism of action and tolerability, the antiproteinuric effect at this low dose should be investigated in elderly or frail nephrotic patients who are not eligible for kidney biopsy or immunosuppressive protocols.
Disclosure
The authors report no conflicts of interest in this work.
References
- BlackDARoseGBrewerDBControlled trial of prednisone in adult patients with the nephrotic syndromeBr Med J197034214264916790
- CattranDCDelmoreTRoscoeJA randomized controlled trial of prednisone in patients with idiopathic membranous nephropathyN Engl J Med19893202102152643046
- [No authors listed]A controlled study of short-term prednisone treatment in adults with membranous nephropathy. Collaborative Study Of The Adult Idiopathic Nephrotic SyndromeN Engl J Med197930113011306388220
- ChenYSchieppatiACaiGImmunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trialsClin J Am Soc Nephrol2013878779623449768
- PonticelliCZucchelliPPasseriniPA randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathyN Engl J Med19893208132642605
- PonticelliCZucchelliPPasseriniPA 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathyKidney Int199548160016048544420
- LindskogAEbeforsKJohanssonMEMelanocortin 1 receptor agonists reduce proteinuriaJ Am Soc Nephrol2010211290129820507942
- GongRDworkinLDACTH (Acthar gel) prevents proteinuria and renal injury in the remnant kidney: evidence for direct podocyte protectionJ Am Soc Nephrol201021548A20299354
- GongRThe renaissance of corticotropin therapy in proteinuric nephropathiesNat Rev Nephrol2011812212822143333
- LausonHDFormanCWMcNamaraHMattarGBarnettHLThe effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndromeJ Clin Invest19543365766413152205
- CameronJSNephrotic syndromeBr Med J197043503535472808
- [No authors listed]Treatment of nephrotic syndromeBr Med J1970358594316980
- CoppoRNon-steroidal and non cytotoxic therapies for nephritic syndromeNephrol Dial Transplant2008231793179618441003
- BergALNilsson-EhlePArnadottirMBeneficial effects of ACTH on serum lipoprotein profile and glomerular function in patients with membranous nephropathyKidney Int1999561534154310504505
- BergALArnadottirMACTH-induced improvement in nephritic syndrome in patients with a variety of diagnosesNephrol Dial Transplant2004191305130715102969
- BergALRafnssonATJohannssonMDallongevilleJArnadottirMThe effects of adrenocorticotrophic hormone and equivalent dose of cortisol on the serum concentrations of lipids, lipoproteins, and apolipoproteinsMetabolism2006551083108716839845
- RauenTMichealisAFleogeJMertensPRCase series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24Clin Nephrol20097163764219473632
- BombackASCanettaPABeckLHJrAyalonRRadhakrishnanJAppelGBTreatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trialAm J Nephrol201236586722722778
- TumulinJAGalphinCMRovinBHAdvanced diabetic nephropathy with nephrotic range proteinuria: a pilot study of the long-term efficacy of subcutaneous ACTH gel on proteinuria, progression of CKD, and urinary levels of VEGF and MCP-1J Diabetes Res2013201348986924159603
- HladunewichMACattranDBeckLHA pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (HP Acthar® gel) in nephrotic syndrome due to idiopathic membranous nephropathyNephrol Dial Transplant2014291570157724714414
- Beloff-ChainAMortonJDunmoreSTaylorGWMorrisHREvidence that the insulin secretagogue, beta-cell-tropin, is ACTH 22–39Nature19833012552586296695
- PonticelliCPasseriniPSalvadoriMA randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathyAm J Kidney Dis20064723324016431252
- KDIGO Clinical practice guideline for glomerulonephritisKidney Int Suppl (2011)2012213927425028634
- BombackASTumulinJABaranskiJTreatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gelDrug Des Devel Ther20115147153
- HoganJBombackASMehtaKTreatment of idiopathic FSGS with adrenocorticotropic hormone gelClin J Am Soc Nephrol201382072208124009220
- GongRLeveraging melanocortin pathways to treat glomerular diseasesAdv Chronic Kidney Dis20142113415124602463
- GongRThe renaissance of corticotropin therapy in proteinuric nephropathiesNat Rev Nephrol2012812212822143333
- GhiggeriGMBruschiMCandianoGDepletion of clusterin in renal diseases causing nephrotic syndromeKidney Int2002622184219412427144
- BrzoskaTLugerTAMaaserCAbelsCBöhmMAlfa-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseasesEndocr Rev20082958160218612139
- SiJGeYZhuangSWangLJChenSGongRAdrenocorticotropic hormone ameliorates acute kidney injury by steroidogenic-dependent and -independent mechanismsKidney Int20138363564623325074